Introduction
Notable advances have been made in pediatric oncology curative treatments because of the dedicated use of treatment standards containing prescriptive approaches for medical interventions aimed at aggressively treating cancer and managing or preventing physical complications. These treatment approaches are often personalized to the child depending upon the individual response to treatment. Similar advances would likely be achieved in psychosocial palliative care if prescriptive approaches to the psychosocial care of children and adolescents with cancer and their families were formulated and implemented with similar diligence. Disparate sources of evidence are available to support this approach to psychosocial palliative care in the context of childhood cancer, but need to be reviewed and synthesized. As care providers are encouraged to offer attentiveness toward individual preferences of patients and their families facing life-limiting illnesses,1 pediatric and adolescent age oncology patients and their families have identified their psychosocial care needs as both complex and unique from adult psychosocial care needs;2–5 a claimed uniqueness warranting the specific attentiveness of care providers. While many descriptive reports speak to the benefits of earlier integration of palliative care in pediatric and adolescent oncology,6,7 currently there is a paucity of synthesized data. The purpose of this integrated review was to review and coordinate landmark pediatric palliative cancer papers to contribute to the development of the Clinical Practice Guidelines for optimal psychosocial palliative care of children with cancer. To complete this comprehensive integrative review, the study team mapped what is known about the role of palliative care and psychosocial services in pediatric and adolescent cancer care through systematic review and synthesis of published data for navigation of best practices and guideline development.
Lack of standardized psychosocial palliative care guidelines in childhood cancer care may result in inconsistent access to assessments and interventions for pediatric cancer patients and their families. The risks of not standardizing psychosocial palliative care include not knowing what therapeutic approach to use in clinical circumstances, not knowing the basis for psychosocial palliative care outcomes, not being able to explain one’s practice or outcomes, and misapplying a therapeutic approach that causes harm.8
To continue the successful tradition in pediatric oncology, care teams may next consider a standardized approach to psychosocial palliative care support in oncology that benefits the whole and yet can be tailored to the individual.
Methods
Sampling the Literature
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines framed reporting of this review.9,10 Methods of the analysis and inclusion criteria were specified in advance and registered in the PROSPERO systematic review database as Protocol CRD42014009926 (http://www.crd.york.ac.uk/PROSPERO/, accessed 14 December 2014). The integrated approach used in this systematic review drew on primary data extracted from diverse study types: inductive and deductive, theoretical and empirical, experimental and non-experimental. Types of studies included randomized and nonrandomized trials with or without comparison groups; qualitative, quantitative, and mixed method data; prior reviews; expert opinion; and consensus reports. Only published studies were included with dates limited to January 1, 2000 through May 1, 2014. No language restrictions applied. Children, adolescents and young adults with oncologic diagnoses were included as study subjects in addition to their family members. Palliative care studies including patients with non-malignant diagnoses were included only if data for the cancer population was specifically summarized.
The search utilized four databases: PubMed, Cochrane, PsycINFO and SCOPUS (2000 to 2014). Search terms included “palliative care” OR “palliative” OR “hospice” OR “end of life” OR “bereavement” AND “psychosocial” OR “communication” OR “support” OR “quality of life” AND “cancer” OR “neoplasm” AND “child” OR “adolescent” OR “young adult” OR “family” OR “sibling” (using indexed MeSH terms). Two medical librarians independently screened the search strategies. The last search was run on May 20, 2014. The team hand searched issues of two journals not fully indexed in the databases. The reference lists of all included studies were checked for additional studies. The study team reported the risk of bias and quality rating of each manuscript according to quality standard unique to the study type (Table 1). With recognized diversity in included study type, the study team acknowledged each study format as carrying unique vantage point worth inclusion with equal weight. Specifically, qualitative papers offered rare vantage of patient voice, quantitative papers brought numeric insight, and consensus reports carried expert opinion. All included studies were treated with equal weight in determining their contribution to the data synthesis.
Table 1.
Second Order Findings Reported by Publication Type
| ORIGINAL RESEARCH – MIXED METHOD STUDIES | |||
|---|---|---|---|
| Study | Participants | Risk of Bias | Second Order Findings by Care Grouping |
| Edwards, 2008 – United States One-time point cross-sectional interview and survey to determine alignment of parental understanding of prognosis and treatment goals at diagnosis and EoL |
Bereaved parental dyads (n=76 parents) | 1. Yes 2. Yes 3. Yes 4. No 5. No 6. No |
SC: Within couples disagreement on goals at EoL correlated with both parents who reported greater suffering for child (p=0.03). FS: Many families reported their child’s QoL fair/poor and described the child’s death as not very peaceful. DM: Poor agreement was noted about primary goal of care within couples (k=0.07). Agreement within couples not critical factor in allowing both parents to feel they had accomplished their goals for child (78% parents felt goals accomplished). However, both parents were more likely to achieve their goals when at least one parent focused on lessening suffering. |
| Heath, 2009 – Australia Self-report questionnaires and semi-structured interviews on perceived quality of EoL care |
Bereaved parents (n=96 interviewed, n=89 completed surveys) | 1. No 2. Yes 3. No 4. No 5. No 6. Yes |
FS: Of those parents who believed their children were old enough to participate in discussions with their doctor, 90% felt that the primary oncologist included their child to the right extent while 10% of parents felt that their child was not involved enough in discussions. Parents rated the primary oncologist’s care positively when believed the primary oncologist gave bad news in a sensitive and caring manner (p<0.01), gave clear information about what to expect in EoL period (p<0.01), provided a feeling of preparedness for EoL period (p<0.01), and communicated directly with the child (p<0.01). |
| Hinds, 2012 – United States Experimental, non-randomized feasibility study which involved timely placement of a summary of parent decision-making and good parent definition in the medical record as a parent-clinician EoL communication intervention |
Parents (n=62) of 58 children and their oncologists (n=126) | 1. No 2. Yes 3. No 4. No 5. No 6. No |
FS: Six out of tensatisfaction and preferences measures had >97% agreement within three days of intervention (overall parent satisfaction); remained high three months after intervention. SS: Physicians reported intervention helped to reduce tension among clinicians. Soc: The largest category of clinician response (n=75) indicated improved professional interaction (efforts to support parents) and many clinicians (n=22) described increased personal connection with parents through intervention. |
| Jones, 2006 – United States Pilot mixed-method survey on social workers’ perceptions regarding EoL needs |
Pediatric oncology social workers (n=131) | 1. Yes 2. No 3. No 4. No 5. No 6. No |
DM: Social workers recognized that adolescents need control over treatment decisions and choice of where to die. Q: Social workers ranked pain control and symptom management, ability to talk freely about feelings and fears, consistent caregivers, normal childhood activities, companionship, and assistance with telling parents and siblings their concerns as highest child needs. Psych: Supportive counseling and emotional support at EoL recognized as a need; traditional counseling services as well as companionship and guidance. Soc: Social workers emphasized attentiveness to child’s unique and widely affected social network. |
| Saad, 2011 – Lebanon Self-report questionnaires and semi-structured interviews on perceived quality of EoL care |
Bereaved parents (n=29) | 1. No 2. Yes 3. Yes 4. No 5. No 6. No |
DM: Only one third of parents reported involvement in resuscitation and home-based care decision-making options. SC: Children experienced an average of 8.3 symptoms (range 2–12) with fatigue, anorexia, depression, and pain highest prevalence. Parents reported child suffered “a lot/great deal” from ≥1 (93%) and ≥5 symptom (69%) at EoL. FS: While parents rated care as “very good/excellent” (93.1%); 83% of parents identified deficiencies in communication. CB: Barriers to the parent’s adjustment included fear of clinical deterioration (50%) and perceived failure as parent (25%) Q: Themes for improving care included service accessibility and empathy. Psych: Three-fourths of parents suggested improved psychological support, developing social and spiritual support for both children and parents, and care coordination. Soc: Support was the most prevalent facilitator theme (55% mentioned spiritual support, 48% mentioned family support, and 10.3% mentioned friends/health care team relationships). |
| Wiener, 2008 – United States Survey and interview investigation into AYA readiness to discuss advanced care planning using Five Wishes tool. |
AYA patients (n=20); n=10 with cancer and n=10 with HIV | 1.No 2. Unclear 3. No 4. No 5. No 6. No |
DM: 95% of participants reported the advance directive document was “helpful” or “very helpful”; 90% believed that the document would be helpful to others. Participants were more interested in items of how they wished to be remembered or treated (example: “What I want my loved ones to know”) than items of decision-making. |
| Wiener, 2012 – United States Survey and interview to assess perceived usefulness, helpfulness, and stress associated with two advanced care directive tools |
AYA patients (n=52); n=26 with cancer and n=26 with HIV | 1. No 2. Unclear 3. No 4. No 5. No 6. No |
DM: All wishes were rated by at least >85% of respondents as being helpful. Most participants preferred advance care document formatting that included both closed choices and open-ended questions that would reflect their voice. |
| ORIGINAL RESEARCH – QUALITATIVE STUDIES | |||
|---|---|---|---|
| Study | Participants | Quality Rating | Second Order Findings by Care Groupings |
| Bousso, 2012 – Brazil Semi-structured individual interviews with families of a child receiving palliative care at home |
Family caregivers (n=14) of 11 children receiving outpatient palliative care; n=6/11 with cancer | 1. Yes 2. Yes 3. Yes 4. Yes 5. Yes 6. Yes 7. Unclear 8. Yes |
HU: Families preferred taking care of child at home rather than inpatient setting. DM: Mothers primarily bore responsibility for providing care and making decisions. Mothers’ described family agreement as key element to making decisions less burdensome. SC: Family members described feeling overwhelmed by the responsibility of managing symptoms at home, although they worked toward symptom control to keep child home (viewed as helpful to maintain the normality of family environment). CB: Mothers described feeling overwhelmed and alone. Psych: Parents were fearful of what family life would feel like following the death of the child. |
| Cataudella, 2012 – Canada Semi-structured focus group interviews for investigation into the psychosocial experiences of children with brain tumors at EoL. |
Bereaved parents (n=24); neuro-oncology specific | 1. Yes 2. Yes 3. Yes 4. Yes 5. Yes 6. Yes 7. Yes 8. Yes |
Psych: Depression in the neuro-oncology pediatric population was related to decreased QoL, communication difficulties, and change in appearance. Anxiety was related to pain and awareness of death. Themes of post-traumatic growth were described by parents the child taking an active role in care, humor, maturity, empathy, hopefulness, and goal-setting. Soc: Parents recalled their children wanting to maintain peer relationships, attend school, achieve developmental goals, and maintain a sense of control. Moments of remaining connected with others and being treated as non-sick were recalled by bereaved parents as the most meaningful interactions for children with brain tumors at EoL. |
| Contro, 2002 – United States Family interviews as prelude to establishing pediatric palliative care service |
Bereaved family members (n=68); n=28/64 cancer-specific | 1. Yes 2. Unclear 3. Unclear 4. Yes 5. Yes 6. Yes 7. Unclear 8. Unclear |
SC: Family members reported their loved ones were in pain at the EoL, simultaneously then rating pain management as adequate. The authors speculated that parents assumed everything that could be done was being done (or, that parents could not live with the idea that more could have been done to control the child’s pain at EoL). Family members reported home hospice workers were not well trained in pediatric-specific pain management. FS: Families preferred direct, honest, accurate, compassionate communication. Families also felt it essential to feel connected to the provider who talked to them about the impending death of their child. A one-time communication event perceived as insensitive/uncaring was freshly recalled with pain even years after the conversation. Q: Areas of unsatisfactory health delivery were preventable oversights and lack of coordination of services. Soc: Parents reported failure to include Spanish-speaking family members, to meet the needs of siblings, and inconsistent bereavement follow-up. |
| Heath, 2012 – Australia Semi-structured parent interviews on use of CAM in children with cancer during EoL |
Bereaved parents (n=96) | 1. Yes 2. Yes 3. Yes 4. Yes 5. No 6. Yes 7. Unclear 8. Unclear |
SC: Thirty percent (n=27) of children used CAM during EoL. Primary goal of CAM use was to lessen suffering. Perceived benefits for child: relaxation, energy, less pain, positive attitude, more hope, and spiritual strength. FS: Sixty-three percent of parents who used CAM thought the care their child received was excellent-to-good (not significantly different from group not using CAM, p=0.891). Parents who used CAM were significantly more likely to strongly agree/agree that child’s oncologist had provided clear explanations about treatment alternatives (p<0.001). |
| Hinds, 2009 – United States Face-to-face interviews for descriptive, content analysis definition of good parent role. |
Parents (n=62) of children with advanced cancer who had made a non-curative treatment decision within 72 hrs | 1. Yes 2. Yes 3. Yes 4. Yes 5. Yes 6. Yes 7. Yes 8. Yes |
FS: Parents identified positive and supportive staff care efforts: providing comfort, knowing child/family, liking child, pleasant, coordinating care, giving facts, asking about faith, telling parents they are good parents. CB: Achieving internal definition of being a good parent helped parents emotionally survive the dying and death of their child. Psych: To help parents explore their definition of being a good parent may offer parents/clinicians insight into parent choices and preferences and therefore foster psychological congruence. |
| Robert, 2012 – United States Exploratory focus group interview method to perceived quality of care at EoL. |
Bereaved parents (n=14) | 1. No 2. Yes 3. Yes 4. Yes 5. Yes 6. Yes 7. Yes 8. Yes |
CB: Parents felt unable to support their other children and spouse. Q: “Standards of care” arose as an unexpected interview theme depicting processes and negotiation (relationships over rules) and the need for personalized accommodations for caregivers and visitors (including younger siblings). Psych: Emotional care included personalized prognostic communication and EoL discussions tailored to participants. In hindsight, parents described family need for anticipatory grief counseling. Soc: Parents described the need for social support (maintaining social relationships and connections) during the course of a child’s serious illness. Long-term relationships and effective communication with their child’s health care providers improved perception of care. Development of trusted relationships with providers arose as an interview focus. |
| Zelcer, 2010 – Canada Three semistructured focus group interviews to explore EoL experience of children with brain tumors and their families. |
Bereaved parents (n=25); neuro-oncology specific | 1. Yes 2. Yes 3. Yes 4. Yes 5. Yes 7. Yes 8. Yes |
HU: Parents spoke of the importance of access to home health services and feasibility of home as a location of death. SC: Parents described distress of neurologic deterioration and uncontrolled symptoms (seizures) with mention of need for early anticipatory symptom guidance. CB: Parents spoke of the challenge of balancing care of the child with the parents’ own internal struggles and the reality of competing home responsibilities. Q: Home care challenges included suboptimal symptom management, financial and practical hardships, and difficulty with arranging home health services. Psych: Children were described as aware of their deterioration with parental recognition that the child felt frustrated, sad, and depressed by inability to play or partake. Loss of play was described as hardest on child psychologically and painful for parents to witness. Soc: Families who were linked with supportive community physician and care services believed they were supported well at home, whereas families without such network access often felt “lost and abandoned”. |
| ORIGINAL RESEARCH – QUANTITATIVE STUDIES | |||
|---|---|---|---|
| Study | Participants | Risk of Bias | Second Order Findings by Care Groupings |
| Bell, 2010 – United States Retrospective chart review to explore the experience of adolescents dying from cancer |
Charts from adolescents (n=103) who died of cancer | 1. No 2. Unclear 3. No 4. No 5. No 6. No |
HU: The majority of adolescents (n=58) died in the hospital. Nearly half (n=24) of the hospital deaths occurred in the ICU. DM: Discussions about death occurred in most cases (n=80) although n=23 charts were without documentation of EoL discussions. Documentation rarely identified whether the adolescent was included in the discussion. Half of the documented conversations began in the last 30 days of life. EOL discussions more likely to occur in the last 7 days of life (p=0.002) for adolescents with leukemia/lymphoma. Psych: More than a third of adolescents used anxioltyics at EoL. Anxiolytic use was significantly higher during late adolescence (p=0.037). Feelings of loneliness and anxiety interfered with a peaceful death. |
| Bona, 2011 – United States Written survey investigating experiences and satisfaction with a state-funded Pediatric Palliative Care Network (PPCN) |
Caregivers (n=227) of children receiving or having received PPCN services; one-third with cancer diagnosis | 1. Yes 2. No 3. Unclear 4. No 5. No 6. No |
HU: PPCN prevented unnecessary transfers/hospital admissions. CE: Volunteers provided 2296 hrs in 2010; low state administrative overhead noted ($680,850) with 87% direct contract funds to hospice. SC: Families highly valued 24-hour emergency symptom management as part of PPCN. FS: Parents (n=31/36) described their child’s quality of life as “mostly better” as a result of PPCN. CB: Families valued respite services, requesting additional respite coverage. Psych: PPCN included counseling for parents, grandparents, and siblings in addition to providing psychological, social, and/or spiritual support for the child. Soc: Bereavement services provided by PPCN included caregivers, siblings, and other family members for up to 13 months. |
| Bradford, 2012 – Australia Database analysis of implemented toll-free, after-hours PPC telephone service |
Caregivers of n=106 children with cancer | 1. No 2. Yes 3. No 4. Unclear 5. No 6. Yes |
HU: A total of 1,954 after-hour phone calls were placed over eight years with mean duration 11 minutes. Paper alluded to the telephone service sparing unnecessary emergency room visits for these families. SC: Service improved management of symptoms at home with 21% of calls for symptom support. FS: Families appreciated availability, clear communication, and reassurance through phone service. SS: Regional clinic staff felt supported by ability to reach palliative providers more familiar with complex patients. Psych: Parents reported comfort knowing service was available (regardless of phone use): 41% of calls were for communication support; 18% for practical advice; 20% for emotional support. Soc: Phone service reduced families’ sense of isolation when caring for child at home. |
| Dussel, 2009 and 2010 – United States Retrospective questionnaire in 2009 paper; inclusion of vignettes to investigate hastening death discussions in 2010 paper |
Bereaved parents (n=140 in 2009 paper and n=141 in 2010 paper) | 1. No 2. Yes 3. Yes 4. No 5. No 6. No |
HU (2009): Planning LOD associated with more home deaths (72% vs 8%, p<0.001). SC (2010): 34% of parents reported that they would have considered hastening child’s death had the child been in uncontrollable pain while ≤15% would consider this for nonphysical suffering (95% CI, 26%–42%). If the vignette involved a child in uncontrolled pain compared with coma, parents more likely to endorse hastening death (OR, 1.4; 95% CI, 1.1–1.8). The authors emphasized importance of intensive pain management at EoL. Q: “Opportunity to plan LOD” emphasized as an outcome associated with high-quality palliative care. Psych (2009): Parents who planned LOD were more likely to feel very prepared for child’s death (33% vs 12%, p=0.007) and very comfortable with LOD (84% vs 40%, p<0.001) with less decisional regret when LOD when planning/communication had occurred. |
| Hays, 2006 – United States Pre- and post-intervention survey (baseline and 3-month follow-up) to evaluate PPC intervention focused on education for HCPs, shared decision-making, and co-case management |
Families (n=41) of pediatric patients expected to live ≤ 1 year; 34% with cancer | 1. Yes 2. Unclear 3. No 4. No 5. No 6. Yes |
CE: Co-case manager improved families’ appraisal of responsiveness of their health plan, including ease of accessing services, ease of ensuring share of the costs, and clarity of insurance benefits (p<0.05). FS: Parents rated the providers’ ability to keep the child comfortable as significantly better post-intervention (p=<0.05). Q: Parents reported significant improvement in quality of information received by the child regarding condition and prognosis, child’s understanding, care transitions, and promptness of provider response to patient needs (p<0.05). Parents reported significant improvement in emotional support, comprehension, provider communication and sensitivity, and joint decision-making (p<0.05). Psych: Emotional domain of HR-QoL improved post-intervention (p=0.021). |
| Hechler, 2008 – Germany Semi-structured interview investigating quality of EoL care. |
Bereaved parents (n=56) | 1. No 2. Yes 3. No 4. No 5. No 6. Yes |
HU: DNR was more frequent in those who discussed EOL with team (p=0.009). Half of parents were informed of home care option. DM: Two-thirds of parents reported having an EoL planning discussion with care team; 48% of the children died at home even though 88% of the parents chose “at home” as the most appropriate locale in hindsight. SC: All children reported to have experienced at least one “distressing” EoL symptom. CB: 92% of parents experienced “significant impact” on lives after child’s death; n=29 parents observed significant change religious, employment, partnership/marital status, or social contacts and n=15 reported significant financial burdens. Psych: High proportion of children suffered from depression or anxiety at EoL. Majority of children still perceived as happy, displaying good mood and peacefulness. Parental mention of “good/very good” quality of care from psychologist/social worker and spiritual mentor for both child and parents. Soc: n=41 parents reported team contacted them after child’s death while 7/48 reported (15%) not being contacted. |
| Hilden, 2001 – United States Survey of pediatric oncologists to assess attitudes, practices, and challenges associated with EoL care |
Pediatric oncologists (n=228) | 1. No 2. No 3. No 4. No 5. No 6. No |
SC: The majority (91%) of pediatric oncologists scored their pain management skills as 4 or 5 in competency [on 5-point scale] and reported that most of their patients do not die in pain. The authors reflected on discrepancy between physicians’ perception of the child’s pain and the child’s perception of that pain. FS: Oncologists perceived themselves as competent at communicating with dying children and their families and at discussing the transition to palliative care. The authors referred to literature suggesting bereaved families may have found physician communications at EoL vague and confusing. CB: For pediatric oncologists, the function and condition of the child, the presence of severe pain, and the family’s caregiving burden were less of an influence in shifting from curative to palliative intent than was the availability of an effective therapy. Psych: Many surveyed oncologists described ready access to psychosocial staff, but suggested that a multidisciplinary approach to terminal care was not yet incorporated into mainstream. Overall, pediatric oncologists did not feel competent managing depression in children with 58% rated their skill level as less than competent. Feelings of anxiety about having to manage “difficult symptoms” in a dying child were reported by 48%. Some pediatric oncologists reported a sense of personal failure at the prospect of patient’s death. |
| Hunt, 2006 – Sweden Questionnaire to examine the impact of care and illness factors on maternal and paternal thoughts about child’s death |
Bereaved parents (n=449) | 1. No 2. Unclear 3. No 4. No 5. No 6. No |
DM: Maternal thought that death would be best for the child was related to the child’s ability to communicate during the final month of life, the child being confined to bed, parent awareness of the child’s pending death, having talked to the child about what is important to the child, and whether the child had ever experienced unrelieved pain. Fathers thought the child’s death would be best for the child when the father was emotionally aware of the time for the child’s death, age of the child at death (<10 years), the context of children who had been ill for six or more years, and the child having unrelieved pain (p<0.05). Psych: The child’s fear of death was prioritized in parental consideration. |
| Johnston, 2008 – Global Cross-sectional survey exploring the institutional practices and resources surrounding EOL care at COG institutions in 2005 |
Principal Investigator or his/her designee (n=187) | 1. No 2. No 3. No 4. No 5. Nov 6. No |
Q: Only 58% of the COG institutions had palliative care teams in 2005. The team consisted of physicians (91% of institutions); social workers (78%); spiritual care workers (70%); nurses (60%); nurse practitioners (59%); bereavement counselors (39%); psychologists (37%); volunteers (27%); expressive therapists (26%); nutritionists (21%); and child life workers (10%). A hospice service was available in 65% of the institutions. SC: The majority of institutions (83%) allowed children to receive both chemotherapy and to be enrolled onto Phase I, II or III clinical trials while also receiving palliative care services. Institutions with a palliative care team were more likely to offer CAM therapies (P = 0.03) and have a pain service (P = 0.02). Psych: A psychosocial support team was available in 80% of the institutions with teams dedicated to pediatric oncology patients 81% of the time. Soc: Bereavement programs dedicated to pediatric oncology patients were available at 59% of the institutions. |
| Kreicsberg, 2004 – Sweden Questionnaire to investigate whether parents had talked with their child about death and regret associated with decision |
Bereaved parents (n= 449 answered survey, 429 stated whether or not they had talked to their child about death) | 1. No 2. Unclear 3. Yes 4. No 5. No 6. No |
CB: None of the 147 parents who talked with their child about death regretted conversation. In contrast, 69/258 parents (27%) who did not talk with their child about death regretted decision. Parents who sensed child was aware of imminent death were more likely to regret not having talked about death (47%) than were those who had not sensed this awareness (13%) with RR 3.7. Psych: Authors recognized that children are often aware of their imminent death; emphasized fostering the child’s inner life (awareness of their imminent death) and the outer reality (information received from HCP and parents). |
| Lyon, 2013 – United States Experimental, randomized control trial in which the intervention group received three 60-minute family centered ACP sessions |
AYA patients with surrogate decision maker (n=17 intervention dyads and n = 13 control dyads) | 1. No 2. Unclear 3. No 4. No 5. No 6. No |
HU: Intervention dyads were more likely to limit treatment than controls. PS: The intervention AYAs self-reported as better informed about EoL decisions than control group adolescents (p=0.007). Soc: All intervention AYAs endorsed that the surrogate “do what he/she thinks is best at the time, considering my wishes” whereas only 62% control group adolescents endorsed this (p=0.009). ACP allowed families to understand adolescents’ wishes. |
| Mack, 2005 – United States Survey of parents and questionnaire of clinicians to ascertain factors associated with quality EoL care |
Bereaved parents (n=144) and their child’s primary oncologist (n=52) | 1. No 2. Yes 3. No 4. No 5. No 6. No |
FS: High EoL care quality ratings were associated with a parental perception that the primary oncologist: gave bad news in a sensitive and caring manner (p<0.001), provided clear information about what to expect (p<0.001), elicited trust (p<0.001), provided a feeling of preparedness for circumstances surrounding death (p=0.001), and communicated directly with the child (p<0.001). Medical outcomes (including time spent in the hospital and pain control in the last month of life) were not important determinants of parental ratings of quality. SS: Factors associated with physician ratings of care were parent report of pain in the last month of life (p=0.01) and a hospital stay of 10 days or more in the last month of life (p<0.001). |
| McCarthy, 2010 – Australia Structured interview by trained clinical psychologist to examine factors related to burden of illness during EoL care as potential predictors of parental grief and depression outcomes |
Bereaved parents (n=58) | 1. No 2. Unclear 3. No 4. No 5. No 6. No |
Q: Child QoL during treatment and preparedness for death independently predicted depression. Perceived quality of physician care and time since death independently predicted grief. Psych: Parents fulfilled criteria for diagnosis of prolonged grief disorder (10.3%), traumatic distress clusters (16%), separation distress (41.4%). Prevalence of clinically significant levels of depression was reported at 22.4%. Separation distress and traumatic distress (subcomponents of the Grief Inventory) and total grief were all significantly correlated with depression. |
| Mitchell, 2005 – United Kingdom Inventory of psychosocial support service provisions available at pediatric oncology centers |
Pediatric oncology treatment centers (n=21) and adolescent care units (n=3) for 24 total centers | 1. No 2. Yes 3. Unclear 4. No 5. No 6. No |
DM: Only 6/24 centers reported involving siblings and 1/24 involved grandparents in decision-making processes. Psych: Formal psychosocial assessment of patients not routine, as only 3/24 centers formally assessed every patient. Most (20/24 centers) carried out informal assessments of new patients and then followed-up if need identified. Some (7/24 centers) reported regular reviews of psychosocial assessments. Only 4/24 centers reported the input of psychologists in treatment preparations. Lack of standard practices and procedures for psychosocial support documented by study. Soc: Support groups could be accessed at 21/24 centers with wide variety of meeting frequency. Social workers (16/24) and nursing staff (15/24) reported regularly providing bereavement support, usually as home visits. At 14/24 centers, staff also referred families to external bereavement agencies. |
| Tomlinson, 2011 (3 papers) – Canada Interviews using hypothetical scenarios to investigate chemotherapy versus supportive care alone decision-making (couple and HCP concurrence considered in separate papers) |
Parents (n=77) of children with cancer determined to have <5% chance of survival and their HCPs (n=128); separate study of fewer parents (n=73) | 1. Unclear 2. Yes 3. Unclear 4. No 5. No 6. No |
DM: Parents identified hope (OR 1.339), increased survival time (OR 0.868), and child’s QoL (OR 0.596) as the three most important considerations in deciding between aggressive chemotherapy and supportive care alone. HCP factored child’s QoL, followed by survival time and other family members’ QoL. HCPs placed greater emphasis on families’ financial considerations than parents. Soc: Unmarried/single parents may be more resistant to giving up aggressive treatment if they lack supportive family structure. Psych: Concordance between parents was poor for interpretation of child’s psychosocial health, emotional function, treatment anxiety, communication, and cognitive fatigue; authors conclude one parent’s assessment may not be considered synonymous with other parents’ assessment and thus encourage inclusive communication. |
| Van der Geest, 2014 – Netherlands Retrospective questionnaire to explore impact of parental perception of EoL care on parental grief |
Bereaved parents (n=89) | 1. Yes 2. Unclear 3. No 4. No 5. No 6. No |
Psych: Higher parental ratings on communication quality (p =0.03) and care continuity (p=0.01) were associated with lower levels of long-term parental grief. Severity of child’s dyspnea (p=0.05), anxiety to be alone (p < 0.01), anxiety about the future (p < 0.01), anger (p< 0.01), and uncontrolled pain (p< 0.01) were associated with higher levels of parental long-term grief. |
| Von Lutzau, 2012 – Germany Semi-structured questionnaire to investigate EoL care experience |
Bereaved parents (n=48) | 1. Yes 2. No 3. No 4. No 5. No 6. No |
HU: 38/48 parents report having been informed of home care. 92% chose to accept home care and ultimately 62% received home care. 82.2% of the children had a DNR. Half of the children died at home and 10% in the ICU. SC: Parents report symptoms successfully treated more than 65% of time per parents with fatigue and pain most frequent reported symptom occurrence. Psych: Almost half (43.8%) of children received psychosocial aid during end-of-life care. However, study reported that 64.3% of the children who suffered from anxiety were not treated. Soc: Author endorsed reasonableness of assumption that “parents’ perception of their child’s suffering would have an impact on the psychosocial functioning of the entire family.” |
| Wolfe, 2008 – United States Retrospective time lapse cohort study from one institution using parent survey and chart data to evaluate changes in patterns of care, ACP, and symptom control among children with cancer at EoL |
Parents of children who died of cancer between 1990-1997 (n=102, Cohort 1) and parents of children who died of cancer between 1997-2004 (n=119, Cohort 2) | 1. Unclear 2. Unclear 3. No 4. No 5. No 6. No |
HU: Cohort 2 had more hospice discussions (p<0.001) and earlier hospice discussions (p <.002) and earlier documentation of DNR orders (p=0.03) ICU and hospital deaths deceased significantly in Cohort 2 (p=0.024). SC: Parents reported less suffering in terms of pain and dyspnea in Cohort 2. FS: More parents in Cohort 2 felt prepared for their child’s death than in Cohort 1. Psych: Parents in Cohort 2 reported less anxiety in child at EoL. |
| Zhukovsky, 2009 – United States Retrospective chart review of consecutive PPC consults at cancer center |
Charts from children (n=15) with palliative care referrals | 1. Yes 2. Yes 3. No 4. No 5. No 6. Yes |
DM: In chart review of documentation of communication about EoL issues at the time of consult, documented involvement of the child occurred in n=2 (13%) cases. Psych: The most commonly recommended interventions from the palliative consult documentation were pharmacologic (14 patients) followed by patient and family counseling (11 patients). Soc: Well-being of siblings was not documented by primary oncologist (n=0) and was documented by palliative care consultant in n=4 (27%) cases. |
| ORIGINAL RESEARCH – REVIEW PAPERS | |||
|---|---|---|---|
| Study and Purpose | Included Papers | Quality Report | Second Order Findings by Care Groupings |
| Hinds, 2007 Systematic Review – Identify empirical papers that included patient reported outcomes for pediatric oncology patients at EoL |
26 | 1. Yes 2. Yes 3. Unclear 4. Yes 5. Yes |
PS: Nearly 85% of completed studies do not include patient reported outcomes. Q: Patient-reported outcomes facilitated patient perspectives and indicators of quality of care at EoL |
| Mack, 2006 Narrative Review – Explore impact of early integration of palliative care for children with life-limiting illnesses |
20 | 1. Yes 2. Yes 3. Unclear 4. Yes 5. Yes |
DM: Pediatric deaths occurring in intensive care unit at the EOL lent to earlier consideration of expected trajectory of illness and communication of goals. Discussion about EoL preferences may be appropriate as early as the time of diagnosis. PS: Children with cancer may wish to talk about the meaning of being ill and physician involvement in these conversations may be important for individual children FS: Families bring a combination of a need for information about EoL and combination for need for sensitive and caring nature of communication (criteria for value of communication). Psych: Integration of palliative care at the time of diagnosis can allow children and families to make decisions about care that fit their needs and values and maintain better psychological health for the child and family. |
| Rosenberg, 2012 Systematic Review – Review of existing studies that used validated instruments to measure psychosocial outcomes among bereaved parents of children who had died from cancer. |
13 | 1. Yes 2. Yes 3. Yes 4. Unclear 5. Yes |
Psych: Bereaved parents of children with cancer versus non-bereaved parents had increased anxiety, depression, prolonged grief, and poor psychological QoL. Outcome predictors included: parental history of loss, economic hardship, duration and intensity of treatment, satisfaction with care team, location of death, and child’s QOL prior to death. Factors associated with psychosocial morbidity included parental history of loss, financial hardship, duration and intensity of cancer-therapy, perception of care, child’s QoL, location of death, and time since death. Parents fared worse with less preparative time before their child’s death or if the parent carried dual morbidities (i.e., grief plus anxiety). |
| Wiener, 2013 Narrative Review – Explore and review how culture and religion informs and shapes PPC. |
37 | 1. Yes 2. Yes 3. Unclear 4. Yes 5. Yes |
HU: Cultural and religious factors influenced palliative care utilization; ethnic minorities tended to underutilize PPC. DM: While many cultures may prefer not to disclose life-threatening diagnoses to children, this may lead to emotional distance at a time when emotional closeness is needed. Paper discussed opportunities to include the family and patient in planning advanced directives. Q: Trained medical interpreters, careful choice of words, and attention to non-verbal cues noted as necessary to ensure the patient/family and provider understand each other. Psych: In order to provide culturally competent care, the HCP must first acknowledge his/her own beliefs about culture and religion. Cultural education of staff should be balanced with individual conversations with patients/families to assess family psychosocial needs. Patients and families facing the EoL often contemplate the meaning in their illness and life. |
Abbreviations: AYA = adolescent young adults; EoL = end of life; 1=insufficient sample size; 2=lack of blinding; 3=selective reporting; 4=incorrect analysis; 5=stopped early; 6=large losses to follow-up. HU=healthcare utilization; CE=cost effectiveness; DM = decision-making; SC=symptom control; PS=patient satisfaction; FS=family satisfaction; SS=staff satisfaction; CB=caregiver burden; Q=quality of care delivery; Psych=Psychological impact; Soc=social support. All participants were cancer-specific study populations, unless otherwise noted.
Abbreviations: ACP = advanced care planning; AYA = adolescent young adult; CAM; complementary and alternative medicines; EoL = end of life; QoL = quality of life; 1=research question clearly stated; 2=qualitative approach clearly justified; 3=study context clearly described; 4=role of the researcher clearly described; 5=sampling strategy appropriate for research question; 6=method of data collection clearly described; 7=method of data analysis clearly described; 8=analysis appropriate for research question; HU=healthcare utilization; CE=cost effectiveness; DM = decision-making; SC=symptom control; PS=patient satisfaction; FS=family satisfaction; SS=staff satisfaction; CB=caregiver burden; Q=quality of care delivery; Psych=Psychological impact; Soc=social support. All participants were cancer-specific study populations, unless otherwise noted.
Abbreviations: ACP = advanced care planning; AYA = adolescent young adult; CAM; complementary and alternative medicines; CI = confidence interval; COG = Children’s Oncology Group; EoL = end of life; HCP= health care professionals; HR-QoL = Health-related quality of life; LOD = location of death; NA = not available; OR = odds ratio; PPC = pediatric palliative care; QoL = quality of life; RR = relative risk; USA = United States of America. 1=insufficient sample size; 2=lack of blinding; 3=selective reporting; 4=incorrect analysis; 5=stopped early; 6=large losses to follow-up. HU=healthcare utilization; CE=cost effectiveness; DM = decision-making; SC=symptom control; PS=patient satisfaction; FS=family satisfaction; SS=staff satisfaction; CB=caregiver burden; Q=quality of care delivery; Psych=Psychological impact; Soc=social support.
Abbreviations: EoL = end of life; HCP = health care professional; PPC = pediatric palliative care; QoL = quality of life. 1=Right types of papers included; 2=Important, relevant studies included; 3=Appropriately assessed for quality of studies; 4=Reasonable to combine results in this way; 5=Important outcomes considered. HU=healthcare utilization; CE=cost effectiveness; DM = decision-making; SC=symptom control; PS=patient satisfaction; FS=family satisfaction; SS=staff satisfaction; CB=caregiver burden; Q=quality of care delivery; Psych=Psychological impact; Soc=social support.
Procedure for Reviewing the Literature
The team developed a data extraction sheet (Appendix 1), which underwent a pilot test on five randomly selected included studies. Items on the extraction sheet included: study methodology, study length, study setting, population and control/comparison description and size, study setting, and findings. For review papers, review question, study group descriptions, inclusion and exclusion criteria, synthesis format, outcome measures, and findings were reported. For expert opinion or consensus papers, recommendations were extracted. Additionally, the data extraction sheet included items for study team members to indicate their judgments about interventions described in the reports, including barriers to implementation, tools for application, cost of application, monitoring or auditing processes, and possible harms of intervention.
Two reviewers independently performed eligibility assessment at abstract level utilizing a pre-determined eligibility checklist. These independent reviewers [MW, KH] reached consensus for exclusion/inclusion decision with 96% inter-rater agreement. Six articles were discussed to reach inclusion/exclusion consensus. Additional non-duplicate articles were added from references of included studies with group consensus on these articles. A team of eight reviewers [MW, KH, AG, KPK, RC, AW, CB, PH] from fields of oncology, psychology, nursing, and social work then systematically reviewed articles at full text level. Members of the study team did not serve as reviewers for papers they had authored. Two team members independently reviewed the same published paper with inter-rater agreement for exclusion/inclusion decision reached at 94%. French and German articles (n=3) underwent review by one study team member. A total of five articles were discussed for consensus with two requiring primary author contact for further clarification prior to consensus agreement.
An electronic folder was created for reviewers to access standard bias definitions for quality assessment scores. Criteria for risk of bias for nonrandomized trials was assessed by each reviewer by applying the Effective Public Health Project tool (www.ephpp.ca/tools.html, accessed 1 October 2014). Similarly, qualitative studies were assessed for bias by reviewers by using the Consolidated Criteria for Reporting Qualitative Research (COREQ) criteria9 and additional studies with reporting per Critical Appraisal Skills Programme (CASP) standards (http://www.caspinternational.org/?o=1020, accessed 1 October 2014). Funding source and author recognition of possible bias were analyzed.
Each reviewer entered the data from completed data extraction sheets into an online extraction template designed by two study team members [MW,KH] to enable consistent data formatting for team analysis. Two study team members independently completed the data extraction sheet per article and a minimum of one additional study team member checked data extraction to recognize differences of opinion and re-circulate these findings back to primary and secondary reviewers for agreement.
Data Analysis
Data analysis followed a pre-determined four-step process of data display, data grouping, data categorizing, and data synthesizing as per integrative review methodology (Figure 1).11 This step-by-step approach facilitated recognition of patterns, variations, and relationships from extracted data. Verification of validity occurred by systematically re-checking themes against the primary literature within and across groupings.
Figure 1.
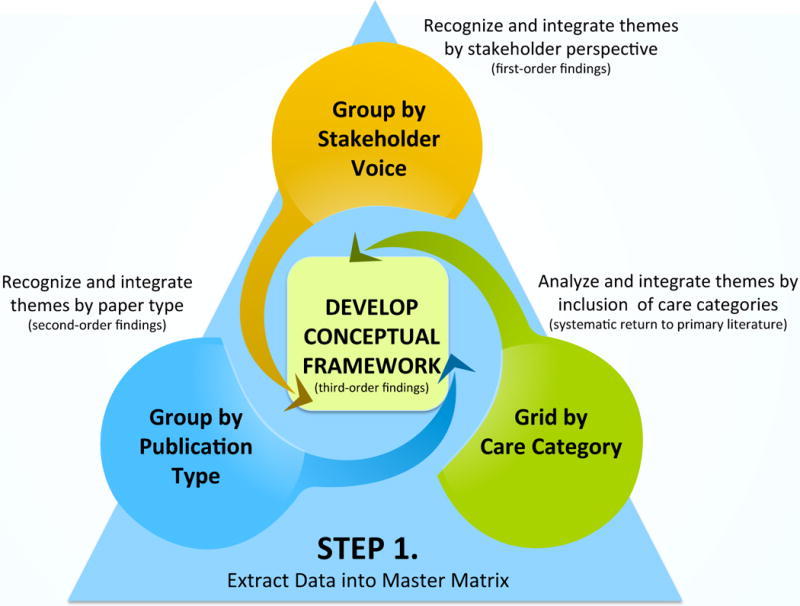
Triangulated Steps of Data Analysis and Integration
Step 1 (data display)
A master matrix was created to display the comprehensive set of data extracted for each article.
Step 2 (data grouping)
All included articles were then organized into non-exclusive groups: 1) stakeholder voice and 2) type of publication. One article could appear in either or both groupings. These groupings facilitated review of first-order findings (themes arising directly from participant such as patient, family member, or staff perspectives) and second-order findings (themes arising from primary author interpretations of the research as described in the outcomes and discussions sections of primary publication type). NVivo software was utilized to analyze direct quotes from patient, family member, or staff for first order findings. By then re-grouping articles according to publication type, the data were analyzed based on primary author discussion as second order findings (Table 1).
Step 3 (data categorizing)
The team created a grid of care category findings for systematic return to the primary data by care category across literature formats (Table 2). Due to the complex inter-play of physical, psychological, social, and communication needs during cancer care, study content was monitored by outcome categories.
Table 2.
Care Category Inclusion and Stakeholder Voice
| Study | Voice Inclusion | Care Access | Psychological Assessment | Symptom Assessment | Communication | Spiritual/Existential | Care Quality | Social Support | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P=patient, F=family, C=staff caregiver | Early integration across illness contiuum | Developmentally informed assessments | Mental health need or intervention | Patient psychological reaction | Parent psychological reaction | Staff psychological reaction | Using patient perspective | Using family perspective | Using staff perspective | Between patient and staff | Between patient and family | Between family and staff | Enabling patients to make informed decisions | Enabling family members to make informed decisions | Legacy making | Spiritual/religious resources | Truth telling | Incorporation of music or art | Patient preferences for goals of care/treatment options | Surrogate preferences for goals of care/treatment options | Advanced care planning | Therapeutic relationships | Incorporating cultural needs | Pediatric specific social context | Grief/bereavement outreach | Sibling support | |
| Aasgard, 2001 | P | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||||||
| Bell, 2009 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||
| Ben-Arush, 2011 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||
| Bona, 2011 | F | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||
| Bousso, 2012 | F | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||||||
| Bradford, 2012 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||||
| Cataudella, 2012 | F | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||||
| Coccia, 2012 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||
| Collins, 2002 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||
| Contro, 2002 | F | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||||
| DAgostino, 2011 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||||
| Devlin, 2006 | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||||||
| Duncan, 2007 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||
| Dussel, 2009 | F | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||||||
| Dussel, 2010 | F | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||||||
| Edwards, 2008 | F | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||||||
| ElShami, 2011 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||
| Foster, 2010 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||
| Furioli, 2011 | F | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||
| George, 2003 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||
| Hatano, 2011 | PFC | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||
| Hays, 2006 | F | ✔ | ✔ | ✔ | |||||||||||||||||||||||
| Heath, 2009 | F | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||
| Heath, 2012 | F | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||||
| Hechler, 2008 | F | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||
| Hilden, 2001 | C | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||||||
| Hinds, 2007 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||
| Hinds, 2009 | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||||||
| Hinds, 2010 | C | ✔ | |||||||||||||||||||||||||
| Hinds, 2012 | F C | ✔ | ✔ | ✔ | |||||||||||||||||||||||
| Hunt, 2006 | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||||||||
| Hurwitz, 2004 | PFC | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||
| Johnston, 2008 | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||||||
| Jones, 2006 | C | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||
| Jones, 2014 | F | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||
| Kreicsberg, 2004 | F | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||||
| Lyon, 2013 | P F | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||
| Mack, 2005 | F | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||
| Mack, 2006 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||
| Mack, 2014 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||
| Matthews, 2006 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||
| Maurucio, 2010 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||
| McCarthy 2010 | F | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||||||
| Mitchell, 2005 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||
| OShea, 2013 | P F | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||||
| Otis-Green, 2013 | ✔ | ✔ | ✔ | ||||||||||||||||||||||||
| Penson, 2005 | C | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||||
| Postovsky, 2004 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||||
| Pritchard, 2011 | PFC | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||
| Robert, 2012 | F | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||
| Rosenburg, 2012 | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||||||||
| Saad, 2011 | F | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||
| Seigneur, 2011 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||
| Schrijvers, 2006 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||
| Tadmor, 2004 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||
| Tomlinson, 2011a | FC | ✔ | ✔ | ||||||||||||||||||||||||
| Tomlinson, 2011b | F | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||||||
| Tomlinson, 2011c | FC | ✔ | ✔ | ✔ | |||||||||||||||||||||||
| Van de Wetering, 2011 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||||
| Van der Geest, 2014 | F | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||||
| Vern-Gross, 2011 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||
| VonLutzau, 2012 | F | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||||||
| Waldman, 2013 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||
| Wein, 2010 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||
| Wiener, 2008 | P | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||||
| Wiener, 2012 | P | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||||
| Wiener, 2013 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||
| Wolfe, 2002 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||
| Wolfe, 2008 | F | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||||
| Zebrack, 2011 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||||||
| Zelcer, 2010 | F | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||
| Zhukovsky, 2009 | C | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||
Step 4 (data synthesis)
Based on iterative integration of first order and second order findings, the study team developed a conceptual framework (Figure 2). To develop the conceptual model, each reviewer was assigned either a horizontal row (quality care factor) or vertical column (stakeholder perspective). Rows and columns were assigned to reviewers based on the thematic content of their reviewed manuscripts and/or based on professional expertise. Each cell in the conceptual model therefore received an intersecting “expert opinion” with one study team member representing the quality care factor (one reviewer) and a different study team member representing the stakeholder perspective (different reviewer). The phrases for each cell were grounded in the integrative review’s foundational evidence base. Study team members were encouraged to incorporate what they hear and witness through their daily inter-disciplinary work as part of the development of third order findings. To protect the conceptual model phrases from extending beyond the original research, two reviewers [KPK, MW] checked phrases against data from the original research to ensure the conceptual model was consistent with and grounded by the original research findings. Group consensus was reached through discussion among reviewers.
Figure 2.
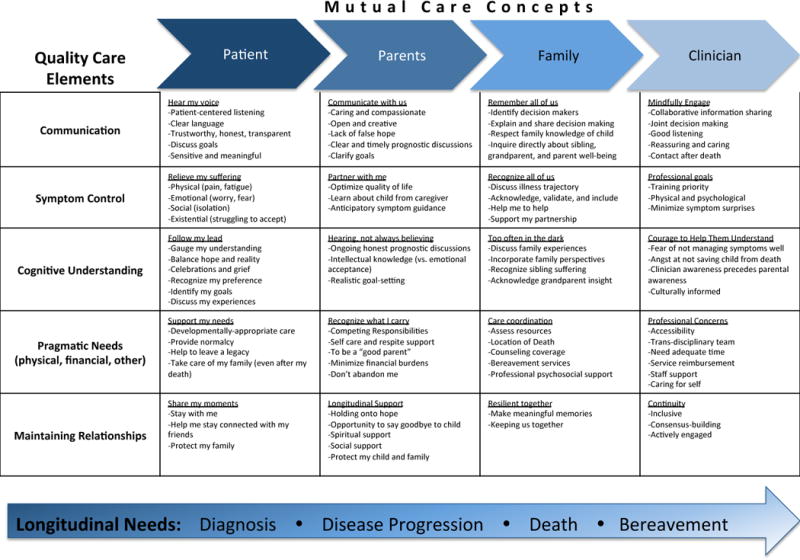
Conceptual Framework for Psychosocial Pediatric Palliative Cancer Care
Findings
A total of 216 studies were identified for inclusion in the review. After removing duplicates, 182 remained. Of these, 67 studies were discarded at abstract level. Approximately 32 abstracts were excluded due to lack of palliative care theme, 24 due study participant age exclusive of pediatric, adolescents, or young adults, 7 due to lack of oncologic diagnosis, and 4 due to publication format. The remaining 115 citations were examined at full text level. Forty-three studies did not meet inclusion criteria, yielding 72 included papers. The most common reason for exclusion at the full text level included study population exclusive of pediatric or adolescent age groups (n=31). PRISMA flow diagram is available as Figure 3.
Figure 3.
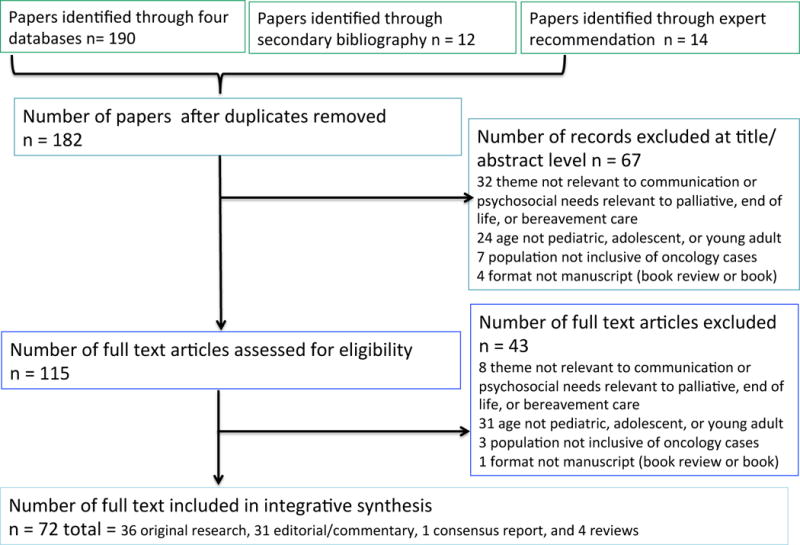
PRISMA: Preferred Reporting for Systematic Reviews and Meta-Analyses
Publication types included 36 original research articles,2,3,12–45 31 editorial or expert opinion papers,4,5,46–74 one consensus report,75 and four reviews.76–79 Of the original research articles, seven were mixed method, seven were qualitative design, and 22 were quantitative design (Table 1). All studies were available and reviewed in English other than two in French53,67 and one in German.80 Fourteen studies were multi-institutional investigations. Manuscripts represented data from nine countries: Australia,24,26,31,36 Brazil,12 Canada,2,23,37,38,43,66 Germany,32,40 Lebanon,18 Netherlands,44 Sweden,33,39 United Kingdom,17 the United States,13–16,19,20,22,25,28,29,45 and one multi-country paper.41 The primary study subject included pediatric ages,42 the adolescent young adult (AYA) population ages 10–21,2214–21,34 16–28,19,28 and 15–25 years;66 caregivers of patients receiving palliative care;3,12,13,15,24,29,37,38,43,81 bereaved parents;2,14,18,20,23,25–27,31–33,35,36,39,40,44,45 social workers;16 principal investigators;41 palliative team members;17 and pediatric oncologists.30 Five original research studies reported on a specific intervention other than the development of a palliative care service: use of family-centered ACP training,34 parental decision rationale reported in timely summary for medical teams,15 introduction of co-case management and shared decision-making education,29 a 24-hour available palliative telephone service,24 and advanced practice palliative staff training for transitions from critical care settings.60 Four studies included longitudinal design with 12-week,15 3-month,29 eight-year24 measures and one extended pre-post cohort study.45 Of included review articles, two were systematic76,78 and two were narrative.77,79 Of included editorials, two authors referred to specific theory as Family Management Style Framework12 and Perceived Personal Control Crisis Model.69
Literature-specific bias and quality scales are summarized in Table 1. For expert opinion/commentary papers, two manuscripts included author mention of potential personal bias or how personal perspective may influence writing.61,69
Synthesis of First Order Findings
As has been previously recognized, end of life research contains a startling paucity of patient reported outcomes.76 Only four papers contained quotes spoken directly by patients46,55,57,61 with five additional manuscripts using patient input as direct data point,19,28,34,66,76 thus 9/72 (13%) papers provided patient perspectives. Thirteen papers2–4,12–14,23,27,53,57,61,66,81 included quotes spoken by family members with 20 additional articles15,18,20,25,26,29,31,32,34–40,42–45,66 including family member input as a direct data source, thus 33/72 (46%) papers provided access to family perspective. Five articles (7%) included direct quotes from surveyed staff.15,16,56,57,64
Quotes spoken by stakeholders (patient, parent, provider) were obtained from primary sources with phrases coded in NVivo 10.0 software. In analyzing the content of quotes spoken by patient, parent, and health provider, shared themes were manifest across stakeholder perspectives (Table 3). The study team recognized a phenomenon of mutual care. Mutual care was defined by the study team as the realization that the ill child and parent experience similar concerns for each other including the desire to protect each other from sad realities and negative emotions and outcomes which could contribute to not openly addressing worries, and a similar intention to look out for or advocate for each other. Parents spoke of struggling by the child’s side as the child struggles12,81 and staying strong for the child while finding strength in the child.12 Patients expressed worry about family members left behind57 as parents expressed worry about leaving child alone.12 Patients spoke of protecting their parents while their parents protect them.57 Patients spoke of carrying the “secrecy” of death and prognosis as part of protecting a silent family.57 Relationships between patients and staff and patients and family revealed mutual elements of striving to care for, protect, and advocate for the other party.
Table 3.
First Order Findings Reported by Stakeholder Perspective (using NVivo 10.0 Software for Theme Coding)
| Patient Perspective | Family Member Perspective | Care Team Perspective | |
|---|---|---|---|
| Communication | Desiring clear and honest communication1 | Desiring consistent,2 honest,2 and factual communication3 Preferring to communicate in family’s first language4,2 Needing space for silence5 Dreading Child’s loss of ability to communicate5 Still suffering from insensitive communication2 |
Desiring honesty1,6 and consistency7 Including patient in conversations1 Ensuring opportunity for patient to ask questions1 Speaking openly and listening actively7 Avoiding offensive/improper questions7 Offering silence6 Providing compassionate and sincere communication8 |
| Symptom Control | Seeking relief from pain9 | Prioritizing comfort10 Lessening suffering3,5,10 Feeling responsible for symptom management5 Wanting staff to hear/respond to symptom concerns2 |
Providing comfort6 Feeling responsible for symptom management1,7 |
| Feelings | Feeling fear1 Worrying about family members left behind1 Blaming self11 |
Feeling fear5 Feeling frightened to leave the child alone5 Blaming self and/or spouse5 Anticipating grief1,12,13 Feeling helpless while watching child suffer2 Feeling incapable and ill-prepared5 Feeling pleasure/joy in caring for child5 |
Acknowledging patient–s fears6 Recognizing personal fears6 Tending to familys emotional needs1 Feeling privileged to care for the family1 Helping child feel secure that family will be okay8 |
| Spiritual/Existential | Maintaining hope1 Hiding and Emerging9 |
Maintaining hope2,5,10,11,14 Finding a place for humor11 Seeking spiritual direction3,13 |
Maintaining hope1,8 Honoring a familys values7,8 Offering a healing presence6 |
| Cognitive | Understanding illness and implications15 Looking for reasons (causation)11 |
Acquiring new information about child and learning from experiences with child5 Knowing everything about child5 |
Investigating familys level of knowledge7 Relaying medical knowledge/seeking options7 |
| Decisional | Picking from choices1 | Making decisions5,13 and sharing decisions5 Prioritizing the child in all choices/decisions5 Acknowledging different decisional approaches within couples5 Needing time to make decisions3 Wanting staff respect for family decisions3 |
Sharing information before decisions8 Hosting empathy for family’s difficult decisions7 |
| Practical Needs | Human interaction9 Play and distraction9 Physical comfort9 |
Respite support for caregiver4 Care continuation through adulthood4 Provision of complementary/alternative therapies4 Improved quality of home care (symptom support)2,14 Material items and support options3 Care coordination3,13 Adaptation of policies to meet the family’s needs13 Relief from financial costs14 |
[Tangible] support and resources7 Improved transition of care between services8 |
| Bereavement | Concern about family’s well-being after death1 | Interpreting Child’s death as a personal failure10 Regretting not talking to child about death14 Wanting to be remembered by staff2,3 Finding a way to survive after Child’s death4 Feeling broken/abandoned/isolated after death of child14,16 |
Viewing Child’s death as professional failure6 Grieving the loss of the child1 Hosting rituals of remembrance1 Helping families feel less alone8 |
| Mutual | Protecting parent while parent is protecting child1 | Struggling by Child’s side as child struggles3,5 Staying strong for child and finding strength in child5 Addressing own feelings and responding to Child’s feelings10,14,17 |
Caring for self to best care for child6
Mustering professional courage at work6 Recognizing the child tries to protect the parent8 |
| Tasks | Overcoming boredom9 Engaging in normal childhood activities1,9 Carrying “secrecy” of diagnosis1 Realizing the reality of the future15 |
Advocating for child3,11 Engaging child in normal childhood activities13,14,17 Monitoring child’s growth and strength17 Protecting child from harm5 Protecting child from knowledge of parents suffering5 Learning the health routine (growing through experiences)5 Growing in patience5 and growing into a new normal5 Preparing for surprises5 Recognizing child’s awareness of impending death17 Measuring time as precious5,10 Living with uncertainty1 and unknowns5,12 Realizing the child may accept death before parent ready14 |
Maximizing each day for child1 |
| Relational | Maintaining relationships9 Receiving comfort from interactions9 |
Care team relationship/interaction with family Recognizing staff as an extension of family12 Finding comfort in staff support5,13 and continuity2–4 Developing trusted relationships with staff10,13 Sibling relationship/interaction Wanting siblings to know/remember each other12 Recognizing that siblings friends/peers dont understand16 Appreciating role of siblings2 Family relationship/interaction Struggling to balancing other family responsibilities5,12,14 Sharing one anothers burdens and struggles5 Relationship/interaction with child Conveying love for child3,5,14 Maintaining child’s connections with others17 Treating the child with tenderness5 and kindness5 Viewing the child (and self) as special5 |
Wanting to make a difference for child and family1 Wanting to doing well by child and colleagues18 Knowing the “team” matters16 Recognizing patient as special/remarkable1 Including sibling in care circle8 Growing in compassion7 |
Hurwitz CA, Duncan J, Wolfe J. Caring for the child with cancer at the close of life: “there are people who make it, and I’m hoping I’m one of them”. Jama. 2004;292(17):2141–2149.
Contro N, Larson J, Scofield S, Sourkes B, Cohen H. Family perspectives on the quality of pediatric palliative care. Arch Pediatr Adolesc Med. 2002;156(1):14–19.
Hinds PS, Oakes LL, Hicks J, Powell B, Srivastava DK, Spunt SL, et al. “Trying to be a good parent” as defined by interviews with parents who made phase I, terminal care, and resuscitation decisions for their children. J Clin Oncol. 2009;27(35):5979–5985.
Bona K, Bates J, Wolfe J. Massachusetts Pediatric Palliative Care Network: successful implementation of a novel state-funded pediatric palliative care program. J Palliat Med. 2011;14(11):1217–1223.
Bousso RS, Misko MD, Mendes-Castillo AM, Rossato LM. Family management style framework and its use with families who have a child undergoing palliative care at home. Journal of family nursing. 2012;18(1):91–122.
Penson RT. Between Parent and Child: Negotiating Cancer Treatment in Adolescents. The Oncologist. 2002;7(2):154–162.
Hinds PS, Oakes LL, Hicks J, Powell B, Srivastava DK, Baker JN, et al. Parent-clinician communication intervention during end-of-life decision making for children with incurable cancer. J Palliat Med. 2012;15(8):916–922.
Jones BL. Companionship, control, and compassion: a social work perspective on the needs of children with cancer and their families at the end of life. J Palliat Med. 2006;9(3):774–788.
Aasgaard T. An ecology of love: aspects of music therapy in the pediatric oncology environment. J Palliat Care. 2001;17(3):177–181.
Edwards KE, Neville BA, Cook EF, Jr., Aldridge SH, Dussel V, Wolfe J. Understanding of prognosis and goals of care among couples whose child died of cancer. J Clin Oncol. 2008;26(8):1310–1315.
O’Shea ER, Bennett Kanarek R. Understanding pediatric palliative care: what it is and what it should be. J Pediatr Oncol Nurs. 2013;30(1):34–44.
Furioli J, Le Guehennec J, Desouches MM, Dureuil L, Thebault J. [Lois or the end of life of a child suffering from cancer]. Soins Pediatrie, puericulture. 2011(260):21–23.
Robert R, Zhukovsky DS, Mauricio R, Gilmore K, Morrison S, Palos GR. Bereaved parents’ perspectives on pediatric palliative care. Journal of social work in end-of-life & palliative care. 2012;8(4):316–338.
Zelcer S, Cataudella D, Cairney AE, Bannister SL. Palliative care of children with brain tumors: a parental perspective. Arch Pediatr AdolescMed. 2010;164(3):225–230.
Hatano Y, Yamada M, Fukui K. Shades of truth: cultural and psychological factors affecting communication in pediatric palliative care. J Pain Symptom Manage. 2011;41(2):491–495.
Jones BL, Contro N, Koch KD. The duty of the physician to care for the family in pediatric palliative care: context, communication, and caring. Pediatrics. 2014;133 Suppl 1:S8–15.
Cataudella DA, Zelcer S. Psychological experiences of children with brain tumors at end of life: parental perspectives. J Palliat Med. 2012;15(11):1191–1197.
Hinds PS. “I know that you said you are studying my patient, but aren’t you really studying me?”: the question posed by pediatric oncology clinicians in end-of-life studies. Cancer Nurs. 2010;33(6):481–482.
Organization of primary quotes according the themes stated by patients, families, and providers revealed important and potentially unifying shared priorities: goals of maintaining hope,3,12,14,23,57,61 giving and receiving honest communication,3,57,64 and relieving pain.46,12,14,81,82 Whether through humor61 or interactive play,46 child and parent appreciated moments of memory making. Although experienced in different ways, fear was voiced across all stakeholder groups,12,57,82 as was the theme of carrying blame and perception of death as a self-failure.12,61,82 Patients appreciated the opportunity to pick from care choices;57 parents appreciated the process of sharing decisions,12,27 and providers recognized information sharing as an essential step to enable patients and parents to participate in decision making.16
Parents and providers both voiced the need for improved care transitions16,27,81 and fewer fiscal barriers to quality care for families.15,23 Parents recognized self-care and respite support as necessary for their care of the ill child and extended family unit.13 Health providers also recognized the need to care for self and colleagues in order to better care for the family and patient.82
Synthesis of Second Order Findings
While analyzing second order findings, an interesting phenomenon emerged: the positive influence of a well-addressed care category benefitted care outcomes in another care category. Similarly, under-addressed areas of care risked extending negative outcomes to other care categories. For example, quality communication with the child at the end of life resulted in improved psychosocial outcomes for bereaved parents.3,35 Whereas, uncontrolled physical symptoms for the child at the end of life translated into parental long-term grief.44 Care categories carry a mutual influence on one another and a synergistic role on perceived quality of overall care.
Care access
Families often preferred to take care of their child in the home setting,12,23 although half of n=56 surveyed bereaved parents in one study32 and 10 out of 48 bereaved parents in a second study40 notably could not recall having ever been informed of home care options. Three parent focus groups23 described subpar home care provisions keeping home from being a feasible care location.3 Opportunities for families to plan the location of their child’s death impacted the enablement of home as the family’s preferred location of death.25 Family-centered ACP resulted in patient and parent dyads more inclined to limit expensive, invasive treatment options at end of life.34 One well-integrated palliative care program resulted in 100% of deaths occurring in the location requested by families13 while another integrated palliative program significantly decreased inpatient and intensive care location deaths (Risk Difference, 16%; p=0.024).45 Extended access to palliative support, such as a 24-hour call line, minimized emergency room visits while still allowing families to feel safe and supported at home.24
Cost analysis
Cost of palliative recommendations were quantified in only one paper, with depiction of the “relatively low cost” for a state-sponsored palliative network.13 Cost of a 24-hour telephone intervention was labeled “economically viable” by the authors.24 Placement of advanced practice palliative care staff at critical transitions was described as a low cost intervention.60 Family-centered advanced care planning (ACP) resulted in patient and parent dyads more inclined to limit expensive, invasive treatment options at end of life.34 As the literature revealed family members often give up employment to care for a child at end of life, they may carry the heavy weight of end-of-life care costs. As reported by 41% of bereaved parents in a Lebanese study, the last month of the child’s life had an impact on the family’s financial status as parents had to quit work or reduce hours.18 Fifteen out of 48 bereaved parents reported to have carried “significant financial burdens” surrounding their child’s death.32 Families were reported to struggle with not only the direct medical costs but also indirect cost of lost income for provision of care.18,32,37 Co-case management interventions were reported by families as helpful in accessing available insurance reimbursement for necessary services.29 Primary sources referred to palliative care as cost savings to tax payers and spoke to the collective fiscal benefits of upstream palliative service provisions.66
Social support
Parents expressed a perceived failure in their role as a parent during the child’s end of life,18 described feeling overwhelmed and alone,12 and felt unable to adequately attend to other responsibilities.23,27 The perceived isolation and emotional burdens carried by siblings3,4,13,31,44,48,51,52,57,68,69,72 and grandparents17 were noted by primary sources as under-recognized and under-supported. Adolescent patients were revealed as a population warranting creative social support enablement, such as web-based networks66 as attentiveness to social factors is essential to their needs at this developmental stage.5,49,68,73 Although the literature contained rich evidence on the need for bereavement care and recognized bereavement services as a fulfillment of nonabandonment,4 papers did not detail family-individualized bereavement assessment as has been feasibly-modeled in intensive care settings.83 Interventions varied from memorial attendance70 to family contact on memorable days74 with communication using personal notes,71 phone calls,62 or home visits.17,62 Only one paper mentioned a specific ideal time frame for the duration of bereavement counseling coverage (13 months),13 otherwise bereavement care coverage was stated as a long term commitment.72,74
Symptom assessment and intervention
Uncontrolled pain was associated with child anxiety,2 parental thoughts on hastening child’s death33 and parental long-term grief.44 Pain was the most frequently reported symptom in need of improved management through palliative interventions.16,18,30,40 One center documented improved pain control through integration of comprehensive palliative consult services.45 Parents described the distress of uncontrolled symptoms and the need for earlier anticipatory symptom guidance.23 Depression and anxiety were depicted as current realities at end of life for patients,2,23,32,40,65 particularly in adolescent patients.22,47,66,75 Care actions during the child’s life clearly impact psychological symptom burden for bereaved family members.23,36,78 Silence surrounding unaddressed fears was associated with patient anxiety.39,64 Fifty-eight percent of surveyed pediatric oncologists (n=282 surveyed) reported not feeling personally competent to manage depression in children,30 further emphasizing the essential role of interdisciplinary palliative teams for comprehensive support.51,63,72 The current approach to psychosocial screening assessments was depicted as inconsistent and locally determined,17 and warranting development of standardized questionnaires for anxiety and psychosocial screening.66 Bereaved parents suggested the need for improved psychological6 and spiritual12 support for both children and family members while recognizing a need for earlier introduction to professional counseling.27,48 The most common needs at the time of new pediatric cancer palliative care consults were pharmaceutical interventions for physical and psychological symptoms and referrals for professional counseling.42 Comprehensive care of oncology patients includes prompt referrals to mental health professionals75 and inclusion of family member referrals for supportive counseling.4,16 Emotional domains were statistically improved with introduction of comprehensive palliative care interventions.29,45
Communication
Inclusion of patients in decision-making conversations between the health care team and parents was under-documented in medical records, as the documentation “rarely” mentioned inclusion of adolescents with cancer22 and “rarely” mentioned inclusion of children.42 The primary literature clarified the essential role of family-centered decisional approaches, recognizing that discordant decisional goals within couples influenced bereaved parental perception of child suffering14 and personal suffering.12,38 The literature emphasized the essential role of enabling and empowering adolescents in medical decision-making16 including ACP.19,28,34 Inclusion of patients in end-of-life conversations was noted to be remembered without regret by parents.33 Parental perception of care quality correlated with measures of communication quality with prioritization of sensitive and caring,35 compassionate,3 honest,3 and prognostic27,58 conversations. Quality communication was associated with lower levels of long-term parental grief.23
Care quality
As described, quality of communication consistently correlated with family perception of overall quality of end of life care. Perceived quality of care at end of life was also impacted by accessibility of services,18 care accommodations,27 and coordination3 of services particularly at times of care transition.23,29,60
Barriers to implementation of palliative care processes were not mentioned in 32/72 (44%) papers. Authors identified barriers as restrictive hospice enrollment rules,13 limited access to mental health professionals,2,17 lack of qualified support services,25 lack of formalized training,3,16,48,59,64 or knowledge,4,26,42 cost,4,18,30,35,52,54,57,65,74,75 lack of communication with the medical setting,34,66,75 perceived lack of time,17,30,34,35,63,74 physician discomfort19,20,58,76,77 or provider misconceptions,51,73,81 lack of a comprehensive (beyond curative-directed) care culture,49,50,62,63,69,72 and lack of implementable guidelines.79
Synthesis of Third Order Findings
Early integration and continuation of quality palliative services provide the potential to achieve better physical and psychological health for both the child and family.77 The literature revealed a need for improved physical and psychological symptom assessments and interventions longitudinally in cancer care. Longitudinal needs exist across the care continuum from diagnosis through disease progression, and into bereavement. Quality care factors were collapsed from second order care category findings into those determined by the study team as psychosocial priorities for standardized longitudinal interventions (still amenable to individualization): communication, symptom control, cognitive understanding, pragmatic needs, and maintaining relationships. The available stakeholder voices provided a perspective of shared mutuality strengthened by varying vantage points. Stakeholder perspectives were then separated according to patient, parents only (or primary guardians), extended family (including siblings and grandparents), and clinician. The conceptual model (Figure 2) depicts that while the focus of palliative interventions is rightfully on impact to patients, enhancing decision-making, discussing prognosis honestly, or decreasing symptom burden honors not only the goals of patients but also the mutual goals of family and staff. Similarly, improving respite care and bereavement outreach for family members honors the patient’s concern for his/her family while meeting family needs.
Discussion
While solely relying on expert recommendations has been the basis for other psychosocial cancer guidelines, this inclusive method enabled the weighing of primarily empirical findings. Given that care of the ill child occurs within the greater context of family, the methodology is strengthened by linking parent and child reports of the illness experience and perceived outcomes. The organized, step-wise approach enabled intimate familiarity with the literature while organizing and integrating data into a conceptual framework. Exclusion of pertinent evidence or premature analytic closure was avoided. Integrative review approach uncovered pediatric palliative cancer care’s diverse and exponentially growing research base while accounting for the complexity of care. A limitation to this study included the lack of global generalization, as the available literature did not include a low-income country perspective despite lack of geographic or language restrictions in the search strategy. Additional bias across studies included lack of diversity in perspective, as ethnic minorities may underutilize palliative services79 and over half of the original research papers utilized “English-speaking” as a participant inclusion criteria. The most common reported bias across studies was selection bias. Absence of patient voice limited findings, leading the study team to wonder whether absence of this essential perspective was due to physical inability to participate or exclusion from participation. Potential systematic barriers to including children and adolescents in research may include inconsistent review board standards for inclusion, the marginalization of pediatric patients in a hierarchical system, and the possible presumption that youth are not capable of meaningful insight.84 The recently released Institute of Medicine Report on Dying for America boldly calls for policies whereby all individuals, including children with the capacity to do so, participate actively in their health care decisions with receipt of services “consistent with their values, goals, and informed preferences.”1 Standard research practice should warrant clear documentation of reason when child voice is not included.76
The study team’s interpretation of the primary literature called for a systematic approach to palliative care42,70 that recognized the urgent need for procedural guidelines,16 standards,17,47 and clinical tools25 as enabling factors toward improved change. Recommended tools for monitoring care included data gathering on consults,62 intervention use,24 and comparative costs.13 For accountability, studies suggested weighing palliative care quality in decisions regarding hospital reimbursement59 and even hospital accreditation.66 Other tools for advancing the area included pediatric reported outcome assessments,76 structured psychosocial assessments for all patients at critical time points,17 and community partnerships.4 The reality that sibling and grandparent perspectives were consistently absent in palliative care reveals the importance of inclusive strategies individualized to each family, as prematurely limiting focus to only include patients and parents risk excluding essential care and support relationships. Authors, such as exampled in an art therapy paper, encouraged monitoring for processes (e.g., the extent to which the child felt psychologically safe while painting) rather than simply measuring end products (the number of painted papers).50 ACP was encouraged to be considered not simply as a one-timepoint conversation but as a process of ongoing communication and trust.34 Primary manuscripts revealed a ripple effect of process improvement in one care category creating positive waves in other care domains.
Conclusion
Navigating toward earlier and enhanced integration of palliative care in pediatric and adolescent cancer settings requires a standardized approach to psychosocial support informed by realistic evaluation of distance from here to there, cultural contexts, and the resources required to achieve optimal care. Youth with cancer and their families should be introduced to palliative care concepts, which are fundamentally important for making choices consistent with goals of care early in the disease process, regardless of disease status. They should receive access to longitudinal psychosocial support and developmentally appropriate end of life care in a standardized format with the flexibility of individualization, as supported by the data that formed the foundation of this integrative review.
Supplementary Material
Acknowledgments
The study team would wish to thank the Palliative Special Interest Group members. Gratitude to Ms. Susan Keller (research librarian). This work is supported in part by ONS Foundation/Genentech, Inc.
Footnotes
Conflict of Interest: The authors declare that there is no conflict of interest.
References
- 1.Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Institute of Medicine of The National Academies; 2014. [PubMed] [Google Scholar]
- 2.Cataudella DA, Zelcer S. Psychological experiences of children with brain tumors at end of life: parental perspectives. J Palliat Med. 2012;15:1191–7. doi: 10.1089/jpm.2011.0479. [DOI] [PubMed] [Google Scholar]
- 3.Contro N, Larson J, Scofield S, Sourkes B, Cohen H. Family perspectives on the quality of pediatric palliative care. Arch Pediatr Adolesc Med. 2002;156:14–9. doi: 10.1001/archpedi.156.1.14. [DOI] [PubMed] [Google Scholar]
- 4.Jones BL, Contro N, Koch KD. The duty of the physician to care for the family in pediatric palliative care: context, communication, and caring. Pediatrics. 2014;133(Suppl 1):S8–15. doi: 10.1542/peds.2013-3608C. [DOI] [PubMed] [Google Scholar]
- 5.Zebrack BJ. Psychological, social, and behavioral issues for young adults with cancer. Cancer. 2011;117:2289–94. doi: 10.1002/cncr.26056. [DOI] [PubMed] [Google Scholar]
- 6.Ferrell B, Connor SR, Cordes A, et al. The national agenda for quality palliative care: the National Consensus Project and the National Quality Forum. J Pain Symptom Manage. 2007;33:737–44. doi: 10.1016/j.jpainsymman.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics. Committee on Bioethics and Committee on Hospital Care. Palliative care for children. Pediatrics. 2000;106:351–7. [PubMed] [Google Scholar]
- 8.Wiener L, Viola A, Koretski J, Perper ED, Patenaude AF. Pediatric psycho-oncology care: standards, guidelines, and consensus reports. Psychooncology. 2014 doi: 10.1002/pon.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The EQUATOR Network. Enhancing the QUAlity and Transparency Of health Research. 2013 http://www.equator-network.org/
- 10.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 11.Whittemore RKK. The Integrative Review: Updated Methodology. J Adv Nurs. 2005;52:546–53. doi: 10.1111/j.1365-2648.2005.03621.x. [DOI] [PubMed] [Google Scholar]
- 12.Bousso RS, Misko MD, Mendes-Castillo AM, Rossato LM. Family management style framework and its use with families who have a child undergoing palliative care at home. Journal of family nursing. 2012;18:91–122. doi: 10.1177/1074840711427038. [DOI] [PubMed] [Google Scholar]
- 13.Bona K, Bates J, Wolfe J. Massachusetts’ Pediatric Palliative Care Network: successful implementation of a novel state-funded pediatric palliative care program. J Palliat Med. 2011;14:1217–23. doi: 10.1089/jpm.2011.0070. [DOI] [PubMed] [Google Scholar]
- 14.Edwards KE, Neville BA, Cook EF, Jr, Aldridge SH, Dussel V, Wolfe J. Understanding of prognosis and goals of care among couples whose child died of cancer. J Clin Oncol. 2008;26:1310–5. doi: 10.1200/JCO.2007.13.4056. [DOI] [PubMed] [Google Scholar]
- 15.Hinds PS, Oakes LL, Hicks J, et al. Parent-clinician communication intervention during end-of-life decision making for children with incurable cancer. J Palliat Med. 2012;15:916–22. doi: 10.1089/jpm.2012.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones BL. Companionship, control, and compassion: a social work perspective on the needs of children with cancer and their families at the end of life. J Palliat Med. 2006;9:774–88. doi: 10.1089/jpm.2006.9.774. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell W, Clarke S, Sloper P. Survey of psychosocial support provided by UK paediatric oncology centres. Arch Dis Child. 2005;90:796–800. doi: 10.1136/adc.2004.065177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saad R, Huijer HA, Noureddine S, Muwakkit S, Saab R, Abboud MR. Bereaved parental evaluation of the quality of a palliative care program in Lebanon. Pediatr Blood Cancer. 2011;57:310–6. doi: 10.1002/pbc.23082. [DOI] [PubMed] [Google Scholar]
- 19.Wiener L, Zadeh S, Battles H, et al. Allowing adolescents and young adults to plan their end-of-life care. Pediatrics. 2012;130:897–905. doi: 10.1542/peds.2012-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dussel V, Joffe S, Hilden JM, Watterson-Schaeffer J, Weeks JC, Wolfe J. Considerations about hastening death among parents of children who die of cancer. Arch PediatrAdolesc Med. 2010;164:231–7. doi: 10.1001/archpediatrics.2009.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinds PS, Oakes LL, Hicks J, et al. “Trying to be a good parent” as defined by interviews with parents who made phase I, terminal care, and resuscitation decisions for their children. J Clin Oncol. 2009;27:5979–85. doi: 10.1200/JCO.2008.20.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell CJ, Skiles J, Pradhan K, Champion VL. End-of-life experiences in adolescents dying with cancer. Support Care Cancer. 2010;18:827–35. doi: 10.1007/s00520-009-0716-1. [DOI] [PubMed] [Google Scholar]
- 23.Zelcer S, Cataudella D, Cairney AE, Bannister SL. Palliative care of children with brain tumors: a parental perspective. Arch Pediatr Adolesc Med. 2010;164:225–30. doi: 10.1001/archpediatrics.2009.284. [DOI] [PubMed] [Google Scholar]
- 24.Bradford N, Irving H, Smith AC, Pedersen LA, Herbert A. Palliative care afterhours: a review of a phone support service. J Pediatr Oncol Nurs. 2012;29:141–50. doi: 10.1177/1043454212446023. [DOI] [PubMed] [Google Scholar]
- 25.Dussel V, Kreicbergs U, Hilden JM, et al. Looking beyond where children die: determinants and effects of planning a child’s location of death. J Pain Symptom Manage. 2009;37:33–43. doi: 10.1016/j.jpainsymman.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heath JA, Oh LJ, Clarke NE, Wolfe J. Complementary and alternative medicine use in children with cancer at the end of life. J Palliat Med. 2012;15:1218–21. doi: 10.1089/jpm.2012.0150. [DOI] [PubMed] [Google Scholar]
- 27.Robert R, Zhukovsky DS, Mauricio R, Gilmore K, Morrison S, Palos GR. Bereaved parents perspectives on pediatric palliative care. Journal of social work in end-of-life & palliative care. 2012;8:316–38. doi: 10.1080/15524256.2012.732023. [DOI] [PubMed] [Google Scholar]
- 28.Wiener L, Ballard E, Brennan T, Battles H, Martinez P, Pao M. How I wish to be remembered: the use of an advance care planning document in adolescent and young adult populations. J Palliat Med. 2008;11:1309–13. doi: 10.1089/jpm.2008.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hays RM, Valentine J, Haynes G, et al. The Seattle Pediatric Palliative Care Project: effects on family satisfaction and health-related quality of life. J Palliat Med. 2006;9:716–28. doi: 10.1089/jpm.2006.9.716. [DOI] [PubMed] [Google Scholar]
- 30.Hilden JM, Emanuel EJ, Fairclough DL, et al. Attitudes and practices among pediatric oncologists regarding end-of-life care: results of the 1998 American Society of Clinical Oncology survey. J Clin Oncol. 2001;19:205–12. doi: 10.1200/JCO.2001.19.1.205. [DOI] [PubMed] [Google Scholar]
- 31.Heath JA, Clarke NE, McCarthy M, Donath SM, Anderson VA, Wolfe J. Quality of care at the end of life in children with cancer. Journal of paediatrics and child health. 2009;45:656–9. doi: 10.1111/j.1440-1754.2009.01590.x. [DOI] [PubMed] [Google Scholar]
- 32.Hechler T, Blankenburg M, Friedrichsdorf SJ, et al. Parents perspective on symptoms, quality of life, characteristics of death and end-of-life decisions for children dying from cancer. Klin Padiatr. 2008;220:166–74. doi: 10.1055/s-2008-1065347. [DOI] [PubMed] [Google Scholar]
- 33.Hunt H, Valdimarsdottir U, Mucci L, Kreicbergs U, Steineck G. When death appears best for the child with severe malignancy: a nationwide parental follow-up. Palliat Med. 2006;20:567–77. doi: 10.1177/0269216306069671. [DOI] [PubMed] [Google Scholar]
- 34.Lyon ME, Jacobs S, Briggs L, Cheng YI, Wang J. Family-centered advance care planning for teens with cancer. JAMA Pediatr. 2013;167:460–7. doi: 10.1001/jamapediatrics.2013.943. [DOI] [PubMed] [Google Scholar]
- 35.Mack JW, Hilden JM, Watterson J, et al. Parent and physician perspectives on quality of care at the end of life in children with cancer. J Clin Oncol. 2005;23:9155–61. doi: 10.1200/JCO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy MC, Clarke NE, Ting CL, Conroy R, Anderson VA, Heath JA. Prevalence and predictors of parental grief and depression after the death of a child from cancer. J Palliat Med. 2010;13:1321–6. doi: 10.1089/jpm.2010.0037. [DOI] [PubMed] [Google Scholar]
- 37.Tomlinson D, Bartels U, Hendershot E, Maloney AM, Ethier MC, Sung L. Factors affecting treatment choices in paediatric palliative care: comparing parents and health professionals. Eur J Cancer. 2011;47:2182–7. doi: 10.1016/j.ejca.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 38.Tomlinson D, Hendershot E, Bartels U, et al. Concordance between couples reporting their child’s quality of life and their decision making in pediatric oncology palliative care. J Pediatr Oncol Nurs. 2011;28:319–25. doi: 10.1177/1043454211418666. [DOI] [PubMed] [Google Scholar]
- 39.Kreicbergs U, Valdimarsdottir U, Onelov E, Henter JI, Steineck G. Talking about death with children who have severe malignant disease. N Engl J Med. 2004;351:1175–86. doi: 10.1056/NEJMoa040366. [DOI] [PubMed] [Google Scholar]
- 40.von Lutzau P, Otto M, Hechler T, Metzing S, Wolfe J, Zernikow B. Children dying from cancer: parents perspectives on symptoms, quality of life, characteristics of death, and end-of-life decisions. J Palliat Care. 2012;28:274–81. [PubMed] [Google Scholar]
- 41.Johnston DL, Nagel K, Friedman DL, Meza JL, Hurwitz CA, Friebert S. Availability and use of palliative care and end-of-life services for pediatric oncology patients. J Clin Oncol. 2008;26:4646–50. doi: 10.1200/JCO.2008.16.1562. [DOI] [PubMed] [Google Scholar]
- 42.Zhukovsky DS, Herzog CE, Kaur G, Palmer JL, Bruera E. The impact of palliative care consultation on symptom assessment, communication needs, and palliative interventions in pediatric patients with cancer. J Palliat Med. 2009;12:343–9. doi: 10.1089/jpm.2008.0152. [DOI] [PubMed] [Google Scholar]
- 43.Tomlinson D, Bartels U, Gammon J, et al. Chemotherapy versus supportive care alone in pediatric palliative care for cancer: comparing the preferences of parents and health care professionals. Cmaj. 2011;183:E1252–8. doi: 10.1503/cmaj.110392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Geest IM, Darlington AS, Streng IC, Michiels EM, Pieters R, van den Heuvel-Eibrink MM. Parents experiences of pediatric palliative care and the impact on long-term parental grief. J Pain Symptom Manage. 2014;47:1043–53. doi: 10.1016/j.jpainsymman.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Wolfe J, Hammel JF, Edwards KE, et al. Easing of suffering in children with cancer at the end of life: is care changing? J Clin Oncol. 2008;26:1717–23. doi: 10.1200/JCO.2007.14.0277. [DOI] [PubMed] [Google Scholar]
- 46.Aasgaard T. An ecology of love: aspects of music therapy in the pediatric oncology environment. J Palliat Care. 2001;17:177–81. [PubMed] [Google Scholar]
- 47.Ben-Arush MW. Current status of palliative care in Israel: a pediatric oncologist’s perspective. J Pediatr Hematol Oncol. 2011;33(Suppl 1):S56–9. doi: 10.1097/MPH.0b013e3182122047. [DOI] [PubMed] [Google Scholar]
- 48.Collins JJ. Palliative care and the child with cancer. Hematol Oncol Clin North Am. 2002;16:657–70. doi: 10.1016/s0889-8588(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 49.D’Agostino NM, Penney A, Zebrack B. Providing developmentally appropriate psychosocial care to adolescent and young adult cancer survivors. Cancer. 2011;117:2329–34. doi: 10.1002/cncr.26043. [DOI] [PubMed] [Google Scholar]
- 50.Devlin B. The art of healing and knowing in cancer and palliative care. Int J Palliat Nurs. 2006;12:16–9. doi: 10.12968/ijpn.2006.12.1.20391. [DOI] [PubMed] [Google Scholar]
- 51.ElShami M. Palliative care: concepts, needs, and challenges: perspectives on the experience at the Childrens Cancer Hospital in Egypt. J Pediatr Hematol Oncol. 2011;33(Suppl 1):S54–5. doi: 10.1097/MPH.0b013e3182122035. [DOI] [PubMed] [Google Scholar]
- 52.Foster TL, Lafond DA, Reggio C, Hinds PS. Pediatric palliative care in childhood cancer nursing: from diagnosis to cure or end of life. Seminars in oncology nursing. 2010;26:205–21. doi: 10.1016/j.soncn.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Furioli J, Le Guehennec J, Desouches MM, Dureuil L, Thebault J. Lois or the end of life of a child suffering from cancer. Soins Pediatrie, puericulture. 2011:21–3. [PubMed] [Google Scholar]
- 54.George R, Hutton S. Palliative care in adolescents. Eur J Cancer. 2003;39:2662–8. doi: 10.1016/j.ejca.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 55.Hatano Y, Yamada M, Fukui K. Shades of truth: cultural and psychological factors affecting communication in pediatric palliative care. J Pain Symptom Manage. 2011;41:491–5. doi: 10.1016/j.jpainsymman.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 56.Hinds PS. “I know that you said you are studying my patient, but aren’t you really studying me?”: the question posed by pediatric oncology clinicians in end-of-life studies. Cancer Nurs. 2010;33:481–2. doi: 10.1097/NCC.0b013e3181f713eb. [DOI] [PubMed] [Google Scholar]
- 57.Hurwitz CA, Duncan J, Wolfe J. Caring for the child with cancer at the close of life: “there are people who make it, and I’m hoping I’m one of them”. Jama. 2004;292:2141–9. doi: 10.1001/jama.292.17.2141. [DOI] [PubMed] [Google Scholar]
- 58.Mack JW, Joffe S. Communicating about prognosis: ethical responsibilities of pediatricians and parents. Pediatrics. 2014;133(Suppl 1):S24–30. doi: 10.1542/peds.2013-3608E. [DOI] [PubMed] [Google Scholar]
- 59.Matthews K, Gambles M, Ellershaw JE, et al. Developing the Liverpool Care Pathway for the dying child. Paediatric nursing. 2006;18:18–21. [PubMed] [Google Scholar]
- 60.Mauricio RV, Okhuysen-Cawley R. The caring continuum: role of the pediatric critical care advanced practice nurse in palliative care program development. Critical care nursing quarterly. 2010;33:292–7. doi: 10.1097/CNQ.0b013e3181ecd5a2. [DOI] [PubMed] [Google Scholar]
- 61.O’Shea ER, Bennett Kanarek R. Understanding pediatric palliative care: what it is and what it should be. J Pediatr Oncol Nurs. 2013;30:34–44. doi: 10.1177/1043454212471725. [DOI] [PubMed] [Google Scholar]
- 62.Duncan J, Spengler E, Wolfe J. Providing pediatric palliative care: PACT in action. MCN Am J Matern Child Nurs. 2007;32:279–87. doi: 10.1097/01.NMC.0000287997.97931.5f. [DOI] [PubMed] [Google Scholar]
- 63.Otis-Green S, Yang E, Lynne L. ACE Project—advocating for clinical excellence: creating change in the delivery of palliative care. Omega (Westport) 2013;67:5–19. doi: 10.2190/OM.67.1-2.b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Penson RT, Partridge RA, Shah MA, Giansiracusa D, Chabner BA, Lynch TJ., Jr Fear of death. Oncologist. 2005;10:160–9. doi: 10.1634/theoncologist.10-2-160. [DOI] [PubMed] [Google Scholar]
- 65.Postovsky S, Arush MWB. CARE OF A CHILD DYING OF CANCER: The Role of the Palliative Care Team in Pediatric Oncology. Pediatric Hematology-Oncology. 2004;21:67–76. [PubMed] [Google Scholar]
- 66.Pritchard S, Cuvelier G, Harlos M, Barr R. Palliative care in adolescents and young adults with cancer. Cancer. 2011;117:2323–8. doi: 10.1002/cncr.26044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seigneur E. How to discuss death with children and families? Bull Cancer. 2011;98:581–8. doi: 10.1684/bdc.2011.1363. [DOI] [PubMed] [Google Scholar]
- 68.Schrijvers D, Meijnders P. Palliative care in adolescents. Cancer treatment reviews. 2007;33:616–21. doi: 10.1016/j.ctrv.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Tadmor CS. Preventive Intervention for Children With Cancer and Their Families at the End-of-Life. J Prim Prev. 2004;24:311–23. [Google Scholar]
- 70.van de Wetering MD, Schouten-van Meeteren NY. Supportive care for children with cancer. Semin Oncol. 2011;38:374–9. doi: 10.1053/j.seminoncol.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 71.Vern-Gross T. Establishing communication within the field of pediatric oncology: a palliative care approach. Current problems in cancer. 2011;35:337–50. doi: 10.1016/j.currproblcancer.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 72.Waldman E, Wolfe J. Palliative care for children with cancer. Nat Rev Clin Oncol. 2013;10:100–7. doi: 10.1038/nrclinonc.2012.238. [DOI] [PubMed] [Google Scholar]
- 73.Wein S, Pery S, Zer A. Role of palliative care in adolescent and young adult oncology. J Clin Oncol. 2010;28:4819–24. doi: 10.1200/JCO.2009.22.4543. [DOI] [PubMed] [Google Scholar]
- 74.Wolfe J, Friebert S, Hilden J. Caring for children with advanced cancer integrating palliative care. Pediatric clinics of North America. 2002;49:1043–62. doi: 10.1016/s0031-3955(02)00034-2. [DOI] [PubMed] [Google Scholar]
- 75.Coccia PF, Altman J, Bhatia S, et al. Adolescent and young adult oncology. Clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2012;10:1112–50. doi: 10.6004/jnccn.2012.0117. [DOI] [PubMed] [Google Scholar]
- 76.Hinds PS, Brandon J, Allen C, Hijiya N, Newsome R, Kane JR. Patient-reported outcomes in end-of-life research in pediatric oncology. J Pediatr Psychol. 2007;32:1079–88. doi: 10.1093/jpepsy/jsm004. [DOI] [PubMed] [Google Scholar]
- 77.Mack JW, Wolfe J. Early integration of pediatric palliative care: for some children, palliative care starts at diagnosis. Current opinion in pediatrics. 2006;18:10–4. doi: 10.1097/01.mop.0000193266.86129.47. [DOI] [PubMed] [Google Scholar]
- 78.Rosenberg AR, Baker KS, Syrjala K, Wolfe J. Systematic review of psychosocial morbidities among bereaved parents of children with cancer. Pediatr Blood Cancer. 2012;58:503–12. doi: 10.1002/pbc.23386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wiener L, McConnell DG, Latella L, Ludi E. Cultural and religious considerations in pediatric palliative care. Palliat Support Care. 2013;11:47–67. doi: 10.1017/S1478951511001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kronberger M. Communication with palliative care patients: truth and hope–a contradiction? Wien Med Wochenschr. 2010;160:319–24. doi: 10.1007/s10354-010-0776-3. [DOI] [PubMed] [Google Scholar]
- 81.Hinds PS, Oakes LL, Hicks J, et al. “Trying to be a good parent” as defined by interviews with parents who made phase I, terminal care, and resuscitation decisions for their children. J Clin Oncol. 2009;27:5979–85. doi: 10.1200/JCO.2008.20.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Penson RT. Between Parent and Child: Negotiating Cancer Treatment in Adolescents. The Oncologist. 2002;7:154–62. doi: 10.1634/theoncologist.7-2-154. [DOI] [PubMed] [Google Scholar]
- 83.Meert KL, Templin TN, Michelson KN, et al. The Bereaved Parent Needs Assessment: a new instrument to assess the needs of parents whose children died in the pediatric intensive care unit*. Crit Care Med. 2012;40:3050–7. doi: 10.1097/CCM.0b013e31825fe164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bishop K. Challenging research: completing participatory social research with children and adolescents in a hospital setting. Herd. 2014;7:76–91. doi: 10.1177/193758671400700205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


