Abstract
Many adult smokers are intermittent smokers (ITS) who do not smoke daily. Prior analyses suggested that, compared to daily smokers (DS), ITS’ smoking was, on average, more linked to particular situations, such as alcohol consumption. However, such particular associations assessed in common across subjects may underestimate stimulus control over smoking, which may vary across persons, due to different conditioning histories. We quantify such idiographic stimulus control using separate multivariable logistic regressions for each subject to estimate how well the subject’s smoking could be predicted from a panel of situational characteristics, without requiring that other subjects respond to the same stimuli. Subjects were 212 ITS (smoking 4-27 days/month) and 194 DS (5-30 cigarettes daily). Using ecological momentary assessment, subjects monitored situational antecedents of smoking for 3 weeks, recording each cigarette on an electronic diary. Situational characteristics were assessed in a random subset of smoking occasions (n = 21,539), and contrasted with assessments of non-smoking occasions (n = 26,930) obtained by beeping subjects at random. ITS showed significantly stronger stimulus control than DS across all context domains: mood, location, activity, social setting, consumption, smoking context, and time of day. Mood and smoking context showed the strongest influence on ITS smoking, food and alcohol consumption the least. ITS’ smoking was under very strong stimulus control; significantly more so than DS’, but DS’ smoking also showed considerable stimulus control. Stimulus control may be an important influence in maintaining smoking and making quitting difficult for all smokers, but especially among ITS.
Keywords: smoking, ecological momentary assessment, non-daily smoking, daily smoking, stimulus control
Nicotine dependence is considered the primary determinant of persistent cigarette smoking. This helps explain why most smokers smoke frequently throughout the day, every day, which functions to prevent nicotine levels from sinking below a level where nicotine withdrawal sets in (Benowitz, 2010; Stolerman & Jarvis, 1995). Maintaining nicotine levels (“trough maintenance”; Russell, 1971) is best accomplished by smoking at very regular intervals, but some models allow room for variations from this, e.g., smoking in response to situational cues. However, this leeway is limited, as nicotine withdrawal can set in within a few hours of abstinence (Benowitz, 2008).
However, nondaily smoking is becoming increasingly prevalent among US adults (Cooper et al., 2010; Schane, Glantz, & Ling, 2009; Shiffman, 2009b; Shiffman, Tindle, et al., 2012). As many as 38% of US adult smokers are now non-daily or intermittent smokers (ITS; U.S. Department of Health and Human Services, 2014). ITS smoke an average of 4-5 cigarettes per day on the days they smoke (Gilpin, Cavin, & Pierce, 1997; Shiffman, Tindle, et al., 2012; Wortley, Husten, Trosclair, & Chrismon, 2003), but their defining characteristic is that they frequently go for several days running without smoking (Shiffman, Tindle, et al., 2012), and thus clearly do not maintain nicotine levels (Benowitz, 2008). ITS may be seeking the reinforcing effects of acute doses of nicotine (“peak-seeking”; Russell, 1971), rather than trying to maintain a minimal level to avoid withdrawal (“trough-avoidance”). Nor is this necessarily just a transient phase en route to dependence: Zhu, Sun, Hawkins, Pierce, and Cummings (2003) reported that a substantial proportion of ITS maintained that status over two years. Similarly, we have studied a sample of ITS who have been smoking for an average of 19 years, and have consumed an average of more than 40,000 cigarettes (Shiffman, Tindle, et al., 2012), yet show little or no dependence (Shiffman, Ferguson, Dunbar, & Scholl, 2012; Shiffman, Tindle, et al., 2012). Nevertheless, ITS have surprising trouble quitting smoking, with failure rates of 78%, only slightly lower than those of daily smokers (DS; Tindle & Shiffman, 2011).
What might account for ITS’ difficulty quitting? One factor might be stimulus control. A behavior (in this case smoking) is said to be under stimulus control when the presence of a given stimulus (or stimuli) changes the likelihood of that behavior occurring. Such relationships are believed to be established through various learning processes. Stimuli could influence smoking by serving as discriminative stimuli, indicating that smoking will be reinforcing, acting as priming stimuli, and/or as conditioned stimuli eliciting responses instilled by prior associations with stimuli, including the effects of smoking itself (Bickel & Kelly, 1988). If ITS’ smoking is strongly associated with certain situational cues, exposure to such cues might promote continued smoking and pose a significant barrier to abstinence in the face of exposure to relevant cues. Strong stimulus control is a common feature of casual drug use (Bickel & Kelly, 1988), and we have hypothesized that its diminution is an important step in the development of tobacco dependence (Shiffman & Paty, 2006; Shiffman, Waters, & Hickcox, 2004), as use shifts from particular settings to nicotine maintenance via frequent nicotine intake. Consistent with this, ITS’ questionnaire responses on a scale assessing smoking motives (Piper et al., 2004) identify responsiveness to cues as their most important motivation to smoke (Shiffman, Dunbar, Scholl, & Tindle, 2012), and smoking in chippers – very light smokers – has been shown to be under greater stimulus control than that seen in heavy smokers (Shiffman & Paty, 2006).
A useful way to assess individual’s smoking patterns is via Ecological Momentary Assessment (EMA; Shiffman, 2009a; Stone & Shiffman, 1994) – collection of real-time, real-world data on multiple occasions. Collecting data in subjects’ real-world settings ensures ecological validity, and collecting it in real time avoids problems of recall bias. Collecting data on both smoking and non-smoking occasions allows one to characterize the associations between smoking and situational antecedents (Paty, Kassel, & Shiffman, 1992; Shiffman, 2009a). This method has been used to study situational associations with smoking in a variety of populations (e.g., Beckham et al., 2008; Cronk & Piasecki, 2010; Mermelstein, Hedeker, Flay, & Shiffman, 2007; Shiffman et al., 2002; Shiffman & Paty, 2006).
We recently used EMA data to compare the particular stimuli associated with smoking for ITS and DS, and found that ITS’ smoking was more likely to be associated with cues such as being away from home, being in a bar, drinking alcohol, socializing, being with friends and acquaintances, and where others were smoking (Shiffman et al., 2014a). However, these analyses, while contributing to our understanding of ITS’ smoking, only identify the smoking triggers that most ITS share in common; they do not fully reflect the degree of control that various stimuli exert over individual ITS’ smoking.
To quantify stimulus control, one must abstract from relationships between smoking and the cues that are shared by ITS (or DS) in general, to examine idiographic associations with cues within each person, as these associations can be idiosyncratic, with different smokers even having different, even opposite, reactions to the same cue. For example, if some subjects smoke when feeling good, while others smoke when feeling bad, a group-wise analysis of individual moods may show no effect, even though mood exercises stimulus control over smoking for both groups of subjects. Indeed, data from an EMA study of DS showed such effects, in that the overall group-wise relationship between smoking and mood was estimated as zero (Shiffman et al., 2002), yet the distribution showed wide variation, with relationships in both directions, and these variations proved meaningful in predicting subsequent relapse (Shiffman et al., 2007). Also, different subjects may respond to different stimuli, even within a given domain. For example, some subjects might respond to how good or bad they feel and others to how aroused they feel. Both might be considered equally under stimulus control by mood, but the associations, too, would be missed or diluted in the analyses of single cues that are typically done (e.g., Shiffman et al., 2014a). Yet, such variable idiographic relationships between smoking and antecedent stimuli are to be expected if the associations are due to conditioning (Niaura et al., 1988), since individuals’ learning histories would likely vary.
Accordingly, in this paper, we go beyond assessing directional group-wide associations between situational stimuli and smoking to quantify the degree of stimulus control, using EMA data to estimate how well various situational characteristics can account for each individual’s smoking idiographically, based on analyses within each individual subject. Subsequent comparisons compare the resulting parameters between ITS and DS.
Method
Subjects
Subjects were 212 ITS and 194 DS recruited via advertisement. Participants had to be at least 21 years old, report smoking for at least three years, smoking at their current rate for at least three months, and not be planning to quit within the next month. DS had to report smoking every day, averaging 5 to 30 cigarettes per day. ITS had to report smoking 4 to 27 days per month, with no restrictions on number of cigarettes. We oversampled African-American smokers, because national surveys indicate they are more likely to be ITS (Trinidad et al., 2009); data were weighted to balance ethnic representation. Analyses of association of smoking and particular cues were reported for this sample in Shiffman et al. (2014a), and the sample largely overlaps with that reported in several analyses of other data (Shiffman, Dunbar, Kirchner, Li, Tindle, Anderson, & Scholl, 2013; Shiffman, Dunbar, Kirchner, Li, Tindle, Anderson, Scholl, et al., 2013; Shiffman et al., 2012; Shiffman, Ferguson, et al., 2012; Shiffman, Tindle, et al., 2012).
Briefly, DS were 41 years old, 55% male, smoked 15 cigarettes per day, and had been smoking for 26 years on average. ITS were slightly younger (37 years old), 49% male, smoked 4-5 cigarettes per day on smoking days, smoked 4-5 days per week, and had been smoking for 19 years on average (see Shiffman et al., 2014a for additional details).
Procedures
The EMA methods for this study have been described in detail in Shiffman et al. (2014a), and are similar to those in previous studies (Ferguson & Shiffman, 2011; Shiffman, 2009a; Shiffman et al., 2002). Briefly, subjects were provided with a palmtop computer that they used to monitor smoking for three weeks (average 21.60 ± 4.11 days). Subjects were to record all cigarettes, but, to avoid excessive burden, the computer administered an assessment of the surrounding circumstances only for a portion of those smoking occasions, selected at random.
To assess the circumstances of non-smoking moments, as a necessary contrast for smoking occasions (Paty et al., 1992; Shiffman, 2009a), the computer “beeped” subjects at random about four times per day (but never within 15 minutes of smoking), and administered a nearly-identical assessment. Subjects in the analysis received 3-4 prompts per day on average (DS: M = 3.52; ITS: M = 3.93) and responded to 88% of them (DS: 87.6%; ITS: 88.2%).
Assessment
All assessments were administered on the computer’s touch-screen, with structured responses (no open-ended text) consisting of 0-100 point Visual Analog Scales for mood items and single or multiple selections for other domains. The content of the domains is shown in Table 1: (a) Mood: ratings of 14 adjectives (listed in Table 1 note) addressing mood, arousal, and attention , respectively, were summarized as four factor scores: Negative Affect, Positive Affect, Arousal, and Attention Disturbance, each of which was analyzed including both linear and quadratic components; (b) Location (if subjects had moved to smoke, they were asked to describe the setting that first prompted them to smoke, otherwise current location was described); (c) Activity; (d) Social setting; (e) Smoking setting: whether others were smoking (and whether those were part of the group of people they were with or just someone in view), and whether smoking was restricted; (f) Consumption of food or drink in the past 15 minutes; and, (g) Time of day: automatically recorded by the palmtop computer. We also assessed craving, on a 0-100 scale.
Table 1.
Summary of stimulus domains
| Domain | Description | Response Options |
|---|---|---|
|
| ||
| Consumption | Food or drink consumption within the past 15 minutes (yes / no items; multiple endorsements allowed) | Eating food |
| Drinking caffeinated drinks | ||
| Drinking non-caffeinated drinks | ||
| Drinking alcohol | ||
| No eating or drinking a | ||
|
| ||
| Time | Time of day block, coded categorically (single item; mutually exclusive and exhaustive) | 4am- <10:00am |
| 10am-< 1:00pm | ||
| 1pm-< 5:00pm | ||
| 5pm-< 9:00pm | ||
| 9pm-<11:00pm | ||
| 11pm-< 4:00am | ||
|
| ||
| Social Setting | Alone or others present; relationship to others (yes / no items; multiple endorsements allowed) | Alone a |
| Friends | ||
| Acquaintances | ||
| Family members | ||
| Co-workers | ||
| Spouse/partner | ||
|
| ||
| Location | Physical location (one variable: mutually exclusive and exhaustive) b | Home |
| Workplace | ||
| Other’s home | ||
| Bar | ||
| Restaurant | ||
| Outside | ||
| Vehicle | ||
| Casino | ||
| Other | ||
|
| ||
| Activity | Type of activity (yes / no items; multiple endorsements allowed) | Working/Chores |
| Inactive/Leisure | ||
| Interacting with others | ||
| Eating/Drinking (last 15 min) | ||
| Between activities | ||
| Other activities | ||
|
| ||
| Mood | Affect ratings (factor scores) c | Negative Affect |
| Positive Affect | ||
| Arousal | ||
| Attention Disturbance | ||
|
| ||
| Smoking Context | Social smoking context and smoking regulations (yes / no items; multiple endorsements allowed) | Others’ Smoking (yes / no items; multiple endorsements allowed) |
| Others smoking within social group | ||
| Others smoking in view | ||
| No others smoking a | ||
| Smoking Regulations (one variable: mutually exclusive and exhaustive) | ||
| Smoking allowed | ||
| Smoking restricted by own rule | ||
| Smoking restricted by others’ rules | ||
| Smoking restricted by the law | ||
Notes.
While multiple endorsements were allowed, subjects could not endorsing this option together with any other selection.
If subjects had moved to smoke, they were asked to describe the setting that first prompted them to smoke, otherwise current location was described.
Based on ratings (0-100) of individual items (able to focus; active; angry/frustrated; bored; calm/relaxed; difficulty concentrating; enthusiastic; happy; irritable; miserable; nervous/tense; quiet/sleepy; restless; sad), and with ratings of overall affective tone (negative to positive) and overall arousal (low to high).
Analysis
Dataset construction is described in detail in Shiffman et al. (2014a). The dataset comprised 406 subjects (212 ITS; 194 DS), each contributing an average of 53.02 (SD = 33.00) smoking assessments (ITS: 36.66 [SD = 30.81]; DS: 70.90 [SD = 25.14]) and 66.28 (SD = 19.78) non-smoking assessments (ITS: 72.07 [SD = 18.32]; DS: 59.94 [SD = 19.42]).
To illustrate the relevance of idiographic analyses, we report the range across subjects of the association between smoking and four illustrative variables: (1) a summary score of emotional state, as captured by a 5-point bipolar item in which subjects indicated how good or bad they were feeling (very bad, bad, neutral, good very good); (2) a factor score indexing degree of arousal; (3) an indicator of drinking coffee in the previous 15 minutes (0/1); and (4) an indicator of being alone (0/1). For each subject, the association of the variables with smoking was estimated by a within-subject correlation coefficient (point-biserial for mood, phi for ‘alone’). We display the distributions for DS and ITS separately, and also note the standard deviation of the correlations.
Stimulus control was assessed for each situational domain. The analysis proceeded in two steps (Raudenbush & Bryk, 1992; see Shiffman & Paty, 2006): 1) within-subject idiographic analyses performed separately for each subject, and 2) between-group analyses of the estimated parameters, by smoker type. We first assessed the degree to which each participant’s smoking was under stimulus control of the variables in each of several domains of situational context by conducting separate multivariable logistic regressions for each subject to determine how well the situational variables predicted smoking (in contrast to non-smoking observations). In other words, for each subject and for each domain, we ran a separate logistic regression with smoking (yes/no) as the dependent variable, and the domain variables (see Table 1) as predictors. To account for potential over-fitting of models, analyses omitted cases that demonstrated complete or quasi-complete separation (<5% of all cases in each domain). In addition to fitting models for each domain, we also fitted for each subject an omnibus model including all the variables listed in Table 1. The within-subject logistic models did not take into account the autocorrelation among a subject’s data; the estimates generated are used descriptively. To quantify the degree of prediction (and thus stimulus control) achieved by each of these models, for each subject and domain, we calculated the area under the curve for the receiver operating characteristic curve (AUC-ROC, also sometimes described as the c-statistic). Like an R2 value for ordinary regression, higher AUC-ROC values indicate better prediction. AUC-ROC is interpretable as the probability of correctly identifying a smoking (vs. non-smoking) observation, given the situational predictors. Thus, AUC-ROC ranges from 0.5 (random guessing) to 1.0 (perfect prediction; Hanley & McNeil, 1982). Thus, each subject had an AUC-ROC value for each domain, which quantified the degree of ‘predictability’ of smoking from the variables in that domain, for that subject.
In the second step, to assess whether DS differed from ITS, we tested the between-group differences (DS vs. ITS) in AUC-ROC for each domain, using mixed regression models (SAS Proc Mixed) specifying variance components autocorrelation structure. At this second level, each estimate was weighted by the inverse of its standard error (SE), so that more prescise estimates received greater weight (Hanley & McNeil, 1982). The SEs of AUC-ROC values decrease as the number of observations increases, and also decrease as the estimated magnitude of the AUC-ROC increases (Hanley & McNeil, 1982). [Note: Although DS reported more smoking events, DS and ITS did not differ in average AUC-ROC SE across any situational domains, though ITS had lower SEs for omnibus model with all the variables included.] Analyses were also weighted by race in order to account for oversampling of African American participants. To assess whether the AUC-ROCs in each domain differed between ITS and DS, we computed mixed regression models, treating AUC-ROC values across situational domains as a within-subjects random effect. The analyses also examined whether the DS-ITS differences varied by domain, by assessing the interaction between smoker type and situational domain. We used a mixed model to accommodate cases where subjects had missing estimates for a particular domain (e.g., due to complete separation).
We also report the AUC-ROC for the relationship between craving and smoking, and test whether the group differences in this relationship mediate the group differences in AUC-ROC for each of the stimulus domains. We used the Sobel test (Preacher & Hays, 2004) to assess the significance of these mediational relationships using separate ordinary least square regression models, first assessing smoker type as a predictor of craving AUC-ROC (α Path) and then examining subjects’ craving AUC-ROC (β Path) co-varying for smoker type as a predictor of the AUC-ROC for each stimulus domain. The product of the α and β coefficients was used to assess evidence for mediation of stimulus control within each domain (Preacher & Hays, 2004).
Results
Idiographic variations in associations of smoking with contexts
Figure 1 shows the distribution of within-subject correlations between smoking and several illustrative variables; the correlations each quantify how each variable relates to smoking for each subject. In all cases, the average correlations are near zero, indicating at most modest association, on average, although some of these associations were significant in analyses reported in Shiffman et al. (2014a). However, this mean value masks the fact that the distributions extend on either side of 0, indicating that there are individuals who show positive associations, as well as others who show negative associations. For example, as seen in Figure 1, for some ITS, being alone was correlated -0.60 with smoking (i.e., they were considerably more likely to smoke when with others); for others, being alone was correlated as high as 0.90 with smoking (i.e., they were much more likely to smoke when alone). The average correlation among ITS was -0.02, indicating no relationship with smoking, on average (see also Shiffman et al., 2014a). Notably, the spread of the correlations was consistently wider among ITS, as demonstrated by the higher SDs. (This was true of almost all variables, not just those shown in Figure 1.) Furthermore, for all variables, both positive and negative correlations were observed, with the range of subject-specific correlations averaging 1.0 [e.g., -0.5 to +0.5 or -0.4 to +0.6].)
Figure 1. Correlations between smoking and various situational characteristics, among daily smokers (DS) and intermittent smokers (ITS).
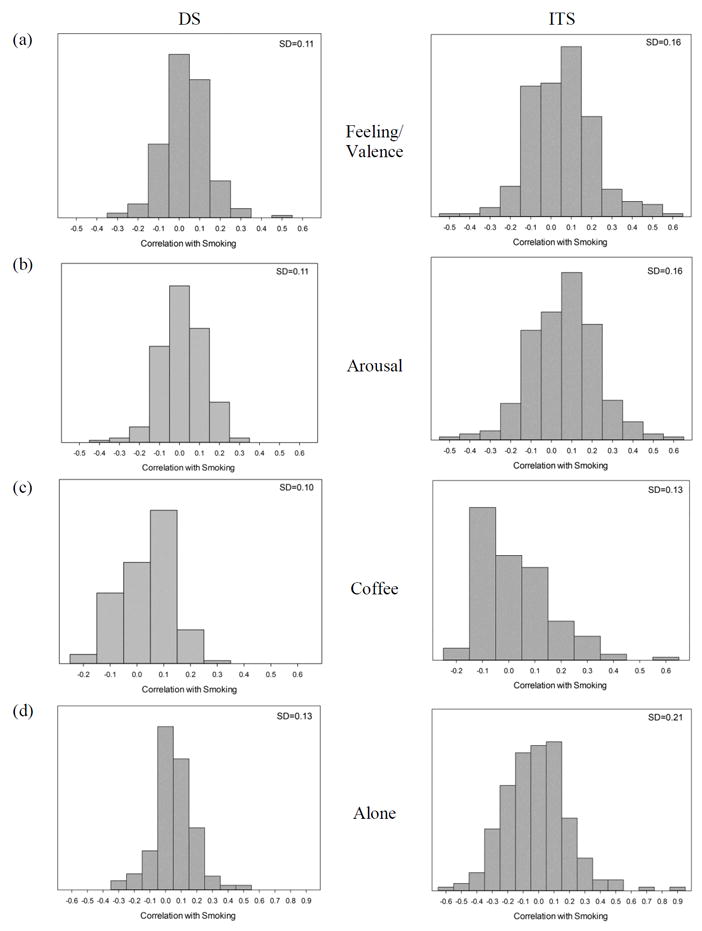
The histograms show the range of correlations, at the level of individual subjects, between selected situational characteristics and smoking, shown separately for DS and ITS. The variables shown are (a) Feeling / valence (a rating of feelings from negative to positive); (b) Arousal (a factor score whose constituent items include “active,” “calm,” “quiet/sleepy,” and “energetic.”) (c) drinking coffee (no/yes) and (d) alone (no/yes). The figures illustrate that the associations vary widely, even when the average association is near 0. Note that the range of the x-axes is kept identical for DS and ITS within a given variable, but the scales differ across variables, to accommodate different ranges of observed correlations. Each graph also shows the standard deviation of the correlation coefficients shown in the histogram, illustrating that the associations observed among ITS are consistently more variable than those observed among DS.
Stimulus control of smoking
When all the situational variables are considered simultaneously, ITS stimulus control is nearly perfect, with AUC-ROC averaging 0.95; that is, smoking and non-smoking occasions are distinguishable 95% of the time based on the situation descriptors. This was significantly higher than the average AUC-ROC for DS, but it was also very high at 0.86. Figure 2 shows the AUC-ROC values for particular stimulus domains, and shows that both groups demonstrate considerable stimulus control in all domains, with all ROC values significantly higher than the null value of 0.5. However, ITS show significantly stronger stimulus control over smoking in all stimulus domains; there was an overall group main effect, and ITS’ values were higher than DS’ overall, and in every domain.
Figure 2. ROC values across situational domain and smoker group.
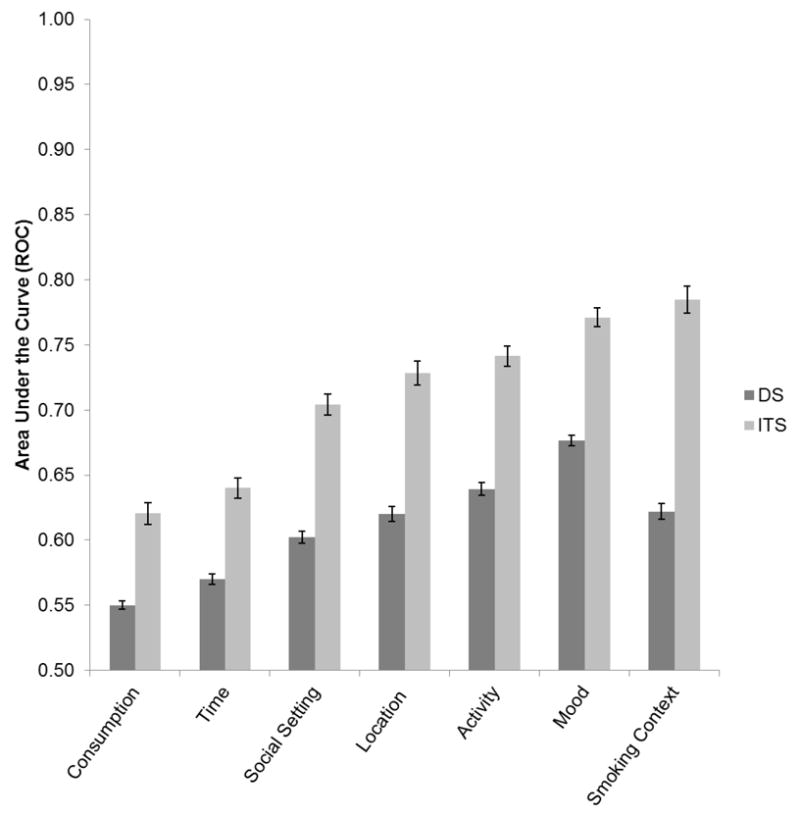
Average values for the Area Under the Curve of the Receiver Operating Curve (ROC), expressing the predictability of smoking from situational domains. ITS’ values were significantly higher for every domain, and all values were significantly greater than 0.5, the null value.
To test whether the ITS-DS difference in AUC-ROCs varied by domain, we evaluated the group x domain interaction, which was significant (p < .0001). As shown in Figure 2, the differences were greatest for the smoking context domain and smallest for consumption. Within each smoker group, analyses revealed a significant main effect of domain, indicating that some domains are more tightly linked to smoking than others. Within-group differences in AUC-ROC values between domains were nearly all significant. (The exceptions were that, among ITS, AUC-ROC values did not differ between activity and location; among DS, values did not differ between location and smoking context). The AUC-ROC values indicated that social setting, mood, and activity exercised the greatest stimulus control over smoking in both groups. In addition, smoking context was the strongest predictor of ITS’ smoking; this was not the case for DS’.
Mediation of Group Differences by Craving Responsiveness
An AUC-ROC analysis evaluated the relationship between craving and smoking. ITS had a significantly higher value (0.79 vs 0.63, p < .0001), indicating that their smoking was more closely linked to craving (see also Shiffman et al., 2014a). Co-varying the craving AUC-ROC in separate ordinary least squares regression analyses of smoker type effects on AUC-ROCs within each stimulus domain suggested that craving attenuated but did not fully account for ITS and DS group differences in stimulus control (all group differences remained significant at p < .0001). However, tests of mediation suggested that differences in the craving-smoking link partially mediated smoker group effects in nearly all situational domains (Sobel test p’s < .01), with the exception of mood (Sobel test p = .12).
Discussion
Detailed data on smoking contexts, collected by real-time EMA methods, demonstrated that situational contexts exercise greater influence over ITS’ compared to DS’ smoking. ITS’ smoking consistently demonstrated significantly greater stimulus control in every situational domain considered: time of day, social setting, affect, restrictions, location, activity, and consumption of food and drink. The absolute magnitude of the associations was striking. For example, just knowing the person’s emotional state allowed one to correctly predict, with over 75% accuracy, whether an ITS was smoking or not. In short, ITS’ smoking seems to be under tight stimulus control.
Even more striking was the estimated level of stimulus control when all variables were considered: the analysis indicated that one could achieve 95% accuracy in identifying smoking situations among ITS. Notably, DS also showed strong stimulus control in this analysis, implying 86% accuracy in identifying smoking situations. However, the figures from this omnibus analysis should be treated with some caution, because the models included all 26 variables in Table 1, and so may have been over-fitted, perhaps achieving spurious levels of prediction.
The finding of stronger stimulus control among ITS across a range of domains is consistent with the hypothesis that stimulus control helps to maintain ITS’ smoking and make quitting difficult in the face of cues associated with smoking, and may help explain why ITS are not much better able to quit than DS (Tindle & Shiffman, 2011), despite the fact that ITS do not maintain nicotine levels, and do not suffer craving or withdrawal when they abstain (Shiffman, Dunbar, Tindle, & Ferguson, 2014). It is also consistent with our previously reported finding that ITS smoking is more responsive to craving than DS smoking (Shiffman, et al. 2014b): ITS may experience craving, and hence smoke, when in the presence of certain stimuli, but in the absence of such stimuli they do not experience a drive to smoke. In a sense, strong stimulus control over use may represent another kind of dependence that keeps users of psychoactive drugs from easily stopping. Given that non-daily use is quite common for other addictive drugs (Substance Abuse and Mental Health Administration Office of Applied Studies, 2003), this mode of dependence may be important for understanding the range of drug use behaviors.
While the observed degree of stimulus control among ITS was particularly striking, DS’ smoking also showed a substantial amount of stimulus control – more than would be expected under a strict nicotine regulation model, which implies smoking at regular intervals, determined by the ebb of nicotine, rather than in response to external stimuli. Further, the pattern of stimulus control across stimulus domains (Figure 2) was strikingly similar for DS and ITS: across the seven situational domains examined, the profiles of AUC-ROC values for DS and ITS correlated 0.90. Thus, stimulus control among DS appears to be qualitatively similar to that in ITS, but consistently weaker.
It is widely understood that even DS’ smoking is initially under stimulus control during early stages of smoking (Russell, 1971), but the emerging need for nicotine maintenance is thought to supplant stimulus control as a driver of smoking (Shiffman & Paty, 2006). These data suggest that stimulus control remains important even for established adult DS. Perhaps the influence of context is not supplanted, but simply diluted, as smokers begin to smoke more of their cigarettes in order to maintain nicotine levels above the withdrawal threshold, independent of the situation. In this conceptualization, both ITS and DS respond to similar cues, but, whereas this is the dominant influence on smoking among ITS, its influence on DS is masked by the addition of cigarettes smoked for nicotine maintenance. This account is consistent with the boundary model (Kozlowski & Herman, 1984), which conceptualizes dependence as demanding a certain minimum rate of smoking, while allowing for additional smoking that might be prompted by situational influences.
This two-factor model of smoking (withdrawal-avoidance and stimulus control) may also have implications for understanding smoking cessation and relapse among DS, who face a dual challenge when quitting smoking. Firstly, they must overcome withdrawal and background craving (West & Schneider, 1987), which can be mitigated by pharmacological treatment (Ferguson & Shiffman, 2009). But they also must also overcome the influence of stimulus control, which is unmasked during cessation, and triggers cue-elicited craving upon exposure to cues (Ferguson & Shiffman, 2009). The role of stimulus control among DS is evident in lapse situations, which are marked by cueing stimuli like the ones seen in our analyses: e.g., exposure to other smokers, consumption alcohol, etc. (Bliss, Garvey, Heinold, & Hitchcock, 1989; Shiffman et al., 1997; Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996; Shiffman & Waters, 2004). The re-emergent role of cues also helps explain why smokers relapse (albeit at lower rates) even when their nicotine requirements are met by nicotine replacement. In a study where 100% of baseline nicotine levels were met by high-dose patch (Shiffman, Ferguson, Gwaltney, Balabanis, & Shadel, 2006), and withdrawal was completely suppressed, 62% of smokers still lapsed within six weeks (vs. 75% on placebo; Shiffman et al., 2006), doing so when cued by the typical situational triggers (Ferguson & Shiffman, 2010, 2014). Thus, two factors appear to maintain smoking and make quitting difficult for DS: the need to maintain nicotine levels to avoid withdrawal and abstinence-induced craving, and the influence of cues that trigger cue-induced craving and smoking (i.e., stimulus control).
Previous analyses (Shiffman et al., 2014b) showed that ITS smoking was more tightly linked to craving, because ITS reported very little craving when they were not smoking. Analyses in the present paper showed more broadly that ITS’ smoking was more sensitive to craving, but mediational analyses showed that this did not account for the difference between ITS and DS in stimulus control of smoking. The actual elicitation of smoking by situational stimuli may still be due to their stimulation of craving; the analysis only suggests that once craving is elicited, differential responsiveness to that craving does not explain differences in stimulus control.
The idiographic n = 1 analyses used here revealed patterns not seen in group-wise nomothetic analyses. It was particularly striking that nomothetic analyses showed almost no relationship between emotional state and smoking among DS, either in this study or in others (Shiffman et al., 2014a; Shiffman et al., 2002; Shiffman, Paty, Gwaltney, & Dang, 2004), and, consistent with this, Figure 1 shows little or no relationship between emotional state and smoking, on average. Yet, considered idiographically, emotional state was among the most important situational influences on DS’ and ITS’ smoking, suggesting that emotion does influence smoking, but not in a simple consistent way. Importantly, the observed influence of affect on smoking is not readily attributable to withdrawal effects, because it includes cases where smoking was associated with positive emotional states. Indeed, in traditional analyses of the role of affect in smoking, smoking was more likely to occur when subjects – both ITS and DS – were feeling better, rather than worse (Shiffman et al, 2014a).
The study’s limitations include reliance on self-report of smoking status and situational characteristics, potential for reactivity, and possible biasing effects of non-compliance and of smoking restrictions (see Shiffman, 2009a). Particularly when there were few smoking observations, the individual logistic regressions could have exploited chance relationships; this was particularly the case for the omnibus models, as they included many predictors. Also, differences in AUC-ROC values across domains could have been due to differences in how domains were assessed, rather than true differences in their influence on smoking. Some stimulus domains may not have been covered as comprehensively or assessed as reliably as others, perhaps resulting in lower average AUC-ROCs due to these measurement factors. Finally, unlike traditional animal studies of stimulus control, we did not control the pairing of specific antecedent stimuli and our target behavior (smoking) and as such we cannot draw causal conclusions about the associations observed; that is, while the patterns observed are consistent with stimulus control, we cannot conclude that they are caused by it.
The study’s strengths included the use of real-time EMA methods, and a non-treatment-seeking sample with diverse smoking behavior. An important aspect of our analysis was its ability to expand the scope of the analysis from uni-directional and univariate nomothetic relationships that were similar across subjects (e.g., all subjects tending to smoke when feeling worse emotionally) to encompass the fact that different individuals have different, indeed opposite associations (e.g., some subjects smoke when feeling worse emotionally, and some smoke when feeling better; Figure 1). The analysis by domains, encompassing several related situational characteristics, also allowed the analysis to encompass variation across subjects in which particular variables were influential. For example, if some subjects tended to smoke more when drinking alcohol, and others to smoke more when drinking coffee, such effects might be diluted, perhaps to the point of being invisible, in traditional analyses treating alcohol and coffee as separate cues. In contrast, both effects would be included in our analysis of the stimulus control exerted by consumption. Particularly because such heterogeneity in influential variables, and in their direction of influence, is to be expected if these individual differences result from idiosyncratic learning histories (Niaura et al., 1988), this mode of analysis seems important for assessing the influence of situational variables on smoking, i.e., stimulus control.
In summary, ITS’ smoking demonstrated very strong stimulus control, which may be a dominant driver of their smoking, and may account for their surprising difficulty quitting. DS also showed substantial stimulus control, suggesting that stimulus control also plays a significant role in driving and maintaining smoking even among DS. DS’ smoking may be maintained by two factors – withdrawal-avoidance and stimulus control – whereas ITS’ smoking may be maintained primarily by stimulus control.
Acknowledgments
This work was supported by grant R01-DA020742 (Shiffman) from the National Institutes of Health, National Institute on Drug Abuse.
Contributor Information
Saul Shiffman, Department of Psychology, University of Pittsburgh, 130 N. Bellefield Avenue, Suite 510, Pittsburgh, PA 15213, USA.
Michael S. Dunbar, Department of Psychology, University of Pittsburgh, 130 N. Bellefield Avenue, Suite 510, Pittsburgh, PA 15213, USA
Stuart G. Ferguson, School of Medicine, University of Tasmania, Private Bag 34, Hobart TAS 7001, Australia
References
- Beckham JC, Wiley MT, Miller SC, Dennis MF, Wilson SM, McClernon FJ, Calhoun PS. Ad lib smoking in post-traumatic stress disorder: An electronic diary study. Nicotine & Tobacco Research. 2008;10(7):1149–1157. doi: 10.1080/14622200802123302. [DOI] [PubMed] [Google Scholar]
- Benowitz N. Clinical pharmacology of nicotine: Implications for understanding, preventing, and treating tobacco addiction. Clinical Pharmacology and Therapeutics. 2008;83(4):531–541. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Benowitz N. Nicotine addiction. New England Journal of Medicine. 2010;362:2295–2203. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Kelly TH, editors. The relationship of stimulus control to the treatment of substance abuse. Vol. 84. Washington, D. C.: National Institute on Drug Abuse, U.S Government Printing Office; 1988. [Google Scholar]
- Bliss RE, Garvey AJ, Heinold JW, Hitchcock JL. The influence of situation and coping on relapse crisis outcomes after smoking cessation. Journal of Consulting and Clinical Psychology. 1989;57(3):443–449. doi: 10.1037//0022-006x.57.3.443. [DOI] [PubMed] [Google Scholar]
- Cooper TV, Taylor T, Murray AA, DeBon MW, Vander Weg MW, Klesges RC, Talcott GW. Differences between intermittent and light daily smokers in a population of U.S. Military recruits. Nicotine and Tobacco Research. 2010;12:465–473. doi: 10.1093/ntr/ntq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronk NJ, Piasecki TM. Contextual and subjective antecedents of smoking in a college student sample. Nicotine & Tobacco Research. 2010;12(10):997–1004. doi: 10.1093/Ntr/Ntq136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrandpre RJ, Bickel WK. Stimulus control and drug dependence. The Psychological Record. 1993;43(4):651–666. [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. Journal of Substance Abuse and Treatment. 2009;36(3):235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. Effect of high-dose nicotine patch on the characteristics of lapse episodes. Health Psychology. 2010;29:358–366. doi: 10.1037/a0019367. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. Using the Methods of Ecological Momentary Assessment in Substance Dependence Research-Smoking Cessation as a Case Study. Substance Use & Misuse. 2011;46(1):87–95. doi: 10.3109/10826084.2011.521399. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. Effect of high-dose nicotine patch on craving and negative affect leading up to lapse episodes. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-013-3429-6. [DOI] [PubMed] [Google Scholar]
- Gilpin E, Cavin S, Pierce J. Adult smokers who do not smoke daily. Addiction. 1997;92(4):473–480. doi: 10.1111/j.1360-0443.1997.tb03379.x. [DOI] [PubMed] [Google Scholar]
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiving operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Herman CP. The interaction of psychosocial and biological determinants of tobacco use: More on the Boundary Model. Journal of Applied Social Psychology. 1984;14(3):244–256. doi: 10.1111/j.15559-1816.1984.tb02234x. [DOI] [Google Scholar]
- Mermelstein R, Hedeker D, Flay B, Shiffman S. Real-time data capture and adolescent cigarette smoking: Moods and smoking. In: Stone AA, Shiffman S, Atienza A, Nebeling L, editors. The Science of Real-Time Data Capture: Self-Reports in Health Research. New York: Oxford University Press; 2007. pp. 117–135. [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. Journal of Abnormal Psychology. 1988;97(2):133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Paty JA, Kassel JD, Shiffman S. The importance of assessing base rates for clinical studies: An example of stimulus control of smoking. In: deVries MW, editor. The Experience of Psychopathology: Investigating Mental Disorders in their Natural Settings. New York: Cambridge University Press; 1992. pp. 347–352. [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC. A multiple motives approach to tobacco dependence: The Wisconsin Inventory of Smoking Dependence Motives. Journal of Consulting and Clinical Psychology. 2004;72:139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Newbury Park, CA: Sage Publications; 1992. [Google Scholar]
- Russell MA. Cigarette smoking: The natural history of a dependence disorder. British Journal of Medical Psychology. 1971;44(1):1–16. doi: 10.1111/j.2044-8341.1971.tb02141.x. [DOI] [PubMed] [Google Scholar]
- Schane RE, Glantz SA, Ling PM. Nondaily and social smoking: An increasingly prevalent pattern. Archives of Internal Medicine. 2009;169:1742–1744. doi: 10.1001/archinternmed.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Ecological Momentary Assessment (EMA) in studies of substance abuse. Psychological Assessment. 2009a;21:486–497. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Light and intermittent smokers: Background and perspective. Nicotine and Tobacco Research. 2009b;11:122–125. doi: 10.1093/ntr/ntn020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Ecological momentary assessment. In: Sher K, editor. The Oxford Handbook of Substance Use Disorders. Oxford University Press; in press. [Google Scholar]
- Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, Paton SM. Prediction of lapse from associations between smoking and situational antecedents assessed by Ecological Momentary Assessment. Drug and Alcohol Dependence. 2007;91:159–168. doi: 10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar M, Kirchner T, Li X, Tindle H, Anderson S, Scholl S. Smoker reactivity to cues: Effects on craving and on smoking behavior. Journal of Abnormal Psychology. 2013;122(1):264–280. doi: 10.1037/a0028339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Kirchner TR, Li X, Tindle HA, Anderson SJ, Ferguson SG. Cue reactivity in non-daily smokers: Effects on craving and on smoking behavior. Psychopharmacology. 2013;226(2):321–333. doi: 10.1007/s00213-012-2909-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Li X, Scholl SM, Tindle HA, Anderson SJ, Ferguson SG. Smoking patterns and stimulus control in intermittent and daily smokers. PLoS ONE. 2014a;9(3):e89911. doi: 10.1371/journal.pone.0089911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Li X, Scholl SM, Tindle HA, Anderson SJ, Ferguson SG. Craving in intermittent and daily smokers during ad libitum smoking. Nicotine and Tobacco Research. 2014b doi: 10.1093/ntr/ntu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Tindle HA, Ferguson SG. Non-daily smokers’ experience of craving on days they do not smoke. 2014 doi: 10.1037/abn0000063. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Scholl SM, Tindle HA. Smoking motives of daily and non-daily smokers: A profile analysis. Drug and Alcohol Dependence. 2012;126:362–368. doi: 10.1016/j.drugalcdep.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Engberg J, Paty JA, Perz W, Gnys M, Kassel JD, Hickcox M. A day at a time: Predicting smoking lapse from daily urge. Journal of Abnormal Psychology. 1997;106:104–116. doi: 10.1037/0021-843X.106.1.104. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Dunbar MS, Scholl SM. Tobacco dependence among intermittent smokers. Nicotine & Tobacco Research. 2012;14(11):1372–1381. doi: 10.1093/ntr/nts097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis M, Shadel W. Reduction of abstinence-induced withdrawal and craving using high dose nicotine replacement therapy. Psychopharmacolgy. 2006;184:637–644. doi: 10.1007/s00213-005-0184-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis M, Liu KS, Paty JA, Kassel JD, Gnys M. Immediate antecedents of cigarette smoking: An analysis from ecological momentary assessment. Journal of Abnormal Psychology. 2002;111(4):531–545. doi: 10.1037/0021-843X.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA. Smoking patterns and non-dependent smokers: Contrasting chippers and dependent smokers. Journal of Abnormal Psychology. 2006;115(3):509–523. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JD, Hickcox M. First lapses to smoking: Within-subjects analyses of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64:366–379. doi: 10.1037/0022-006X.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gwaltney CJ, Dang Q. Immediate antecedents of cigarette smoking: An analysis of unrestricted smoking patterns. Journal of Abnormal Psychology. 2004;113(1):116–171. doi: 10.1037/0021-843X.113.1.166. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Tindle H, Li X, Scholl S, Dunbar M, Mitchell-Miland C. Characteristics and smoking patterns of intermittent smokers. Experimental and Clinical Psychopharmacology. 2012;20(4):264–277. doi: 10.1037/a0027546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: A prospective analysis. Journal of Consulting and Clinical Psychology. 2004;72(2):192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ, Hickcox M. The Nicotine Dependence Syndrome Scale: A multi-dimensional measure of nicotine dependence. Nicotine and Tobacco Research. 2004;6:327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine. 1994;16(3):199–202. [Google Scholar]
- Substance Abuse and Mental Health Administration Office of Applied Studies, N. Results from the 2002 National Survey on Drug Use and Health: National findings. Rockville, MD: DHHS Publications; 2003. [Google Scholar]
- Tindle HA, Shiffman S. Smoking cessation behavior among intermittent smokers versus daily smokers. American Journal of Public Health. 2011;101(7):e1–3. doi: 10.2105/AJPH.2011.300186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinidad DR, Perez-Stable EJ, Emery SL, White MM, Grana RA, Messer KS. Intermittent and light daily smoking across racial/ethnic groups in the United States. Nicotine and Tobacco Research. 2009;11(2):203–210. doi: 10.1093/ntr/ntn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking 50 Years of Progress: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. [Google Scholar]
- West R, Schneider N. Craving for cigarettes. British Journal of Addiction. 1987;82:407–415. doi: 10.1111/j.1360-0443.1987.tb01496.x. [DOI] [PubMed] [Google Scholar]
- Wortley PM, Husten CG, Trosclair A, Chrismon J. Nondaily smokers: A descriptive analysis. Nicotine and Tobacco Research. 2003;5(5):755–759. doi: 10.1080/1462220031000158753. [DOI] [PubMed] [Google Scholar]
- Zhu S, Sun J, Hawkins S, Pierce J, Cummings S. A population study of low-rate smokers: Quitting history and instability over time. Health Psychology. 2003;22(3):245–252. doi: 10.1037/0278-6133.22.3.245. [DOI] [PubMed] [Google Scholar]


