Introduction
Oral direct inhibitors of activated factor II (FIIa), such as dabigatran, and of activated factor X (FXa), such as rivaroxaban, have the potential to interfere with the great majority of clot-based coagulation assays and some chromogenic assays, whereas these drugs would not be expected to interfere in antigen-based assays1–4.
Data concerning the influence of anti-FIIa and anti-FXa on functional assays for determination of activated protein C resistance (APCr) are still rather scarce and available studies have mainly been conducted using tests based on the activated partial thromboplastin time (aPTT). Moreover the data were obtained from in vitro studies performed on plasma from normal individuals, which was spiked with variable amounts of the drugs1–4. For dabigatran there is evidence that aPTT-based APCr methods over-estimate the APCr ratio, which may lead to patients with heterozygous factor V Leiden (FVL) being misclassified as normal1–4. In contrast, for rivaroxaban there is evidence that aPTT-based APCr methods may under-estimate the APCr ratio2,3.
As previously reported, in our laboratory we adopted a prothrombinase-based method to study the APCr5. Data concerning the influence of dabigatran and rivaroxaban on this assay are still rather scarce. We, therefore, report the influence of FIIa and FXa direct oral inhibitors on the APCr, evaluated with a prothrombinase assay, in a small number of FVL patients.
Materials and methods
Patients
We considered seven patients, three male and four females, aged between 61 and 84 years old. All these subjects were heterozygous for FVL. The patients, after a basal evaluation, were treated with dabigatran (3 patients) or rivaroxaban (4 patients). The patients’ blood samples were taken in the morning before administration of the drugs. Dabigatran was administered at 220 mg/die in two doses, rivaroxaban was administered at 20 mg/die in one dose.
Laboratory methods
The seven patients considered were evaluated for thrombophilia risk factors using the standard protocol adopted in our institution6: complete blood cell count, liver and kidney function tests, and a serum homocysteine assay. Coagulation parameters were studied using a Sysmex CA7000 analyser, supplied by Dasit (Cornaredo, Italy), to determine the thrombin time (TT), prothrombin time (PT), aPTT, fibrinogen, antithrombin, protein C, and free protein S. Dabigatran was quantified using a TT diluted assay5 whereas rivaroxaban was quantified using a chromogenic assay6; both these tests were supplied by Hyphen BioMed (Neuville sur l’Oise, France). The APCr was determined using a Sysmex CA7000 analyser and a PT-based assay (Pefakit APC-R) supplied by Penthafarm (Basel, Switzerland). For this assay, the following interpretation criteria were adopted: APCr ratio >2.2 normal subjects, from 1.2 to 2.2 heterozygous, <1.2 homozygous3. Genetic investigations for FV G1691A and FII G20210A and GPro were performed using a Cepheid GeneXpert analyser with GeneXpert HemosIL factor II and factor V cartridges7.
Statistical analysis
Means were compared with the Student’s t test for coupled data using specific software (MedCalc 8.0). p values <0.05 are considered statistically significant.
Results
Results obtained for the main coagulation assays before and after treatment with direct inhibitors of FIIa and FXa are reported in Table I.
Table I.
Influence of dabigatran and rivaroxaban on some coagulation assays.
| Patients treated with dabigatran (n=3) | Patients treated with rivaroxaban (n=4) | |||
|---|---|---|---|---|
|
|
||||
| Baseline | With dabigatran | Baseline | With rivaroxaban | |
| PT (sec) | 16.7±3.11 | 20.17±3.24* | 18.1±2.21 | 35.43±3.69** |
| aPTT (sec) | 26.84±2.98 | 36.41±6.45* | 27.99±3.12 | 35.05±5.82* |
| Fibrinogen (mg/dL) | 383±84 | 360±33 | 348±96 | 327±90 |
| TT (sec) | 15.92±1.26 | 533±150*** | 16.73±1.01 | 19.21±1.61 |
| APCr (ratio) | 1.67±0.31 | 2.87±0.29** | 1.81±0.27 | 1.35±0.21* |
| Antithrombin (%) | 98±12 | 92±14 | 85±21 | 68±19* |
| Protein C (%) | 93±27 | 99±22 | 95±24 | 98±21 |
| Protein S (%) | 102±19 | 102±21 | 91±23 | 88±17 |
| Drug (ng/mL) | 167±97 | 299±131 | ||
Results are reported as the mean ±1 SD.
Reference values: PT=10.7–14.6 sec; aPTT=23.2–30.9; TT=12–20.5 sec; fibrinogen=200–450 mg/dL; APCr= >2.2 normal subjects, from 1.2 to 2.2 in heterozygotes, <1.2 in homozygotes; antithrombin=80–120%; protein C=80–120%; free protein S=80–120%.
p<0.05,
p<0.01,
p<0.0001.
PT: prothrombin time; aPTT: activated partial thromboplastin time; TT: thrombin time; APCr: activated protein C resistance.
Three patients were treated with dabigatran. In these subjects we observed slight increases of PT (p<0.05) and aPTT (p<0.05), and a large increase of TT (p<0.001); the quantification of fibrinogen, antithrombin, protein C and free protein S was unaffected by dabigatran assumption.
Four patients were treated with rivaroxaban. In these subjects we observed a marked increase of PT (p<0.01) and a slight increase of aPTT (p<0.05). The quantification of fibrinogen, protein C and free protein S and the TT were unaffected by rivaroxaban assumption. A decrease in antithrombin was noted (p<0.05).
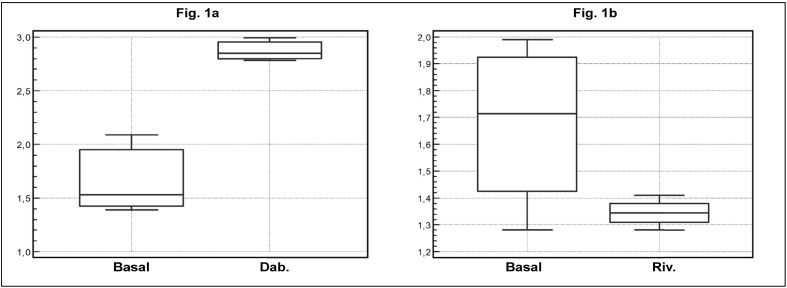
In the first three patients, the APCr ratios observed at baseline, prior to initiation of medication with direct inhibitors of FIIa, were 2.09, 1.39 and 1.53 (1.67±0.31); these results were consistent with a state of heterozygosity for FVL. During treatment with dabigatran the APCr ratios were 2.85, 2.78 and 2.99 (2.87±0.29), which are significantly higher than those observed at baseline (p<0.01). Moreover these values were consistent with a state of wild-type homozygosity for FV. There data are illustrated in Figure 1a.
Figure 1.
Figure 1a - Interference of Dabigatran with a prothrombinase base assay for APCr detection.
Figure 1b - Interference of Rivaroxaban with a prothrombinase base assay for APCr detection.
We considered seven FVL heterozygous subjects. In patients (three) treated with Dabigatran basal APCr ratio was 1.67+0.31, APCr ratio observed during treatment was 2.87+0.29. This difference was statistically significant (p<0.01).
In patients (four) treated with Rivaroxaban basal APCr ratio was 1.81+0.27, APCr ratio observed during treatment was 1.31+0.21. This difference was statistically significant (p<0.05).
APCr: activated protein C resistance.
In the other four patients the APCr ratios observed at baseline, before initiating medication with direct inhibitors of FXa, were 1.57, 1.99, 1.86 and 1.58 (1.81±0.27); these results were consistent with a state of heterozygosity for FVL. During treatment with rivaroxaban the APCr ratios observed were 1.35, 1.28, 1.34 and 1.41 (1.31±0.21) which were significantly lower (p<0.05) than the basal values. These ratios were consistent with a state of heterozygosity for FVL although they were lower than those measured at baseline. These data are also illustrated in Figure 1b.
Discussion
The data available concerning the interference of direct inhibitors of FXa and FIIA with functional assays for evaluating the APCr are still rather scarce. Furthermore, most studies investigating this problem have been in vitro investigations and usually evaluated assays based on the aPTT ratio. For example Adcock et al.1 and Lindahl et al.8 observed, in two in vitro studies performed using aPTT-based functional assays, that dabigatran was able to increase the APCr ratio. However, only subjects homozygous for the wild-type FV were included in those studies. There are no studies evaluating the effect of inhibitors of FIIa on the determination of APCr ratio performed using prothrombinase-based functional assays. Our results, although extremely preliminary, seem to suggest that, in vivo, medication with dabigatran is able to significantly increase the APCr ratio in FVL heterozygous patients, until the ratio lies within the reference values for normal subjects.
In contrast, in two in vitro studies performed using aPTT-based functional assays, Hillarp et al.2 and Douxfils et al.3 observed that rivaroxaban was able to decrease the APCr ratio slightly. Hillarp et al.2 also evaluated the influence of rivaroxaban on the APCr ratio determined using a prothrombinase-based functional assay. The conclusion of this in vitro study, performed by adding the inhibitor of FXa to plasma of normal subjects, was that rivaroxaban did not seem to interfere with the determination of the APCr ratio using a prothrombinase-based functional assay. Our results, although extremely preliminary, would seem to suggest that in vivo, in FVL heterozygous patients, treatment with rivaroxaban does decrease the APCr ratio significantly.
Obviously, this study has some limitations. The first is the limited number of patients who could be evaluated, the second is that it was not possible to study subjects homozygous for FVL. Nevertheless, we believe that our preliminary data may have some points of interest as they represent information obtained in vivo from subjects in our daily clinical practice.
Conclusions
In patients heterozygous for FVL being treated with oral direct inhibitors of activated clotting factors, we evaluated APCr ratios using a prothrombinase based functional test. Our results were obtained in vivo and not in vitro by adding drugs to normal plasma samples. To our knowledge, this is the first report on the in vivo effect of dabigatran and rivaroxaban on the APCr ratio determined using a test based on prothrombinase in subjects heterozygous for FVL.
The results obtained in this study seem to suggest that, in FVL heterozygous subjects, the administration of dabigatran can increase APCr ratios determined using a prothrombinase-based assay until they reach values typical for homozygous wild-type subjects, thus causing a potential misclassification of patients. In contrast, rivaroxaban administration in FVL heterozygous subjects tends to reduce APCr ratios determined using a prothrombinase-based assay, but the observed APCr ratios seem to remain in all cases within the typical range of values observed in FVL heterozygous patients. Obviously, given the preliminary nature of this report of two very small series of patients, larger studies will be needed to evaluate the in vivo effect of direct inhibitors of FXa and FIIA on different functional tests for the presence of APCr.
Footnotes
Authorship contributions
GG conceived the study, coordinated the group and prepared the manuscript. SV and FG performed the laboratory assays and the statistical analysis. RV revised the manuscript and enrolled patients.
Disclosure of conflicts of interest
The Authors are employees of the Italian national health service and have no economic relationship with the manufacturers or distributors of pharmaceutical and diagnostic products mentioned in the manuscript.
References
- 1.Adcock D, Gosselin R, Kitchen S, Dwyre D. The effect of dabigatran on select specialty coagulation assays. Am J Clin Pathol. 2013;139:102–9. doi: 10.1309/AJCPY6G6ZITVKPVH. [DOI] [PubMed] [Google Scholar]
- 2.Hillarp A, Baghaei F, Fagerberg Blixter I, et al. Effects of the oral, direct factor Xa inhibitor rivaroxaban on commonly used coagulation assays. J Thromb Haemost. 2011;9:133–9. doi: 10.1111/j.1538-7836.2010.04098.x. [DOI] [PubMed] [Google Scholar]
- 3.Douxfils J, Mullier F, Loosen C, et al. Assessment of the impact of rivaroxaban on coagulation assays: laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb Res. 2012;130:956–66. doi: 10.1016/j.thromres.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Douxfils J, Mullier F, Robert S, et al. Impact of dabigatran on a large panel of routine specific coagulation assays. Thromb Haemost. 2012;107:985–97. doi: 10.1160/TH11-11-0804. [DOI] [PubMed] [Google Scholar]
- 5.Gessoni G, Valverde S. Clinical evaluation of a functional prothrombin time based assay for identification of factor V Leiden carriers in a group of Italian patients with venous thrombosis. Blood Coagul Fibrinolysis. 2007;18:603–10. doi: 10.1097/MBC.0b013e3282891e2f. [DOI] [PubMed] [Google Scholar]
- 6.Gessoni G, Valverde S, Canistro R, et al. Laboratory assessment of hypercoagulable state. A study in a group of patients with venous thromboembolism born in Chioggia. Minerva Med. 2007;98:89–93. [PubMed] [Google Scholar]
- 7.Gessoni G, Valverde S, Canistro R, Manoni F. Factor V Leiden in Chioggia: a prevalence study in patients with venous thrombosis, their blood relatives and the general population. Blood Transfus. 2010;8:193–5. doi: 10.2450/2010.0157-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindahl TL, Baghaei F, Blixter IF, et al. Effects of the oral, direct thrombin inhibitor dabigatran on five common coagulation assays. Thromb Haemost. 2011;105:371–8. doi: 10.1160/TH10-06-0342. [DOI] [PubMed] [Google Scholar]