Dear Sir,
There is a concern that transfusions of stored red blood cells can cause a significant hypercoagulation effect. One of the reasons for this effect might be the increase in microvesiculation and decrease in utilisation of the formed microvesicles under blood bank conditions.
In order to evaluate the presence and dynamics of antithrombin activity in microvesicles derived from erythrocytes during 27 days of storage, whole blood was collected from 35 healthy donors. Red blood cells were separated into two parts. One part was washed three times with saline, then a preservative solution was added to both parts and they were stored at 4 °C. Microvesicles were separated from the stored red blood cells by ultracentrifugation at 100,000 g for 60 minutes1. As microvesiculation increases during the storage of red cells2 (especially washed ones) the number of microvesicles was standardised in the samples (5,000 microparticles/μL) in order to eliminate the effect of the amount of microvesicles on antithrombin activity. Antithrombin activity of stored red blood cells and the microvesicles derived from them was assessed using a coagulation method and an optical method.
In the coagulation method3 the slowdown of fibrin clot formation from a solution of fibrinogen was determined in the presence of heparin (heparin-induced antithrombin activity) and without heparin, when a mixture containing a suspension of microvesicles or red blood cells and thrombin was incubated for 2 hours before adding a solution of fibrinogen (slow, progressive inhibition of thrombin). In the optical method the target of action of thrombin was not fibrinogen, but a chromogenic substrate selective for thrombin.
All data were subjected to appropriate statistical analyses.
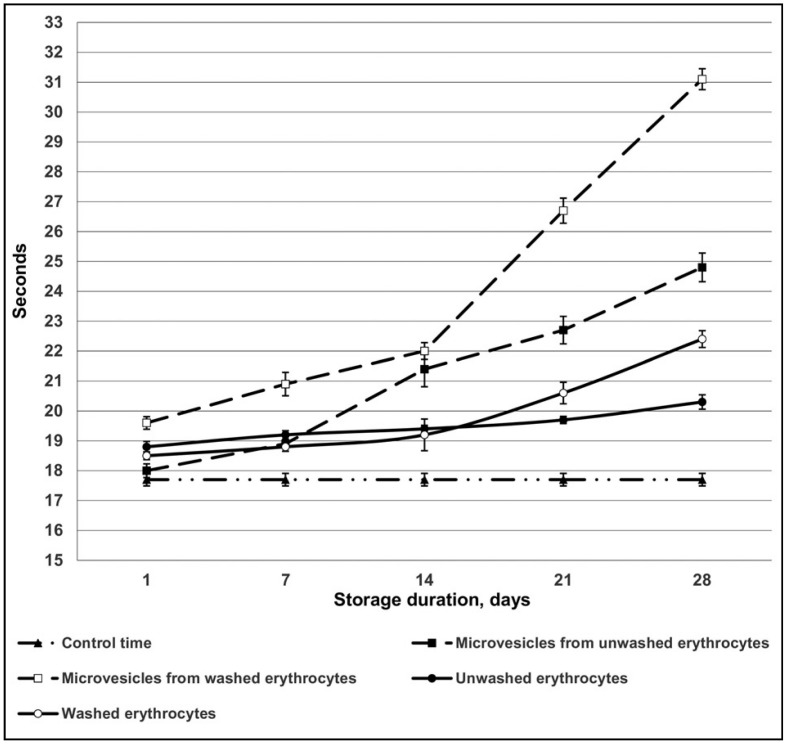
It was found that erythrocyte-derived microvesicles slowed down fibrin clot formation from fibrinogen in the presence of exogenous thrombin and heparin. With prolonged red blood cell storage, microvesicles released from the erythrocytes decreased the speed of fibrin clot formation even further. Slowdown of fibrin clot formation was more prominent when microvesicles from washed red blood cells were used, especially those stored for a prolonged period (Figure 1).
Figure 1.
Antithrombin activity of erythrocytes and microvescicles with heparin according to U. Abildgaard3.
We also studied how red blood cells, from which the microvesicles were isolated, affected the speed of fibrin clot formation. We observed that, under the same conditions (stimulation of antithrombin activity by heparin), red blood cells after storage slowed down fibrin clot formation to a lesser extent than the microvesicles isolated from them.
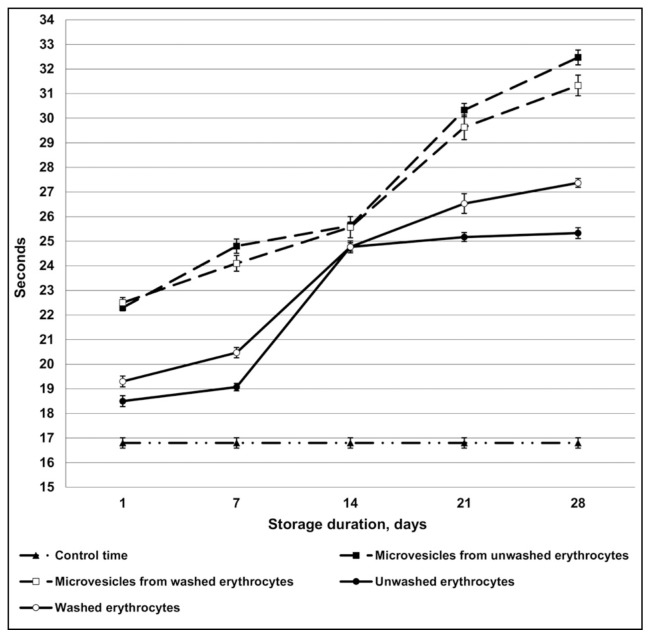
The antithrombin activity of red blood cells and microvesicles isolated from them without using heparin as the catalyst was also studied (slow progressive inhibition of thrombin). There was no relevant difference in the results of the experiments with and without heparin (Figure 2).
Figure 2.
Antithrombin activity of erythrocytes and microvescicles without heparin according to U. Abildgaard3.
Our studies investigating antithrombin activity of red blood cells (washed and unwashed) and microvesicles derived from them using the optical method confirmed the results obtained by the coagulation method.
In our opinion, an important reason for lower antithrombin activity of microvesicles isolated from unwashed red blood cells may be the thrombin generation occurring in these microvesicles. Thrombin formation requires the presence of factors Xa and Va4. During the washing of red blood cells, factors Xa and Va are largely removed and thrombin formation decreases.
It is known that thrombin generation itself leads to activation of protein C. Changes in the concentration of protein C in plasma (for example, in sickle-cell anaemia) can be accompanied by the changes in its concentration on erythrocyte and microvesicle membranes5. However, in our experiments protein C was not activated, mainly due to the absence of thrombomodulin. That is why its role in the slowdown of fibrin clot formation was not covered in our research.
It was shown that during the storage of red blood cells, the antithrombin activity of microvesicles derived from them increases, partly because of the increase in antithrombin activity of the red blood cells themselves. Thus, the antithrombin activity of erythrocytes and erythrocyte-derived microvesicles can be explained by two factors: the presence of heparin-dependent and heparin-independent inhibitors on their membranes as well as by the properties of the membrane itself, the surface of which allows the generation of tenase and prothrombinase complexes, and the change in the ability of thrombin to cleave fibrinogen or the chromogenic substrate.
Overall, our findings give grounds to believe the transfusion of stored red blood cells causes less thrombotic complications than might have been assumed when taking into account only procoagulant activity of red blood cells and microvesicles derived from them. A possible hypercoagulation effect is reduced to a certain extent by the antithrombin activity of erythrocyte-derived microvesicles.
Footnotes
The Authors declare no conflict of interest.
References
- 1.Chung SM, Bae ON, Lim KM, et al. Lysophosphatidic acid induces thrombogenic activity through phosphatidylserine exposure and procoagulant microvesicle generation in human erythrocytes. Arterioscler Thromb Vasc Biol. 2007;27:414–21. doi: 10.1161/01.ATV.0000252898.48084.6a. [DOI] [PubMed] [Google Scholar]
- 2.Bosman GJ, Werre JM, Willekens FL, Novotny VM. Erythrocyte ageing in vivo and in vitro: structural aspects and implications for transfusion. Transfus Med. 2008;18:335–47. doi: 10.1111/j.1365-3148.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 3.Abildgaard U. Biological action and clinical significance of antithrombin III. Haematologia. 1984;17:77–9. [PubMed] [Google Scholar]
- 4.Rubin O, Delobel J, Prudent M, et al. Red blood cell-derived microparticles isolated from blood unitsinitiate and propagate thrombin generation. Transfusion. 2013;53:1744–54. doi: 10.1111/trf.12008. [DOI] [PubMed] [Google Scholar]
- 5.Piccin A, Murphy C, Eakins E, et al. Protein C and free protein S in children with sickle cell anemia. Ann Hematol. 2012;91:1669–71. doi: 10.1007/s00277-012-1447-9. [DOI] [PubMed] [Google Scholar]