Abstract
Photoreceptor outer segments (OS) in the vertebrate retina undergo a process of continual renewal involving shedding of disc membranes that are cleared by phagocytic uptake into the retinal pigment epithelium (RPE). In dystrophic Royal College of Surgeons (RCS) rats, OS phagocytosis is blocked by a mutation in the gene encoding the receptor tyrosine kinase MERTK. To identify proteins tyrosine-phosphorylated downstream of MERTK in the RPE, MALDI-mass spectrometry with peptide-mass fingerprinting was used in comparative studies of RCS congenic and dystrophic rats. At times corresponding to peak phagocytic activity, the RAB GTPase effector GDP dissociation inhibitor alpha (GDI1) was found to undergo tyrosine phosphorylation only in congenic rats. In cryosections of native RPE/choroid, GDI1 colocalized with MERTK and the intracellular tyrosine-kinase SRC. In cultured RPE-J cells, and in transfected heterologous cells, MERTK stimulated SRC-mediated tyrosine phosphorylation of GDI1. In OS-fed RPE-J cells, GDI1 colocalized with MERTK and SRC on apparent phagosomes located near the apical membrane. In addition, both GDI1 and RAB5, a regulator of vesicular transport, colocalized with ingested OS, but exhibited labeling patterns that were coincident in some areas and mutually exclusive in others. Taken together, these findings identify a novel role of MERTK signaling in membrane trafficking in the RPE that is likely to subserve mechanisms of phagosome formation.
Keywords: phagocytosis, RAB GTPase, retinal dystrophy, retinal pigment epithelium, tyrosine phosphorylation
1. Introduction1
A critical function of the retinal pigment epithelium (RPE) is the phagocytic uptake of outer segment (OS) membranes that are shed from the distal tips of the photoreceptor cells (Kevany and Palczewski, 2010). In Royal College of Surgeons (RCS) dystrophic rats, disruption of the phagocytic process results in the formation of a debris field between the RPE and retina that blocks the supply of oxygen and nutrients to the photoreceptor cells, leading to profound retinal degeneration (LaVail, 2001). The disease in the RCS rat is caused by a loss-of-function mutation in the gene encoding MERTK (D'Cruz et al., 2000). MERTK was established as a human disease gene by the identification of disease-associated mutations in individuals with autosomal recessive retinitis pigmentosa (Gal et al., 2000).
MERTK, TYRO3, and AXL together comprise the TAM family of receptor tyrosine kinases that regulate multiple cellular functions, including heterophagic elimination of apoptotic cells, and play critical roles in tissue homeostasis, inflammation, autoimmune responses, and innate immunity (Hafizi and Dahlback, 2006). Activation of TAM family receptor tyrosine kinases involves interaction with growth-arrest-specific protein 6 (Gas6) or the anti-coagulant protein S (ProS) which act as bridging-ligands that recognize phosphatidyl serine on effete membranes (Stitt et al., 1995; Nagata et al., 1996). In double-knockout mice, the combined loss of Gas6 and ProS has been shown to mimic the retinal phenotype of the RCS rat (Burstyn-Cohen et al., 2012) and the Mertk-deficient mouse (Duncan et al., 2003). Other potential MERTK-activating ligands include tubby, tubby-like protein 1, and galectin-3 (Caberoy et al., 2010; Caberoy et al., 2012). In RPE phagocytosis, MERTK activation has been shown to drive the redistribution of myosin II (Strick et al., 2008) potentially involved in formation and closure of the phagocytic cup (Olazabal et al., 2002; Araki, 2006). MERTK has also been shown to associate with the actinremodeling protein annexin II (Law et al., 2009), suggesting that MERTK signaling contributes to the regulation of motor protein activity. In addition, MERTK has been shown to act in concert with αvβ5 integrin to enable normal binding and uptake of OS (Finnemann and Nandrot, 2006; Nandrot et al., 2012).
Ligand activation of MERTK stimulates its intrinsic tyrosine kinase activity, resulting in autophosphorylation of the receptor intracellular domain and the recruitment and activation of SH2-domain-containing signal-transduction proteins (Georgescu et al., 1999). Our previous studies identified multiple MERTK-interacting SH2-domain proteins in the RPE, including the signal-transducing proteins GRB2, VAV3, phosphoinositide-3-kinase, as well as the intracellular protein kinase SRC (Shelby et al., 2013). SRC is the founding member of the family of SRC family kinases (SFKs), powerful intracellular tyrosine kinases that regulate multiple pathways through changes in the phosphorylation status of target proteins (Martin, 2001). The activity of SRC itself is regulated by phosphorylation, with formation of pTyr416-SRC resulting in activation, and formation of pTyr527-SRC resulting in inactivation (Smart et al., 1981; Cooper et al., 1986; Liu and Pawson, 1994). The formation of active pTyr416-SRC in the RPE has been shown to correlate with peak phagocytic activity occurring in vivo and in cultured cells (Shelby et al., 2013).
As an approach to understanding the role of SRC activation in the RPE downstream of MERTK signaling, we compared the profiles of tyrosine phosphorylated proteins present in congenic and dystrophic RCS rats before and after light onset. At times corresponding to peak phagocytic uptake, we observed a unique pattern of MERTK-dependent tyrosine phosphorylation of GDP dissociation inhibitor alpha (GDI1). GDI1 is an effector of RAB GTPases involved in regulating membrane trafficking events such as tethering, docking and fusion through direct effects on RAB partitioning between membrane compartments (Gilbert and Burd, 2001; Alory and Balch, 2003; Hutagalung and Novick, 2011). GDIs are closely related to RAB escort protein 1 (REP1/CHM) that is mutated in choroideremia (Merry et al., 1992; Seabra et al., 1992). Our findings point to a novel role of MERTK in regulating membrane trafficking in the RPE, and suggest a direct link to mechanisms of membrane remodeling necessary for RPE phagocytosis.
2. Materials and Methods
2.1. Animals
C57BL/6J and Balb/C mice were obtained by breeding animals acquired from the Jackson Laboratories. Pigmented dystrophic (RCS-p+) and congenic control (RCS-rdy+p+) rats were obtained by breeding animals from previously described strains (LaVail et al., 1975; LaVail, 1981). Mice and rats were housed in a 12-h/12-h light–dark cycle (~300 lux room light) and were euthanized by CO2 inhalation. All animal procedures were carried out in accordance with protocols approved by the institutional animal care and use committee of the University of Michigan (UCUCA) and with the recommendation of the Panel on Euthanasia of the American Veterinary Medical Association.
2.2. Materials
Complete protease inhibitors, PhosSTOP phosphatase inhibitors, protein-G agarose, FuGENE, AmpliTaq Gold polymerase, and sequencing grade trypsin were from Roche Applied Science (Indianapolis, IN). Ni2+-NTA resin, RNeasy kits, Superscript II, and oligo-dT were from Qiagen (Valencia, CA). Pfu Ultra polymerase was from Agilent Technologies (Santa Clara, CA). SRC family kinase inhibitors 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP1) and 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) were from BIOMOL Research Laboratories, Inc. (Plymouth Meeting, PA). 4-amino-7-phenylpyrazol[3,4-d]pyrimidine (PP3) was from Calbiochem (La Jolla, CA). Sybr safe was from Life Technologies (Grand Island, NY). RPE-J and HEK-293T cells were from ATCC (Manassas, VA). Antibodies recognizing the following were from the sources indicated: SRC, pTyr416-SRC, and HCK (Cell Signaling Technology, Boston, MA); FYN (Sigma St. Louis, MO); YES (Thermo Scientific, Hanover Park, IL); GDI (mouse) (Synaptic Systems, Goettingen, Germany); GDI (rabbit) (Santa Cruz Biotechnology, Dallas, TX); RAB5 (Abcam, Cambridge, MA); MERTK (Feng et al., 2002); rhodopsin 4D2 (mouse) (Hicks and Molday, 1986) (EMD Millipore, Darmstadt, Germany); rhodopsin (rabbit) (Khorana et al., 1988); phosphotyrosine (Margolis et al., 1989); and Xpress, GAPDH, and secondary antibodies AlexaFluor 555-conjugated ant-mouse IgG (Life Technologies, Grand Island, NY), AlexaFluor 488-conjugated anti-rabbit or anti-mouse IgG, and AlexaFluor 647-conjugated anti-rabbit or anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA).
2.3. Western analysis
RPE/choroid was dissected from groups of 3 dystrophic and 3 congenic RCS rats at 1 month of age, euthanized 1.0 h before or 1.5 h after light onset. At this age, retinal degeneration was not yet evident in the dystrophic animals. The tissue samples from the animals in each group were combined and homogenized in 20 mM MOPS, 2 mM EGTA, 5 mM EDTA, 1% Triton X-100, 1 mM DTT, plus protease and phosphatase inhibitors. Cellular debris was removed by low speed centrifugation and protein concentrations of supernates were determined by modified Lowry assay (Peterson, 1977). For two-dimensional analysis, protein samples (300 µg) were separated in the first dimension by isoelectric focusing and in the second dimension by SDS-PAGE, and the proteins transferred onto polyvinylidene difluoride. Blots were blocked, incubated with a monoclonal anti-phosphotyrosine antibody, then with horse radish peroxidase-conjugated secondary antibody, developed with 3,3′-diamino benzidine, and exposed to film. For reprobing, these blots were stripped, blocked, incubated with anti-GDI primary antibody, washed, incubated with alkaline phosphatase-conjugated secondary antibody, and developed using 5-bromo-4-chloro-3'-indolyphosphate p-toluidine and nitro-blue tetrazolium chloride.
2.4. MALDI-mass spectrometry and peptide mass fingerprinting
For in-gel digestion, protein spots were transferred to clean tubes and hydrated in water. The gel pieces were washed twice with 100 µl 0.05 M Tris, pH 8.5/ 30% acetonitrile for 20 min with shaking, then with 100% acetonitrile for 1–2 min. After drying for 30 min under vacuum, the samples were digested by adding 0.08 µg sequencing-grade trypsin in 13–15 µl of 25 mM Tris, pH 8.5, and incubating overnight at 32°C. Peptides were extracted two times with 50 µl of 50% acetonitrile/ 2% TCA, and the combined extracts were dried. Matrix solution was prepared by making a 10 mg/ml solution of 4-hydroxy-α-cyanocinnamic acid in 50% acetonitrile/ 0.1% TCA and adding two internal standards, angiotensin and ACTH 7–38 peptide. The dried digest was dissolved in 3 µl matrix/standard solution and 0.5 µl was spotted onto the sample plate, completely dried, and washed twice with water. MALDI-mass spectrometric analysis was performed on the digest using an Applied Biosystems Voyager DE Pro mass spectrometer in the linear mode. For peptide mass search, peptide masses were entered into search programs to search the SwissProt database for a protein match. Programs used were Mascot (www.matrixscience.com) and MS-Fit (http://prospector.ucsf.edu).
2.5. Immunohistochemical analysis
Four-week-old Balb/C albino mice were euthanized by CO2 inhalation, and the eyes were enucleated and post-fixed in 4% paraformaldehyde for 15 min at room temperature, washed with phosphate buffered saline (PBS), transitioned to sucrose/OCT, and flash frozen. Cryosections of retina/RPE/choroid were generated (10 µm), permeabilized with PBS-Triton X-100 (0.125%), blocked with 1% bovine serum albumin (BSA), 10% normal goat serum, and 0.125% Triton X-100, then incubated with primary antibody for overnight at 4°C, washed, and incubated with secondary antibody for 1 h, washed and cover mounted using Prolong Gold containing DAPI (4',6-diamidino-2-phenylindole). Antibody dilutions were as follows: mouse anti-GDI (1:200); rabbit anti-MERTK (1:300); rabbit anti-SRC (1:200); rabbit anti-FYN (1:300); rabbit anti-YES (1:200); rabbit anti-HCK (1:200); AlexaFluor 488 anti-rabbit IgG (1:1500); and AlexaFluor 555 anti-mouse IgG (1:1500). Images were obtained using indirect fluorescence microscopy (Leica DM6000).
2.6 Immunocytochemical analysis of outer segment fed RPE-J cells
Rat RPE-J cells (Nabi et al., 1993) were maintained in DMEM supplemented with 4% fetal bovine serum (FBS) and 1 mM non-essential amino acids at 33°C in 5% CO2. Cells were cultured for 4–5 days on four-well chamber slides and then incubated with bovine rod outer segments (Papermaster, 1982) at a ratio of 10 OS per RPE cell for 2 h at 33°C. OS were either unlabeled or labeled with DyLight 405 nm N-hydroxysuccinimide ester dye at a ratio of 1.5×109 OS/mg of dye according to manufacturer's instructions (Life Technologies). Unbound OS were removed by washing the cells 3 times with calcium and magnesium containing Hanks Buffered Saline Solution, and the cells were fixed in paraformaldehyde (4%) for 15 min at room temperature. Cells were permeabilized by incubating for 5 min with PBS containing 0.2% saponin, and all subsequent processing solutions contained 0.1% saponin in PBS. Blocking solution included 3% bovine serum albumin (BSA), 10% normal goat serum, and 0.3 M glycine. Antibody dilution and wash solutions contained 3% BSA. Cells were incubated with primary antibodies overnight at 4°C, washed, and incubated with secondary antibodies for 40 min, and washed. Antibody dilutions were as follows: rabbit anti-SRC (1:100); rabbit anti-RAB5 (1:200); rabbit anti-MERTK (1:300); mouse anti-GDI (1:200); mouse 4D2 anti-rhodopsin (1:1000); rabbit anti-rhodopsin (1:500); rabbit anti-MERTK (1:200). Secondary antibodies for the green channel were AlexaFluor 488 anti-rabbit or anti-mouse IgG (1:600, diluted in 50% glycerol), for the red channel were AlexaFluor 555 anti-mouse IgG (1:1500), and for the far red channel were AlexaFluor 647 anti-mouse IgG (1:600, diluted in 50% glycerol). Secondary antibodies were cross-subtracted against the immunoglobulins of any other species present during the immunocytochemistry protocol. Slides were cover mounted using Prolong Gold with or without DAPI, and images were obtained using confocal fluorescence microscopy (Leica SP5) with a 1.4NA, 63x oil objective and a 0.75µm Z-sampling rate. For all multilabeled images, slides were prepared in which each primary antibody or dye-labeled OS was systematically omitted from the immunostaining. To confirm no significant spectral bleed-through in the colocalization analysis, these control conditions were evaluated alongside experimental conditions on the confocal microscope using a single universal laser power and photomultiplier tube sensitivity for each color channel. Further, all lasers were allowed to warm up in a temperature-stable room for at least an hour before imaging to ensure minimal image-to-image variability in fluorescence sensitivity. Finally, the Leica SP5’s spectral detector prism was used on representative colocalizing images to confirm that the detected emission spectrum matched the expected emission spectrum of the putative colocalizing dye-labeled secondary antibodies or outer segments.
2.6. Generation of constructs
Constructs encoding full-length human MERTK or kinase-dead MERTK-R844C in pcDNA 3.1+ were generated previously (McHenry et al., 2004). Expression constructs encoding full-length human RAB5 or GDI1 with an Xpress leader sequence tag in pcDNA3.1/His were generated using reverse transcriptase-coupled polymerase chain reaction (RT-PCR) with gene-specific primers to amplify first-strand cDNAs generated using Superscript II, oligo-dT, and total RNA isolated from human RPE/choroid using the RNAeasy kit. RAB5B: Forward primer 5’-TGGATCCAATCTGGCCACGACTAGCAGAAG-3’; Reverse primer 5’-TGAATTCAGCCACCCCCTCAGTTGCTACAA-3’. GDI1: Forward primer 5’-GGATCCCGAGGCCTGACCACGGACGAGGAAT-3’; Reverse primer 5’-CTTAAGGCGGCCACAA TCACTGCTCAGCT-3’. To generate GDI1 mutants of known tyrosine phosphorylation sites, site-directed mutagenesis using the QuikChange Kit (Agilent Technologies, Santa Clara, CA) was performed using the following forward primers, and complementary reverse primers: Y249F: 5’-TGCCATCTATGGGGGGACATTTATGCTGAACAAACCTGTG-3’;Y333F: 5’-CAGGAAGTCAGACATCTTCGTGTGCATGATCTCCT-3’; Y339F: 5’-ACGTGTGCATGAT CTCCTTTGCACACAACGTGG-3’. The changed nucleotides are in bold and underlined, and the mutations were validated and verified by DNA sequencing the expression constructs.
2.7. Cell culture, transfection, and immunoprecipitation
HEK-293T cells were maintained in DMEM supplemented with 10% FBS, 1 mM sodium pyruvate, and 1 mM penicillin/streptomycin at 37°C in 5% CO2. The cultures were transiently transfected using FuGENE according to the manufacturer’s recommendations with the expression vector constructs alone or in pairs. Thirty hours after transfection, cells were serum starved for 18 h to reduce activation of MERTK observed in transiently transfected HEK-293T cells in the presence of serum (McHenry et al., 2004). In selected experiments, cells were treated for 60 min with the SFK inhibitors PP1 and PP2, or their inactive analog PP3 (Hanke et al., 1996; Traxler et al., 1997). MERTK was reactivated by adding back serum to the cells for 10 min before cell lysis. At 48 h post-transfection, cell lysates were prepared as described above for tissue samples. Aliquots of 200 µg protein were incubated overnight at 4°C with 5 µg primary antibody, Xpress antibody (to detect Xpress-tagged RAB5 or GDI1), or control IgG, then with 15 µl protein G-agarose beads for 2 h at 22°C. The beads were washed three times in 150 mM NaCl and 25 mM Tris at pH 7.2; eluted with 0.1 M glycine-HCl pH 3.0; pH adjusted by addition of 1 M Trizma base, pH 10.0, and made 1×SDS-sample buffer. Western analysis was performed on immunoprecipitates and cell lysates, visualized using alkaline phosphatase-conjugated secondary antibodies and detection reagents.
2.8. Expression analysis using RT-PCR
Total RNA was isolated from congenic and dystrophic RCS rat RPE/choroid, retina, and from rat RPE-J cells as described in the previous section. Sequences encoding RAB family proteins were amplified using AmpliTaq Gold polymerase with pairs of gene-specific primers flanking at least one intron in the genomic sequence. RAB 5A: Forward primer 5’-CTTCAAAGGCAAGCAAGTCC-3’; Reverse primer 5’-CTTCCTCTGGCTGAGTTTGC-3’.RAB 5B: Forward primer 5’-ACAAAGCTGACCTTGCCAAC-3’; Reverse primer 5’-TTCTGGGGTTCACTCTTTGG-3’. RAB 5C: Forward primer 5’-CAAGCCTACGCAGATGACAA-3’; Reverse primer 5’-AGGTAAGGGGCTCAGTTGCT-3’. Cycling conditions were: 1 cycle at 95°C for 10 min, followed by 28 cycles at 95°C for 2 min, 60°C for 45 sec, 72°C for 2 min. Amplifications of hypoxanthine-guanine phosphoribosyltransferase (Hprt) Forward primer 5’-GCAGACTTTGCTTTCCTTGG-3’; Reverse primer 5’-CCGCTGTCTTTTAGGCTTTG-3’ served as a control. PCR products were analyzed by electrophoresis on agarose gels with Sybr safe.
3. Results
3.1. Differential phosphorylation of GDI1 in RPE/choroid
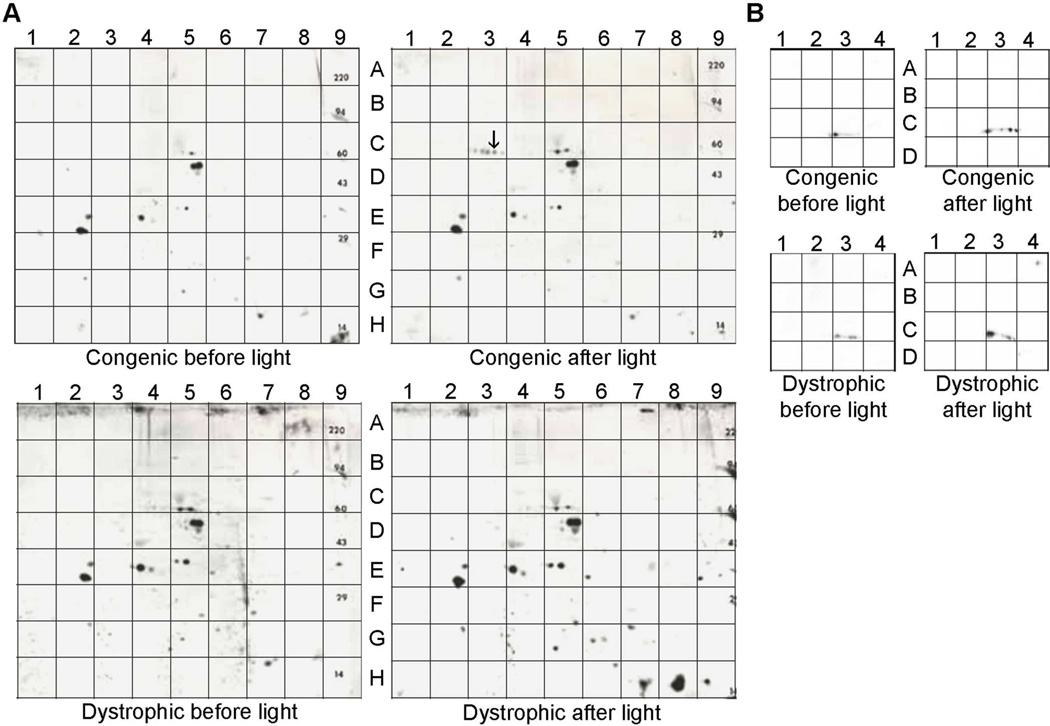
The fundamental role of MERTK signaling in OS phagocytosis suggests that protein-tyrosine phosphorylation is likely to regulate key regulatory mechanisms in the RPE. To evaluate tyrosine phosphorylation associated with phagocytic uptake, two-dimensional (2-D) western analysis was performed on RPE/choroid homogenates from cohorts of pigmented dystrophic (RCS-p+) and congenic control (RCS-rdy+p+) rats. Animals were euthanized 1.0 h before, or 1.5 h following, the time of light onset in the vivarium - times that precede, or coincide with, peak levels of daily phagocytic activity, respectively (LaVail, 1980). Comparison of anti-phosphotyrosine immunoreactivity on 2-D blots of RPE/choroid proteins showed some differences in signal intensity, but overall there was significant overlap in the labeling patterns obtained for dystrophic and congenic rats (Fig. 1A). One striking exception was seen on blots from congenic rats euthanized after light onset, which consisted of a series of closely spaced dots of immunoreactivity suggestive of a protein phosphorylation series (Fig. 1A, upper right panel).
Fig. 1. Tyrosine-phosphorylated proteins present in RPE/choroid of 4 wk old RCS congenic and dystrophic rats.
(A) Protein homogenates were subjected to 2-D polyacrylamide-gel electrophoresis followed by western analysis to visualize anti-phosphotyrosine reactivity on blots from animals euthanized 1.0 h before light onset or 1.5 h after light onset. The spot denoted with an arrow in quadrant C3 of “congenic after light” corresponds to the location of the protein that was evaluated by MALDI-MS analysis (Table 1). (B) Regions A–D/1–4 of the blots in A) probed for anti-GDI1 immunoreactivity.
To determine the identity of the protein(s) corresponding to these dots of immunoreactivity, the area of gel corresponding to the darkest spot in this cluster (indicated by an arrow) was excised and subjected to MALDI-mass spectrometry and peptide-mass fingerprinting. This analysis resulted in the identification of 15 tryptic peptides corresponding to RAB GDP dissociation inhibitor 1 (GDI1), also known as RAB GDI-alpha (Table A.1). The combined masses of the 15 identified peptides correspond to 57% coverage of the sequence of GDI1, including areas that distinguish it from the sequence of closely related and similarly sized GDI2. GDI1 and GDI2 are RAB effectors that participate in regulating membrane trafficking by stabilizing and extracting the GDP-bound form of RAB proteins from various membrane targets (Pfeffer et al., 1995). Previous studies have shown that GDI1 is expressed mainly in neural and sensory tissues, whereas GDI2 is ubiquitously expressed (Nishimura et al., 1994; Bachner et al., 1995).
3.2. GDI1 expression and localization in the RPE/retina
When the 2-D blots of RPE/choroid proteins shown in Figure 1 were stripped and probed with antibodies recognizing RAB-GDIs, the labeling in samples from light-exposed congenic rats appeared to comigrate with the spots of phosphotyrosine immunoreactivity (Fig. 1B). Immunoreactivity was also present in the corresponding areas of the blots from dark-adapted congenic rats, as well as from dystrophic rats. Although the antibodies do not distinguish between GDI1 and GDI2, the identification of GDI1 in the RPE using MALDI-mass spectrometry suggests that the immunoreactivity observed is likely to correspond in whole or in part to the presence of GDI1, and is referred to as such throughout the remainder of the manuscript. In both congenic and dystrophic animals, a discontinuous pattern of GDI1 immunolabeling was seen that likely reflects heterogeneity at the posttranslational level, as both GDI1 and GDI2 have been reported to undergo phosphorylation on multiple serine and tyrosine residues (Shisheva et al., 1999; Cavalli et al., 2001; Rush et al., 2005; Ballif et al., 2008).
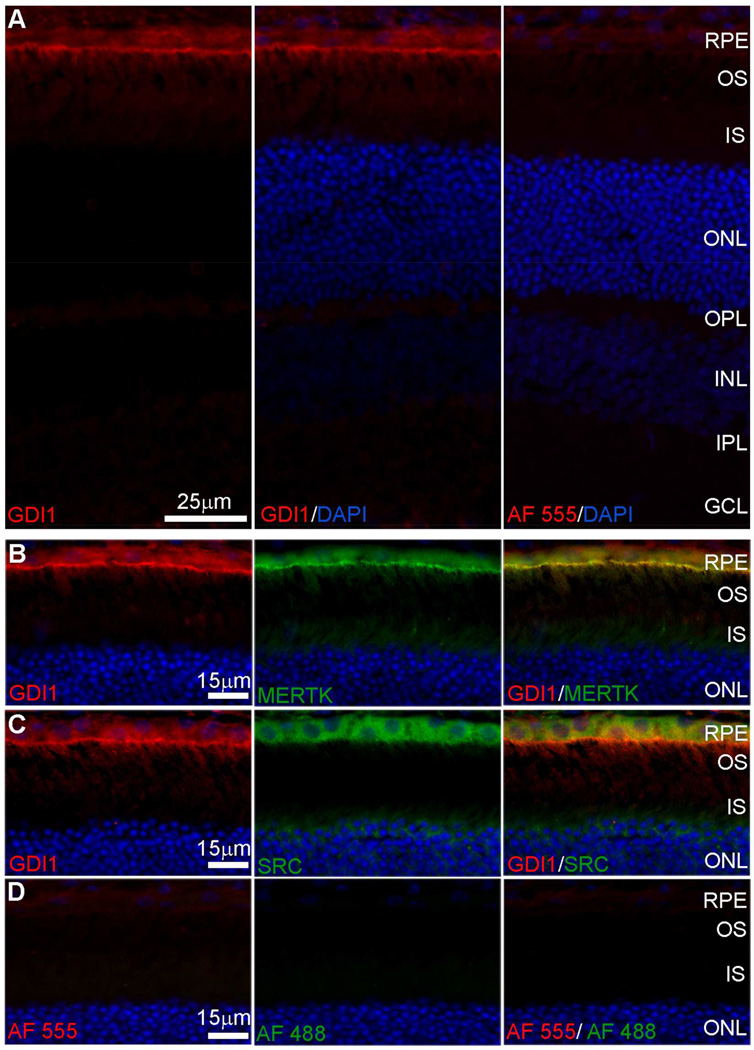
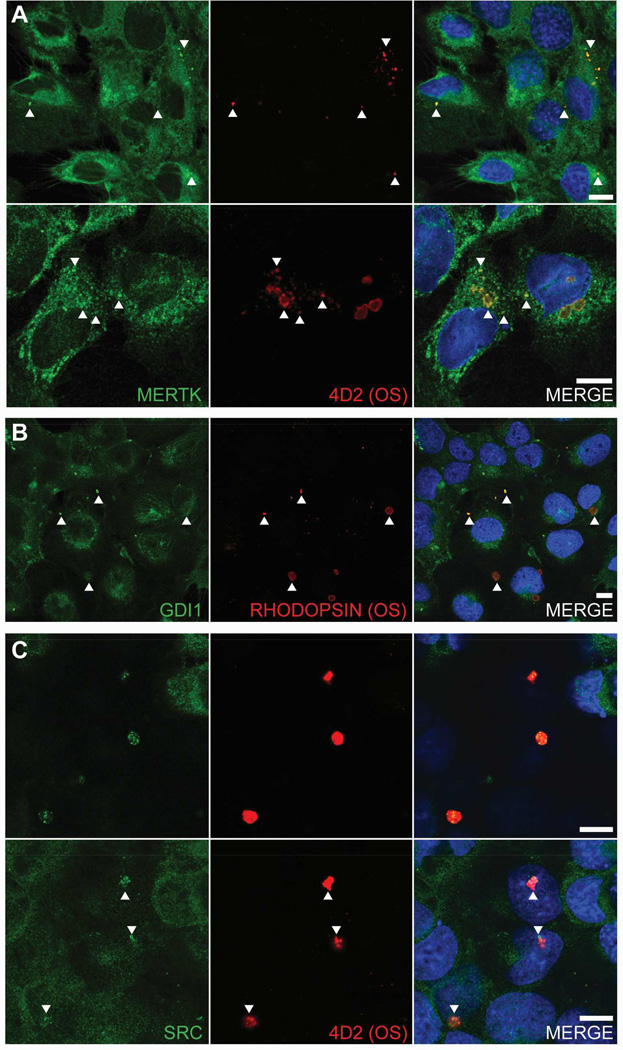
Analysis of GDI1 localization using indirect fluorescence microscopy to image retina/RPE/choroid cryosections showed little immunolabeling of the neural retina, whereas pronounced immunolabeling was seen along the apical aspect of the RPE cell layer (Fig. 2A). Double-labeling with antibodies specific for MERTK that localizes to the RPE apical membrane (Prasad et al., 2006) showed a high degree of overlap with GDI1 immunoreactivity (Fig. 2B). Double-labeling with antibodies that recognize the intracellular tyrosine kinase SRC showed strong expression throughout the RPE cytoplasm that overlapped with GDI1 expression in the apical region (Fig. 2C). Comparison of negative controls and cryosections labeled with specific primary antibodies demonstrated the specificity of GDI1 colocalization with MERTK and SRC (Fig. 2D). These findings confirm the presence of all three proteins in the apical compartment of the RPE in a position to participate in the phagocytic mechanism.
Fig. 2. Indirect fluorescence microscopy of GDI1 localization in cryosections of mouse retina/RPE/choroid.
(A) Anti-GDI1 immunoreactivity visualized using AlexaFluor 555 anti-mouse IgG (red) with DAPI staining of nuclei (blue). (B, C) High magnification images of the outer retina/RPE showing anti-GDI1 immunoreactivity (red) and (B) anti-Mertk immunoreactivity or (C) anti-SRC immunoreactivity visualized using AlexaFluor 488 anti-rabbit IgG (green). (D) Control sections from which primary antibodies were omitted. RPE, retinal pigment epithelium; OS, outer segments; IS, inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
3.3. Endogenous GDI1 tyrosine phosphorylation
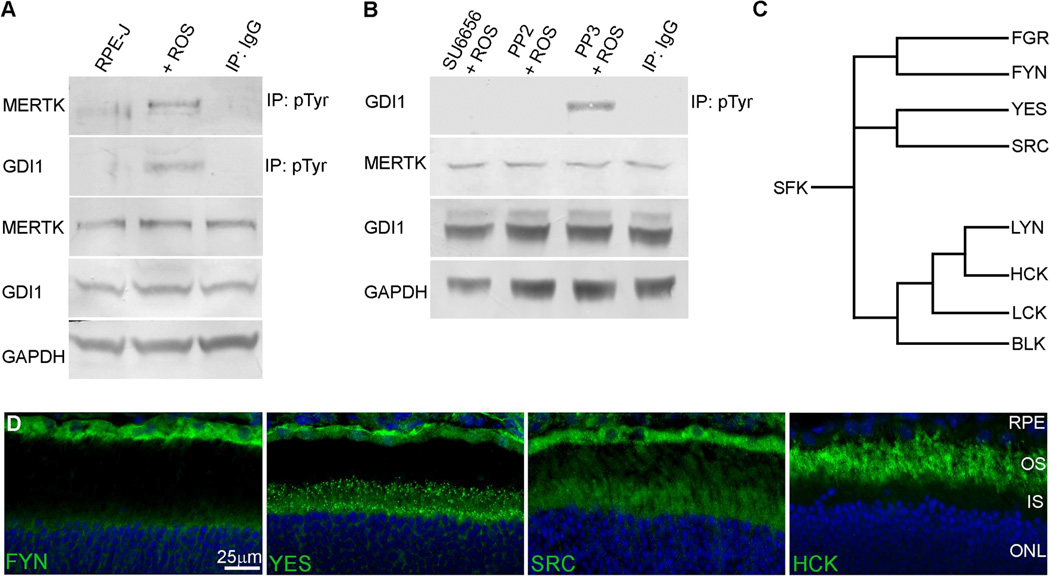
Previous studies have shown that the membrane association of GDI1 and its affinity for RAB proteins are regulated by the phosphorylation of GDI1 serine and tyrosine residues (Shisheva et al., 1999; Cavalli et al., 2001; Rush et al., 2005; Ballif et al., 2008). To evaluate the role of MERTK activity in stimulating GDI1 tyrosine phosphorylation in the RPE, cultures of rat RPE-J cells which retain expression of endogenous MERTK (Nabi et al., 1993) were fed isolated OS to activate phagocytic uptake, and immunoprecipitations were performed using an anti-phosphotyrosine antibody. Western analysis of the recovered proteins using protein-specific antibodies showed that tyrosine phosphorylation of both endogenous MERTK and GDI1 occurred in response to ROS feeding (Fig. 3A).
Fig. 3. Tyrosine phosphorylation of GDI1 in RPE-J cells after outer segment engulfment is mediated by SFK.
(A) Anti-phosphotyrosine immunoprecipitates obtained from OS-fed RPE-J cells and evaluated by western analysis show tyrosine phosphorylation of endogenous MERTK and GDI1. (B) SFK inhibitors SU6656 and PP2, but not the inactive analog PP3, block tyrosine phosphorylation of GDI1 in OS-fed RPE-J cells. (C) Phylogenetic tree showing the nearest neighbor relationships of the SFKs. (D) Indirect fluorescence microscopy of cryosections of mouse retina/RPE/choroid showing anti-FYN, anti-YES, anti-SRC, and anti-HCK immunoreactivity in the outer retina/RPE visualized using AlexaFluor 488 anti-rabbit IgG (green), with DAPI staining of nuclei (blue). Abbreviations as in Fig. 2.
To evaluate the role of SRC activity in GDI1 phosphorylation, cultured rat RPE-J cells were fed isolated OS in the presence or absence of SRC inhibitors under conditions that inhibit formation of its active phosphorylated form, pTyr416-SRC (Jin et al., 2008). Western analysis of anti-phosphotyrosine immunoprecipitates showed that SRC inhibitors SU6656 and PP2, but not the inactive analog PP3, inhibited GDI1 tyrosine phosphorylation (Fig. 3B). Within the family of SFKs in eukaryotes, SRC is most closely related to YES, FYN, and FGR, whereas LYN, HCK, LCK, and BLK form a second branch of the family (Fig. 3C). SFKs inhibited by SU6656 include SRC, FYN, YES, and LYN (Blake et al., 2000), whereas the closely-related inhibitors PP1 and PP2 block the activity of SRC, FYN, HCK, and LCK (Hanke et al., 1996). Previous studies of the SFK-encoding transcripts present in mouse RPE/choroid showed relatively high abundance of SRC, YES, FYN, and HCK (Shelby et al., 2013). Analysis of SFK expression at the protein level in retina/RPE/choroid cryosections showed strong immunolabeling of SRC, YES, and FYN in mouse RPE, whereas HCK labeling was seen mainly in the neural retinal (Fig. 3D). Although the specificity of the inhibitors used does not exclude the possibility of FYN involvement, the results of the expression and inhibitor studies are consistent with an important role of SRC in the MERTK-mediated tyrosine phosphorylation of GDI1 in RPE phagocytosis.
3.4. GDI1 tyrosine phosphorylation in transfected cells
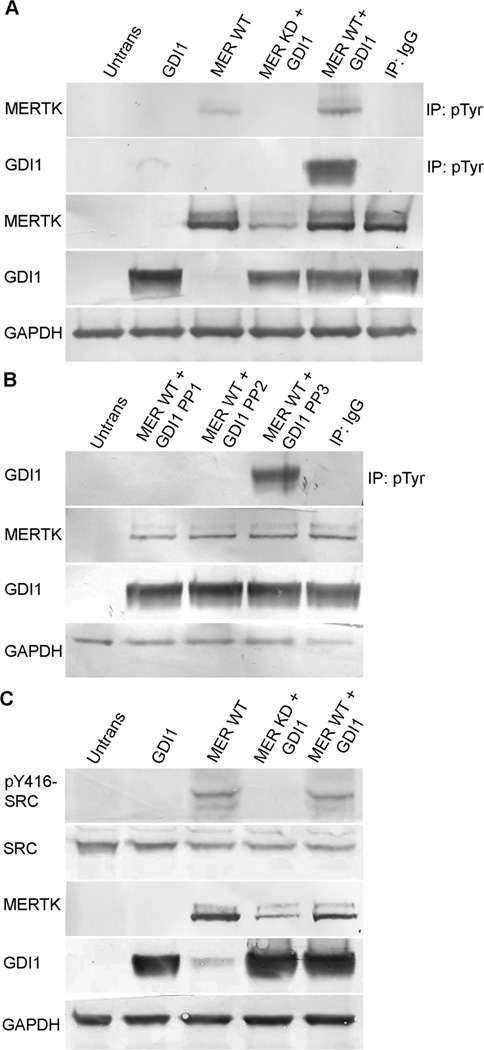
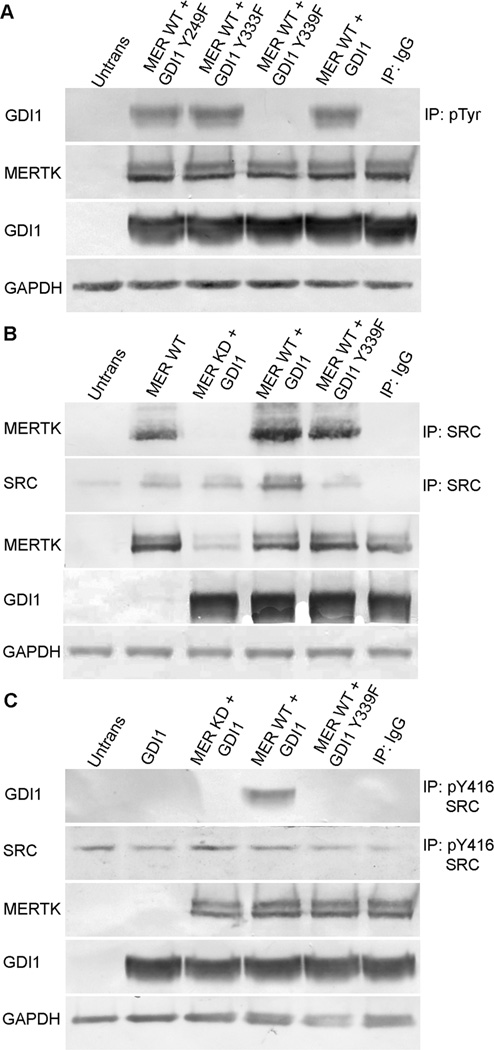
To establish a system in which to further evaluate the role of MERTK in the tyrosine phosphorylation of GDI1, HEK-293T cells were co-transfected with an Xpress-tagged GDI1-fusion construct together with constructs encoding either wild-type MERTK or a kinase-dead form of MERTK containing an Arg844Cys mutation (MERTK-R844C). Previous studies showed that overexpression of wild-type MERTK in heterologous cells resulted in receptor tyrosine kinase activity that was not seen in cells transfected with the MERTK-R844C mutant (McHenry et al., 2004). In the present study, western analysis of anti-phosphotyrosine immunoprecipitates from co-transfected cells showed that tyrosine phosphorylation of GDI1 was stimulated by wild-type, tyrosine-phosphorylated MERTK, but not by the MERTK-R844C kinase-dead mutant (MER KD) (Fig. 4A). In addition, MERTK-induced tyrosine phosphorylation of GDI1 was blocked by SFK inhibitors, but not by an inactive analog (Fig. 4B). Furthermore, formation of the activated form of SRC, pTyr416-SRC, was seen only in immunoblots of cells expressing wild-type MERTK (Fig. 4C). Thus the mechanism of GDI1 tyrosine phosphorylation occurring in HEK-293T cells involves MERTK-mediated activation of SRC and is consistent with that proposed to result from OS-feeding of RPE-J cells.
Fig. 4. Tyrosine phosphorylation of MERTK, SRC, and GDI1 in transfected HEK-293T cells.
Constructs encoding an Xpress-tagged GDI1 fusion protein and wild-type MERTK or a kinase-dead MERTK-R844C mutant were cotransfected into HEK-293T cells and protein expression was evaluated in lysates by western blotting. (A, B) Anti-phosphotyrosine immunoprecipitates evaluated by western blotting using Xpress antibody (for GDI1) or anti-MERTK. (A) In cells coexpressing GDI1 and wild-type MERTK, but not a kinase-dead MERTK mutant, both GDI1 and MERTK were tyrosine phosphorylated. (B) The tyrosine phosphorylation of GDI1 induced by wild-type MERTK was blocked by the SFK inhibitors PP1 or PP2, but not the inactive analog PP3. (C) SRC phosphorylation evaluated by western blotting with antibodies specific for the activated form of SRC, pTyr416-SRC. Activation of SRC was seen in cells expressing wild-type MERTK, but not a kinase-dead MERTK mutant. The cotransfection of GDI1 with MERTK did not augment SRC activation further, suggesting SRC is upstream of GDI1. IB, immunoblot; IP, immunoprecipitate; Mer WT, full-length wild-type MERTK; Mer KD, kinase-dead mutant MERTK-R844C; and IgG, IgG control.
3.5. SRC activation and interaction with GDI1
To further address the mechanism of MERTK-mediated tyrosine phosphorylation of GDI1, HEK-293T cells were co-transfected with GDI1 constructs encoding either wild-type or mutant proteins in which known sites of GDI1 tyrosine phosphorylation were disrupted (Shisheva et al., 1999; Rush et al., 2005; Ballif et al., 2008). Western analysis of anti-phosphotyrosine immunoprecipitates showed that MERTK-induced phosphorylation of GDI1 was unaffected by mutations affecting GDI1 residues Tyr249 (GDI1 Y249F) and Tyr333 (GDI1 Y333F), but was abolished by mutation of Tyr339 (GDI1 Y339F) (Fig. 5A). In addition, western analysis of anti-SRC immunoprecipitates showed that wild-type but not kinase-dead MERTK was associated with SRC, and this association was unaffected by GDI1 phosphorylation status (Fig. 5B). Furthermore, for cells co-transfected with wild-type, but not kinase-dead MERTK, western analysis of anti-pTyr416-SRC immunoprecipitates showed that the active form of SRC was associated with wild-type GDI1, but not the phosphorylation defective GDI1 Y339F mutant (Fig. 5C). Viewed together, these findings are consistent with a regulatory mechanism in which MERTK activation results in the formation of pTyr416-SRC that phosphorylates GDI1 on one or more sites, including Tyr339.
Fig. 5. MERTK, SRC, and GDI1 interaction in transfected HEK-293T cells depends on specific phosphorylation sites in each protein.
Constructs encoding Xpress-tagged wild-type GDI1 or GDI1 tyrosine mutants were co-transfected with wild-type MERTK or a kinase-dead MERTK-R844C mutant, and expression was evaluated in HEK-293T cell lysates by western blotting. (A) Anti-phosphotyrosine immunoprecipitates evaluated for recovery of GDI1 by western blotting using Xpress antibody show that MERTK-induced tyrosine phosphorylation of GDI1 was unaffected for GDI1-Y249F and GDI1-Y333F mutants, but was abolished for GDI1-Y339F. (B) Anti-SRC immunoprecipitates evaluated for recovery of MERTK by western blotting show that SRC associates with wild-type MERTK, but not kinase-dead MERTK, and this association does not require phosphorylation of GDI1. (C) Immunoprecipitates obtained using anti-pTyr416-SRC antibody evaluated for recovery of GDI1 by western blotting with Xpress antibody show that transfections with wild-type MERTK, but not with kinase-dead MERTK, result in association of the activated form of SRC (pTyr416 SRC) with GDI1, but with not the phosphorylation defective mutant GDI1-Y339F. Abbreviations as in Fig. 4; Y249F, GDI1-Y249F; Y333F, GDI1-Y333F; Y339F, GDI1-Y339F.
3.6. MERTK, SRC and GDI1 association with phagosomes
To assess the subcellular localization of GDI1 during phagocytic uptake, indirect immunofluorescence confocal microscopy was performed on OS-fed RPE-J cells. Phagosomes were identified by double labeling with antibodies against rhodopsin and MERTK. In images taken near the apical aspect of the cells at 2 h post feeding, diffuse MERTK labeling extended across the cell surface, but clearly appeared as puncta associated with rhodopsin-positive particles (Fig. 6A, upper panel). In high magnification images (Fig. 6A, lower panel), MERTK labeling was seen on both large and small rhodopsin-positive particles, apparently corresponding to phagosomes at different stages in processing. Punctate labeling of rhodopsin-positive particles was also observed using antibodies recognizing GDI1 (Fig. 6B) or SRC (Fig. 6C). The nearness of the double-labeled particles to the apical cell surface can be appreciated when comparing the images in the top and bottom rows of Fig. 6C that show the same X-Y view of SRC-labeling at two different Z slices. Although the SRC-positive puncta appear to be above the cell surface in the top row images (most apical), the continuity of the SRC-positive puncta with the cell surface is clearly seen in the bottom row images (mid-apical), thus establishing that the labeling is not attributable to artifact or extracellular debris. For all double-staining experiments above, secondary antibodies conjugated with fluorophores having widely separated peak emission spectra (AlexaFluor 488 and AlexaFluor 647) were employed to avoid spectral bleed-through between color channels.
Fig. 6. MERTK, GDI1, and SRC colocalization in OS-fed RPE-J cells.
Densely grown cultures were incubated with 10 OS per cell for 2 h, paraformaldehyde fixed unless otherwise noted, saponin permeabilized, and antibody labeled, and indirect fluorescence confocal microscopy was used to obtain images of the very apical cell surface. (A) Left panels show MERTK immunolabeling, middle panels show rhodopsin immunolabeling, and right panels show merge with DAPI staining of nuclei (blue). Top row images are at lower magnification relative to bottom row images. Cells in bottom row image were fixed in 100% methanol at −20°C for 15 min. (B) Left panel shows GDI1 immunolabeling, middle panel shows rhodopsin immunolabeling, and right panel shows merge with DAPI staining (blue). (C) Left panels show SRC immunolabeling, middle panels show rhodopsin immunolabeling, and right panels show merge with DAPI staining, where upper and lower panels show same X-Y field of view at different Z slices. Secondary antibodies were AlexaFluor-488 for the green channel and AlexaFluor-647 for the red channel. Separate experiments omitting the green channel primary antibody only, or the red channel primary antibody only, confirmed there was no spectral bleed-through between channels and the absence of spurious secondary antibody staining (not shown). Arrowheads point to representative areas of colocalization for each antibody pair. Scale bars are 10 µm.
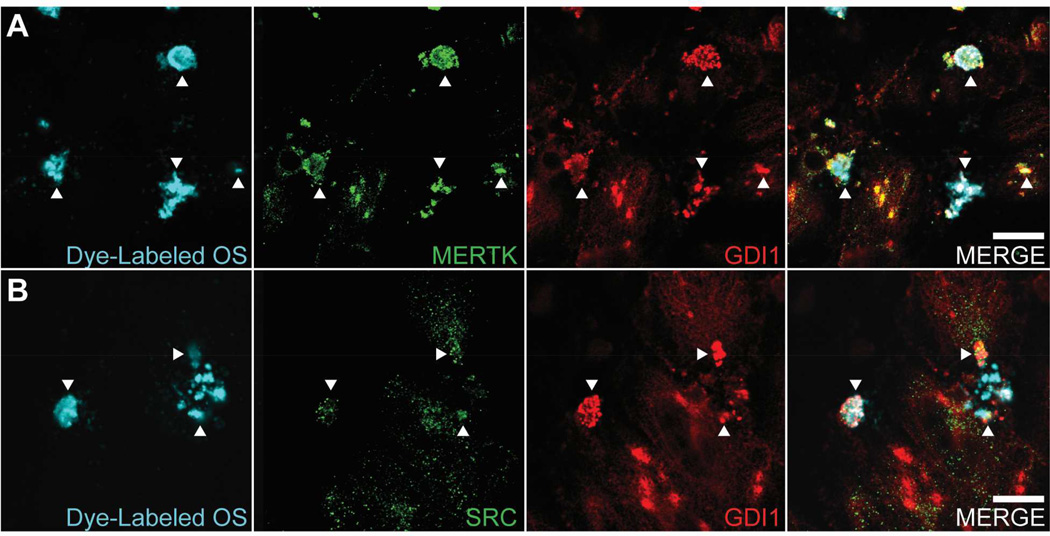
To evaluate GDI1 co-localization with MERTK on phagosomes, confocal microscopy was performed using RPE-J cells that were fed OS covalently labeled with a fluorophore (DyLight 405 nm) whose peak emission spectrum is widely separated from those of the secondary antibodies used (AlexaFluor 488 and AlexaFluor 647). In high magnification images taken at the very apical cell surface, multiple overlapping MERTK-and GDI1-positive puncta were seen associated with fluorescently-labeled OS (Fig. 7A). Controls in which dye-labeled OS or MERTK antibody (green channel) or GDI1 antibody (red channel) were omitted demonstrate the lack of spectral bleed-through between channels (Supplemental Fig.1). Colocalization of GDI1 with SRC on phagosomes was similarly evaluated, showing co-localization of small SRC-positive puncta with larger GDI1-positive puncta on fluorescently-labeled OS (Fig. 7B). All OS exhibiting colocalization of GDI1 with MERTK, and colocalization of GDI1 with SRC, were present only in images taken at the very apical surface. The absence of observed colocalization deeper in the cells suggests that the association of GDI1 with the phagocytic machinery occurs on the apical plasma membrane.
Fig. 7. Colocalization of GDI1 with MERTK and SRC on phagosomes.
Cultures of RPE-J cells were fed OS labeled with fluorescent dye (DyLight 405 nm) for 2 hours, fixed, immunostained for GDI1 and MERTK or GDI1 and SRC, and then subjected to indirect immunofluorescence confocal microscopy as in Fig. 6. (A) Panels from left to right show OS fluorescence, MERTK immunolabeling, GDI1 immunolabeling, and merge of OS, MERTK, and GDI1. (B) Panels from left to right show OS fluorescence, SRC immunolabeling, GDI1 immunolabeling, and merge of OS, SRC, and GDI1. Secondary antibodies were AlexaFluor-488 for the green channel and AlexaFluor-647 for the red channel. Controls which omitted dye-labeled OS, or MERTK/SRC antibody (green channel), or GDI1 antibody (red channel) confirmed the absence of spectral bleed-through between channels (see Supplemental Fig. 1). Arrowheads point to representative areas of colocalization for each antibody pair. All the colocalization seen is exclusively on the apical surface, with no colocalization apparent deeper in the cells, consistent with the association of GDI1 with MERTK and SRC on the plasma membrane. Scale bars are 10 µm.
3.7. GDI1 interaction with RAB5
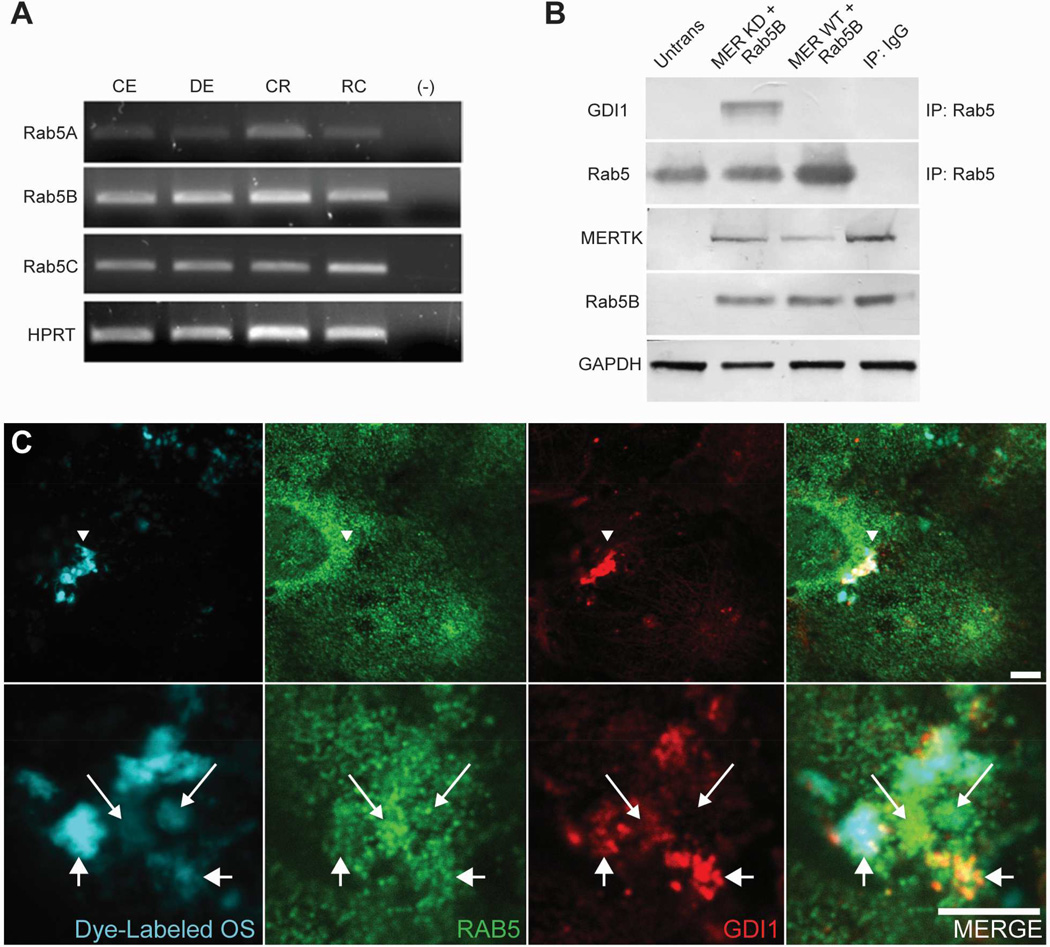
Previous studies have shown that the small G-protein RAB5 regulates the kinetics of membrane traffic in the early endocytic pathway (Bucci et al., 1992). In phagocytes, RAB5 has been shown to associate transiently with forming phagosomes and enhance the engulfment of apoptotic cells (Nakaya et al., 2006), to function in the recruitment of RAB7, and to promote phagolysosome maturation (Vieira et al., 2003). RAB5 has also been identified in phagosome fractions from RPE-J cells (Hoppe et al., 2001). As our earlier work showed that transient SRC activation correlates with onset of RPE phagocytosis (Shelby et al., 2013), it was of interest to evaluate the potential impact of SRC activity on the interaction of GDI1 with RAB proteins during phagocytic uptake. Transcripts encoding all three RAB5 isoforms (A, B, C) could be amplified from congenic and dystrophic RCS rat RPE/choroid, congenic RCS rat retina, and RPE-J cells (Fig. 8A).
Fig. 8. RAB5 expression in the RPE and interaction with GDI1 during MERTK activation.
(A) Expression of RAB5 isoforms in the RPE and retina assayed using RT-PCR analysis. Transcripts were amplified from total RNA isolated from congenic rat RPE/choroid (CE); dystrophic rat RPE/choroid (DE); congenic rat retina (CR); RPE-J cells (RC); no cDNA (–). Amplification of ubiquitously expressed enzyme HPRT served as control. (B) GDI1 interactions with RAB5 assayed in immunoprecipitates from HEK-293T cells co-transfected with constructs encoding RAB5B, wild-type MERTK (MER WT) and kinase-dead MERTK-R844 (MER KD). Using anti-RAB5 antibodies, GDI1 was recovered in pulldowns from cells transfected with kinase-dead MERTK, but not wild-type MERTK. Control lanes show western analysis of recombinant MERTK (anti-MERTK antibodies) and RAB5B (anti-Xpress epitope) expression, as well as GAPDH. (C) GDI1 and RAB5 immunoreactivity in RPE-J cultures fed fluorescently labeled OS (DyLight 405 nm) imaged using fluorescence confocal microscopy. To avoid spectral bleed-through using AlexaFluor 555 for RAB5 detection and AlexaFluor 647 for GDI1 detection, emission bandpass for each color was narrowly set. Absence of bleed-through was confirmed for samples in which either labeled OS, RAB5 primary antibody, or GDI1 primary antibody were omitted (data not shown). Low magnification views (top row) show marked colocalization of GDI1 and OS in regions without obvious concentration of RAB5. High magnification views (bottom row) show colocalization of both RAB5 and GDI1 with OS, sometimes to mutual exclusion, with some OS showing relatively high GDI1 accumulation (shorter arrows with larger arrowheads) and other OS showing relatively high RAB5 accumulation (longer arrows with smaller arrowheads). Scale bars are 5 µm.
To evaluate the effect of MERTK signaling on the interaction of GDI1 with RAB5, HEK-293T cells were cotransfected with expression constructs encoding RAB5B and either wild-type MERTK (MER WT) or the MERTK-R844C kinase-dead mutant (MER KD), and immunoprecipitations of endogenous GDI1 were performed. Western analysis of the recovered proteins showed that sustained interaction of GDI1 and RAB5 did not occur in the presence of wild-type MERTK, but could be seen in its absence (i.e. in transfections with kinase-dead MERTK) (Fig. 8B).
The interaction of GDI1 with RAB5 was also evaluated in RPE-J cells by concurrently imaging fluorescently-labeled OS (DyLight 405), GDI1, and RAB5 (via immunolabeling with AlexaFluor 555 and AlexaFluor 647 secondary antibodies, respectively). Using a confocal microscope, some apical fields of view demonstrated OS and GDI1 colocalization without any specific RAB5 accumulation (Fig. 8C, top row), consistent with the phosphorylated form of GDI1 dissociating from RAB5. In other apical fields of view, both RAB5 and GDI1 were seen to colocalize with fluorescently-labeled OS, exhibiting distinct patterns of immunolabeling sometimes to mutual exclusion (Fig. 8C, bottom, compare different sized-arrows) and resulting in multiple regions in which only GDI1, or in which only RAB5, appears adjacent to the OS. Consistent with our pulldown data of GDI1 and RAB5, these microscopy images suggest that while both RAB5 and GDI1 participate in outer segment phagocytosis, GDI1 activation triggers dissociation from RAB5.
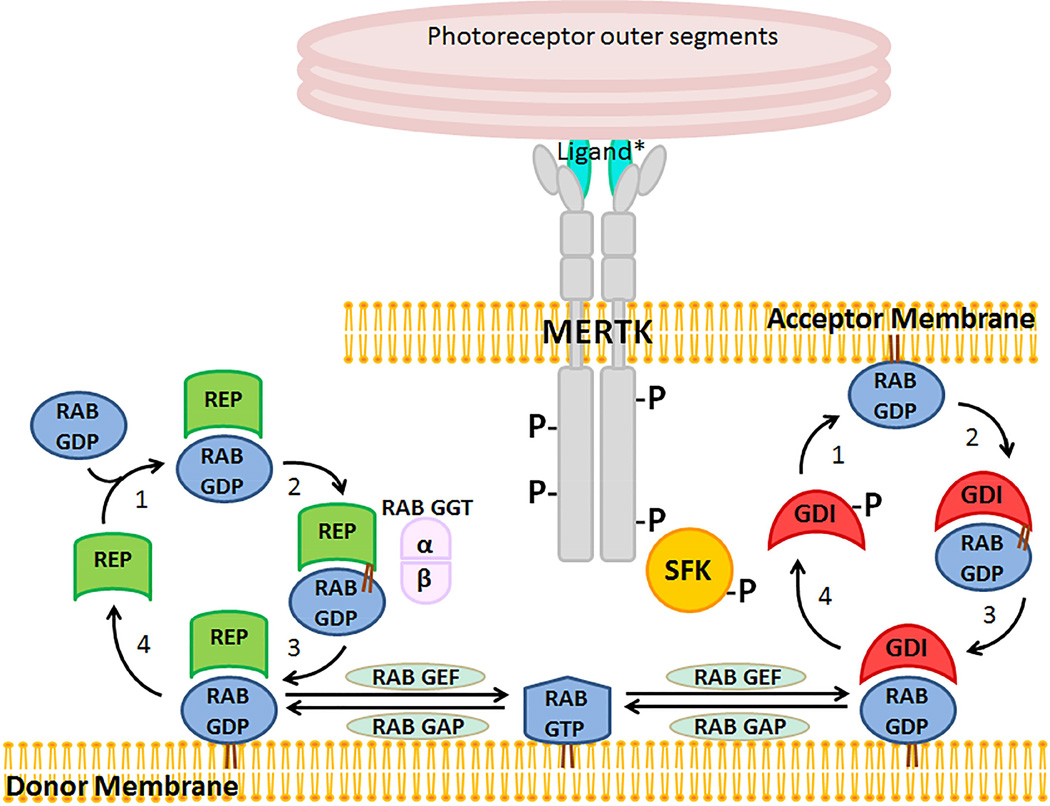
Viewed in aggregate, our findings are consistent with a mechanism in which MERTK-mediated activation of SRC results in tyrosine-phosphorylation of GDI1, thereby decreasing its affinity for RAB5 and potentially other RAB proteins acting early in RPE phagocytosis. The schematic in Fig. 9 illustrates the proposed contribution of MERTK to the mechanism of RAB cycling between donor and acceptor membranes. This cycling is potentially involved in specifying membrane neededfor phagocytic cup formation and uptake in the RPE. Also shown is the linked pathway of RAB prenylation and activation requiring RAB geranylgeranyl transferase activation by the choroideremia gene REP1/CHM from the GDI/CHM family of RAB GTPase effectors.
Fig. 9. Proposed role of MERTK in regulating RAB GTPase mediated membrane trafficking in the RPE.
Newly synthesized RABs associate with RAB escort proteins (REPs) that present them to RAB geranylgeranyl transferase (GGT) for prenylation and escort the modified proteins their destination membranes. GDP dissociation inhibitors (GDIs) bind to the GDP-bound form of RABs, thus facilitating their delivery and retrieval from donor and target membranes. MERTK activation of SRC results in phosphorylation of GDI1, thus decreasing its affinity for RAB5 and promoting recycling. In the RPE, donor membranes are likely to be ER-derived endosomes, and target membranes are likely to include forming phagocytic cups and nascent phagosomes. Modified from Seabra, M.C. et al., Trends Mol. Med. 2002:8; 23–30.
4. Discussion
The present study establishes that GDI1 is tyrosine phosphorylated coincident with peak phagocytic uptake in the RPE, and places GDI1 in the apical region of the RPE with MERTK and the intracellular tyrosine kinase SRC. GDI1 also localizes with MERTK and SRC on phagosomes in OS-fed RPE-J cells in culture. In heterologous cells expressing recombinant proteins, SRC phosphorylation of GDI1 requires MERTK tyrosine kinase activity, and RAB5 associates with phosphorylation-defective, but not wild-type, GDI1. Coupled with the known role of GDI proteins in regulating intracellular vesicle traffic, these observations are consistent with a role for GDI1 in modulating the membrane association of RAB proteins involved in RPE phagocytosis, and identify a novel regulatory role for MERTK signaling.
By preventing the dissociation of GDP, GDIs limit GTP binding and activation of RAB proteins in the cytoplasmic pool and thus regulate their association with cellular membranes (Goody et al., 2005). Changes in GDI phosphorylation status play a key role in regulating RAB protein-GDI interactions (Steele-Mortimer et al., 1993; Cavalli et al., 2001). Phosphorylation of GDI1 by p38 MAPK on serine/threonine residues has been shown to decrease the affinity of GDI1 for RAB5 and early endosomal antigen 1 on endosomes during endocytosis (Cavalli et al., 2001). In addition, tyrosine phosphorylation of the closely related isoform, GDI2, has been shown to modulate its affinity for RAB proteins (Shisheva et al., 1999). The present finding that MERTK-induced activation of SRC results in tyrosine phosphorylation of GDI1 and its decreased interaction with RAB5 suggests that this signaling is central to the RPE phagocytic mechanism. This view, as detailed in Fig. 9, is consistent with previous reports showing that RAB5 associates with early phagosomes (Vieira et al., 2003; Kinchen et al., 2008) and is involved in the formation and maturation of phagosomes in the RPE (Hoppe et al., 2001).
Members of the RAB superfamily participate in multiple aspects of membrane trafficking, including the coordination of vesicle formation, motility, and fusion (Hutagalung and Novick, 2011). The importance of RAB proteins in the specialized functions of the RPE is well established. Complexes comprised of RAB27A, myosin Va, myosin VIIa and RAB-interacting protein facilitate melanosome transport within the cell layer (Futter et al., 2004; Klomp et al., 2007; Lopes et al., 2007a; Ramalho et al., 2009). RAB38-mutant “chocolate mice” have defective melanosome stability, which can be partially rescued by RAB32 (Lopes et al., 2007b; Wasmeier et al., 2008). RAB escort protein 1 (REP1) is essential for the trafficking of melanosomes and other membranes in the RPE (Alory and Balch, 2001). REP1 participates in the activation of geranylgeranyl transferase type II that carries out the prenylation of RAB proteins required for their membrane association (Farnsworth et al., 1994; Desnoyers et al., 1996). Mutations in the CHM gene encoding REP1 cause choroideremia, a degenerative disease affecting the choroid, RPE, and photoreceptor cells (Cremers et al., 1990; Merry et al., 1992; Seabra et al., 1992; Cremers et al., 1994). In choroideremia, unprenylated RAB27A accumulates in the RPE along with undegraded OS in phagosomes (Gordiyenko et al., 2010). The eye phenotype of choroideremia likely reflects the inability of the closely related isoform, REP2, to compensate for REP1 function in the outer retina, as it does in most other tissues (Cremers et al., 1994).
In contrast to the limited phenotype associated with mutations in CHM, mutations in the gene encoding GDI1 result in severe developmental defects leading to X-linked mental retardation (Bienvenu et al., 1998; D'Adamo et al., 1998). In knockout mice, GDI1 loss-of-function results in impaired synaptic function in the forebrain needed to form temporal associations (Ishizaki et al., 2000; D'Adamo et al., 2002). Thus, an interesting question is whether patients with X-linked mental retardation due to mutations in GDI1 may exhibit an ocular phenotype. In studies reported so far, this is does not seem to be the case (Strobl-Wildemann et al., 2011). In addition, Gdi1-knockout mice at 9 months-of-age do not exhibit significant retinal degeneration relative to control mice (Thompson and D’Adamo, unpublished observations). These findings are consistent with the presence of mechanisms that compensate for GDI1 loss in the outer retina, potentially including, but not limited to, substitution by the closely related isoform GDI2.
Previous studies have shown that SRC activation downstream of MERTK occurs during phagocytic uptake of apoptotic cells by macrophages, resulting in SRC-mediated tyrosine phosphorylation and activation of focal adhesion kinase FAK, its recruitment to αvβ5 integrin, and formation of the RAC1 activating complex p130(CAS)/CRKII/DOCK180 (Wu et al., 2005). In macrophages, MERTK signaling has been shown to synergize with that of αvβ5 integrin in driving RAC1 activation, cytoskeletal remodeling and phagocytic uptake of apoptotic cells (Wu et al., 2005). In the RPE, ligand activation of αvβ5 integrin has been shown to initiate the activation of FAK (Finnemann, 2003; Mao and Finnemann, 2012). Future studies will be needed to determine the extent to which the activation of SRC downstream of αvβ5 integrin impacts GDI1 phosphorylation, and conversely, the extent to which activation of SRC downstream of MERTK contributes to the formation of the active CRKII/DOCK180/RAC1 module in the RPE. It is interesting to note that GDI1 is closely related to RHO GDI that inhibits the dissociation of GDP from RAC, RHO, and CDC42 GTPases that regulate actin polymerization (DerMardirossian and Bokoch, 2005). Rho GDI activity is also modulated by SRC-mediated tyrosine phosphorylation (DerMardirossian et al., 2006).
In summary, a mechanism in which MERTK activation of SRC results in tyrosine phosphorylation of GDI1 is proposed to regulate the association of RAB5 with phagosomes in the RPE, suggesting a direct link between MERTK activity and membrane remodeling. First indications are that this regulatory mechanism functions at the level of the forming phagocytic cup and nascent phagosome. Future studies will be needed to determine the extent to which this mechanism may further impact phagosome maturation and trafficking. It will also be of interest to explore the possibility that MERTK signaling may impact RAB proteins involved in additional aspects of RPE function, including melanosome transport and membrane trafficking. Together, these new insights constitute an important advance in defining the mechanism of OS phagocytosis, and expand our understanding of the critical role played by the RAB-GDI/CHM superfamily in the specialized functions of the RPE.
Supplementary Material
Control conditions were evaluated for the experiment shown in Fig. 7, in which RPE-J cells were fed OS labeled with fluorescent dye (DyLight 405 nm) and immunostained for MERTK (AlexaFluor-488 secondary antibody; green channel) and GDI1 (AlexaFluor-647 secondary antibody; red channel). (A) Top row in which OS were omitted shows the absence of spectral bleed-through into the OS channel. Note also that MERTK and GDI1 occasionally colocalize in the absence of outer segments, suggesting either a common membrane domain they cycle through or alternate ligands that activate MERTK and subsequently colocalize and activate GDI1. (B) Middle row in which MERTK antibody was omitted shows the absence of spectral bleed-through into the green channel. This absence of bleed-through also serves as a control for the SRC image in Fig. 7B. Note the colocalization of OS and GDI1. C) Bottom row in which GDI1 antibody was omitted shows the absence of spectral bleed-through into the red channel. Given the similar fluorescent intensities of MERTK and SRC staining, the lack of bleed-through in (C) also serves as a control for the SRC image in Fig. 7B. Note the colocalization of OS and MERTK. The absence of fluorescence in the negative controls and the unique fluorescence patterns seen in each test panel both confirm the absence of spectral bleed-through and further document the strong colocalization of MERTK and GDI1 with OS. Scale bars are 10 µm.
Tryptic peptides from spot 4DA measured by MALD-TOF. The protein was identified as RAB GDP dissociation inhibitor (GDI1), Uniprot P50398. Measured masses from the MALDI-TOF spectrum are compared to calculated values. Masses listed represent 57% sequence coverage. MS-Fit MOWSE score: 1.38e+06; Mascot score 142, expect value 1.6e-10. amu = atomic mass unit (Daltons).
The RAB GTPase effector GDI1 is tyrosine phosphorylated coincident with peak phagocytic uptake in the RPE.
GDI1 colocalizes with MERTK and SRC on phagosomes in the RPE.
MERTK activation of SRC results in tyrosine phosphorylation of GDI1 and decreased interaction with RAB5.
MERTK signaling in the RPE is directly linked to mechanisms of membrane remodeling.
Acknowledgements
We thank Kendrick Laboratories, Inc. (Madison, WI) for assistance with two-dimensional electrophoresis and western analysis; Mary Ann Gawinowicz (Columbia University Protein Core Facility) for MALDI-mass spectrophotometry analysis; Christina L. McHenry (University of Michigan) for MERTK expression constructs; Ron Bush (National Eye Institute) for providing dystrophic (RCS-p+) and congenic control (RCS-rdy+p+) rats; Patrizia D'Adamo (Dulbecco Telethon Institute at San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy) for Gdi1-knockout mouse eyes; Christin Carter-Su (University of Michigan) for gifts of SRC family kinase inhibitors PP1 and PP2, and PP3 inactive analog; Douglas Vollrath (Stanford University) for anti-MERTK antibodies; Benjamin Margolis, (University of Michigan) for anti-phosphotyrosine antibodies; and Steve Lentz, Austra Liepa, Frank Mei, and Mitchell Gillett (University of Michigan, Kellogg Eye Center) for technical assistance.
This work has been supported by grants from the Midwest Eye Bank and Transplantation Center (DAT) and Research to Prevent Blindness (DAT is the recipient of a Senior Scientific Investigator Award); University of Michigan Rackham Merit Fellowship, King-Chavez-Parks Initiative, and Future Faculty Fellowship Program (SJS); University of Michigan Department of Ophthalmology and Visual Sciences Pre-Residency Research Fellowship (JMM); and the National Institutes of Health University of Michigan Vision Training Program (T32 EY13934) (SJS, JMM), Vision Core (P30 EY007003), and the Diabetes Research and Training Center (P60DK020572).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: No conflicting relationship exists for any author
Abbreviations: DAPI (4',6-diamidino-2-phenylindole); GDI(s), GDP dissociation inhibitor(s); OS, outer segment; PP1, 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; PP2, 4-amino-5-(4-chlorophenyl)-7-(tbutyl) pyrazolo[3,4-d]pyrimidine; PP3, 4-amino-7-phenylpyrazol[3,4-d]pyrimidine; RPE, retinal pigment epithelium; RCS, Royal College of Surgeons; SFK(s), SRC family kinase(s).
References
- 1.Alory C, Balch WE. Organization of the Rab-GDI/CHM superfamily: the functional basis for choroideremia disease. Traffic. 2001;2:532–543. doi: 10.1034/j.1600-0854.2001.20803.x. [DOI] [PubMed] [Google Scholar]
- 2.Alory C, Balch WE. Molecular evolution of the Rab-escort-protein/guanine-nucleotide-dissociation-inhibitor superfamily. Mol Biol Cell. 2003;14:3857–3867. doi: 10.1091/mbc.E03-04-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araki N. Role of microtubules and myosins in Fc gamma receptor-mediated phagocytosis. Front Biosci. 2006;11:1479–1490. doi: 10.2741/1897. [DOI] [PubMed] [Google Scholar]
- 4.Bachner D, Sedlacek Z, Korn B, Hameister H, Poustka A. Expression patterns of two human genes coding for different rab GDP-dissociation inhibitors (GDIs), extremely conserved proteins involved in cellular transport. Hum Mol Genet. 1995;4:701–708. doi: 10.1093/hmg/4.4.701. [DOI] [PubMed] [Google Scholar]
- 5.Ballif BA, Carey GR, Sunyaev SR, Gygi SP. Large-scale identification and evolution indexing of tyrosine phosphorylation sites from murine brain. J Proteome Res. 2008;7:311–318. doi: 10.1021/pr0701254. [DOI] [PubMed] [Google Scholar]
- 6.Bienvenu T, des Portes V, Saint Martin A, McDonell N, Billuart P, Carrie A, Vinet MC, Couvert P, Toniolo D, Ropers HH, Moraine C, van Bokhoven H, Fryns JP, Kahn A, Beldjord C, Chelly J. Non-specific X-linked semidominant mental retardation by mutations in a Rab GDP-dissociation inhibitor. Hum Mol Genet. 1998;7:1311–1315. doi: 10.1093/hmg/7.8.1311. [DOI] [PubMed] [Google Scholar]
- 7.Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, Courtneidge SA. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000;20:9018–9027. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 9.Burstyn-Cohen T, Lew ED, Traves PG, Burrola PG, Hash JC, Lemke G. Genetic dissection of TAM receptor-ligand interaction in retinal pigment epithelial cell phagocytosis. Neuron. 2012;76:1123–1132. doi: 10.1016/j.neuron.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caberoy NB, Alvarado G, Bigcas JL, Li W. Galectin-3 is a new MerTK-specific eat-me signal. J Cell Physiol. 2012;227:401–407. doi: 10.1002/jcp.22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caberoy NB, Zhou Y, Li W. Tubby and tubby-like protein 1 are new MerTK ligands for phagocytosis. EMBO J. 2010;29:3898–3910. doi: 10.1038/emboj.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavalli V, Vilbois F, Corti M, Marcote MJ, Tamura K, Karin M, Arkinstall S, Gruenberg J. The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol Cell. 2001;7:421–432. doi: 10.1016/s1097-2765(01)00189-7. [DOI] [PubMed] [Google Scholar]
- 13.Cooper JA, Gould KL, Cartwright CA, Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986;231:1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- 14.Cremers FP, Armstrong SA, Seabra MC, Brown MS, Goldstein JL. REP-2, a Rab escort protein encoded by the choroideremia-like gene. J Biol Chem. 1994;269:2111–2117. [PubMed] [Google Scholar]
- 15.Cremers FP, Sankila EM, Brunsmann F, Jay M, Jay B, Wright A, Pinckers AJ, Schwartz M, van de Pol DJ, Wieringa B, et al. Deletions in patients with classical choroideremia vary in size from 45 kb to several megabases. Am J Hum Genet. 1990;47:622–628. [PMC free article] [PubMed] [Google Scholar]
- 16.D'Adamo P, Menegon A, Lo Nigro C, Grasso M, Gulisano M, Tamanini F, Bienvenu T, Gedeon AK, Oostra B, Wu SK, Tandon A, Valtorta F, Balch WE, Chelly J, Toniolo D. Mutations in GDI1 are responsible for X-linked non-specific mental retardation. Nat Genet. 1998;19:134–139. doi: 10.1038/487. [DOI] [PubMed] [Google Scholar]
- 17.D'Adamo P, Welzl H, Papadimitriou S, Raffaele di Barletta M, Tiveron C, Tatangelo L, Pozzi L, Chapman PF, Knevett SG, Ramsay MF, Valtorta F, Leoni C, Menegon A, Wolfer DP, Lipp HP, Toniolo D. Deletion of the mental retardation gene Gdi1 impairs associative memory and alters social behavior in mice. Hum Mol Genet. 2002;11:2567–2580. doi: 10.1093/hmg/11.21.2567. [DOI] [PubMed] [Google Scholar]
- 18.D'Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- 19.DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 20.DerMardirossian C, Rocklin G, Seo JY, Bokoch GM. Phosphorylation of RhoGDI by Src regulates Rho GTPase binding and cytosol-membrane cycling. Mol Biol Cell. 2006;17:4760–4768. doi: 10.1091/mbc.E06-06-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desnoyers L, Anant JS, Seabra MC. Geranylgeranylation of Rab proteins. Biochem Soc Trans. 1996;24:699–703. doi: 10.1042/bst0240699. [DOI] [PubMed] [Google Scholar]
- 22.Duncan JL, LaVail MM, Yasumura D, Matthes MT, Yang H, Trautmann N, Chappelow AV, Feng W, Earp HS, Matsushima GK, Vollrath D. An RCS-like retinal dystrophy phenotype in mer knockout mice. Invest Ophthalmol Vis Sci. 2003;44:826–838. doi: 10.1167/iovs.02-0438. [DOI] [PubMed] [Google Scholar]
- 23.Farnsworth CC, Seabra MC, Ericsson LH, Gelb MH, Glomset JA. Rab geranylgeranyl transferase catalyzes the geranylgeranylation of adjacent cysteines in the small GTPases Rab1A, Rab3A, and Rab5A. Proc Natl Acad Sci U S A. 1994;91:11963–11967. doi: 10.1073/pnas.91.25.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng W, Yasumura D, Matthes MT, LaVail MM, Vollrath D. Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J Biol Chem. 2002;277:17016–17022. doi: 10.1074/jbc.M107876200. [DOI] [PubMed] [Google Scholar]
- 25.Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. Embo J. 2003;22:4143–4154. doi: 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finnemann SC, Nandrot EF. MerTK activation during RPE phagocytosis in vivo requires alphaVbeta5 integrin. Adv Exp Med Biol. 2006;572:499–503. doi: 10.1007/0-387-32442-9_69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Futter CE, Ramalho JS, Jaissle GB, Seeliger MW, Seabra MC. The role of Rab27a in the regulation of melanosome distribution within retinal pigment epithelial cells. Mol Biol Cell. 2004;15:2264–2275. doi: 10.1091/mbc.E03-10-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, Vollrath D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet. 2000;26:270–271. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- 29.Georgescu MM, Kirsch KH, Shishido T, Zong C, Hanafusa H. Biological effects of c-Mer receptor tyrosine kinase in hematopoietic cells depend on the Grb2 binding site in the receptor and activation of NF-kappaB. Mol Cell Biol. 1999;19:1171–1181. doi: 10.1128/mcb.19.2.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert PM, Burd CG. GDP dissociation inhibitor domain II required for Rab GTPase recycling. J Biol Chem. 2001;276:8014–8020. doi: 10.1074/jbc.M008845200. [DOI] [PubMed] [Google Scholar]
- 31.Goody RS, Rak A, Alexandrov K. The structural and mechanistic basis for recycling of Rab proteins between membrane compartments. Cell Mol Life Sci. 2005;62:1657–1670. doi: 10.1007/s00018-005-4486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordiyenko NV, Fariss RN, Zhi C, MacDonald IM. Silencing of the CHM gene alters phagocytic and secretory pathways in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2010;51:1143–1150. doi: 10.1167/iovs.09-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hafizi S, Dahlback B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev. 2006;17:295–304. doi: 10.1016/j.cytogfr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 35.Hicks D, Molday RS. Differential immunogold-dextran labeling of bovine and frog rod and cone cells using monoclonal antibodies against bovine rhodopsin. Exp Eye Res. 1986;42:55–71. doi: 10.1016/0014-4835(86)90017-5. [DOI] [PubMed] [Google Scholar]
- 36.Hoppe G, Marmorstein AD, Pennock EA, Hoff HF. Oxidized low density lipoprotein-induced inhibition of processing of photoreceptor outer segments by RPE. Invest Ophthalmol Vis Sci. 2001;42:2714–2720. [PubMed] [Google Scholar]
- 37.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishizaki H, Miyoshi J, Kamiya H, Togawa A, Tanaka M, Sasaki T, Endo K, Mizoguchi A, Ozawa S, Takai Y. Role of rab GDP dissociation inhibitor alpha in regulating plasticity of hippocampal neurotransmission. Proc Natl Acad Sci U S A. 2000;97:11587–11592. doi: 10.1073/pnas.97.21.11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin H, Lanning NJ, Carter-Su C. JAK2, but not Src family kinases, is required for STAT, ERK, and Akt signaling in response to growth hormone in preadipocytes and hepatoma cells. Mol Endocrinol. 2008;22:1825–1841. doi: 10.1210/me.2008-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda) 2010;25:8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khorana HG, Knox BE, Nasi E, Swanson R, Thompson DA. Expression of a bovine rhodopsin gene in Xenopus oocytes: demonstration of light-dependent ionic currents. Proc Natl Acad Sci U S A. 1988;85:7917–7921. doi: 10.1073/pnas.85.21.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinchen JM, Doukoumetzidis K, Almendinger J, Stergiou L, Tosello-Trampont A, Sifri CD, Hengartner MO, Ravichandran KS. A pathway for phagosome maturation during engulfment of apoptotic cells. Nat Cell Biol. 2008;10:556–566. doi: 10.1038/ncb1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klomp AE, Teofilo K, Legacki E, Williams DS. Analysis of the linkage of MYRIP and MYO7A to melanosomes by RAB27A in retinal pigment epithelial cells. Cell Motil Cytoskeleton. 2007;64:474–487. doi: 10.1002/cm.20198. [DOI] [PubMed] [Google Scholar]
- 44.LaVail MM. Circadian nature of rod outer segment disc shedding in the rat. Invest Ophthalmol Vis Sci. 1980;19:407–411. [PubMed] [Google Scholar]
- 45.LaVail MM. Photoreceptor characteristics in congenic strains of RCS rats. Invest Ophthalmol Vis Sci. 1981;20:671–675. [PubMed] [Google Scholar]
- 46.LaVail MM. Legacy of the RCS rat: impact of a seminal study on retinal cell biology and retinal degenerative diseases. Prog Brain Res. 2001;131:617–627. doi: 10.1016/s0079-6123(01)31048-8. [DOI] [PubMed] [Google Scholar]
- 47.LaVail MM, Sidman RL, Gerhardt CO. Congenic strains of RCS rats with inherited retinal dystrophy. J Hered. 1975;66:242–244. doi: 10.1093/oxfordjournals.jhered.a108621. [DOI] [PubMed] [Google Scholar]
- 48.Law AL, Ling Q, Hajjar KA, Futter CE, Greenwood J, Adamson P, Wavre-Shapton ST, Moss SE, Hayes MJ. Annexin A2 regulates phagocytosis of photoreceptor outer segments in the mouse retina. Mol Biol Cell. 2009;20:3896–3904. doi: 10.1091/mbc.E08-12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X, Pawson T. Biochemistry of the Src protein-tyrosine kinase: regulation by SH2 and SH3 domains. Recent progress in hormone research. 1994;49:149–160. doi: 10.1016/b978-0-12-571149-4.50011-8. [DOI] [PubMed] [Google Scholar]
- 50.Lopes VS, Ramalho JS, Owen DM, Karl MO, Strauss O, Futter CE, Seabra MC. The ternary Rab27a-Myrip-Myosin VIIa complex regulates melanosome motility in the retinal pigment epithelium. Traffic. 2007a;8:486–499. doi: 10.1111/j.1600-0854.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopes VS, Wasmeier C, Seabra MC, Futter CE. Melanosome maturation defect in Rab38-deficient retinal pigment epithelium results in instability of immature melanosomes during transient melanogenesis. Mol Biol Cell. 2007b;18:3914–3927. doi: 10.1091/mbc.E07-03-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mao Y, Finnemann SC. Essential diurnal Rac1 activation during retinal phagocytosis requires alphavbeta5 integrin but not tyrosine kinases focal adhesion kinase or Mer tyrosine kinase. Mol Biol Cell. 2012;23:1104–1114. doi: 10.1091/mbc.E11-10-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Margolis B, Rhee SG, Felder S, Mervic M, Lyall R, Levitzki A, Ullrich A, Zilberstein A, Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989;57:1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- 54.Martin GS. The hunting of the Src. Nat Rev Mol Cell Biol. 2001;2:467–475. doi: 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- 55.McHenry CL, Liu Y, Feng W, Nair AR, Feathers KL, Ding X, Gal A, Vollrath D, Sieving PA, Thompson DA. MERTK arginine-844-cysteine in a patient with severe rod-cone dystrophy: loss of mutant protein function in transfected cells. Invest Ophthalmol Vis Sci. 2004;45:1456–1463. doi: 10.1167/iovs.03-0909. [DOI] [PubMed] [Google Scholar]
- 56.Merry DE, Janne PA, Landers JE, Lewis RA, Nussbaum RL. Isolation of a candidate gene for choroideremia. Proc Natl Acad Sci U S A. 1992;89:2135–2139. doi: 10.1073/pnas.89.6.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nabi IR, Mathews AP, Cohen-Gould L, Gundersen D, Rodriguez-Boulan E. Immortalization of polarized rat retinal pigment epithelium. J Cell Sci. 1993;104(Pt 1):37–49. doi: 10.1242/jcs.104.1.37. [DOI] [PubMed] [Google Scholar]
- 58.Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem. 1996;271:30022–30027. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- 59.Nakaya M, Tanaka M, Okabe Y, Hanayama R, Nagata S. Opposite effects of rho family GTPases on engulfment of apoptotic cells by macrophages. J Biol Chem. 2006;281:8836–8842. doi: 10.1074/jbc.M510972200. [DOI] [PubMed] [Google Scholar]
- 60.Nandrot EF, Silva KE, Scelfo C, Finnemann SC. Retinal pigment epithelial cells use a MerTK-dependent mechanism to limit the phagocytic particle binding activity of alphavbeta5 integrin. Biol Cell. 2012;104:326–341. doi: 10.1111/boc.201100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishimura N, Nakamura H, Takai Y, Sano K. Molecular cloning and characterization of two rab GDI species from rat brain: brain-specific and ubiquitous types. J Biol Chem. 1994;269:14191–14198. [PubMed] [Google Scholar]
- 62.Olazabal IM, Caron E, May RC, Schilling K, Knecht DA, Machesky LM. Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcgammaR, phagocytosis. Curr Biol. 2002;12:1413–1418. doi: 10.1016/s0960-9822(02)01069-2. [DOI] [PubMed] [Google Scholar]
- 63.Papermaster DS. Preparation of retinal rod outer segments. Methods Enzymol. 1982;81:48–52. doi: 10.1016/s0076-6879(82)81010-0. [DOI] [PubMed] [Google Scholar]
- 64.Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 65.Pfeffer SR, Dirac-Svejstrup AB, Soldati T. Rab GDP dissociation inhibitor: putting rab GTPases in the right place. J Biol Chem. 1995;270:17057–17059. doi: 10.1074/jbc.270.29.17057. [DOI] [PubMed] [Google Scholar]
- 66.Prasad D, Rothlin CV, Burrola P, Burstyn-Cohen T, Lu Q, Garcia de Frutos P, Lemke G. TAM receptor function in the retinal pigment epithelium. Mol Cell Neurosci. 2006;33:96–108. doi: 10.1016/j.mcn.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 67.Ramalho JS, Lopes VS, Tarafder AK, Seabra MC, Hume AN. Myrip uses distinct domains in the cellular activation of myosin VA and myosin VIIA in melanosome transport. Pigment Cell Melanoma Res. 2009;22:461–473. doi: 10.1111/j.1755-148X.2009.00567.x. [DOI] [PubMed] [Google Scholar]
- 68.Rush J, Moritz A, Lee KA, Guo A, Goss VL, Spek EJ, Zhang H, Zha XM, Polakiewicz RD, Comb MJ. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat Biotechnol. 2005;23:94–101. doi: 10.1038/nbt1046. [DOI] [PubMed] [Google Scholar]
- 69.Seabra MC, Brown MS, Slaughter CA, Sudhof TC, Goldstein JL. Purification of component A of Rab geranylgeranyl transferase: possible identity with the choroideremia gene product. Cell. 1992;70:1049–1057. doi: 10.1016/0092-8674(92)90253-9. [DOI] [PubMed] [Google Scholar]
- 70.Shelby SJ, Colwill K, Dhe-Paganon S, Pawson T, Thompson DA. MERTK Interactions with SH2-Domain Proteins in the Retinal Pigment Epithelium. PLoS One. 2013 doi: 10.1371/journal.pone.0053964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shisheva A, Chinni SR, DeMarco C. General role of GDP dissociation inhibitor 2 in membrane release of Rab proteins: modulations of its functional interactions by in vitro and in vivo structural modifications. Biochemistry. 1999;38:11711–11721. doi: 10.1021/bi990200r. [DOI] [PubMed] [Google Scholar]
- 72.Smart JE, Oppermann H, Czernilofsky AP, Purchio AF, Erikson RL, Bishop JM. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src) Proc Natl Acad Sci U S A. 1981;78:6013–6017. doi: 10.1073/pnas.78.10.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steele-Mortimer O, Gruenberg J, Clague MJ. Phosphorylation of GDI and membrane cycling of rab proteins. FEBS Lett. 1993;329:313–318. doi: 10.1016/0014-5793(93)80244-o. [DOI] [PubMed] [Google Scholar]
- 74.Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–670. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- 75.Strick DJ, Feng W, Vollrath D. Mertk Drives Myosin II Redistribution during Retinal Pigment Epithelium Phagocytosis. Invest Ophthalmol Vis Sci. 2008;50:2427–2435. doi: 10.1167/iovs.08-3058. [DOI] [PubMed] [Google Scholar]
- 76.Strobl-Wildemann G, Kalscheuer VM, Hu H, Wrogemann K, Ropers HH, Tzschach A. Novel GDI1 mutation in a large family with nonsyndromic X-linked intellectual disability. Am J Med Genet A. 2011;155A:3067–3070. doi: 10.1002/ajmg.a.34291. [DOI] [PubMed] [Google Scholar]
- 77.Traxler P, Bold G, Frei J, Lang M, Lydon N, Mett H, Buchdunger E, Meyer T, Mueller M, Furet P. Use of a pharmacophore model for the design of EGF-R tyrosine kinase inhibitors: 4-(phenylamino)pyrazolo[3,4-d]pyrimidines. J Med Chem. 1997;40:3601–3616. doi: 10.1021/jm970124v. [DOI] [PubMed] [Google Scholar]
- 78.Vieira OV, Bucci C, Harrison RE, Trimble WS, Lanzetti L, Gruenberg J, Schreiber AD, Stahl PD, Grinstein S. Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol Cell Biol. 2003;23:2501–2514. doi: 10.1128/MCB.23.7.2501-2514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wasmeier C, Hume AN, Bolasco G, Seabra MC. Melanosomes at a glance. J Cell Sci. 2008;121:3995–3999. doi: 10.1242/jcs.040667. [DOI] [PubMed] [Google Scholar]
- 80.Wu Y, Singh S, Georgescu MM, Birge RB. A role for Mer tyrosine kinase in alphavbeta5 integrin-mediated phagocytosis of apoptotic cells. J Cell Sci. 2005;118:539–553. doi: 10.1242/jcs.01632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Control conditions were evaluated for the experiment shown in Fig. 7, in which RPE-J cells were fed OS labeled with fluorescent dye (DyLight 405 nm) and immunostained for MERTK (AlexaFluor-488 secondary antibody; green channel) and GDI1 (AlexaFluor-647 secondary antibody; red channel). (A) Top row in which OS were omitted shows the absence of spectral bleed-through into the OS channel. Note also that MERTK and GDI1 occasionally colocalize in the absence of outer segments, suggesting either a common membrane domain they cycle through or alternate ligands that activate MERTK and subsequently colocalize and activate GDI1. (B) Middle row in which MERTK antibody was omitted shows the absence of spectral bleed-through into the green channel. This absence of bleed-through also serves as a control for the SRC image in Fig. 7B. Note the colocalization of OS and GDI1. C) Bottom row in which GDI1 antibody was omitted shows the absence of spectral bleed-through into the red channel. Given the similar fluorescent intensities of MERTK and SRC staining, the lack of bleed-through in (C) also serves as a control for the SRC image in Fig. 7B. Note the colocalization of OS and MERTK. The absence of fluorescence in the negative controls and the unique fluorescence patterns seen in each test panel both confirm the absence of spectral bleed-through and further document the strong colocalization of MERTK and GDI1 with OS. Scale bars are 10 µm.
Tryptic peptides from spot 4DA measured by MALD-TOF. The protein was identified as RAB GDP dissociation inhibitor (GDI1), Uniprot P50398. Measured masses from the MALDI-TOF spectrum are compared to calculated values. Masses listed represent 57% sequence coverage. MS-Fit MOWSE score: 1.38e+06; Mascot score 142, expect value 1.6e-10. amu = atomic mass unit (Daltons).