Abstract
People routinely make poor choices, despite knowledge of negative consequences. We found that individuals with Anorexia Nervosa, who make maladaptive food choices to the point of starvation, engaged the dorsal striatum more than healthy controls when making choices about what to eat, and that activity in fronto-striatal circuits was correlated with their actual food consumption in a meal the next day.
A fundamental challenge in the study of human behavior is to understand why people persistently make choices that are bad for them, even when they seem to know better. This paradox has intrigued philosophers and scientists for centuries and has led to many theories regarding the cognitive and psychological processes by which individual choices are made. Yet many open questions remain about the neurobiological mechanisms underlying persistent maladaptive choices and how such mechanisms relate to choices in everyday life1.
In Anorexia Nervosa, repeated, maladaptive food choices result in starvation accompanied by significant morbidity and mortality. While Anorexia Nervosa is a complex illness with many features, one highly stereotyped phenomenon is the persistent selection of low-calorie and low-fat food2-4. Clinically, this pattern of behavior has often been understood as the manifestation of a remarkable but misguided ability to override primary drives; that is, as an expression of single-minded, goal-directed self-control5. Yet, when goals change, as when individuals elect to enter treatment in order to gain weight, their ability to alter their pattern of food choice is exceedingly poor and, given the opportunity, they continue to choose low-fat and low-calorie foods6,7. Thus, Anorexia Nervosa provides a unique and compelling model of persistent maladaptive behavior.
To examine the neurobiological mechanisms underlying persistent maladaptive food choices in Anorexia Nervosa, we used fMRI to compare BOLD activity among women newly hospitalized for treatment of Anorexia Nervosa to that of healthy, female controls (Supplementary Table 1). Participants engaged in a food choice task that captures the salient eating behavior of Anorexia Nervosa—restrictive caloric intake8. The task was adapted from Hare et al.9 toprovide a range of foods that are part of a normal diet. In the first two of three phases, participants rated the healthiness and the tastiness of 76 food items (Fig. 1a, Supplementary Table 2). After completion of the ratings, a “Reference” item was randomly selected for each participant from items the participant had rated as “Neutral” in both taste and health. In the third phase, each participant made a series of choices between the Reference item and each of the other foods. This task allowed an individual's ratings of food to determine her Reference item, which was critical in comparing behavior of healthy controls with that of individuals with Anorexia Nervosa, as the two groups were expected to rate the health and taste values of food differently10 (Supplementary Fig. 1). Choices were incentive-compatible—one of the foods chosen in the task was randomly selected for the participant to consume as a snack after scanning. To determine whether choice responses in the task were related to actual eating behavior, we compared responses on the food choice task with caloric intake in a lunch-time meal the next day, using a validated procedure in which participants are presented with a buffet-style array of foods and asked to eat whatever they wish11.
Figure 1. Food choice task and behavioral results.

(a) The task consisted of three phases: Health rating, Taste rating, and Food choice. In the Health and Taste Rating phases (order counterbalanced across participants), each of 76 food items was rated on a 5-point Likert scale. On each trial in the Choice phase, participants indicated their preference for a food item (shown on the right) relative to a repeated Reference item (previously rated neutral on Health and Taste; shown on the left). After completion of the fMRI scan, participants were provided with a snack-sized portion of the food they had chosen in one randomly selected trial.(b) Food choices (over the Reference item) differed between individuals with Anorexia Nervosa (AN, n=21) and healthy controls (HC, n=21) for low- and high-fat foods (Group × Food Type interaction: F(1,40)=32.16, P = 0.000001, mixed 2 × 2 ANOVA). Individuals with AN selected a smaller proportion of high-fat foods (t(40)=7.28, P = 7.6*10-9, two-tailed), but a similar proportion of low-fat foods (t(40)=1.47, P = 0.15, two-tailed). Data are mean±s.e.m. (c) During a lunch meal the following day, calorie intake in the AN group was correlated with the proportion of high-fat food choices made in the experimental task (r(14)=0.61, P =0.01, two-tailed, n = 16). This association was not significant for the HC group (r(19)=0.17, P = 0.47, two-tailed, n = 21), possibly because healthy individuals do not manifest the stereotyped eating patterns exhibited by individuals with AN, whose eating behavior is expected to be more consistent from one day to the next.
Food choices were quantified as the proportion of choices of the food item presented on each trial vs. the Reference item, for high-fat (>30% of total calories from fat) and low-fat foods. As anticipated, individuals with Anorexia Nervosa were significantly less likely than healthy controls to choose high-fat foods over the Reference item (t(40)=7.28, P = 7.6*10-9). The groups did not differ in their tendency to select low-fat foods over the Reference item (t(40)=1.47, P = 0.15; Fig. 1b, Supplementary Fig. 1 and 2). This pattern of food choice replicated our previous findings using the same task8 and is consistent with observational studies of eating behavior in individuals with Anorexia Nervosa11,12.
Next, we assessed whether food choices of individuals with Anorexia Nervosa were related to their eating behavior in the buffet lunch meal the following day. Indeed, the frequency with which individuals with Anorexia Nervosa chose high-fat foods in the task was significantly correlated with caloric intake at lunch the following day (r(14)=0.61, P = 0.01; Fig. 1c). Two patients declined to participate in the meal. Three patients reported a subjective loss of control over their eating during the meal (binge eating). Individuals with Anorexia Nervosa who sometimes engage in such binge eating typically restrict their food intake outside of such episodes13,14. Since our focus was on restrictive eating behavior, consistent with prior studies11, data from these three meals were excluded from analyses that included lunch meal measures; data from these individuals were included in all other behavioral and fMRI analyses. Robust regression including the participants who binge-ate also yielded a significant association between task performance and eating behavior (t(17)=2.71, P = 0.015). Thus, the food choice task both captured the hallmark maladaptive restrictive food choice behavior in Anorexia Nervosa and related to actual eating behavior.
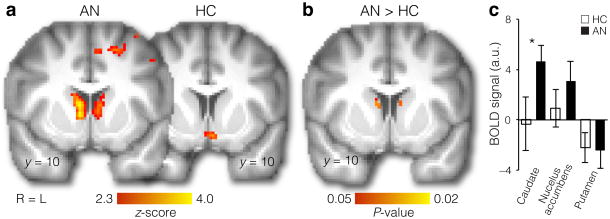
To investigate the neural mechanisms underlying food choice in Anorexia Nervosa, we performed a parametric analysis of BOLD activity during the Choice phase, using choice preferences (as indicated on a 5-point Likert scale) and comparing activity correlated with choice preferences between healthy controls and individuals with Anorexia Nervosa. We hypothesized that the persistent maladaptive eating behavior of individuals with Anorexia Nervosa is associated with imbalances in striatal sub-regions and in fronto-striatal circuits. Emerging evidence suggests that there are abnormalities in Anorexia Nervosa within these circuits15-18. Here, we focused on the striatum, known for its role in reinforcement learning and action selection and control19. We were particularly interested in determining whether ventral versus dorsal sub-regions of the striatum were differentially associated with food choice in individuals with Anorexia Nervosa. The parametric Choice phase analysis indicated that, for individuals with Anorexia Nervosa, food choices were related to neural activity in the dorsal striatum (caudate; Fig. 2a), significantly more than for healthy controls (Fig. 2b). In contrast, we found no between-group differences in BOLD activity in the ventral striatum (Fig. 2b and c). These results suggest that the dorsal striatum, but not the ventral striatum, contributes to maladaptive food choices in Anorexia Nervosa. Notably, the difference in response in the dorsal striatum was selective to the Choice phase: we found no differences between the groups in dorsal striatal activity during the earlier Taste or Health rating phases (Supplementary Fig. 3a). There were also no differences in dorsal striatum BOLD activity between high- vs. low-fat foods within each group(Supplementary Fig. 3b).
Figure 2. Distinct neural systems engaged in food choice.
(a) Regions correlated with choice values in AN (left, n = 21) and HC (right, n = 21) (FWE-corrected P < 0.05 whole-brain, cluster-forming threshold Z>2.3). In AN (left), a correlation with choice values was observed in dorsal striatum, whereas no above threshold correlation was observed in HC (right). Additionally, no above-threshold correlations were observed in ventral striatum in either group, and no differences were found between HC and AN groups, even at a liberal threshold (P < 0.1 tfce in bilateral nucleus accumbens). (b) Parametric analysis of choice strength showed significantly greater activity in the dorsal striatum in the AN than in the HC group (left, P < 0.05 tfce in bilateral caudate). Age was included as a covariate in this analysis, but the same pattern of results was obtained when not including age as a covariate.(c) To illustrate the pattern across dorsal and ventral striatum, in an independent analysis, we extracted data from the right caudate, right nucleus accumbens, and right putamen (all anatomically defined). Differences between the HC and AN groups were seen in the caudate (t(40) = −2.04, P = 0.048), but not in the nucleus accumbens (t(40)= −1.02, P = 0.315) or putamen (t(40) = 0.11, P = 0.913). Data are mean ±s.e.m.
Consistent with previous findings9, we found robust vmPFC responses correlated with choice preferences in both healthy controls and individuals with Anorexia Nervosa (Supplementary Fig. 4 and 5, and Supplementary Table 3). However, vmPFC activity did not differ between groups. Thus, we extend previous work showing choice related activation in the vmPFC across a range of individuals varying in their food restriction, in addition to the novel finding that distinct brain regions contribute to food choices in Anorexia Nervosa.
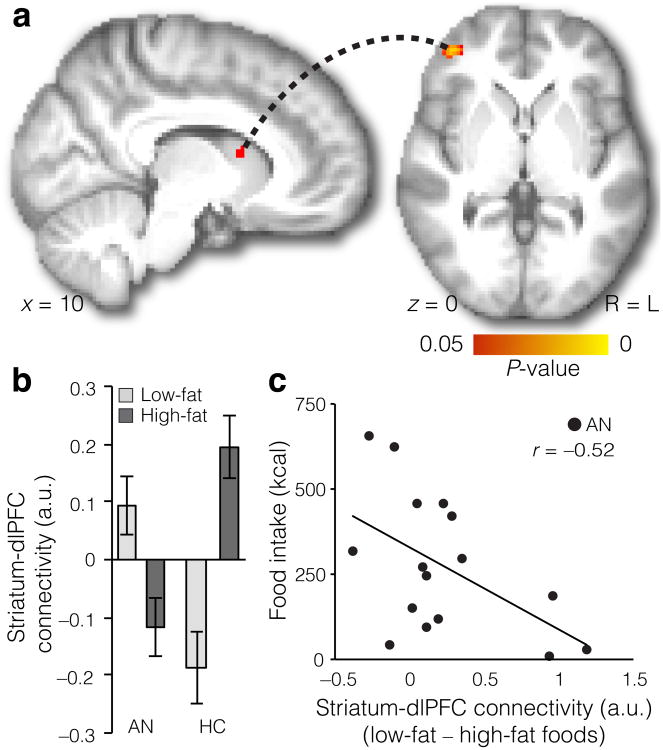
To examine the possible role of fronto-striatal circuits in food choice in Anorexia Nervosa, we performed a functional connectivity analysis (psychophysiological interaction; PPI). Specifically, we examined whether fronto-striatal connectivity differed between trials when participants faced high-fat and low-fat food options. We used the dorsal striatum region identified in the choice analysis above as a seed and high- versus low-fat food stimuli as a categorical psychological modulator. This analysis revealed a peak in dorsolateral PFC, with healthy controls showing stronger connectivity for high- relative to low-fat foods compared to individuals with Anorexia Nervosa (Fig. 3a, Supplementary Table 4). Specifically, individuals with Anorexia Nervosa showed greater connectivity for low-fat foods than for high-fat foods, whereas healthy controls showed the opposite pattern (Fig. 3b). Next, we asked whether connectivity differences for low-vs. high-fat foods were related to actual food intake in the individuals with Anorexia Nervosa. Greater connectivity differences for low-fat versus high-fat food stimuli (i.e., the magnitude of the difference between connectivity in the low-fat condition and the high-fat condition) were associated with lower caloric intake the following day (r(14)=–0.52, P = 0.04; Fig. 3c, Supplementary Fig. 6; robust regression results including all participants with Anorexia Nervosa: t(17)=–2.11, P =0.05). This finding suggests that a circuit between dorsolateral PFC and dorsal striatum may play an important role in maladaptive food choices in Anorexia Nervosa.
Figure 3. Food choice is related to functional connectivity between the striatum and the dlPFC.
(a) Differential connectivity between dorsal striatum and prefrontal cortex in HC (n = 21) vs. AN (n = 21)for high- vs. low-fat foods (FWE-corrected P < 0.05 tfce in PFC). Age was included as a covariate in this analysis, but the same pattern of results was obtained when not including age as a covariate.(b) In AN, connectivity was greater for low-fat foods, whereas in HC connectivity was greater for high-fat foods. (Data were extracted from 6 mm radius sphere located at the peak voxel for the HC > AN contrast, MNI = [42 48 0], for illustration purposes). Data are mean ±s.e.m. (c) In AN, the difference in connectivity between low- and high-fat foods was correlated with actual food intake (kcals) eaten in an experimental meal the following day (r(14)=−0.52, P = 0.04, two-tailed, n = 16).
This study examined the neural correlates of maladaptive food choices in individuals with Anorexia Nervosa. Prior neuroimaging studies of Anorexia Nervosa have examined the neural responses to passive viewing of food17,20 without examining the neurobiological mechanisms underlying active food choices. Our results suggest that a direct examination of food choice may be particularly useful in probing the neural basis of the persistent maladaptive behavior that is a salient characteristic of this disorder.
As restrictive food intake is a persistent and clinically challenging problem in Anorexia Nervosa, these findings provide new insight into the neural mechanisms underlying this enigmatic illness. Furthermore, as human and animal data have documented that the dorsal striatum plays a critical role in the establishment and expression of action control and learned automatic behaviors, the current results are consistent with the possibility that the persistent, maladaptive food choice in Anorexia Nervosa is subserved by fronto-striatal networks that are crucial for the development of habitual behavior19. More broadly, the current study adds to the growing evidence that disturbances in fronto-striatal circuits play a crucial role in persistent maladaptive human behaviors.
Online Methods
Participants
Participants were 21 inpatients with Anorexia Nervosa (AN) at the New York State Psychiatric Institute/Columbia University Medical Center Eating Disorders Research Clinic, studied within 24 hours of hospital admission, and 21 healthy controls (HC) (Supplementary Table 1). No statistical methods were used to predetermine sample sizes but our sample sizes are similar to those reported in previous publications9,16. This study was approved by the New York State Psychiatric Institute Institutional Review Board, and all participants provided written informed consent. Eligible patients met DSM-521 criteria for AN, restricting (AN-R, n=10) or binge-purge (AN-BP, n=11) subtype, and were receiving inpatient treatment. Diagnosis was made by the Structured Clinical Interview for DSM-IV (SCID)22, and the Eating Disorder Examination (EDE)23. HC called the clinic in response to advertisements. Individuals were excluded if they were taking psychotropic medications, had a known history of a neurological disorder or injury, reported drug or alcohol abuse in the last 6 months, were currently dieting, or had significant weight fluctuations in the past 6 months. HC were included if they had no current or past psychiatric illness, including any history of an eating disorder, and had a BMI between 18 kg/m2 and 25 kg/m2. Additional exclusion criteria for HC were significant medical illness or dietary restrictions (such as vegetarianism, or religious restrictions that would impact food choice in the task). All participants were right-handed, female, aged 16-39 years. Inpatient treatment at NYSPI is provided at no cost for those interested in and eligible for participation in research. HC were compensated $125 for their time.
Procedure
Study procedures occurred over 2 days. Pre-procedure food intake was standardized on each day and participants were instructed to have nothing to eat or drink, except water, between the standardized meal and study participation. Day 1 began with a research lunch at 12 pm consisting of ∼550 kcal (turkey sandwich, Nutrigrain bar, 8 oz water) followed by the food choice task with fMRI scanning at 2 pm. At the end of the task, one of the trials was randomly selected, and the food item chosen on that trial was identified. Immediately after the scan session, at ∼3 pm, participants received a snack-sized portion of this food item to eat, observed by staff. Day 2 began with a research breakfast consisting of ∼300 kcal (8 oz juice, English muffin, pat of butter) at 8 am, followed by a laboratory Multi Item Meal (see below) at 1 pm, providing an objective measure of food choice.
Height and weight were measured on a beam balance scale (Detecto, Webb City, MO) in the morning of Day 1 and Day 2. Prior to scanning on Day 1, participants were administered a demographics questionnaire, the Eating Disorder Examination Questionnaire (EDE-Q)24, and the Three Factor Restraint Questionnaire (TFEQ)25. Other psychological measures at the time of scanning included Spielberger Anxiety Inventory, State version (STAI-S) and the Profile of Mood States (POMS).
Experimental food task (scanned)
The food choice task8 was adapted from Hare et al.9 to include images with a greater range of caloric density and macronutrient content. The food items were chosen to be representative of a range of dietary choices that would be encountered in the environment (Supplementary Table 2), instead of emphasizing “junk foods” and “healthy snack foods,” as in the original task.
The task consisted of 3 phases: Health rating, Taste rating, and Food Choice (see Fig. 1a). Seventy-six food items were presented in each phase (38 high-fat, 38 low-fat). We defined “low-fat” as items with < 30% of total calories from fat, as determined by our staff research nutritionist. The foods represented a range of dietary choices as well as a range from low to high fat content. In each phase, participants were presented with a series of images of food and asked to rate each one. Food images were high-resolution color photographs of food arranged on white plates on a black background and were prepared by a food photographer. Participants first rated the food items for tastiness and healthiness on a 5-point Likert scale in two separate phases. The rating scale appeared at the bottom of the screen for each item and participants were instructed that they could rate it as “Neutral” or along the scale. For the Health phase, the anchors were identified as “Unhealthy” to “Healthy” (Fig. 1a, left panel). For the Taste phase, the anchors were “Bad” to “Good” (Fig. 1a, middle panel). For the Taste phase, participants were additionally instructed to rate it only for tastiness. All task parameters (order of the rating phases, direction of rating scale, and trial orders) were counterbalanced and randomized across participants.
After completion of the rating scales, a “Reference” item was selected for each participant that had been rated by that participant as “Neutral” in both Taste and Health phases. The computer program randomly selected one item among items rated “Neutral” in both Health and Taste phases. If an individual did not identify an item as neutral on both scales, an item neutral on Health and positive on Taste was selected, if possible. This method aimed to minimize biasing choices based on taste value. Most participants (20 HC and 19 AN) had Reference items rated neutral on both scales. One HC and 1 individual with AN had a Reference item neutral on Health and rated 4 on Taste; 1 individual with AN had a Reference item neutral on Health and rated 2 on Taste. The choice behavior of these three participants was similar to that of other members of their group.
Participants were then presented with the Choice phase of the task in which they were instructed to identify on each trial whether they chose to eat the Reference item or the other food item presented. The Reference item remained the same throughout the Choice phase and, on each trial, was presented on the left of the screen with a different food item on the right side of the screen. The images were presented side by side to ensure that participants were aware that they were making a choice relative to a Reference food and to minimize the risk of individuals with AN answering according to a valuation of each individual food (i.e. rejecting food rather than choosing food). On each trial, the participant indicated their preference via a Likert Scale on the bottom of the screen, anchored by Strongly Prefer on the left for the Reference item and Strongly Prefer on the right for the other item (Fig. 1a, right panel).
Importantly, participants were instructed that they would be served a snack-sized portion of one of their choices, selected at random, as a snack following the task. Thus, participants' responses should reflect actual preferences. At 3 pm, a snack portion of the selected food item was provided (control participants ate a mean of 88.2±18.5% of their snack). After scanning was completed, participants were debriefed and asked about how difficult they found it to rate the healthiness and tastiness of the food images.
In all phases, the food stimulus was presented for 4 seconds on each trial, during which participants made their response. Each trial was followed by a fixation cross during the inter-trial interval (ITI). The duration of ITIs was jittered for optimization of event-related fMRI design. Stimulus presentation sequence and timing were optimized using the optseq2 algorithm (http://surfer.nmr.mgh.harvard.edu/optseq/). Each learning run lasted 480 s. Mean ITI=2.3 s, median=2 s, and range =1–10 s, across all three phases. All task phases were presented using Matlab (Natick, Massachusetts) and the Psychophysics toolbox (Brainard, 1997).
Multi Item Meal11
On Day 2, participants were escorted to the eating behavior laboratory at 1 pm, after 4.5 hours of fasting. Participants entered the testing room, where a multi-item buffet was arranged on a table. Participants were given the neutral instruction: “This is your lunch for today. Eat as much as you like.” The multi-item buffet was similar to that employed by Mayer et al.11 and consisted of a range of foods including salad, salad dressing, grilled chicken, fried chicken, tuna, bread, fruit salad, Oreo cookies, ice cream, water, soda (details available upon request). Participants signaled the end of the meal by ringing a bell. Food was weighed before and after (Acculab 7200 balance, readability 0.1g) and amount consumed in grams, kcal, and macronutrient content (e.g., % kcal from fat) were calculated. All participants were asked in post-meal debriefing whether the meal had felt like a binge.
Data analysis
Choices on the 5-point scale were converted to binary responses (yes or no preference for the Reference Item versus the trial-unique food item) and neutral responses were omitted. The proportion of choices of the trial-unique food over the Reference item was calculated for high-fat and low-fat foods separately and submitted to analysis. The mean ratings of healthiness and tastiness on the 5-point scale were also averaged for high-fat versus low-fat foods.
Median response times were calculated for high and low fat trials for each participant. Individuals with AN are known to have general motor slowing secondary to malnutrition26. To account for this and to facilitate comparison across task phases, each participant's response times were normalized by dividing by their average response times across all task phases.
Summary data were analyzed using mixed ANOVA [2(HC/AN) × 2 (high-fat/low-fat)] within the IBM SPSS Statistics 22 analysis package.
To understand the influence of health and taste ratings on choices and the relationship between health and taste ratings, we used multilevel regression models (lme4 linear mixed effects package for R)27. Binomial choice data were modeled with multilevel logistic regression, in which participant choice (selection of the trial-unique food item over the Reference food) was the dependent variable. Continuous outcome rating data were modeled using multilevel linear regression. When entered as independent variables, continuous rating data were z-scored. The significance of the partial correlation coefficients was assessed by χ2 statistics (and accompanying P values) which were derived for the estimates from Type-III analysis of variance tables from the Anova function in the car package for R28 or from the esticon function in the doBy package when contrasting regression parameters. All within-subject effects were entered as random by participant29.
Pearson correlation was used to assess the relationship between food task choices and real food intake (lunch meal data) in HC and individuals with AN separately. Two individuals with AN declined to participate in the laboratory meal. Three patients reported a subjective loss of control over their eating during the meal (binge eating; caloric content of these meals was 5377, 4354, and 930 kcals). Because our focus was on restrictive eating behavior, data from these three meals were excluded from analyses that included lunch meal measures but were included in all other behavioral and fMRI analyses. Due to the presence of such outliers in the lunch meal data, we verified results with robust regression analyses implemented using the rlm function in the MASS package with bisquare weighting30. Demographic characteristics were compared between diagnostic groups (AN vs. HC) using independent sample t-tests.
Tests were two-tailed. If unequal variances were indicated, degrees of freedom were adjusted accordingly. Data distribution was assumed to be normal but this was not formally tested.
fMRI data acquisition
Whole-brain imaging was conducted on a 3.0T Phillips MRI system at Columbia University's Program for Imaging and Cognitive Sciences, using a SENSE head coil. Structural images were collected using a high-resolution T1-weighted MPRAGE pulse sequence (1 × 1 × 1 mm voxel size). Functional images were collected using a gradient echo T2*-weighted echoplanar (EPI) sequence with blood oxygenation level-dependent (BOLD) contrast (TR = 2000 ms, TE = 19 ms, flip angle = 77, 3 × 3 × 3 mm voxel size; 46 contiguous axial slices). For each functional scanning run, four discarded volumes were collected prior to the first trial to allow for magnetic field equilibration.
fMRI data analysis
Pre-processing
Imaging data were converted from DICOM to NIFTI format and preprocessed and analyzed using the FSL (http://fsl.fmrib.ox.ac.uk/fsl/) package version 5 [FMRIB's Software Library; Oxford Centre for Functional Resonance Imaging of the Brain (FMRIB), Oxford University, Oxford, U.K. 31]. Functional images were aligned using the MCFLIRT tool32. The skull was removed from functional and structural images using the brain extraction tool (BET)33. Spatial smoothing was applied with a Gaussian kernel of 5 mm (FWHM). Data and design matrix were high-pass filtered with a cutoff period of 100 s. After analysis at the individual level, the results were normalized to a standard template. Given potential brain atrophy in individuals with AN due to malnutrition, we performed the following steps to improve registration. Functional images were first aligned to the T1-weighted MPRAGE using a boundary-based registration method implemented in FSL5 (BBR) and then the MPRAGE to the standard MNI152 2 mm template using FLIRT (12 degrees of freedom) and FNIRT (10 mm warp resolution)32,34.
Analyses
For all analyses, at the level of the individual participant, each event was convolved with a canonical hemodynamic response function (HRF) and entered into a general linear model (GLM) (see below for specific models). In order to account for any residual effects of subject movement, we included the six scan-to-scan head motion parameters estimated during motion correction as well as their derivatives and the squares of the motion parameters and derivatives as confound regressors in our model. Confound regressors in addition to the motion parameters were included to remove the effects of the first volume of each fMRI scan from analyses, as quality controls indicated that discarding 4 dummy scans was insufficient for magnetic field equilibration. Finally, volumes affected by moderate scan-to-scan movement (∼1 mm) were also modeled with confound regressors. This affected 5 HC and 1 individual with AN, with a total of 16 volumes affected across all scans in all participants (out of 126 scans of 240 volumes each). One fMRI scan was excluded completely (a Taste rating scan from 1 HC) due to excessive head motion (> 4mm). No remaining scans had movement exceeding 2mm. Linear contrasts were performed on specific comparisons of interest. These contrasts were used for mixed-effects group analyses using FSL's FLAME 1 (FMRIB's local analysis of mixed effects) tool, using two-sample unpaired t-tests. Higher-level analyses were thresholded using clusters determined by Z> 2.3 and a whole-brain corrected, family wise error (FWE) cluster significance threshold of P = 0.05. Between-group differences in a priori regions of interest (ROIs) were assessed using the threshold-free cluster-enhancement (TFCE) option in the Randomise permutation-testing35 tool (v2.9) in FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise36) with 5000 permutations. The significance threshold was set at P < 0.05, FWE-corrected for multiple comparisons. Additionally, age was included as a covariate in between-group analyses because the AN group was slightly older (Supplementary Table 1). For all analyses, the same pattern of results was obtained when age was not included as a covariate.
Anatomical ROIs for the dorsal striatum (caudate and putamen), ventral striatum (nucleus accumbens), and the prefrontal cortex (PFC) were obtained from the Harvard-Oxford probabilistic atlas included in FSL. All regions were thresholded at 25% probability. For the caudate and putamen, left and right structures were combined to create bilateral ROIs. The PFC ROI was created by combining the following regions bilaterally: Frontal pole, superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus (triangularis and opercularis), frontal medial cortex, subcallosal cortex, paracingulate gyrus, cingulate gyrus anterior division, and frontal orbital cortex. VMPFC ROIs were identified from previous studies of value based choice: a meta-analysis by Bartra et al.37 and the study of Hare et al.9 Additional ROIs were obtained by creating spherical 6 mm radius masks centered on functionally defined regions from the present study or from previous studies. FSL's Featquery tool was used to extract data from ROIs. The main analyses were conducted in a priori striatum and PFC ROIs. Whole-brain analyses were conducted to provide a fuller characterization of results for information purposes and are presented in Fig. 2a, Supplementary Fig. 4a, and Supplementary Table 3.
Parametric choice and rating analyses
Because participants' preferences differ, the conservative strategy to analyzing data under such circumstances is to conduct parametric analyses of each participant's choices so as to eliminate this variation (e.g.38,39). The parametric analysis allows for identification of activity associated with the choice process despite individual variability in behavior. Each person's choices are normalized to their own response range and therefore the analysis is not biased by overall differences in choice preferences.
The GLMs for the choice and rating phases included the following regressors
1) onsets for each trial on which a response was made, 2) onsets for each trial where a response was made parametrically modulated by the rating made on that trial (demeaned), 3) onsets for each trial where a response was made parametrically modulated by the response time on that trial (demeaned), and 4) onsets for missed trials. Regressor 1-3 were modeled with a duration equal to the response time (RT) on each trial, and missed trials with duration equal to the trial length (4 s). The RT regressor was orthogonalized with respect to the parametric rating regressor. Motion and confound regressors were included as outlined above. In order to assess responses separately for low- and high-fat trials, we ran an additional model including separate onsets for low-fat trials, high-fat trials, onsets for low-fat trials parametrically modulated by the ratings made on low-fat trials (demeaned), and onsets for high-fat trials parametrically modulated by the ratings made on high-fat trials (demeaned). A regressor for missed trials and motion and confound regressors were included as well.
Response bin analysis (Choice phase)
To further assess the results from the parametric analysis described above, we performed an additional analysis including onsets for each of the response bins from 1-5. Additionally, a regressor for missed trials and motion and confound regressors were included.
Psychophysiological interaction (PPI) analyses
To assess functional connectivity we estimated three PPI models. Our approach followed that outlined for PPI implementation in FSL's Feat module (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/PPIHowToRun). The seed region for the PPI analyses was the caudate region identified in the parametric choice phase analysis including age as a covariate (Fig. 2b). A mask was created in standard space and transformed into each participant's functional native space. The resulting individual masks were used to extract the time-course from the seed region for each participant.
The first model included a psychological regressor comparing high- versus low-fat foods (coded as 1 and -1, respectively) convolved with an HRF, a physiological regressor (the time-course extracted from the seed region), and the interaction between the two. In addition, we included a regressor with onsets for high- plus low-fat trials and a regressor for missed trials. To follow up on the results from the model comparing connectivity for high- versus low-fat foods, we ran two additional models, assessing high-fat and low-fat trials separately. To assess connectivity on high-fat food trials, our model included a psychological regressor indicating onsets for high-fat trials, the seed region time-course, and their interaction. Additionally, we included a regressor indicating the onsets of low-fat trials and a regressor for missed trials. To assess connectivity on low-fat food trials, our model included a psychological regressor indicating onsets for low-fat trials, the seed region time-course, and their interaction. Additionally, we included a regressor indicating the onsets of high-fat trials and a regressor for missed trials. Motion and confound regressors in all PPI analyses were included as outlined above.
To assess the relationships between connectivity and other experimental measures we extracted data from an ROI centered on the peak voxel resulting from the PPI analysis and created a 6 mm radius sphere surrounding this voxel (MNI=[42 48 0]).
For all results, voxel locations (x-y-z values) are reported in Montreal Neurological Institute (MNI) space. Results are displayed on a study specific mean anatomical image resulting from averaging all participants' normalized high-resolution structural images.
A supplementary methods checklist is available.
Supplementary Material
Acknowledgments
We thank Janet Schebendach, PhD, for guidance and support for the laboratory meals and nutritional analyses. This research was supported in part by the Global Foundation for Eating Disorders and the National Institute for Mental Health (R01 MH079397, K23 MH076195).
Footnotes
Author contributions: K.F, J.S, D.S., and B.T.W. designed the research; J.S. collected the data; K.F. and J.S. analyzed the data; K.F, J.S, D.S., and B.T.W. wrote the manuscript.
Competing Financial Interests: The authors declare no competing financial interests.
References
- 1.Marteau TM, Hollands GJ, Fletcher PC. Changing human behavior to prevent disease: the importance of targeting automatic processes. Science. 2012;337:1492–1495. doi: 10.1126/science.1226918. [DOI] [PubMed] [Google Scholar]
- 2.Walsh BT. The importance of eating behavior in eating disorders. Physiol Behav. 2011;104:525–529. doi: 10.1016/j.physbeh.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadigan CM, et al. Assessment of macronutrient and micronutrient intake in women with anorexia nervosa. Int J Eat Disord. 2000;28:284–292. doi: 10.1002/1098-108x(200011)28:3<284::aid-eat5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.Fernstrom MH, Weltzin TE, Neuberger S, Srinivasagam N, Kaye WH. Twenty-four-hour food intake in patients with anorexia nervosa and in healthy control subjects. Biological psychiatry. 1994;36:696–702. doi: 10.1016/0006-3223(94)91179-7. [DOI] [PubMed] [Google Scholar]
- 5.Bruch H. The Golden Cage: The Enigma of Anorexia Nervosa. Vintage Books; 1978. [Google Scholar]
- 6.Schebendach JE, et al. Dietary energy density and diet variety as predictors of outcome in anorexia nervosa. Am J Clin Nutr. 2008;87:810–816. doi: 10.1093/ajcn/87.4.810. [DOI] [PubMed] [Google Scholar]
- 7.Sysko R, Walsh BT, Schebendach J, Wilson GT. Eating behavior among women with anorexia nervosa. Am J Clin Nutr. 2005;82:296–301. doi: 10.1093/ajcn.82.2.296. [DOI] [PubMed] [Google Scholar]
- 8.Steinglass J, Foerde K, Kostro K, Shohamy D, Walsh BT. Restrictive food intake as a choice-A paradigm for study. Int J Eat Disord. 2015;48:59–66. doi: 10.1002/eat.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 10.Drewnowski A, Halmi KA, Pierce B, Gibbs J, Smith GP. Taste and eating disorders. Am J Clin Nutr. 1987;46:442–450. doi: 10.1093/ajcn/46.3.442. [DOI] [PubMed] [Google Scholar]
- 11.Mayer LE, Schebendach J, Bodell LP, Shingleton RM, Walsh BT. Eating behavior in anorexia nervosa: before and after treatment. Int J Eat Disord. 2012;45:290–293. doi: 10.1002/eat.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoner SA, Fedoroff IC, Andersen AE, Rolls BJ. Food preferences and desire to eat in anorexia and bulimia nervosa. Int J Eat Disord. 1996;19:13–22. doi: 10.1002/(SICI)1098-108X(199601)19:1<13::AID-EAT3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.De Young KP, et al. Restrictive eating behaviors are a nonweight-based marker of severity in anorexia nervosa. Int J Eat Disord. 2013;46:849–854. doi: 10.1002/eat.22163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh BT, Kissileff HR, Cassidy SM, Dantzic S. Eating behavior of women with bulimia. Arch Gen Psychiatry. 1989;46:54–58. doi: 10.1001/archpsyc.1989.01810010056008. [DOI] [PubMed] [Google Scholar]
- 15.Oudijn MS, Storosum JG, Nelis E, Denys D. Is deep brain stimulation a treatment option for anorexia nervosa? BMC psychiatry. 2013;13:277. doi: 10.1186/1471-244X-13-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Decker JH, Figner B, Steinglass JE. On Weight and Waiting: Delay Discounting in Anorexia Nervosa Pretreatment and Posttreatment. Biological psychiatry. 2014 doi: 10.1016/j.biopsych.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uher R, et al. Recovery and chronicity in anorexia nervosa: brain activity associated with differential outcomes. Biological psychiatry. 2003;54:934–942. doi: 10.1016/s0006-3223(03)00172-0. [DOI] [PubMed] [Google Scholar]
- 18.Wagner A, et al. Altered insula response to taste stimuli in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology. 2008;33:513–523. doi: 10.1038/sj.npp.1301443. [DOI] [PubMed] [Google Scholar]
- 19.Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 20.Ellison Z, et al. Functional anatomy of calorie fear in anorexia nervosa. The Lancet v. 1998;3521192 doi: 10.1016/S0140-6736(05)60529-6. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. Fifth. American Psychiatric Association; 2013. [Google Scholar]
- 22.Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV-R (SCID) New York State Psychiatric Institute, Biometrics Research; 1987. [Google Scholar]
- 23.Fairburn CG, Cooper PJ. In: Binge Eating: Nature, Assessment, and Treatment. Fairburn CG, Wilson GT, editors. Guilford Press; 1993. pp. 317–360. [Google Scholar]
- 24.Fairburn CG, Beglin S. In: Cognitive Behavior Therapy and Eating Disorders. Fairburn CG, editor. Guilford Press; 2008. pp. 309–313. [Google Scholar]
- 25.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 26.Croxson MS, Ibbertson HK. Low serum triiodothyronine (T3) and hypothyroidism in anorexia nervosa. The Journal of clinical endocrinology and metabolism. 1977;44:167–174. doi: 10.1210/jcem-44-1-167. [DOI] [PubMed] [Google Scholar]
- 27.Bates D, Maechler M, Bolker B. Vol. Version: 0.999999-2. Technical Report. 2011 [Google Scholar]
- 28.Fox J, Weisberg S. An R Companion to Applied Regression. Sage; 2011. [Google Scholar]
- 29.Schielzeth H, Forstmeier W. Conclusions beyond support: overconfident estimates in mixed models. Behavioral ecology : official journal of the International Society for Behavioral Ecology. 2009;20:416–420. doi: 10.1093/beheco/arn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venables WN, Ripley BD. Modern applied statistics with S. 4th. Springer; 2002. [Google Scholar]
- 31.Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 32.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 33.Smith SM. Fast robust automated brain extraction. Hum Brain Mappv. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersson JLR, Jenkinson M, Smith S. FMRIB technical report TR07JA. 2010;2 [Google Scholar]
- 35.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human brain mapping. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage. 2013;76 doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samuelson PA. A note on the pure theory of consumer's behaviour. Economica. 1938;5:61–71. [Google Scholar]
- 39.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.