Abstract
Objectives
To disclose the associated risk factors for latent tuberculosis infection (LTBI) and the current situation of LTBI in the eastern China.
Methods
A cross-sectional study was undertaken to evaluate the LTBI rate and risk factors.
Results
A total of 5305 subjects were finally included, with the IGRA positive rate of 19.98% (1060/5305). The LTBI rates were increasing with age (ORs were in significance from 6.60 to 20.92). Male gender significantly increased the risk of LTBI by 0.52 fold (OR = 1.52). Both smoking and drinking significantly increased the risk of LTBI (OR = 1.83 and OR = 1.67, respectively). Meanwhile, overweight and close contact with tuberculosis were risk factors for LTBI (OR = 1.36 and OR = 2.38, respectively). However, higher level of education and BCG vaccination lowered the risk of LTBI (OR = 0.16 and OR = 0.39, respectively). The multivariate logistic regression showed that age, male gender, smoking, overweight and close contacting with tuberculosis were risk factors for LTBI, but BCG vaccination was a protective factor for LTBI.
Conclusions
BCG vaccination exerted protective effect on tuberculosis. However, LTBI rate in the Chinese rural area was critical and subjects above 30 years, male, smoking, overweight and close contact with tuberculosis wound be the targets for LTBI screening and source of tuberculosis.
Introduction
Latent tuberculosis infection (LTBI) is defined as a state of persistent mycobacteria-specific T-cell responses without clinical evidence of tuberculosis [1]. It was estimated that one-third of the world population was infected by M. tuberculosis [2], and most of the infected people had no signs and symptoms of tuberculosis. Although LTBI people were not transmitters, some of the infected people were at a risk of developing active disease of tuberculosis during their lifetime, and it is reported that 5–10% of the LTBI people would finally turn out to be of tuberculosis [3]. China has the second largest number of tuberculosis patients in the world [4]. To make things worse, the LTBI population would potentially contribute to the aggressively increased new tuberculosis incidences and which may be greatly counted on the LTBI rate of the population, especially for the rural areas of China because tuberculosis patients from rural areas accounted for a major proportion of tuberculosis incidences. Thus, to accurately elucidate the current LTBI in rural areas of China is of critically important for tuberculosis control. Jiangsu Province was located in the eastern part of China with relatively lower incidence of tuberculosis [5], and it is worthwhile to reveal the situation of LTBI in this area.
Currently, LTBI screening was recommended on those target populations at high risk of developing tuberculosis, such as patients receiving tumor necrosis factor treatment, co-infection with HIV and children aged less than 5 years [6–8]. Meanwhile, prophylaxis treatment method was an alternative for tuberculosis control for the high risk populations [9, 10]. However, the extensive testing LTBI for the general population was not affordable, especially in the health resource limited regions. In our study, we also intend to find out the related risk factors for LTBI in this study, and provide evidences for adopting necessary interventions on the general population to decrease the LTBI rate.
Methods
Study design and participants
This cross-sectional study was undertaken from 1 July to 31 July in 2013 of Danyang County, Jiangsu Province. Two villages of Danyang County were chosen to conduct the study. Meanwhile, two steps of population screening were carried out in our study. Firstly, we interviewed the residence population of the two villages with a face to face way. The residence population of the study site was 7311. Among the residence population, 110 children with age less than 5 years and 4 pregnant women were excluded. There is no present active pulmonary tuberculosis in the residence population. For the first step survey, 1199 of the qualified subjects declined or could not complete in the study period. Thus, the eligible population included in the baseline survey was 5998. For the next step, we conducted investigation on the eligible subjects and the IGRA test for each subject. During the investigation, 520 eligible subjects did not join the investigation. The following 116 subjects who finished the investigation were also excluded from this cross-sectional study (among the 116 subject, 12 subjects presented clinically suspected pulmonary tuberculosis signs and symptoms and self-reported a history of tuberculosis simultaneously): one subject with absent result of IGRA, 16 subjects with absent results of digital chest radiography, 45 subjects reported a history of tuberculosis, 66 subjects presented clinically suspected pulmonary tuberculosis. Finally, 5361 subjects were selected for evaluation of the prevalence of LTBI, with a response rate of 73.33% (5361/7311). The study was approved by the ethics committee of the Institute of Pathogen Biology, Chinese Academy of Medical Sciences, and all the participants provided written informed consent before undergoing the investigation (For those enrolled minors or children above 5 years old, their parents or guardians provided written informed consent on behalf of them before the investigation). Smoking was defined as tobacco consumption more than 5 cigarettes per month and lasted for at least 6 months consecutively. Drinking was defined as alcohol consumption more than 100 mL per month in the last year. Close contact with tuberculosis was considered as contacting tuberculosis patients in household or workplace. The body-mass index (BMI) was classified as underweight (BMI < 18.5 Kg/m2), normal weight (BMI ≥ 18.5 Kg/m2 and BMI < 23 Kg/m2), overweight (BMI ≥ 23 Kg/m2 and BMI < 27.5 Kg/m2) and obesity (BMI ≥ 27.5 Kg/m2) [11].
Determination of LTBI
Each subject provided approximate 3 mL venous blood sample, and the interferon-gamma release assay (QuantiFERON-TB Gold In-Tube [QFT; Qiagen, Valencia, CA, USA]) was used to evaluate the status of LTBI. The procedure of conducting QFT follows the instructions provided by Qiagen and the methodology of QFT can be referred to the review of Whitworth et al. [12]. 5% of the incubation samples were randomly selected to repeat for consistency, and all the results were 100% consistent with the primary results.
Exclusion of pulmonary tuberculosis patients and clinically suspected pulmonary tuberculosis
Pulmonary tuberculosis was diagnosed by positive culture and X-ray manifestation of tuberculosis. Those subjects with abnormal digital chest radiography which indicated active pulmonary tuberculosis but without bacteriological evidence were considered as clinically suspected pulmonary tuberculosis patients. In this study, all the pulmonary tuberculosis cases and clinically suspected pulmonary tuberculosis subjects were excluded for the LTBI rate evaluation.
Statistical analysis
The between-group demographics were compared by person χ2 test or fisher exact test for categorical data. The association of risk factors among QTF positive group and QFT negative group was estimated by computing the odds ratio (OR) and 95% confidence intervals (CI) from both univariate and multivariate logistic regression analyses, and the dummy variable was used for those variables with more than two stratifications. P value less than 0.05 was considered as statistically significant. All the analysis were performed by Stata software (Version 13.0, StataCorp, Texas, USA)
Results
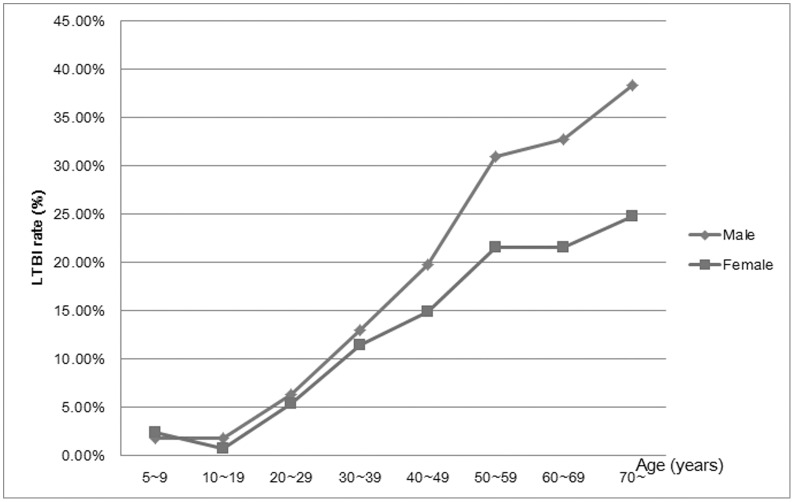
Of the 5361 participant with valid investigation and receiving QTF test, 56 of them came out of indeterminate QFT result. Thus, 5305 subjects were finally analyzed for evaluating the LTBI rate and associated risk factors (S1 Dataset). The positive LTBI rate determined by QFT in our study was 19.98% (1060/5305). As shown in Table 1, all the participants were above 5 years old, so we classified the age in to six groups by every ten years from 10 to 70, and finally the age was categorized into eight groups. When taking the 5–9 years class as the reference group, we found that since 30 years age groups, the risk of LTBI significant increased with ORs from 6.60 to 20.92. In this cross-sectional study, the male subjects constituted 46.5% of the total population. However, we found the proportion of male subjects in LTBI positive subjects was more (54.9%) than those in LTBI negative groups (44.4%), the OR showed that male subjects increased the risk of LTBI by 0.52 fold (OR = 1.52, 95%CI, 1.33–1.75). Meanwhile, as shown in Table 2 and Fig 1, the LTBI rate was increasing among male and female subjects along with age growing, and the LTBI rate among male subjects was significantly increased than female subjects from 40–49 years category. The education distribution showed that the higher education level lowered the risk of LTBI when compared to the primary or lower levels (OR = 0.16, 95%CI, 0.08–0.32 for college or higher levels). Smoking significantly increased the risk of LTBI by 0.83 fold (OR = 1.83, 95%CI, 1.59–2.10), while alcohol drinking also significantly increased the risk of LTBI by 0.67 fold (OR = 1.67, 95%CI, 1.43–1.94). Taking the normal weight as the reference, the BMI below 18.5 Kg/m2 significantly decreased the risk of LTBI by 0.35 fold (OR = 0.65, 95%CI, 0.46–0.92), while the overweight significantly increased the risk of LTBI by 0.36 fold (OR = 1.36, 95%CI, 1.17–1.57). However, the obesity was not in association with LTBI (P = 0.1055). The self-reported close contact with tuberculosis patients was in significant association with LTBI (OR = 2.38, 95%CI, 1.20–4.75), and subjects with bacillus Calmette-Guérin (BCG) scar were at a lower risk of LTBI (OR = 0.39, 95%CI, 0.34–0.46). The family history of tuberculosis was not in association with LTBI in our study (P = 0.1126). In multivariate logistic regression analysis (Table 3), we put the following variables concerning LTBI in the model, and only variables with significant result were finally selected into the model: age (every 10 years), gender (Female = 0, Male = 1), BMI (BMI ≥18.5 Kg/m2 and BMI <23 Kg/m2 (reference) = 0, BMI<18.5 Kg/m2 = 1, BMI ≥23 Kg/m2 and BMI<27.5 Kg/m2 = 2, BMI ≥27.5 Kg/m2 = 3), smoking (No = 0, Yes = 1), drinking (No = 0, Yes = 1), Family history of tuberculosis (No = 0, Yes = 1), close contact with tuberculosis (No = 0, Yes = 1) and BCG scar (No = 0, Yes = 1). As a result, we found the age with every 10 years increase (OR = 1.03, 95%CI, 1.03–1.04), male (OR = 1.32, 95%CI, 1.08–1.43), BMI between 23 and 27.5 Kg/m2 (OR = 1.24, 95%CI = 1.08–1.43), smoking (OR = 1.41, 95%CI, 1.16–1.71) and close contract with tuberculosis (OR = 2.10, 95%CI, 1.03–4.30) were risk factors for LTBI, while only BCG scar was a protective factor for LTBI (OR = 0.79, 95%CI, 0.65–0.97).
Table 1. Baseline characteristics of the enrolled population and the comparisons between QFT positive and QFT negative populations.
| Characteristics | Total | QFT Negative | QFT Positive | OR | P Value | |||
|---|---|---|---|---|---|---|---|---|
| Age (years) | n | % | n | % | n | % | ||
| 5–9 | 98 | 1.8 | 96 | 2.3 | 2 | 0.2 | reference | |
| 10–19 | 295 | 5.6 | 291 | 6.9 | 4 | 0.4 | 0.66(0.12–3.66) | 0.6346 |
| 20–29 | 397 | 7.5 | 374 | 8.8 | 23 | 2.2 | 2.95(0.68–12.74) | 0.1468 |
| 30–39 | 463 | 8.7 | 407 | 9.6 | 56 | 5.3 | 6.60(1.58–27.54) | 0.0096 |
| 40–49 | 1293 | 24.4 | 1073 | 25.3 | 220 | 20.8 | 9.84(2.41–40.22) | 0.0015 |
| 50–59 | 1136 | 21.4 | 840 | 19.8 | 296 | 27.9 | 16.91(4.14–69.04) | <0.0001 |
| 60–69 | 1030 | 19.4 | 751 | 17.7 | 279 | 26.3 | 17.83(4.37–72.82) | <0.0001 |
| ≥70 | 593 | 11.2 | 413 | 9.7 | 180 | 17.0 | 20.92(5.10–85.78) | <0.0001 |
| Gender | ||||||||
| Female | 2838 | 53.5 | 2360 | 55.6 | 478 | 45.1 | reference | <0.0001 |
| Male | 2467 | 46.5 | 1885 | 44.4 | 582 | 54.9 | 1.52(1.33–1.75) | |
| Education | ||||||||
| Primary school or lower | 2356 | 44.4 | 1799 | 42.4 | 557 | 52.5 | reference | |
| Middle school | 2090 | 39.4 | 1709 | 40.3 | 381 | 35.9 | 0.72(0.62–0.83) | <0.0001 |
| High school | 671 | 12.6 | 558 | 13.1 | 113 | 10.7 | 0.65(0.52–0.82) | 0.0002 |
| College or higher | 188 | 3.5 | 179 | 4.2 | 9 | 0.8 | 0.16(0.08–0.32) | <0.0001 |
| Smoking | ||||||||
| Never smoked | 4013 | 75.6 | 3320 | 78.2 | 693 | 65.4 | reference | <0.001 |
| Ever smoked | 1292 | 24.4 | 925 | 21.8 | 367 | 34.6 | 1.83(1.59–2.10) | |
| Alcohol drinking | ||||||||
| No | 4119 | 77.6 | 3377 | 79.6 | 742 | 70.0 | reference | <0.001 |
| Yes | 1186 | 22.4 | 868 | 20.4 | 318 | 30.0 | 1.67(1.43–1.94) | |
| BMI (Kg/m2) | ||||||||
| <18.5 | 330 | 6.2 | 289 | 6.8 | 41 | 3.9 | 0.65(0.46–0.92) | 0.0139 |
| ≥18.5 and <23 | 2184 | 41.2 | 1792 | 42.2 | 392 | 37.0 | reference | |
| ≥23 and <27.5 | 2222 | 41.9 | 1714 | 40.4 | 508 | 47.9 | 1.36(1.17–1.57) | <.0001 |
| ≥27.5 | 569 | 10.7 | 450 | 10.6 | 119 | 11.2 | 1.21(0.96–1.52) | 0.1055 |
| Family history of TB | ||||||||
| No | 5232 | 98.6 | 4192 | 98.8 | 1040 | 98.1 | reference | 0.1126 |
| Yes | 73 | 1.4 | 53 | 1.2 | 20 | 1.9 | 1.52(0.91–2.56) | |
| Close contact with TB patients | ||||||||
| No | 5270 | 99.3 | 4223 | 99.5 | 1047 | 98.8 | reference | 0.0135 |
| Yes | 35 | 0.7 | 22 | 0.5 | 13 | 1.2 | 2.38(1.20–4.75) | |
| BCG Scar | ||||||||
| No | 3410 | 64.3 | 2567 | 60.5 | 843 | 79.5 | reference | <.0001 |
| Yes | 1895 | 35.7 | 1678 | 39.5 | 217 | 20.5 | 0.39(0.34–0.46) |
Abbreviations: BCG, bacillus Calmette-Guérin; BMI, body mass index; CI, confidence interval; QFT, QuantiFERON-TB Gold In-TubeQFT; OR, odds ratio; TB, tuberculosis.
Table 2. The LTBI rate differences among male and female subjects among different age groups.
| Age (years) | Female | Male | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| QFT negative (n) | (%) | QFT positive (n) | (%) | QFT negative (n) | (%) | QFT positive (n) | (%) | |||
| 5–9 | 41 | 97.62 | 1 | 2.38 | 55 | 98.21 | 1 | 1.79 | 0.75(0.05–12.27) | *1.00 |
| 10–19 | 131 | 99.24 | 1 | 0.76 | 160 | 98.16 | 3 | 1.84 | 2.46 (0.25–23.89) | *0.6308 |
| 20–29 | 195 | 94.66 | 11 | 5.34 | 179 | 93.72 | 12 | 6.28 | 1.19(0.51–2.76) | 0.6878 |
| 30–39 | 233 | 88.59 | 30 | 11.41 | 174 | 87.00 | 26 | 13.00 | 1.16(0.66–2.03) | 0.6025 |
| 40–49 | 624 | 85.13 | 109 | 14.87 | 449 | 80.18 | 111 | 19.82 | 1.42(1.06–1.89) | 0.0189 |
| 50–59 | 466 | 78.45 | 128 | 21.55 | 374 | 69.00 | 168 | 31.00 | 1.64(1.25–2.14) | 0.0003 |
| 60–69 | 408 | 78.46 | 112 | 21.54 | 343 | 67.25 | 167 | 32.75 | 1.77(1.34–2.34) | <.0001 |
| ≥70 | 262 | 75.29 | 86 | 24.71 | 151 | 61.63 | 94 | 38.37 | 1.90(1.33–2.70) | 0.0004 |
Abbreviations: CI, confidence interval; OR, odds ratio; LTBI, latent tuberculosis infection; OR, odds ratio; QFT, QuantiFERON-TB Gold In-TubeQFT;
*Fisher exact test;
Fig 1. the LTBI rate between male and female subjects along with age groups.
Abbreviations: LTBI, latent tuberculosis infection
Table 3. Multivariate logistic regression on risk factors for Mycobacterium tuberculosis infection.* .
| Variables | β | Wald Chi-square | P | OR | 95%CI |
|---|---|---|---|---|---|
| Intercept | -2.9102 | 130.0982 | <.0001 | ||
| Age (every 10 years) | 0.0308 | 122.7828 | <.0001 | 1.03 | 1.03–1.04 |
| Gender (male) | 0.2799 | 9.4559 | 0.0021 | 1.32 | 1.11–1.58 |
| BMI (≥23 and <27.5 Kg/m2) | 0.2186 | 9.441 | 0.0021 | 1.24 | 1.08–1.43 |
| Smoking (Yes) | 0.343 | 12.3513 | 0.0004 | 1.41 | 1.16–1.71 |
| Close contact with TB patients (Yes) | 0.3715 | 4.1486 | 0.0417 | 2.10 | 1.03–4.30 |
| BCG Scar (Yes) | -0.2311 | 5.3504 | 0.0207 | 0.79 | 0.65–0.97 |
Abbreviations: BCG, bacillus Calmette-Guérin; BMI, body mass index; CI, confidence interval; OR, odds ratio.
*Age: every 10 years; Gender: female as reference; BMI: BMI ≥18.5 Kg/m2 and BMI <23 Kg/m2 as reference; None smoking status as reference; None closing contact with tuberculosis patients as reference; No BCG Scar as reference.
Discussion
Although simple to perform and inexpensive, the tuberculin skin test (TST) need a return of visit and could not distinguish M. tuberculosis infection from prior BCG vaccination [13]. However, with higher sensitivity and specificity than TST, interferon-gamma release assays were recommended for the diagnosis of LTBI [12, 14], and interferon-gamma release assays have been adopted for confirmation of the status of LTBI in several studies [15, 16]. In our study, the interferon-gamma release assay by QFT was used to test the status of LTBI.
In this cross-sectional study, we evaluated the rate of LTBI in the rural area of eastern China, and revealed associated factors that would be involved in the risk of LTBI. 5305 subjects provided valid results and showed a LTBI of 19.98% in the rural population. Meanwhile, age, male gender, smoking, overweight and close contacting with tuberculosis were risk factors for LTBI, while BCG vaccination was a protective factor for LTBI.
In our study, we found that subjects with BCG scar were protected from LTBI. BCG vaccination was included in the national immunization system of China since 1978, which means those subjects aged 35 years old or younger (calculated by the time of our investigation) would be protected from LTBI if people vaccinated with BCG. Meanwhile, our data showed that since the age categories above from 30–39 years level, the risks of LTBI were in significance when compared with the age category of 5–9 years. This may implicate that those population with age less than 30 years old might be protected by BCG vaccination from LTBI infection. Review of randomized controlled trials showed that BCG was in effective of protection form LTBI until 10 years [17]. Recently, Roy et al. conducted a meta-analysis of 14 studies showed that BCG was protecting children less than 16 years from LTBI and also from tuberculosis infection to tuberculosis disease [18]. Our study supported the findings and provided more evidence that people aged less than 30 years might be also protected from LTBI and with only a slightly increased rate of LTBI among those aged 20–29 years (5.79%).
Although the increasing trend of LTBI rate was overwhelming along with age, the gender difference of LTBI may further implicate clues for LTBI control in target population. It is reported that the ratio of male to female among tuberculosis patients was 2 to 1 [19], which suggested paying more efforts on tuberculosis control on male subjects.
In our study, we found that LTBI rate among male subjects was significantly higher than that among female subject since the age group of 40–49 year. Ting et al. discussed the gender disparity on the risk of LTBI, and they reiterated that male gender was not contributing to the increased risk of LTBI after controlling confounders of age, smoking status and other clinical factors [20]. However, in the multivariate analysis of LTBI risk factors, we found that male gender still increased the risk of LTBI, which need to be paid more attention for latent tuberculosis control. Except the different view of gender on LTBI risk, smoking habit was an independent risk factor for LTBI among Ting’s study and in other previous association studies [21–23]. Our results also supported the findings. Meanwhile, the passive smoking for children also increased the risk of LTBI [24, 25]. Cigarette smoke exposure inhibited the lung T-cell production of IFN-gamma during stimulation in vitro with anti-CD3 and thus increased the susceptibility to M. tuberculosis [26]. Meanwhile, the possible mechanism of smoking inducing increased susceptibility to LTBI was also supported by the evidences found in vitro that smoking impairs macrophage control of M. tuberculosis and the nicotine and acrolein were implicated in smoking induced immunosuppression [27]. Thus, control of cigarette smoking would be benefit to tuberculosis control in settings with high LTBI rate.
According to the classification of BMI for Asian people [11], overweight (BMI between 23 and 27.5 Kg/m2) was found to elevate the risk of LTBI for the general population in the rural area in our study. Many studies have proved that people with lower BMI was at increased risk of developing tuberculosis disease [28]. However, how BMI affected the M. tuberculosis infection was not well described. Studies focused on another particular population, the health care workers, and showed discrepant results of BMI on LTBI risk [29, 30]. Our cross-sectional study with a large sample size provided implications that overweight might be contributed in LTBI increased risk.
Besides the important findings of LTBI in the eastern part of China, several limitations of our study need to be addressed. Firstly, the study population can’t represent the urban population because of the sampling was only conducted in the rural area. Secondly, the QFT method was an indirect method for LTBI diagnosis, and it may not totally represent the existence of M. tuberculosis in vivo, because we don’t know how long the immunology of human body to M. tuberculosis will last. Meanwhile, the sensitivity of QTF will be decreased for those immunosuppression patients or those people receiving immunosuppressive agent [1]. However, the differentiation ability of the QTF is more convincing in detection M. tuberculosis induced infection rather than other Mycobacteria when compared with TST method.
Risk factors for developing tuberculosis have been extensively studied previously. However, the risk factors for LTBI were rarely reported, and the importance of revealing the risk factors for LTBI in high LTBI regions was critical for tuberculosis control. We conducted this cross-sectional study with a large sample size to disclose the current LTBI situation in the eastern rural China and the associated risk factors which should be paid more attention under the current tuberculosis control strategy in China, and the similar results could be referred by regions with similar burden of tuberculosis and economic development.
Supporting Information
(SAS7BDAT)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was supported by the national twelfth five-year mega-scientific projects of infectious diseases of China, National Science and Technology Major Project, grant number: 2013ZX10003004-002 (http://www.nmp.gov.cn/). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mack U, Migliori GB, Sester M, Rieder HL, Ehlers S, Goletti D, et al. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. European Respiratory Journal. 2009. May;33(5):956–73. 10.1183/09031936.00120908 [DOI] [PubMed] [Google Scholar]
- 2. Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. Jama. 1999. August 18;282(7):677–86. [DOI] [PubMed] [Google Scholar]
- 3. Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol. 1974. February;99(2):131–8. [DOI] [PubMed] [Google Scholar]
- 4. WHO. Global Tuberculosis Report 2013. World Health Organization; Genova: 2014. [Google Scholar]
- 5. group Tfnts. Report of the fifth national tuberculosis epidemiological survey. Chinese Journal of Antituberculosis. 2012;34(8):24. [Google Scholar]
- 6. Lee SK, Kim SY, Kim EY, Jung JY, Park MS, Kim YS, et al. Mycobacterial Infections in Patients Treated with Tumor Necrosis Factor Antagonists in South Korea. Lung. 2013. October;191(5):565–71. 10.1007/s00408-013-9481-5 [DOI] [PubMed] [Google Scholar]
- 7.Geneva. Recommendations for investigating contacts of persons with infectious tuberculosis in low- and middleincome countries. World Health Organization. 2012. [PubMed]
- 8.Geneva. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. World Health Organization. 2011.
- 9. World Health Organization Stop TBPCTBS. Chapter 4: childhood contact screening and management. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2007. 2007-January;11(1):12–5. [PubMed] [Google Scholar]
- 10. Sichletidis L, Settas L, Spyratos D, Chloros D, Patakas D. Tuberculosis in patients receiving anti-TNF agents despite chemoprophylaxis. International Journal of Tuberculosis and Lung Disease. 2006. October;10(10):1127–32. [PubMed] [Google Scholar]
- 11. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004. January 10;363(9403):157–63. [DOI] [PubMed] [Google Scholar]
- 12. Whitworth HS, Scott M, Connell DW, Donges B, Lalvani A. IGRAs—the gateway to T cell based TB diagnosis. Methods. 2013. May 15;61(1):52–62. 10.1016/j.ymeth.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 13. Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006. November;10(11):1192–204. [PubMed] [Google Scholar]
- 14. Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K, et al. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2010. 2010-June-25;59(RR-5):1–25. [PubMed] [Google Scholar]
- 15. Hermansen TS, Thomsen VO, Lillebaek T, Ravn P. Non-Tuberculous Mycobacteria and the Performance of Interferon Gamma Release Assays in Denmark. Plos One. 2014. April 4;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song S, Jeon D, Kim JW, Kim YD, Kim S-P, Cho JS, et al. Performance of Confirmatory Interferon-gamma Release Assays in School TB Outbreaks. Chest. 2012. April;141(4):983–8. 10.1378/chest.11-1158 [DOI] [PubMed] [Google Scholar]
- 17. Sterne JA, Rodrigues LC, Guedes IN. Does the efficacy of BCG decline with time since vaccination? Int J Tuberc Lung Dis. 1998. March;2(3):200–7. [PubMed] [Google Scholar]
- 18. Roy A, Eisenhut M, Harris RJ, Rodrigues LC, Sridhar S, Habermann S, et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. Bmj-British Medical Journal. 2014. August 5;349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rhines AS. The role of sex differences in the prevalence and transmission of tuberculosis. Tuberculosis (Edinb). 2013. January;93(1):104–7. [DOI] [PubMed] [Google Scholar]
- 20. Ting WY, Huang SF, Lee MC, Lin YY, Lee YC, Feng JY, et al. Gender disparities in latent tuberculosis infection in high-risk individuals: a cross-sectional study. PloS one. 2014;9(11):e110104 10.1371/journal.pone.0110104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lindsay RP, Shin SS, Garfein RS, Rusch MLA, Novotny TE. The Association between Active and Passive Smoking and Latent Tuberculosis Infection in Adults and Children in the United States: Results from NHANES. PloS one. 2014. March 24;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Horne DJ, Campo M, Ortiz JR, Oren E, Arentz M, Crothers K, et al. Association between smoking and latent tuberculosis in the U.S. population: an analysis of the National Health and Nutrition Examination Survey. PloS one. 2012;7(11):e49050 10.1371/journal.pone.0049050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. den Boon S, van Lill SWP, Borgdorff MW, Verver S, Bateman ED, Lombard CJ, et al. Association between smoking and tuberculosis infection: a population survey in a high tuberculosis incidence area. Thorax. 2005. July;60(7):555–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sridhar S, Karnani N, Connell DW, Millington KA, Dosanjh D, Bakir M, et al. INCREASED RISK OF MYCOBACTERIUM TUBERCULOSIS INFECTION IN HOUSEHOLD CHILD CONTACTS EXPOSED TO PASSIVE TOBACCO SMOKE. Pediatric Infectious Disease Journal. 2014. December;33(12):1303–6. 10.1097/INF.0000000000000506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. du Preez K, Mandalakas AM, Kirchner HL, Grewal HMS, Schaaf HS, van Wyk SS, et al. Environmental tobacco smoke exposure increases Mycobacterium tuberculosis infection risk in children. International Journal of Tuberculosis and Lung Disease. 2011. November;15(11):1490–6. 10.5588/ijtld.10.0759 [DOI] [PubMed] [Google Scholar]
- 26. Feng Y, Kong Y, Barnes PF, Huang F-F, Klucar P, Wang X, et al. Exposure to Cigarette Smoke Inhibits the Pulmonary T-Cell Response to Influenza Virus and Mycobacterium tuberculosis. Infection and Immunity. 2011. January;79(1):229–37. 10.1128/IAI.00709-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shang S, Ordway D, Henao-Tamayo M, Bai X, Oberley-Deegan R, Shanley C, et al. Cigarette Smoke Increases Susceptibility to Tuberculosis-Evidence From In Vivo and In Vitro Models. Journal of Infectious Diseases. 2011. May 1;203(9):1240–8. 10.1093/infdis/jir009 [DOI] [PubMed] [Google Scholar]
- 28. Patra J, Jha P, Rehm J, Suraweera W. Tobacco smoking, alcohol drinking, diabetes, low body mass index and the risk of self-reported symptoms of active tuberculosis: individual participant data (IPD) meta-analyses of 72,684 individuals in 14 high tuberculosis burden countries. PloS one. 2014;9(5):e96433 10.1371/journal.pone.0096433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mathew A, David T, Thomas K, Kuruvilla PJ, Balaji V, Jesudason MV, et al. Risk factors for tuberculosis among health care workers in South India: a nested case-control study. Journal of Clinical Epidemiology. 2013. January;66(1):67–74. 10.1016/j.jclinepi.2011.12.010 [DOI] [PubMed] [Google Scholar]
- 30. Luu Thi L, Nguyen Thi Le H, Kobayashi N, Yanai H, Toyota E, Sakurada S, et al. Prevalence and Risk Factors for Tuberculosis Infection among Hospital Workers in Hanoi, Viet Nam. PloS one. 2009. August 27;4(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(SAS7BDAT)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.