Abstract
Clinical studies suggest that obesity and type 2 (insulin resistant) diabetes impair the structural integrity of medial temporal lobe regions involved in memory and confer greater vulnerability to neurological insults. While eliminating obesity and its endocrine comorbidities would be the most straightforward way to minimize cognitive risk, structural barriers to physical activity and the widespread availability of calorically dense, highly palatable foods will likely necessitate additional strategies to maintain brain health over the lifespan. Research in rodents has identified numerous correlates of hippocampal functional impairment in obesity and diabetes, with several studies demonstrating causality in subsequent mechanistic studies. This review highlights recent work on pathways and cell-cell interactions underlying the synaptic consequences of obesity, diabetes, or in models with both pathological conditions. Although the mechanisms vary across different animal models, immune activation has emerged as a shared feature of obesity and diabetes, with synergistic exacerbation of neuroinflammation in model systems with both conditions. This Review discusses these findings with reference to the benefits of incorporating existing models from the fields of obesity and metabolic disease. Many transgenic lines with basal metabolic alterations or differential susceptibility to diet-induced obesity have yet to be characterized with respect to their cognitive and synaptic phenotype. Adopting these models, and building on the extensive knowledge base used to generate them, is a promising avenue for understanding interactions between peripheral disease states and neurodegenerative disorders.
Keywords: Obesity, diabetes, insulin resistance, hippocampus, long-term potentiation, synapse
Understanding relationships between cellular metabolism and circuit function is a central question for both basic and clinical neuroscience. Changes in energy intake and expenditure influence synaptic plasticity, and this relationship is not exclusive to brain regions classically implicated in food intake and metabolism. Decades of research in animal models has revealed correlations between metabolic efficiency at the systems level and neuroplasticity in the hippocampus and other regions involved in learning and memory (Bedford et al., 1979; Dulloo et al., 1991; Greenwood and Winocur et al., 1990; Neeper et al., 1995). These relationships are considered bidirectional based on studies demonstrating enhancement of hippocampal plasticity with exercise and caloric restriction (van Praag et al., 1999; Fontan-Lozano et al., 2007), and functional impairment in obesity and diabetes (Magariños and McEwen, 2000; Molteni et al., 2002). Associations between metabolism and neuroplasticity are detectable at the systems level and the cellular level, where insulin receptor activation (Lee et al., 2011), glucose transporter expression and localization (Ferreira et al., 2011), and mitochondrial function (Cheng et al., 2012) have all been linked with synaptic mechanisms for learning and memory. Given the substantial metabolic demands required for synaptic transmission, it is perhaps unsurprising that bidirectional regulation of neuroplasticity by energetic challenges would be evident across most, if not all, brain circuits (for review, see Stranahan and Mattson, 2011). The challenge in addressing this question lies in isolating individual systems impacted by complex pathologies, such as obesity and diabetes.
Nearly fifteen years since the first report of increases in dementia risk among diabetics in the Rotterdam study (Ott et al., 1996), obesity and diabetes have yet to be clinically implemented as risk factors for cognitive impairment and dementia. Consequentially, there have been no efforts to develop therapeutics to reduce dementia risk in individuals with diabetes and obesity, and the promise of greater efficacy based on treatments tailored to individual risk factors has yet to be realized. Some of the impediments to translation are likely attributable to variability in the degree to which different animal models of diabetes and obesity mimic features of these conditions in human populations. Type 1 (insulin-deficient) diabetes is typically diagnosed early in life and the most frequent cause is autoimmune destruction of the insulin-producing pancreatic beta cells (Hamman et al., 2014). Type 1 diabetics are not typically overweight or obese, and with adherence to an insulin administration regimen, there is little to no cognitive risk in later life (Lobnig et al., 2006). Type 2 (insulin resistant) diabetes is a progressive disease, with the earliest stages characterized by elevated fasting glucose levels and compensatory increases in insulin production (American Diabetes Association, 2014). Over time, the pancreatic beta cells become exhausted and the patient converts from insulin resistant to insulin deficient diabetes (American Diabetes Association, 2014). Individuals with Type 2 diabetes are frequently, but not always, overweight or obese (Sullivan et al., 2005), and dementia risk is elevated in Type 2 diabetes independent of body mass index (BMI; Xu et al., 2009).
Obesity is a complex disorder that occurs as a consequence of genetic and lifestyle factors (Ogden et al., 2014). While some obese individuals do not develop insulin resistant diabetes, data from twin studies and longitudinal studies indicates that, even in the absence of metabolic and cardiovascular comorbidities, obesity increases risk for multiple forms of dementia, including vascular dementia and Alzheimer's disease (Xu et al., 2011; Whitmer et al., 2008). These reports are consistent with other studies that came to similar conclusions using statistical methods to separate the effects of obesity from those of diabetes (Profenno et al., 2010).
The goal of identifying cellular and systems-level mechanisms for changes in synaptic plasticity and cognition in obesity and diabetes would be significantly advanced by incorporating sophisticated model systems developed in the field of obesity and metabolism. These models include transgenic mice with vulnerability or resistance to the metabolic effects of diet-induced obesity and surgical approaches for manipulating the amount and distribution of adipose tissue. Comparing learning and plasticity measures across model systems with selective deficits in glycemic control or body weight homeostasis could distinguish the effects of diabetes from those of obesity. This approach would enable subsequent studies of synergy between the two conditions and may also assist in refinement of risk criteria in clinical populations. This Review highlights recent developments in the literature on mechanisms for impaired hippocampal neuroplasticity in obesity and diabetes, with reference to the importance of addressing related questions in future studies using metabolic models that have yet to be characterized with respect to their cognitive and synaptic phenotype.
Pharmacological models used to study hippocampal plasticity in obesity and diabetes
Streptozotocin (STZ) is a pancreatic beta-cell toxin injected intravenously or intraperitoneally to create a model of insulin deficient diabetes (Lenzen et al., 2008). Either STZ or alloxan, a related nitrosylurea compound, causes rapid-onset insulin deficient diabetes that is accompanied by reductions in body weight in some, but not all studies (Biessels et al., 1998; Magariños and McEwen, 2000; Stranahan et al., 2008a). Studies of hippocampal plasticity in diabetes make frequent use of STZ as a rapidly inducible model with robust deficits in neurogenesis (Zhang et al., 2008; Ho et al., 2014), synaptic plasticity (Biessels et al., 1998; Stranahan et al., 2008a), and cognition (Kamal et al., 2000; Stranahan et al., 2008b). Although some mechanisms identified in the insulin-deficient STZ model have also been demonstrated in insulin resistant rodents (Clodfelder-Miller et al., 2005; Stranahan et al., 2008a; Kim et al., 2009), many studies using STZ assert that the observed changes in hippocampal function are relevant to both insulin deficient and insulin resistant diabetes without demonstrating that this is the case (Diegues et al., 2014). This element of interpretation is flawed, as insulin resistance develops over years and typically is detected in middle-aged human populations, but insulin deficiency is generally identified in pediatric populations (Hamman et al., 2014). Even when scaled down to the shorter lifespan in rodents, the development of insulin deficiency and hyperglycemia following one to three days of STZ treatment in no way resembles the gradual time course for development of insulin resistance in humans (American Diabetes Association, 2014). The use of STZ as a model of human Type 2 diabetes is similar to the outdated research practice of using kainic acid-induced neurodegeneration as a model of Alzheimer's disease; there are some similarities, but the therapeutic utility of treatments that reverse hippocampal plasticity deficits in STZ-treated rodents is likely to be limited to treating uncontrolled insulin deficient diabetes in humans.
Streptozotocin is also used to model insulin resistant diabetes following repeated low-dose administration in conjunction with a high-fat diet (HFD) regimen. The advantage of using STZ with HFD feeding is that the cognitive and synaptic effects of HFD alone are variable, with some studies reporting deficits in hippocampus-dependent memory after 2-3 months of diet exposure (Molteni et al., 2002; Greenwood and Winocur, 1990), and other studies detecting memory impairment only after extended maintenance on the diet (Stranahan et al., 2008c). Combining HFD with partial damage to the insulin-producing beta cells of the pancreas generates insulin resistance and increases neuronal vulnerability to ischemic insults (Zhang et al., 2009). Although accelerating the onset for changes in hippocampal function allows for more timely completion of research studies, the relevance of rapid-onset metabolic dysregulation to human disease is questionable, as the progression of human obesity to insulin resistance and type 2 diabetes involves protracted periods of obesogenic diet consumption together with genetic vulnerability and lifestyle choices (Walley et al., 2009).
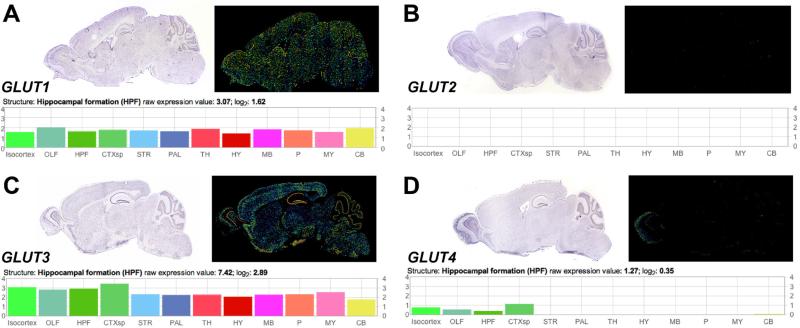
Since the initial reports of an epidemiological correlation between diabetes and dementia in the Rotterdam study (Ott et al., 1996), basic and clinical scientists have begun to refer to Alzheimer's disease (AD) as ‘Type III diabetes’ (Pilcher, 2006). This designation was based in part on mechanistic studies demonstrating that insulin-degrading enzyme (IDE) participates in the removal of both insulin and beta-amyloid from the brain, and the recruitment of IDE for insulin disposal would presumably limit the capacity for disposal of amyloid beta (Yang and Song, 2013). While IDE is an attractive candidate for AD and diabetes comorbidity, a subset of the research on alterations in hippocampal plasticity in diabetes has begun to use intracerebroventricular (ICV) administration of STZ as a model for central insulin resistance (Lannert and Hoyer, 1998; Shoham et al., 2003). Unfortunately, this model bears little resemblance to either AD or insulin resistant diabetes at the neuronal level, and fails to take into account the well-characterized mechanism of action for STZ. The molecular structure of STZ is similar to glucose, allowing STZ to enter cells via the GLUT2 subtype of the glucose transporter (Schnedl et al., 1994). Once inside the cell, STZ causes both DNA damage and protein glycosylation, leading to cell death (Liu et al., 2000). GLUT2 is highly expressed in pancreatic beta cells, but neurons and glia do not exhibit widespread expression GLUT2 in the CNS (Vannucci et al., 1997), making it unlikely that they will take up STZ following intracerebroventricular (ICV) administration. The absence of GLUT2 expression in the CNS is further upheld by interrogation of gene expression data from the Allen Brain Atlas, where no detectable signal for GLUT2 mRNA was observed in any brain region (Lein et al., 2007; Figure 1). The consequences of ICV STZ are therefore likely to be independent of cellular uptake and indirect, with limited consequences for brain insulin sensitivity. Moreover, the acute nature of ICV STZ experiments renders them subject to the same caveats as experiments using systemic STZ treatment, as neither the time course nor the topography of neuroplasticity deficits resembles human aging or dementia (Fotuhi et al., 2009).
Figure 1. Absence of detectable GLUT2 detected in gene expression mapping of the mouse brain.
(A), The astroglial and endothelial glucose transporter GLUT1 is abundantly expressed in the mouse brain. Graph (below) depicts GLUT1 gene expression data from the Allen Brain Atlas. (B), No detectable GLUT2 mRNA was observed in any brain region. (C), The neuronal glucose transporter GLUT3 is widely expressed in the mouse brain. Graph (below) depicts regional comparison of gene expression data generated by the Allen Brain Institute. (D), Gene expression screening revealed lower levels of expression for the insulin-stimulated glucose transporter GLUT4. Graph (below) depicts GLUT4 gene expression data from the Allen Brain Atlas. Images and data were obtained from the Allen Institute for Brain Science website at www.alleninstitute.org.
Non-obese models of insulin resistant diabetes used to study hippocampal plasticity
The Goto-Kakizaki (GK) rat model allows for disambiguation of synaptic regulation by insulin resistance in the absence of obesity. GK rats were generated by artificial selection for impaired glucose tolerance in the Wistar strain (Goto et al., 1976). This strategy produced a polygenic model of spontaneous insulin resistant diabetes with normal body weight and food intake (Galli et al., 1996). Studies in GK rats have demonstrated impairment of hippocampal function in the water maze paradigm and deficits in dendritic spine density among pyramidal neurons in hippocampal area CA1 (Li et al., 2013). These changes were not restricted to the CA1 subfield, as other groups demonstrated alterations in dentate gyrus neurogenesis, with increases in cell proliferation reported at 16 weeks of age (Beauquis et al., 2010), and reductions in cell proliferation and neurogenesis detected at 18 weeks of age (Lang et al., 2009). The apparent disparity between these two observations could reflect methodological differences, as increased cell proliferation was determined by stereological estimates of total cell number (Beauquis et al., 2010) and reductions were determined by quantification of cell density (Lang et al., 2009). Because densitometric measures are by nature confounded by regional volume, which was not reported in either study, it is not possible to directly compare the outcomes of these experiments, but it is likely that the GK rat model exhibits changes in adult neurogenesis in the dentate gyrus in addition to deficits in functional and structural plasticity in hippocampal area CA1.
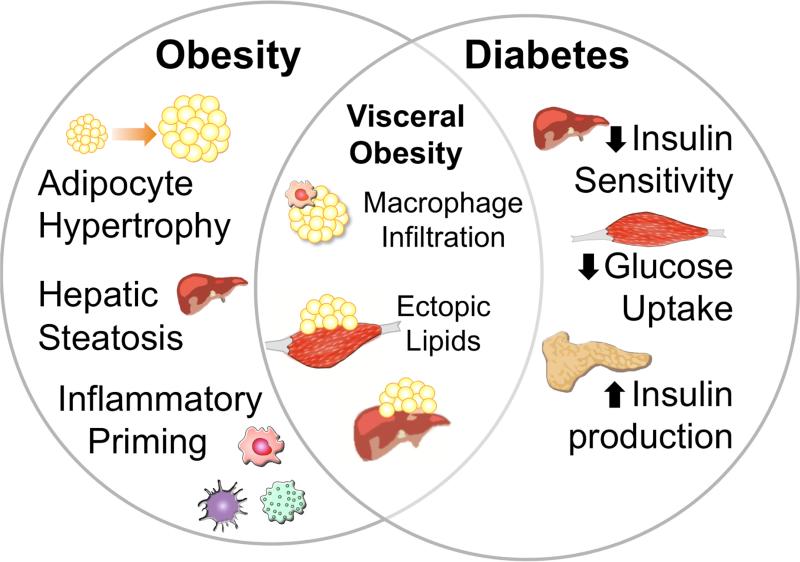
The GK rat model exhibits insulin resistance without obesity, but also has chronically elevated levels of the adrenal steroid corticosterone (Beddow and Samuel, 2012). Given the well-recognized dysregulation of hippocampal plasticity that occurs with chronic exposure to elevated glucorticoids (Joëls and Baram, 2009), the possible contributions of hippocampal glucocorticoid receptor activation to impaired plasticity in GK rats warrants further investigation. Although some reports have described correlations between reductions in hippocampal neurogenesis and elevated corticosterone levels in GK rats (Beauquis et al., 2010), no studies have manipulated corticosterone levels in this model to address a possible causal relationship. The interplay between glucocorticoids and glycemic control complicates the requisite design for such an experiment, since corticosterone acts in the periphery to exacerbate hyperglycemia in GK rats (Beddow and Samuel, 2012). Central manipulation of glucocorticoid signaling in GK rats would therefore be required to specifically examine the role of hippocampal corticosterone in synaptic deficits. Because no published studies have examined the consequences of selectively manipulating hippocampal glucocorticoid signaling in GK rats, the question of whether impaired hippocampal function in this model arises from insulin resistance, hyperglycemia, or elevated corticosterone levels cannot currently be answered. The primary conclusion that can be drawn from publications using the GK rat model is that the endocrine consequences of type 2 diabetes impair cognition and hippocampal plasticity independently of signaling mechanisms associated with obesity. While the specific endocrine factors that participate in hippocampal synaptic dysregulation remain to be identified, non-obese insulin resistant models such as the GK rat are useful to separate the consequences of diabetes from those of obesity (Figure 2).
Figure 2. Distinct and overlapping features of metabolic pathology in obesity and type 2 diabetes.
While there is substantial individual variability in the degree of overnutrition required to induce obesity, caloric excess in the absence of commensurate increases in energy expenditure will eventually lead to hypertrophy of adipose tissue. Most adipose tissue in adults is made up of white fat, and there is also a significant genetic component related to preferential energy storage in subcutaneous white fat or visceral white fat. When the storage capacity of subcutaneous and visceral fat deposits is exceeded, lipids are stored in other tissues. Hepatic steatosis is a feature present in obesity and exaggerated in populations with visceral obesity. Invasion of lipid droplets into other organs contributes to chronic, low-grade inflammation and priming of immune cells in obesity. These features are exacerbated in visceral obesity, particularly when the storage capacity of mesenteric adipocytes is exceeded, causing cell death, which attracts and activates macrophages. Continued demand for energy storage promotes further invasion of ectopic lipid droplets and contributes to the development of insulin resistance. Loss of hepatic insulin sensitivity and reductions in muscle glucose uptake are initially met with compensatory increases in insulin production by pancreatic beta cells. At this point, individuals may meet clinical criteria for type 2 diabetes, and may receive treatment with glucose-lowering drugs, along or in combination with treatments that stimulate insulin production, or with newer drugs that increase the bioavailability of glucagon-like peptide 1. These therapeutics are applied with the goal of preventing or decelerating eventual exhaustion of pancreatic beta cells and conversion to insulin dependent diabetes.
Genetic models used to study hippocampal plasticity in obesity and diabetes
A variety of genetic models with obesity and insulin resistance due to loss-of-function mutations in the gene for the satiety hormone leptin or leptin receptors have been used in studies of hippocampal plasticity. Early research in the Zucker rat, in which obesity and type 2 diabetes arise from the lack of functional leptin receptors, yielded mixed results. Some studies reported deficits in spatial learning using the hippocampus-dependent water maze (Li et al., 2002), but others failed to detect changes in hippocampus-dependent memory using this paradigm (Belanger et al., 2004). This variability could be attributable to the use of four daily massed trials for water maze testing by Li et al., (2002), versus the once-daily spaced trials used by Belanger and colleagues (2004). While the massed-training design places greater demand on working memory, the spaced-training design more effectively elicits lasting recall of the platform location, and differential outcomes may be explained by a more prominent impairment of working memory in Zucker rats. Alternatively, the Zucker rats tested in the spaced design by Belanger et al., (2004) exhibited reductions in swim speed in both the visible and submerged platform versions of the water maze, while the Zucker rats in the study by Li et al., (2002) did not exhibit differences in swim speed. This difference limits the validity of comparisons between the two studies, and the effects of trial spacing on acquisition and persistence of memory in the water maze paradigm remain to be explored in relationship to genetic or dietary obesity.
Relative to studies in the Zucker rat model, the data from similar loss-of-function mutations in the leptin receptor in mice are more consistent. Leptin receptor deficient db/db mice have impaired spatial memory in the water maze (Li et al., 2002), novel object recognition (Stranahan et al., 2008a), and spatial recognition memory tasks (Wosiski-Kuhn et al., 2014). Behavioral deficits in this model are accompanied by reductions in the magnitude of long-term potentiation (LTP) in hippocampal area CA1 (Li et al., 2002) and in the dentate gyrus (Stranahan et al., 2008a). Deficits in synaptic plasticity are likely a result of reductions in the density of dendritic spines, the primary sites for excitatory synaptic contact (Stranahan et al., 2009). Importantly, these measures of loss-of-function among hippocampal neurons are not a direct consequence of the leptin receptor mutation, as previous studies using adrenalectomy and physiological corticosterone replacement (ADX+CORT; Stranahan et al., 2008a) successfully rescued hippocampal plasticity without reversing leptin receptor deficiency. ADX+CORT normalized mRNA for the consensus region shared by all transcript variants for brain-derived neurotrophic factor (BDNF) in db/db mice, with a similar pattern for the high-affinity BDNF receptor TrkB (Stranahan et al., 2011). This pattern was observed in the hippocampus, but not in the hypothalamus, where BDNF and TrkB were similarly reduced in db/db mice that received ADX+CORT or sham operation (Stranahan et al., 2011). The findings from these studies support the existence of regional differences in the mechanisms for synaptic dysfunction in db/db mice. While dysregulation of hypothalamic development and plasticity appears to be a direct consequence of defective leptin signaling (Bouret et al., 2012), hippocampal dysfunction in db/db mice is likely to be an indirect effect mediated by perturbation of other physiological systems (Stranahan et al., 2008a; Stranahan et al., 2011).
Leptin unquestionably modulates aspects of hippocampal synaptic physiology (Shanley et al., 2001), but the consequences of the db/db mutation likely involve both leptin-dependent effects and effects that are secondary to the obese phenotype of this model. The mechanistic complexity of synaptic dysregulation in leptin-deficient models reflects the diversity of their pathological phenotype, which involves elements of both obesity and insulin resistant diabetes (Figure 2). Although obesity and insulin resistance exhibit substantial comorbidity in human populations, leptin deficient rodent models are physiologically extreme, and cases of human obesity due to congenital leptin deficiency are restricted to an extremely small cohort (Ozata et al., 1999). Human obesity is associated with leptin resistance (Caro et al., 1996), but the role of leptin resistance in the development and progression of insulin resistance and Type 2 diabetes remains somewhat controversial, as administration of exogenous leptin to obese human subjects with Type 2 diabetes failed to influence glycemic control or body weight (Chan et al., 2005; Chan et al., 2006). Despite the absence of detectable physiological effects in human insulin resistance, studies in animal models suggest that leptin receptor activation recruits both canonical Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway, and the phosphatidylinositol 3-kinase (PI3K) pathway, which is also a target of insulin receptor signaling (Marino et al., 2011). The ongoing debate over the degree of overlap in mechanisms for diabetes and obesity underscores the importance of selecting appropriate models of one or both disorders in studies of hippocampal plasticity.
Inducible genetic models used to study hippocampal plasticity in obesity and diabetes
The possibility that leptin receptor deficiency would exert indirect effects on hippocampal plasticity is cumbersome, but it is also supported by an elegant series of studies using lentiviral manipulation of hypothalamic sensitivity to metabolic signals as a strategy to induce obesity and cognitive impairment in rats. Injection of lentiviral vectors carrying insulin receptor antisense (IRAS) into the third ventricle resulted in dowregulation of insulin receptors in the hypothalamic arcuate and ventromedial hypothalamic nuclei (Grillo et al., 2007). Changes in hippocampal insulin receptor expression did not occur following IRAS injection, confirming the regional specificity of this manipulation (Grillo et al., 2007). Reductions in hypothalamic insulin receptor expression caused obesity and increased circulating leptin levels, but did not alter glycemic control (Grillo et al., 2007). Elevations in serum leptin led to subsequent demonstrations of impaired leptin sensitivity in hypothalamic neurons in IRAS animals, assessed based on immunoreactivity for the phosphorylated form of signal transducer and activator of transcription 3 (STAT3) following peripheral leptin administration (Grillo et al., 2011a). This series of experiments clearly demonstrates that downregulation of hypothalamic insulin receptors is an inducible and regionally selective strategy to induce obesity.
Obesity in IRAS rats was associated with deficits in hippocampal long-term potentiation in area CA1 that were reversible when the obese phenotype was attenuated by caloric restriction (Grillo et al., 2011b). Impairment of functional plasticity at CA1 synapses was accompanied by reduced contextual fear conditioning, with commensurate deficits in fear conditioning-evoked immediate early gene expression (Grillo et al., 2011a). Hippocampal expression of synaptophysin and postsynaptic density 95 (PSD95) were also reduced as a consequence of obesity in IRAS rats (Grillo et al., 2011a), indicating that obesity weakens hippocampal connectivity even in the absence of changes in insulin sensitivity and glycemic control. This premise is especially striking in light of the complete absence of hyperglycemia and insulin resistance demonstrated in this model (Grillo et al., 2007), and suggests that insulin resistant diabetes and obesity independently suppress hippocampal plasticity, with synergy between the two disorders leading to more aggressive synaptic loss and cognitive impairment.
Direct effects of insulin on hippocampal plasticity in diabetes
Although the IRAS studies identified insulin-independent synaptic deficits in non-diabetic obesity, there is substantial evidence for insulin signaling as an obligatory participant in hippocampal function. This evidence comes from studies using intrahippocampal insulin infusions in non-obese, non-diabetic animals, and from studies that measure insulin and glucose in awake behaving animals using microdialysis. Pre-training (Moosavi et al., 2006) or post-training (Stern et al., 2014; Babri et al., 2007) injections of insulin into the dorsal hippocampus of rats improved inhibitory avoidance learning without altering circulating glucose levels. This relationship was not restricted to avoidance learning tasks, as assessment of spatial working memory in normal rats also revealed enhancement following pre-test hippocampal insulin administration, and impairment following pre-test administration of an insulin-scavenging antibody (McNay et al., 2010). The physiological consequences of intrahippocampal insulin treatment include reduced extracellular glucose and increases in extracellular lactate, both of which are lost in animals with diet-induced obesity (McNay et al., 2010). When examined in parallel with research in normal weight, insulin resistant GK rats, it is possible to conclude that insulin signaling supports learning and memory, and that support is lost in the context of diabetes.
Insulin enters the central nervous system (CNS) via receptor-mediated transport across the blood-brain barrier (Abbott et al., 2006). The transport mechanism for insulin is saturable, adding another layer of regulation to prevent insulin excess in the brain parenchyma (Banks et al., 1997). Given this additional buffer against CNS insulin excess, it is entirely possible that development of peripheral insulin resistance would occur along a shorter timescale than insulin resistance in the brain. There are a number of experimental approaches to measure neuronal insulin sensitivity, including quantification of extracellular insulin and glucose in awake behaving animals by microdialysis (McNay and Gold, 1999; McNay et al., 2010) and ex vivo analysis of insulin sensitivity in synaptosomes (Grillo et al., 2011) or acute slice preparations (Mielke et al., 2005; Ross et al., Dey et al., 2014). Although McNay and colleagues (2010) conclusively demonstrated reductions in both hippocampal insulin sensitivity and systemic insulin sensitivity following five months of obesogenic diet consumption in rats (McNay et al., 2010), a possible delayed onset of impaired insulin neuronal sensitivity, relative to peripheral insulin sensitivity, has never been directly examined. Additional studies will be required to address possible differences in the temporal development of impaired neural insulin sensitivity under conditions of reduced peripheral insulin sensitivity, and the GK rat model of Type 2 diabetes without obesity would be especially suitable for this purpose. Identification of changes in CNS insulin exposure due to normal weight hyperinsulinemia would pave the way for more detailed investigation into the kinetics and anatomical specificity of decrements in neuronal insulin receptor sensitivity.
Diabetic hyper- and hypoglycemia: feast or famine
Individuals with Type 1 diabetes that experience severe hypoglycemic episodes develop structural atrophy in medial temporal regions (Hershey et al., 2010). Patients in the early stages of Type 2 diabetes experience prolonged peripheral hyperglycemia during the early stages of the disease (American Diabetes Association, 2014), and non-insulin drug treatments for lowering glucose levels occasionally overshoot the physiological range and produce episodes of hypoglycemia (Melander, 2004). At later stages of Type 2 diabetes, beta cell exhaustion converts insulin resistance to insulin deficiency (American Diabetes Association, 2014), which is subject to similar risk of hypoglycemia seen in Type 1 diabetes (Hamman et al., 2014). Pathological extremes in circulating glucose levels may initially be buffered by changes in glucose transport across the BBB (Simpson et al., 1999), but prolonged hyper- or hypoglycemia paradoxically leads to the same outcome: impaired synaptic function and eventually, cell death.
Cellular glucose uptake is mediated by the fourteen-member glucose transporter (Glut) family, of which Glut1 is expressed in astrocytes and cerebral endothelial cells (Vannucci et al., 1997; Simpson et al., 1999), and Glut3, Glut4, and Glut8/Glutx1 are expressed by neurons (Reagan et al., 1999; Grillo et al., 2009; Reagan et al., 2001). In normal animals, the expression and localization of neuronal glucose transporters is dynamically regulated by glucose (Reagan et al., 1999; Piroli et al., 2002), glutamate (Ferreira et al., 2011), and in the case of Glut4, by insulin (Grillo et al., 2009). Activity-dependent increases in cell-surface Glut3 in neurons are dependent on activation of the PI3K/Akt pathway (Ferreira et al., 2011), and insulin-stimulated surface expression of Glut4 is also PI3K-dependent (Grillo et al., 2009). The subcellular localization of Glut8/Glutx1 suggests a different role, as peripheral glucose administration in non-diabetic animals promoted trafficking of Glut8/Glutx1 from the cytoplasm to the endoplasmic reticulum (Piroli et al., 2002). Glucose-stimulated translocation of Glut8/Glutx1 was lost in rats with insulin deficiency due to STZ treatment (Piroli et al., 2002), suggesting that neuronal glucose metabolism indeed fluctuates with pathological hyperglycemia.
Diet-induced obesity models used to study hippocampal structure and function
Diet-induced obesity models represent the most relevant system for identifying mechanisms likely to generalize across species. However, experimental variables such as diet composition, duration of exposure, age at onset of diet availability, and variability in individual consumption by experimental animals often complicate the design and interpretation of these studies. The types of diets used to generate obesity in rats and mice vary widely, with some groups using commercially available high-fat diets ranging from 45% to 60% fat (Stranahan et al., 2008c; Kanoski et al., 2010; McNay et al., 2010; Table 1). The ingredients used to supply calories from fat also vary between vendors, with most suppliers supplying additional fat from lard, milkfat, or vegetable oil (Table 1). The fatty acid composition of experimental diets represents an additional source of variability, as the proportion of long-chain saturated fatty acids determines the severity of heptatic steatosis (Geng et al., 2013) which may influence the degree of cognitive impairment in obesity (Greenwood and Winocur, 1990; Ross et al., 2012). Although the majority of studies use diets that differ in fat content, there are a number of commercial diets that combine increases in fat content with carbohydrates from simple sugars rather than complex sources (Table 1). When examining the consequences of commercial obesogenic diets for any endpoint, it is absolutely essential to use control diets purchased from the same source, as typical grain-based diets supplied by most academic and pharmaceutical animal facilities often differ in the source of carbohydrates and in micronutrient content per unit of chow. Use of control diets from a different supplier than the obesogenic diets is a significant confounding variable in many diet-induced obesity studies.
Table 1.
Variations in diet composition across studies of obesity and hippocampal function.
| Fat | Carbohydrate | Protein | |||||
|---|---|---|---|---|---|---|---|
| Citation | Diet | kCal (%) | Source | kCal (%) | Source | kCal (%) | Source |
| Greenwood and Winocur, 1990 | LFD | 5 | Lard | 75 | Cornstarch | 20 | Casein |
| HFD | 40 | Soybean oil | 30 | Cornstarch | 30 | Casein | |
| HSFD | 40 | Beef tallow | 30 | Cornstarch | 30 | Casein | |
| Molteni et al., 2002 | LFD | 13 | Soybean oil | 60 | Cornstarch | 27 | Soy |
| HFD | 40 | Lard | 40 | Sucrose | 20 | Soy | |
| Stranahan et al., 2008c | LFD | 5 | Butter oil | 75 | Cornstarch | 20 | Casein |
| HFD | 20 | Butter oil | 60 | Cornstarch | 20 | Casein | |
| McNay et al., 2010 | LFD | 6 | Lard | 64 | Cornstarch | 30 | Soy |
| HFD | 32 | Butter oil | 51 | Sucrose | 17 | Casein | |
| Kanoski et al., 2010 | LFD | 14 | Soybean oil | 56 | Cornstarch | 30 | Soy |
| HFD | 40 | Lard | 30 | Glucose | 30 | Casein | |
| Ross et al., 2012 | LFD | 10 | Soybean oil | 70 | Cornstarch | 20 | Casein |
| HFD | 10 | Soybean oil | 70 | Fructose | 20 | Casein | |
| Sobesky et al., 2014 | LFD | 17 | Soybean oil | 54 | Sucrose | 29 | Soy |
| HFD | 35 | Lard | 32 | Sucrose | 33 | Casein | |
Abbreviations: LFD, low-fat diet; HFD, high-fat diet; HSFD, high saturated fat diet.
In a similar vein, some studies utilize a ‘cafeteria diet’ that involves providing various types of human food to experimental animals. Food sources used in these experiments range from lard, high-fructose corn syrup, and processed meats, to more complex palatable foods like pastries and processed meats (Beilharz et al., 2014; Reichelt et al., 2014). The advantage of supplying experimental animals with a broad array of varied palatable foods is that they will more readily overconsume those foods relative to high-fat research diets. The disadvantage is the lack of experimental control over the many nutritional variables that differ between rats on a cafeteria diet and rats eating normal rodent chow. Availability of human food sources in addition to standard rodent chow often results in reduced consumption of rodent chow, with the potential for micronutrient deficits that may influence experimental outcomes. Studies using cafeteria diet instead of rodent chow also suffer from this confound to a greater degree, since animals are not provided with standardized food sources known to contain specified quantities of vitamins and minerals, many of which influence hippocampal plasticity (Cocco et al., 2002; Ferri et al., 2006). Particulate or semisolid food sources also limit the accuracy of intake measurements, which are necessary in order to attribute the results to increased caloric intake or changes in macronutrient intake without overall increases in caloric intake. In these respects, although cafeteria diets may appear to more accurately model obesogenic diets consumed by humans, the utility of these diets for mechanistic research is limited due to a lack of experimental control. Future studies would benefit from implementation of custom diet formulations that provide variability in taste and texture while maintaining macro- and micronutrient content.
Disambiguation of hormonal effects on metabolism and cognition in diabetes
Chronic activation of the hypothalamic-pituitary-adrenal axis (HPA axis) is known to exacerbate hyperglycemia and insulin resistance in Type 2 diabetes, based on case reports of improved glycemic control following unilateral adrenalectomy in patients with Type 2 diabetes and adrenal tumors (Blüher et al., 2000, Weisner et al., 2003). Adrenalectomy attenuates hyperglycemia and insulin resistance and completely reverses obesity in db/db mice (Shimomura et al., 1987; Stranahan et al., 2008a). The effects of lowering glucocorticoids on glycemic control in db/db mice therefore represent a potential alternative explanation for improvements in hippocampal function, as the initial studies did not incorporate direct measures of hippocampal insulin sensitivity (Stranahan et al., 2008a). Subsequent experiments revealed that db/db mice exhibit impaired hippocampal insulin sensitivity, based on analysis of insulin receptor phosphorylation after insulin application in hippocampal slice preparations (Dey et al., 2014). However, this deficit was unaffected by pharmacological inhibition of corticosterone synthesis (Dey et al., 2014), which normalized hippocampal plasticity at the structural, physiological, and behavioral levels (Wosiski-Kuhn et al., 2014).
Despite the lack of correlation between hippocampal insulin sensitivity and functional rescue in db/db mice (Dey et al., 2014; Wosiski-Kuhn et al., 2014), the diverse physiological effects of corticosteroid hormones limit the utility of systemic manipulations for mechanistic analysis of effects on hippocampal plasticity. To overcome this limitation, corticosterone was infused intrahippocampally to db/db mice that received concurrent peripheral injections of the glucocorticoid synthesis inhibitor metyrapone (Wosiski-Kuhn et al., 2014). The challenge with central delivery of steroid hormones is their insolubility in aqueous solution, necessitating the use of organic solvents or cholesterol pellets, both of which have the capacity to damage the CNS (Akana et al., 2001; Alfarez et al., 2002). Fortunately, a bioequivalent form of corticosterone conjugated to 2-hydroxypropyl-β-cyclodextrin, which forms a hydrophilic pocket around the hydrophobic steroid, was recently developed and used to evaluate the sufficiency of hippocampal corticosterone exposure for fear memory (Kaouane et al., 2012). Hippocampal infusion of soluble corticosterone recapitulated deficits in synaptic plasticity and cognition in metyrapone-treated db/db mice, (Wosiski-Kuhn et al., 2014), providing support for local elevations in corticosterone as a mechanism for memory impairment. Followup studies using lentiviral knockdown of hippocampal glucocorticoid receptor (GR) expression revealed that over-activation of GR in db/db mice led to sequestration of the transcription factor c-Fos and subsequent transrepression of BDNF expression at promoters I and IV (Wosiski-Kuhn et al., 2014). This observation parallels the underlying molecular cascade for reduction of hippocampal BDNF in normal weight, non-diabetic animals chronically exposed to psychological stress-induced elevations in corticosterone (Hansson et al., 2003).
Similarities in the molecular mechanism for reductions in hippocampal BDNF with psychological stress and genetic obesity suggests that chronic exposure to elevated corticosteroids is a pivotal event for hippocampal dysfunction in both conditions. However, dysregulation of adrenal steroid hormones is not reliably detectable in all models of diabetes, with or without obesity. Pharmacological models of insulin deficiency using STZ or alloxan causes chronic elevations in circulating corticosterone levels (Magariños and McEwen, 2000), and ADX+CORT or systemic treatment with the GR antagonist RU486 prevents impairment of hippocampal plasticity in this model (Stranahan et al., 2008; Revsin et al., 2009). Type 2 diabetic, normal weight GK rats also exhibit chronically elevated corticosterone levels (Beddow and Samuel, 2012), but a potential role in hippocampal synaptic impairment has yet to be evaluated in this model. IRAS rats exhibit behavioral anxiety and reductions in hippocampal BDNF without concurrent alterations in corticosterone levels (Grillo et al., 2011c), suggesting that obesity without insulin resistance or hyperglycemia does not elevate corticosteroids. This idea is also supported by data from diet-induced obesity models, where most studies report blunting of stress-induced elevations in glucocorticoids (Foster et al., 2009; Auvinen et al., 2012).
Taken together, the outcome of studies in diabetic rodent models supports a role for impaired insulin signaling (Biessels et al., 1998; McNay et al., 2010) and increases in glucocorticoids (Stranahan et al., 2008a; Revsin et al., 2009; Wosiski-Kuhn et al., 2014) as mediators of hippocampal synaptic impairment and cognitive deficits. The predominance of either mechanism does not correlate with the presence of comorbid obesity and diabetes, as model systems with both features have been shown to exhibit insulin-dependent (McNay et al., 2010) and insulin-independent, glucocorticoid-mediated hippocampal dysfunction (Dey et al., 2014; Wosiski-Kuhn et al., 2014). The two systems are not mutually exclusive and are likely to interact at the neuronal level and in the periphery. The challenge for understanding these interactions can be met by combining direct manipulation of insulin and corticosterone using in vitro preparations with temporally controlled in vivo manipulation of insulin receptors, glucose transporters, and glucocorticoid receptors.
Neuroimmune regulation of hippocampal function in obesity
Obesity causes chronic, low-grade inflammation (Kanneganti and Dixit, 2012). During the initial phases of adipose tissue hypertrophy, adipocytes synthesize and release cytokines that attract macrophages (Osborn and Olefsky, 2012). Cytokine production by adipose tissues occurs in a regionally specific manner, with visceral white adipose tissue (vWAT) as the predominant driver of inflammation in obesity (Rosen and Spiegelman, 2014). The role of adipose tissue inflammation in the metabolic consequences of obesity has been investigated for over twenty years since the initial demonstration of synthesis and release of the pro-inflammatory cytokine tumor necrosis factor alpha by adipocytes from obese animals (Hotamisligil et al., 1993). Inflammatory signaling was quickly accepted as a mechanism for peripheral neuropathy and retinopathy in diabetes and obesity models (Tsuda et al., 2005; Joussen et al., 2004), but CNS exposure to circulating inflammatory cytokines was considered minimal due to protection from the blood-brain barrier (BBB; Wolburg and Lippoldt, 2002).
Given that certain hypothalamic nuclei lack a functional BBB, it is perhaps unsurprising that initial studies of CNS inflammation in obesity focused on the hypothalamus (Milanski et al., 2009). Immune signaling in the brain is largely carried out by microglia, which resemble macrophages but diverge from a shared precursor during embryonic development (Kierdorf et al., 2013). In parallel with accumulating evidence for hypothalamic microglial activation in models of obesity and diabetes (Thaler et al., 2010), studies in physiologically normal animals revealed direct evidence for internalization of hippocampal synaptic terminals by microglia during developmental pruning (Paolicelli et al., 2011). Reports of correlations between hippocampal inflammation and cognitive impairment subsequently emerged in models of obesity (Andre et al., 2014) and obesity with features of diabetes (Dinel et al., 2011), but the mechanisms were never addressed with selective gain-of-function or loss-of-function approaches. Similarities between neuroinflammation in models of obesity and in mouse models of Alzheimer's disease were noted (Tucsek et al., 2014), but the pathways were not fully characterized, limiting the informative value of these qualitative observations.
Most transgenic models of familial Alzheimer's disease (AD) exhibit progressive microglial activation and increases in hippocampal pro-inflammatory cytokines (Perry et al., 2010). Increasing energy expenditure with wheel-running or treadmill training reliably and repeatedly attenuated this and other aspects of neuropathology in AD mice (Cotman et al., 2007; Stranahan et al., 2012a). db/db mice housed with a running wheel from five weeks of age initially run between 3-5 km/wk, and decrease to less than 1 km/wk by three months of age (Stranahan et al., 2009). Despite the decline in activity, which at maximum remained significantly less than the 10 km/wk distance run by wildtype mice, three-month-old db/db mice retained dendritic spine densities and hippocampal BDNF levels that were comparable with sedentary wildtype mice (Stranahan et al., 2009). Even with their limited amount of activity, db/db mice housed with a running wheel showed attenuation of obesity, hyperglycemia, and other metabolic measures (Stranahan et al., 2009). Subsequent experiments using treadmill training in db/db mice were initiated to maintain activity levels at 4 km/wk, and constrain activity in wildtype mice to comparable levels (Erion et al., 2014).
Mice and rats are typically motivated to run on a treadmill by avoidance of the shock grid located behind the belt on the apparatus (Kregel et al., 2006). To avoid the stress of shock exposure, the treadmill training program for db/db mice used compressed air, delivered manually to mice that failed to stay on the belt. Treadmill training normalized hippocampal function in db/db mice and reduced expression of major histocompatibility complex II (MHCII), a marker of classical activation, among hippocampal microglia (Erion et al., 2014). Reductions in microglial activation in treadmill-trained db/db mice were accompanied by lower levels of the pro-inflammatory cytokine interleukin 1beta in hippocampal lysates (Erion et al., 2014). Given the disparity between the level of running exhibited by wildtype mice in a running wheel (10 km/wk; Stranahan et al., 2009) and the treadmill training protocol (4 km/wk), it is unsurprising that wildtype mice showed no alterations in hippocampal plasticity, neuroinflammation, or adiposity in this study (Erion et al., 2014).
Although microglia are classically thought of as the primary immune cell in the brain, they often interact with astrocytes, which can generate, amplify, or dampen immune signals (Norden et al., 2014). To specifically examine the role of microglial activation in neuroinflammation, primary microglia were isolated from mice in each condition and cells were stimulated with increasing concentrations of lipopolysaccharide (LPS), a component of e. coli bacteria that robustly promotes inflammatory cytokine production (Frank et al., 2006). Microglia that have been activated or ‘primed’ exhibit lower thresholds for cytokine release (Frank et al., 2012), and priming was evident in cells isolated from sedentary db/db mice (Erion et al., 2014). By contrast, cells from treadmill-trained db/db mice showed dose-dependent responses to LPS stimulation that were identical to wildtype mice (Erion et al., 2014).
Treadmill training reduced the amount of visceral adipose tissue and attenuated adipose tissue inflammation in db/db mice (Erion et al., 2014), and to determine whether reductions in visceral fat mediate neuroinflammation and synaptic deficits, subsequent experiments used surgical lipectomy (LPX) and fat transplantation for direct manipulation of adiposity. Lipectomy and transplantation has been extensively used in obesity studies with endpoints outside of the CNS (Mauer et al., 2001). LPX completely normalized hippocampus-dependent memory, long-term potentiation, and dendritic spine density in db/db mice (Erion et al., 2014). By contrast, wildtype transplant recipients developed behavioral and synaptic impairments (Erion et al., 2014). Parallel opposing changes in microglial number and activation were evident, suggesting that signals from visceral fat drive microglial activation in genetic obesity (Erion et al., 2014). Effects on microglial activation were most likely cell autonomous, as primary microglia from mice in each condition showed similar patterns of functional sensitization (Erion et al., 2014).
Although microglia diverge from the monocyte lineage during embryonic development (Kierdorf et al., 2013), most of the markers used to identify microglia detect mixed populations of microglia and perivascular macrophages (Guillemin and Brew, 2004). Perivascular macrophages are derived from circulating monocyte precursors that penetrate the BBB, differentiate, and wrap around the abluminal surface of cerebral blood vessels, giving them a distinct tubular morphology (Audoy-Rémus et al., 2008). Perivascular macrophages promote local inflammation in the hypothalamus and thereby promote HPA axis activation following immune stimulation (Serrats et al., 2010), and can be detected based on expression of CD163, an antigen not present on microglia (Williams et al., 2001). To determine whether perivascular macrophages initiate CNS inflammation due to visceral obesity, activated cells were visualized by immunofluorescence for CD163 and MHCII in hippocampal sections from wildtype transplant recipients, lipectomized db/db mice, and sham-operated mice from each genotype. Changes in CD163/MHCII double-positive cells followed the same pattern observed with the monocyte lineage marker ionized calcium binding adapter protein 1 (IBA1); namely, LPX reversed activation of perivascular macrophages in db/db mice, and fat transplantation increased activation of this population in wildtype mice (Erion et al., 2014). Because the direction and magnitude of changes in activation were comparable among IBA1-positive cells and perivascular macrophages labeled with CD163, the issue of whether a specific immune cell population initiates hippocampal inflammation in obesity remains unresolved.
The issue of cell type specificity for most widely used microglial markers, such as IBA1, CD11b, and F4/80 (Guillemin and Brew, 2004), clouds the interpretation of neuroimmune studies in obesity, diabetes, and many other conditions. While a number of markers exist for intensity-based disambiguation of microglia and macrophages by flow cytometry (Guillemin and Brew, 2004), this approach is not always universally available. Only two antigens have been identified and validated as exclusive to microglia in vivo and in vitro (Butovsky et al., 2014). These antigens are the scavenger receptor Fc receptor-like S (Fcrls), which is expressed in murine microglia but has no human homologue, and the purinergic G-protein coupled receptor P2Y12 (P2ry12), expressed in both mouse and human microglia (Butovsky et al., 2014). Adoption of these more selective antigens, together with broad-based collaborations that bring together expertise in cell sorting and neuroplasticity, would help to advance understanding of microglia/neuron interactions under normal and pathological conditions.
Metabolic models of differential adiposity and resistance to dietary obesity: the next frontier
Obesity and insulin resistant diabetes each evoke a series of signaling cascades leading to impaired hippocampal function, with mechanistic overlap at multiple levels both within and outside of the central nervous system. However, closer examination of the pathological features of obesity and diabetes reveals elements of each disease that can be separately examined using existing animal models (Figure 2). In obesity, the metabolic consequences of increased adiposity depend on the distribution of adipose tissue across the visceral and subcutaneous regions. Increases in visceral white adipose tissue (vWAT) contribute to the detrimental effects of obesity on glycemic control (Després and Lemieux, 2006), while subcutaneous white adipose tissue (scWAT) represents both an active buffer and a protective metabolic ‘sink’ for energy storage outside of the visceral compartment (Rosen and Spiegelman, 2014). The distribution of fat in different areas can be manipulated surgically using lipectomy and fat transplantation (Mauer et al., 2001) or genetically, using available mouse models with transgenes that elicit hypertrophy or atrophy of different depots (Lowell et al., 1993; Masuzaki et al., 2001; Nedergaard et al., 2001). One prominent example of a transgenic model with increased vWAT is the aP2-11betaHSD1 overexpressing mouse, in which the glucocorticoid-amplifying enzyme 11beta-hydroxysteroid dehydrogenase 1 (11betaHSD1) is overexpressed under the adipocyte fatty acid binding protein 2 (aP2) promoter (Masuzaki et al., 2001). Although the aP2 promoter is expressed in both scWAT and vWAT, overexpression of 11betaHSD1 significantly increased the amount of vWAT, with concomitant hyperphagia and impairment of glycemic control. aP2-11betaHSD1 overexpressing mice also exhibit increased circulating corticosterone levels at the level of the hepatic portal vein, but not in the overall circulation (Masuzaki et al., 2001). These features would substantially advance both the glucocorticoid hypothesis of hippocampal synaptic deficits in diabetes, and the vWAT-driven immune hypothesis for obesity-induced synaptic deficits.
In contrast with the energy storage function of white adipose tissue, brown adipocytes make a direct contribution to energy expenditure. Brown adipose tissue (BAT) participates in metabolism in a manner that is more analogous to muscle, with mitochondrial uncoupling protein 1 (UCP1) in BAT as an obligatory contributor to thermogenesis (Rosen and Spiegelman, 2014). The essential role of BAT in body temperature regulation complicates the design of loss-of-function experiments, as surgical removal or genetic ablation of BAT impairs nonshivering thermogenesis (Nedergaard et al., 2001). This limitation has not prevented successful demonstrations of resistance to diet-induced obesity following BAT transplantation (Zhu et al., 2014), nor has it slowed the implementation of transgenic gain-of-function models for studies of metabolism (Seale et al., 2007). These designs could easily lend themselves to endpoints associated with hippocampal synaptic plasticity. With appropriate consideration of the caveats of each model, it may eventually become possible to identify features of synaptic impairment due to obesity, with or without comorbid diabetes. This strategy paves the way for eventual incorporation of body fat distribution and glycemic control criteria for early identification of risk factors leading to cognitive decline.
Conclusion
Patterns consistent with enhanced synaptic function with exercise or caloric restriction, and maladaptive plasticity with obesity or diabetes, are evident in the cerebellum (Green et al., 2011; Sickmann et al., 2010), brainstem (Michelini and Stern, 2009; Landsberg, 2006), hypothalamus (Horvath, 2005; Stranahan et al., 2012b), hippocampus (Neeper et al., 1995; Fontan-Lozano et al., 2007; Stranahan et al., 2008a), and across multiple cortical areas (Ehninger and Kempermann, 2003; Stranahan et al., 2007; Sweetnam et al., 2012). Opposing regulation of plasticity at the neural level by alterations in energy metabolism at the global level therefore appears to be a general principle detectable across multiple brain regions, including those regions not traditionally associated with food intake and metabolism. Any potential relationship between global and cerebral energy metabolism must account for interposition by the BBB, but accumulating evidence suggests that the BBB is less of a barrier and more of a dynamic gateway for access to the central nervous system (Abbott et al., 2006). BBB permeability is heterogeneous in different regions of the cerebral vasculature under normal conditions, with greater resistance along surface arteries, penetrating arterioles, and capillaries, and varying degrees of leakage in venules and along the venous drainage system (Ge et al., 2005). The list of pathological conditions known to compromise BBB integrity is growing, with obesity (Gustafson et al., 2007), aging (Montagne et al., 2015), and Alzheimer's disease (Zlokovic, 2005) shown to cause barrier breakdown in humans. CNS exposure to metabolic dysregulation is therefore probable given the potential for gradual erosion of barrier properties under pathological conditions.
As with any broad conceptual hypothesis, the difficulty lies in integrating the range of mechanisms and correlates reported in different conditions and identifying pathways recruited at either end of the metabolic spectrum. There is some degree of consensus that changes in insulin sensitivity and signaling (Biessels et al., 1998; McNay et al., 2010) and glucocorticoid receptor activation (Stranahan et al., 2008a; Revsin et al., 2009; Wosiski-Kuhn et al., 2014) contribute to hippocampal plasticity deficits in diabetes. In the absence of insulin resistance or deficiency, obesity compromises hippocampal function by promoting inflammation (André et al., 2014). The combination of hyperglycemia, insulin resistance, and obesity promotes inflammation in the hippocampus and periphery (O'Connor et al., 2005; Dinel et al., 2011; Dey et al., 2014), and visceral adipose tissue is the most probable source of inflammatory cytokines that interfere with synaptic plasticity and cognition (Erion et al., 2014). The combination of type 2 diabetes and obesity in clinical populations is therefore likely to induce a synaptic ‘double hit,’ with both endocrine and immune features weakening hippocampal circuits.
Obesity and diabetes both increase the likelihood of cognitive decline and accelerate the conversion of cognitive impairment to full-blown dementia (Xu et al., 2009; Xu et al., 2011; Yang and Song, 2013). However, there is substantial individual variability in the degree of caloric excess required to cause obesity, and in the anatomical distribution of adipose tissue hypertrophy in different areas during the pathogenesis of obesity. Even within the clinical population of obese individuals, there is substantial variability in the rate of glycemic control and progression to full-blown insulin resistance. Prior to implementation of broad-based conclusions regarding obesity, diabetes, and risk of cognitive impairment, it will be necessary to identify features of each condition that lead to neurocognitive deficits. These features can then be used to extrapolate risk, with the goal of preventing cognitive decline in vulnerable populations without exacerbating societal prejudice against overweight and obese individuals.
Research Highlights.
Evidence from human neuroimaging studies indicates that obesity and type 2 diabetes impair brain structure and function
Neurological changes that occur in metabolic pathologies are not restricted to brain regions involved in metabolism
Research in rodent models of diabetes and obesity has begun to examine synaptic mechanisms for memory impairment
Neuroimmune and neuroendocrine systems mediate hippocampal dysfunction in obesity and diabetes
Acknowledgements
This work was supported by grants (K01DK100616 and R03DK101817) from the National Institutes of Health.
Abbreviations
- 11betaHSD1
11beta hydroxysteroid dehydrogenase 1
- AD
Alzheimer's disease
- BAT
brown adipose tissue
- BBB
blood-brain barrier
- GK
Goto-Kakizaki
- HFD
high-fat diet
- ICV
intracerebroventricular
- IRAS
insulin receptor antisense
- PSD95
postsynaptic density-associated protein 95
- scWAT
subcutaneous white adipose tissue
- STAT3
signal transducer and activator of transcription 3
- STZ
streptozocin/streptozotocin
- UCP1
mitochondrial uncoupling protein 1
- vWAT
visceral white adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Akana SF, Chu A, Soriano L, Dallman MF. Corticosterone exerts site-specific and state-dependent effects in prefrontal cortex and amygdala on regulation of adrenocorticotropic hormone, insulin and fat depots. J Neuroendocrinol. 2001;13:625–37. doi: 10.1046/j.1365-2826.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- Alfarez DN, Wiegert O, Joëls M, Krugers HJ. Corticosterone and stress reduce synaptic potentiation in mouse hippocampal slices with mild stimulation. Neuroscience. 2002;115:1119–26. doi: 10.1016/s0306-4522(02)00483-9. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- Audoy-Rémus J, Richard JF, Soulet D, Zhou H, Kubes P, Vallières L. Rod-Shaped monocytes patrol the brain vasculature and give rise to perivascular macrophages under the influence of proinflammatory cytokines and angiopoietin-2. J Neurosci. 2008;28:10187–99. doi: 10.1523/JNEUROSCI.3510-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auvinen HE, Romijn JA, Biermasz NR, Pijl H, Havekes LM, Smit JW, Rensen PC, Pereira AM. The effects of high fat diet on the basal activity of the hypothalamus-pituitary-adrenal axis in mice. J Endocrinol. 2012;214:191–7. doi: 10.1530/JOE-12-0056. [DOI] [PubMed] [Google Scholar]
- André C, Dinel AL, Ferreira G, Layé S, Castanon N. Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: Focus on brain indoleamine 2,3-dioxygenase activation. Brain Behav Immun. 2014;41:10–21. doi: 10.1016/j.bbi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Babri S, Badie HG, Khamenei S, Seyedlar MO. Intrahippocampal insulin improves memory in a passive-avoidance task in male wistar rats. Brain Cogn. 2007;64:86–91. doi: 10.1016/j.bandc.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides. 1987;18:1423–9. doi: 10.1016/s0196-9781(97)00231-3. [DOI] [PubMed] [Google Scholar]
- Beauquis J, Homo-Delarche F, Giroix MH, Ehses J, Coulaud J, Roig P, Portha B, De Nicola AF, Saravia F. Hippocampal neurovascular and hypothalamic-pituitary-adrenal axis alterations in spontaneously type 2 diabetic GK rats. Exp Neurol. 2010;222:125–134. doi: 10.1016/j.expneurol.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Beddow SA, Samuel VT. Fasting hyperglycemia in the Goto-Kakizaki rat is dependent on corticosterone: a confounding variable in rodent models of type 2 diabetes. Dis Model Mech. 2012;5:681–685. doi: 10.1242/dmm.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford TG, Tipton CM, Wilson NC, Oppliger RA, Gisolfi CV. Maximum oxygen consumption of rats and its changes with various experimental procedures. J Appl Physiol. 1979;47:1278–83. doi: 10.1152/jappl.1979.47.6.1278. [DOI] [PubMed] [Google Scholar]
- Beilharz JE, Maniam J, Morris MJ. Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain Behav Immun. 2014;37:134–141. doi: 10.1016/j.bbi.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Belanger A, Lavoie N, Trudeau F, Massicotte G, Gagnon S. Preserved LTP and water maze learning in hyperglycaemic-hyperinsulinemic ZDF rats. Physiol Behav. 2004;83:483–494. doi: 10.1016/j.physbeh.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Kamal A, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH. Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment. Brain Res. 1998;800:125–135. doi: 10.1016/s0006-8993(98)00510-1. [DOI] [PubMed] [Google Scholar]
- Blüher M, Windgassen M, Paschke R. Improvement of insulin sensitivity after adrenalectomy in patients with pheochromocytoma. Diabetes Care. 2000;23:1591–2. doi: 10.2337/diacare.23.10.1591. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Bates SH, Chen S, Myers MG, Jr, Simerly RB. Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. J Neurosci. 2012;32:1244–52. doi: 10.1523/JNEUROSCI.2277-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bree A, Puente E, Daphna-Iken D, Fisher S. Diabetes increases brain damage caused by severe hypoglycemia. American Journal of Physiology, endocrinology and metabolism. 2009;297:E194–201. doi: 10.1152/ajpendo.91041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131–43. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo-Ochoa E, Hernández-Ortega K, Ferrera P, Morimoto S, Arias C. Short-term high-fat-and-fructose feeding produces insulin signaling alterations accompanied by neurite and synaptic reduction and astroglial activation in the rat hippocampus. J Cereb Blood Flow Metab. 2014;34:1001–1008. doi: 10.1038/jcbfm.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–61. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- Chan JL, Matarese G, Shetty GK, Raciti P, Kelesidis I, Aufiero D, De Rosa V, Perna F, Fontana S, Mantzoros CS. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci U S A. 2006;103:8481–6. doi: 10.1073/pnas.0505429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JL, Bullen J, Stoyneva V, Depaoli AM, Addy C, Mantzoros CS. Recombinant methionyl human leptin administration to achieve high physiologic or pharmacologic leptin levels does not alter circulating inflammatory marker levels in humans with leptin sufficiency or excess. J Clin Endocrinol Metab. 2005;90:1618–24. doi: 10.1210/jc.2004-1921. [DOI] [PubMed] [Google Scholar]
- Cheng A, Wan R, Yang JL, Kamimura N, Son TG, Ouyang X, Luo Y, Okun E, Mattson MP. Involvement of PGC-1 in the formation and maintenance of neuronal dendritic spines. Nat Commun. 2012;3:1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clodfelder-Miller B, De Sarno P, Zmijewska AA, Song L, Jope RS. Physiological and pathological changes in glucose regulate brain Akt and glycogen synthase kinase-3. J Biol Chem. 2005;280:39723–31. doi: 10.1074/jbc.M508824200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocco S, Diaz G, Stancampiano R, Diana A, Carta M, Curreli R, Sarais L, Fadda F. Vitamin A deficiency produces spatial learning and memory impairment in rats. Neuroscience. 2002;115:475–482. doi: 10.1016/s0306-4522(02)00423-2. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–72. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- Diegues JC, Pauli JR, Luciano E, de Almeida Leme JA, de Moura LP, Dalia RA, de Araújo MB, Sibuya CY, de Mello MA, Gomes RJ. Spatial memory in sedentary and trained diabetic rats: molecular mechanisms. Hippocampus. 2014;24:703–11. doi: 10.1002/hipo.22261. [DOI] [PubMed] [Google Scholar]
- Dinel AL, André C, Aubert A, Ferreira G, Layé S, Castanon N. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS One. 2011;6:e24325. doi: 10.1371/journal.pone.0024325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulloo AG, Calokatisa R. Adaptation to low calorie intake in obese mice: contribution of a metabolic component to diminished energy expenditures during and after weight loss. Int J Obes. 1991;15(1):7–16. [PubMed] [Google Scholar]
- Ehninger D, Kempermann G. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb Cortex. 2003;13:845–51. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- Erion JR, Wosiski-Kuhn M, Dey A, Hao S, Davis CL, Pollock NK, Stranahan AM. Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J Neurosci. 2014;34:2618–2631. doi: 10.1523/JNEUROSCI.4200-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira JM, Burnett AL, Rameau GA. Activity-dependent regulation of surface glucose transporter-3. J Neurosci. 2011;31:1991–9. doi: 10.1523/JNEUROSCI.1850-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri P, Cecchini T, Ambrogini P, Betti M, Cuppini R, Del Grande P, Ciaroni S. alpha-Tocopherol affects neuronal plasticity in adult rat dentate gyrus: the possible role of PKCdelta. J Neurobiol. 2006;66:793–810. doi: 10.1002/neu.20255. [DOI] [PubMed] [Google Scholar]
- Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, Dallman MF. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology. 2009;150:2325–33. doi: 10.1210/en.2008-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012;8:189–202. doi: 10.1038/nrneurol.2012.27. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nat Rev Neurol. 2009;5:649–58. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun. 2012;26:337–45. doi: 10.1016/j.bbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. J Neurosci Methods. 2006;151:121–30. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Galli J, Li LS, Glaser A, Ostenson CG, Jiao H, Fakhrai-Rad H, Jacob HJ, Lander ES, Luthman H. Genetic analysis of non-insulin dependent diabetes mellitus in the GK rat. Nat Genet. 1996;12:31–7. doi: 10.1038/ng0196-31. [DOI] [PubMed] [Google Scholar]
- Ge S, Song L, Pachter JS. Where is the blood-brain barrier ... really? J Neurosci Res. 2005;79:421–7. doi: 10.1002/jnr.20313. [DOI] [PubMed] [Google Scholar]
- Geng T, Hu W, Broadwater MH, Snider JM, Bielawski J, Russo SB, Schwacke JH, Ross J, Cowart LA. Fatty acids differentially regulate insulin resistance through endoplasm reticulum stress-mediated induction of tribbles homologue 3: a potential link between dietary fat composition and the pathophysiological outcomes of obesity. Diabetologia. 2013;56:2078–87. doi: 10.1007/s00125-013-2973-2. [DOI] [PubMed] [Google Scholar]
- Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med. 1976;119:85–90. doi: 10.1620/tjem.119.85. [DOI] [PubMed] [Google Scholar]
- Green JT, Chess AC, Burns M, Schachinger KM, Thanellou A. The effects of two forms of physical activity on eyeblink classical conditioning. Behav Brain Res. 2011;219(1):165–74. doi: 10.1016/j.bbr.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood CE, Winocur G. Learning and memory impairment in rats fed a high saturated fat diet. Behav Neural Biol. 1990;53:74–87. doi: 10.1016/0163-1047(90)90831-p. [DOI] [PubMed] [Google Scholar]
- Grillo CA, Tamashiro KL, Piroli GG, Melhorn S, Gass JT, Newsom RJ, Reznikov LR, Smith A, Wilson SP, Sakai RR, Reagan LP. Lentivirus-mediated downregulation of hypothalamic insulin receptor expression. Physiol Behav. 2007;92:691–701. doi: 10.1016/j.physbeh.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Junor L, Wilson SP, Mott DD, Wilson MA, Reagan LP. Obesity/hyperleptinemic phenotype impairs structural and functional plasticity in the rat hippocampus. Physiol Behav. 2011a;105:138–144. doi: 10.1016/j.physbeh.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Evans AN, Macht VA, Wilson SP, Scott KA, Sakai RR, Mott DD, Reagan LP. Obesity/hyperleptinemic phenotype adversely affects hippocampal plasticity: effects of dietary restriction. Physiol Behav. 2011b;104:235–241. doi: 10.1016/j.physbeh.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Hendry RM, Reagan LP. Insulin-stimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Res. 2009;1296:35–45. doi: 10.1016/j.brainres.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Kaigler KF, Wilson SP, Wilson MA, Reagan LP. Downregulation of hypothalamic insulin receptor expression elicits depressive-like behaviors in rats. Behav Brain Res. 2011c;222:230–5. doi: 10.1016/j.bbr.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol. 2004;75:388–97. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- Gustafson DR, Karlsson C, Skoog I, Rosengren L, Lissner L, Blennow K. Mid-life adiposity factors relate to blood-brain barrier integrity in late life. J Intern Med. 2007;262:643–50. doi: 10.1111/j.1365-2796.2007.01869.x. [DOI] [PubMed] [Google Scholar]
- Hamman RF, Bell RA, Dabelea D, D'Agostino RB, Jr, Dolan L, Imperatore G, Lawrence JM, Linder B, Marcovina SM, Mayer-Davis EJ, Pihoker C, Rodriguez BL, Saydah S, SEARCH for Diabetes in Youth Study Group The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care. 2014;37:3336–44. doi: 10.2337/dc14-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Sommer W, Rimondini R, Andbjer B, Strömberg I, Fuxe K. c-fos reduces corticosterone-mediated effects on neurotrophic factor expression in the rat hippocampal CA1 region. J Neurosci. 2003;23:6013–22. doi: 10.1523/JNEUROSCI.23-14-06013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey T, Perantie DC, Wu J, Weaver PM, Black KJ, White NH. Hippocampal volumes in youth with type 1 diabetes. Diabetes. 2010;59:236–41. doi: 10.2337/db09-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho N, Brookshire BR, Clark JE, Lucki I. Indomethacin reverses decreased hippocampal cell proliferation in streptozotocin-induced diabetic mice. Metab Brain Dis. 2015;30:555–62. doi: 10.1007/s11011-014-9611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL. The hardship of obesity: a soft-wired hypothalamus. Nat Neurosci. 2005;8:561–5. doi: 10.1038/nn1453. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–66. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–2. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- Kamal A, Biessels GJ, Duis SE, Gispen WH. Learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: interaction of diabetes and ageing. Diabetologia. 2000;43:500–506. doi: 10.1007/s001250051335. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13:707–12. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- Kaouane N, Porte Y, Vallée M, Brayda-Bruno L, Mons N, Calandreau L, Marighetto A, Piazza PV, Desmedt A. Glucocorticoids can induce PTSD-like memory impairments in mice. Science. 2012;335:1510–3. doi: 10.1126/science.1207615. [DOI] [PubMed] [Google Scholar]
- Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Hölscher C, Müller DN, Luckow B, Brocker T, Debowski K, Fritz G, Opdenakker G, Diefenbach A, Biber K, Heikenwalder M, Geissmann F, Rosenbauer F, Prinz M. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16:273–80. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- Kim B, Backus C, Oh S, Hayes JM, Feldman EL. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology. 2009;150:5294–301. doi: 10.1210/en.2009-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel KC, Allen DL, Booth FW, Fleshner MR, Henriksen EJ, Musch TI, O'Leary DS, Toth LA. Resource book for the design of animal exercise protocols. American Physiological Society; Bethesda, MD: 2006. pp. 15–17. [Google Scholar]
- Landsberg L. Feast or famine: the sympathetic nervous system response to nutrient intake. Cell Mol Neurobiol. 2006;26:497–508. doi: 10.1007/s10571-006-9010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang BT, Yan Y, Dempsey RJ, Vemuganti R. Impaired neurogenesis in adult type-2 diabetic rats. Brain Res. 2009;1258:25–33. doi: 10.1016/j.brainres.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannert H, Hoyer S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci. 1998;112:1199–1208. doi: 10.1037//0735-7044.112.5.1199. [DOI] [PubMed] [Google Scholar]
- Lee CC, Huang CC, Hsu KS. Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology. 2011;61:867–79. doi: 10.1016/j.neuropharm.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–26. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- Li XH, Xin X, Wang Y, Wu JZ, Jin ZD, Ma LN, Nie CJ, Xiao X, Hu Y, Jin MW. Pentamethylquercetin protects against diabetes-related cognitive deficits in diabetic Goto-Kakizaki rats. J Alzheimers Dis. 2013;34:755–767. doi: 10.3233/JAD-122017. [DOI] [PubMed] [Google Scholar]
- Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–615. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Liu K, Paterson AJ, Chin E, Kudlow JE. Glucose stimulates protein modification by O-linked GlcNAc in pancreatic beta cells: linkage of O-linked GlcNAc to beta cell death. Proc Natl Acad Sci U S A. 2000;97:2820–5. doi: 10.1073/pnas.97.6.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobnig BM, Krömeke O, Optenhostert-Porst C, Wolf OT. Hippocampal volume and cognitive performance in long-standing Type 1 diabetic patients without macrovascular complications. Diabet Med. 2006;23:32–9. doi: 10.1111/j.1464-5491.2005.01716.x. [DOI] [PubMed] [Google Scholar]
- Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–2. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Experimental diabetes in rats causes hippocampal dendritic and synaptic reorganization and increased glucocorticoid reactivity to stress. Proc Natl Acad Sci U S A. 2000;97:11056–61. doi: 10.1073/pnas.97.20.11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino JS, Xu Y, Hill JW. Central insulin and leptin-mediated autonomic control of glucose homeostasis. Trends Endocrinol Metab. 2011;22:275–85. doi: 10.1016/j.tem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- Mauer MM, Harris RB, Bartness TJ. The regulation of total body fat: lessons learned from lipectomy studies. Neurosci Biobehavioral Rev. 2001;25:15–28. doi: 10.1016/s0149-7634(00)00047-6. [DOI] [PubMed] [Google Scholar]
- Melander A. Kinetics-effect relations of insulin-releasing drugs in patients with type 2 diabetes: brief overview. Diabetes. 2004;53:S151–5. doi: 10.2337/diabetes.53.suppl_3.s151. [DOI] [PubMed] [Google Scholar]
- Michelini LC, Stern JE. Exercise-induced neuronal plasticity in central autonomic networks: role in cardiovascular control. Exp Physiol. 2009;94:947–60. doi: 10.1113/expphysiol.2009.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem. 2010;93:546–553. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, Gold PE. Extracellular glucose concentrations in the rat hippocampus measured by zero-net-flux: effects of microdialysis flow rate, strain, and age. J Neurochem. 1999;72:785–90. doi: 10.1046/j.1471-4159.1999.720785.x. [DOI] [PubMed] [Google Scholar]
- Mielke JG, Taghibiglou C, Liu L, Zhang Y, Jia Z, Adeli K, Wang YT. A biochemical and functional characterization of diet-induced brain insulin resistance. J Neurochem. 2005;93:1568–78. doi: 10.1111/j.1471-4159.2005.03155.x. [DOI] [PubMed] [Google Scholar]
- Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–70. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Barnard RJ, Ying Z, Roberts CK, Gómez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosavi M, Naghdi N, Maghsoudi N, Zahedi Asl S. The effect of intrahippocampal insulin microinjection on spatial learning and memory. Horm Behav. 2006;50:748–752. doi: 10.1016/j.yhbeh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Golozoubova V, Matthias A, Shabalina I, Ohba K, Ohlson K, Jacobsson A, Cannon B. Life without UCP1: mitochondrial, cellular and organismal characteristics of the UCP1-ablated mice. Biochem Soc Trans. 2001;29:756–763. doi: 10.1042/0300-5127:0290756. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gómez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Norden DM, Fenn AM, Dugan A, Godbout JP. TGF produced by IL-10 redirected astrocytes attenuates microglial activation. Glia. 2014;62:881–95. doi: 10.1002/glia.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Satpathy A, Hartman ME, Horvath EM, Kelley KW, Dantzer R, Johnson RW, Freund GG. IL-1beta-mediated innate immunity is amplified in the db/db mouse model of type 2 diabetes. J Immunol. 2005;174:4991–7. doi: 10.4049/jimmunol.174.8.4991. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Childhood and Adult Obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–74. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- Ott A, Stolk RP, Hofman A, van Harskamp F, Grobbee DE, Breteler MM. Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia. 1996;39:1392–1397. doi: 10.1007/s001250050588. [DOI] [PubMed] [Google Scholar]
- Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–95. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–8. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- Pilcher H. Alzheimer's disease could be “type 3 diabetes”. Lancet Neurol. 2006;5:388–9. doi: 10.1016/s1474-4422(06)70434-3. [DOI] [PubMed] [Google Scholar]
- Piroli GG, Grillo CA, Hoskin EK, Znamensky V, Katz EB, Milner TA, McEwen BS, Charron MJ, Reagan LP. Peripheral glucose administration stimulates the translocation of GLUT8 glucose transporter to the endoplasmic reticulum in the rat hippocampus. J Comp Neurol. 2002;452:103–14. doi: 10.1002/cne.10368. [DOI] [PubMed] [Google Scholar]
- Poitout V, Rouault C, Guerre-Millo M, Reach G. Does leptin regulate insulin secretion? Diabetes Metab. 1998;24:321–6. [PubMed] [Google Scholar]
- Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67:505–12. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Reagan LP, Gorovits N, Hoskin EK, Alves SE, Katz EB, Grillo CA, Piroli GG, McEwen BS, Charron MJ. Localization and regulation of GLUTx1 glucose transporter in the hippocampus of streptozotocin diabetic rats. Proc Natl Acad Sci U S A. 2001;98:2820–5. doi: 10.1073/pnas.051629798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan LP, Magariños AM, Lucas LR, van Bueren A, McCall AL, McEwen BS. Regulation of GLUT-3 glucose transporter in the hippocampus of diabetic rats subjected to stress. Am J Physiol. 1999;276:E879–86. doi: 10.1152/ajpendo.1999.276.5.E879. [DOI] [PubMed] [Google Scholar]
- Reichelt AC, Maniam J, Westbrook RF, Morris MJ. Dietary-induced obesity disrupts trace fear conditioning and decreases hippocampal reelin expression. Brain Behav Immun. 2015;43:68–75. doi: 10.1016/j.bbi.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Revsin Y, Rekers NV, Louwe MC, Saravia FE, De Nicola AF, de Kloet ER, Oitzl MS. Glucocorticoid receptor blockade normalizes hippocampal alterations and cognitive impairment in streptozotocin-induced type 1 diabetes mice. Neuropsychopharmacology. 2009;34:747–58. doi: 10.1038/npp.2008.136. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AP, Bruggeman EC, Kasumu AW, Mielke JG, Parent MB. Non-alcoholic fatty liver disease impairs hippocampal-dependent memory in male rats. Physiol Behav. 2012;106(2):133–41. doi: 10.1016/j.physbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Schnedl WJ, Ferber S, Johnson JH, Newgard CB. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes. 1994;43:1326–1333. doi: 10.2337/diab.43.11.1326. [DOI] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrats J, Schiltz JC, García-Bueno B, van Rooijen N, Reyes TM, Sawchenko PE. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65:94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21:1–6. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y, Bray GA, Lee M. Adrenalectomy and steroid treatment in obese (ob/ob) and diabetic (db/db) mice. Horm Metab Res. 1987;19:295–9. doi: 10.1055/s-2007-1011804. [DOI] [PubMed] [Google Scholar]
- Shoham S, Bejar C, Kovalev E, Weinstock M. Intracerebroventricular injection of streptozotocin causes neurotoxicity to myelin that contributes to spatial memory deficits in rats. Exp Neurol. 2003;184:1043–1052. doi: 10.1016/j.expneurol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Sickmann HM, Waagepetersen HS, Schousboe A, Benie AJ, Bouman SD. Obesity and type 2 diabetes in rats are associated with altered brain glycogen and amino-acid homeostasis. J Cereb Blood Flow Metab. 2010;30:1527–37. doi: 10.1038/jcbfm.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson IA, Appel NM, Hokari M, Oki J, Holman GD, Maher F, Koehler-Stec EM, Vannucci SJ, Smith QR. Blood-brain barrier glucose transporter: effects of hypo- and hyperglycemia revisited. J Neurochem. 1999;72:238–47. doi: 10.1046/j.1471-4159.1999.0720238.x. [DOI] [PubMed] [Google Scholar]
- Stern SA, Chen DY, Alberini CM. The effect of insulin and insulin-like growth factors on hippocampus- and amygdala-dependent long-term memory formation. Learn Mem. 2014;21:556–563. doi: 10.1101/lm.029348.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008a;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Mattson MP. Lowering corticosterone levels reinstates hippocampal brain-derived neurotropic factor and Trkb expression without influencing deficits in hypothalamic brain-derived neurotropic factor expression in leptin receptor-deficient mice. Neuroendocrinology. 2011;93:58–64. doi: 10.1159/000322808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–22. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Pistell PJ, Nelson CM, Readal N, Miller MG, Spangler EL, Ingram DK, Mattson MP. Accelerated cognitive aging in diabetic rats is prevented by lowering corticosterone levels. Neurobiol Learn Mem. 2008b;90:479–483. doi: 10.1016/j.nlm.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Martin B, Maudsley S. Anti-inflammatory effects of physical activity in relationship to improved cognitive status in humans and mouse models of Alzheimer's disease. Curr Alzheimer Res. 2012a;9:86–92. doi: 10.2174/156720512799015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Martin B, Chadwick W, Park SS, Wang L, Becker KG, Wood WH, Zhang Y, Maudsley S. Metabolic context regulates distinct hypothalamic transcriptional responses to antiaging interventions. Int J Endocrinol. 2012b;2012:732975. doi: 10.1155/2012/732975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Recruiting adaptive cellular stress responses for successful brain aging. Nat Rev Neurosci. 2012;13:209–216. doi: 10.1038/nrn3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Bidirectional metabolic regulation of neurocognitive function. Neurobiol Learn Mem. 2011;96:507–16. doi: 10.1016/j.nlm.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008c;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PW, Morrato EH, Ghushchyan V, Wyatt HR, Hill JO. Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the U.S., 2000-2002. Diabetes Care. 2005;28:1599–603. doi: 10.2337/diacare.28.7.1599. [DOI] [PubMed] [Google Scholar]
- Sweetnam D, Holmes A, Tennant KA, Zamani A, Walle M, Jones P, Wong C, Brown CE. Diabetes impairs cortical plasticity and functional recovery following ischemic stroke. J Neurosci. 2012;32:5132–43. doi: 10.1523/JNEUROSCI.5075-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Choi SJ, Schwartz MW, Wisse BE. Hypothalamic inflammation and energy homeostasis: resolving the paradox. Front Neuroendocrinol. 2010;31:79–84. doi: 10.1016/j.yfrne.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–7. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Sosnowska D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2014;69:1212–26. doi: 10.1093/gerona/glt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia. 1997;21:2–21. doi: 10.1002/(sici)1098-1136(199709)21:1<2::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Walley AJ, Asher JE, Froguel P. The genetic contribution to non-syndromic human obesity. Nat Rev Genet. 2009;10:431–42. doi: 10.1038/nrg2594. [DOI] [PubMed] [Google Scholar]
- Wiesner TD, Blüher M, Windgassen M, Paschke R. Improvement of insulin sensitivity after adrenalectomy in patients with pheochromocytoma. J Clin Endocrinol Metab. 2003;88:3632–6. doi: 10.1210/jc.2003-030000. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4:103–9. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- Williams K, Alvarez X, Lackner AA. Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia. 2001;36:156–64. doi: 10.1002/glia.1105. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–37. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Wosiski-Kuhn M, Erion JR, Gomez-Sanchez EP, Gomez-Sanchez CE, Stranahan AM. Glucocorticoid receptor activation impairs hippocampal plasticity by suppressing BDNF expression in obese mice. Psychoneuroendocrinology. 2014;42:165–177. doi: 10.1016/j.psyneuen.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes. 2009;58:71–7. doi: 10.2337/db08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Caracciolo B, Wang HX, Winblad B, Bäckman L, Qiu C, Fratiglioni L. Accelerated progression from mild cognitive impairment to dementia in people with diabetes. Diabetes. 2010;59:2928–2935. doi: 10.2337/db10-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011;76:1568–74. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Song W. Molecular links between Alzheimer's disease and diabetes mellitus. Neuroscience. 2013;250:140–150. doi: 10.1016/j.neuroscience.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Zhang T, Pan BS, Zhao B, Zhang LM, Huang YL, Sun FY. Exacerbation of poststroke dementia by type 2 diabetes is associated with synergistic increases of beta-secretase activation and beta-amyloid generation in rat brains. Neuroscience. 2009;161:1045–1056. doi: 10.1016/j.neuroscience.2009.04.032. [DOI] [PubMed] [Google Scholar]
- Zhang WJ, Tan YF, Yue JT, Vranic M, Wojtowicz JM. Impairment of hippocampal neurogenesis in streptozotocin-treated diabetic rats. Acta Neurol Scand. 2008;117:205–210. doi: 10.1111/j.1600-0404.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Spicer EG, Gavini CK, Goudjo-Ako AJ, Novak CM, Shi H. Enhanced sympathetic activity in mice with brown adipose tissue transplantation (transBATation). Physiol Behav. 2014;125:21–29. doi: 10.1016/j.physbeh.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci. 2005;28:202–8. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]