Abstract
Introduction
Whether patients with functional dyspepsia (FD) should receive Helicobacter pylori (H. pylori) eradication therapy remains controversial. The objective of this trial was to evaluate the effect of H. pylori eradication therapy on dyspeptic symptoms of patients with FD.
Material and methods
A prospective, randomized, placebo-controlled trial of H. pylori eradication for FD was conducted. A total of 720 FD patients diagnosed by Rome III criteria were consecutively enrolled. We randomly assigned 186 H. pylori infected patients with FD to receive quadruple therapy for 14 days and 173 such patients to receive identical-appearing placebos. Severity of abdominal symptoms was assessed with the Glasgow Dyspepsia Severity Score (GDSS), and eradication of H. pylori by 13C-urea breath test was evaluated during one year.
Results
The rate of eradication of H. pylori infection was 87.1% in the treatment group and 2.9% in the placebo group at 6 weeks (p = 0.001). The mean GDSS at 12 months was 4.9 ±2.8 in the treatment group, as compared to 5.2 ±3.4 in the placebo group (p = 0.064). The scores in both groups were lower than those at baseline. According to the intention-to-treat analysis, at 12 months, there was no significant difference between groups in the rate of successful treatment (48.6% in the treatment group and 51.2% in the placebo group; p = 0.84). There was no significant difference in mean symptom scores between the two treatment groups at any point during follow-up.
Conclusions
The results of our study provide no evidence that H. pylori eradication leads to relief of symptoms 12 months after treatment, and there is a need for further studies.
Keywords: functional dyspepsia, helicobacter pylori, eradication
Introduction
Dyspepsia is a common clinical condition associated with a complex of upper gastrointestinal symptoms including: upper centered discomfort or pain, feeling of abdominal fullness, early satiety, abdominal distention and bloating, belching, and nausea. It occurs in approximately 25% of the population each year, but most affected people do not seek medical care [1]. Approximately 25% of patients with dyspepsia have an underlying organic cause. However, up to 75% of patients have functional (idiopathic or non-ulcer) dyspepsia with no underlying cause on diagnostic evaluation.
Functional dyspepsia (FD), one of the most common gastrointestinal disorders, has prevalence rates of 11% to 29.2% worldwide [2]. According to the Rome III criteria, FD is defined as the presence of one or more of the following: postprandial fullness, early satiation, epigastric pain or burning and no evidence of structural disease (including on upper endoscopy) that is likely to explain the symptoms [3]. While patients with these symptoms and a negative diagnostic evaluation likely have FD, according to the Rome III guidelines, the criteria should be fulfilled for the last 3 months with symptom onset at least 6 months before diagnosis.
Helicobacter pylori (H. pylori) is an important risk factor for the development of peptic ulcer disease, gastric adenocarcinoma, and primary B cell lymphoma of the stomach. A possible role for H. pylori in the pathogenesis of gastroesophageal reflux disease (GERD) has also been suggested in a growing number of studies. The association between H. pylori infection and FD is unclear, although a minority of patients (from 1% to 15%) may improve after eradication treatment [4, 5]. Dyspepsia continuing after treatment of H. pylori is more likely the result of GERD or FD. Whether patients with FD should receive H. pylori eradication therapy remains controversial. The present study was designed to evaluate the effects of H. pylori eradication therapy on dyspeptic symptoms of patients with FD.
Material and methods
Patients
This study was approved by the Ethics Committee of the Ardabil University of Medical Sciences. This was a prospective, randomized, placebo-controlled study. Patients presenting with FD to the gastroenterology out-patient clinic of Imam Khomeini Hospital in Ardabil, Iran between June 2010 and October 2013 were recruited for the study. Patients who met the following criteria were enrolled in this study: (1) aged 18–45 years; (2) a definitive diagnosis of FD defined by Rome III criteria; (3) a positive H. pylori infection using a rapid urease test (RUT) and histology; (4) not receiving antacids, antibiotics, bismuth, or proton-pump inhibitors within the prior 4 weeks; and (5) consent to participate in the study.
The exclusion criteria included: (1) use of antibiotics, bismuth, or proton-pump inhibitors within the prior 4 weeks; (2) a negative H. pylori infection using RUT or histology; (3) previous H. pylori eradication therapy; (4) past H. pylori infection; (5) patients with previous gastric surgery; (6) the coexistence of serious concomitant illness (e.g., decompensated liver cirrhosis or uremia); (7) patients less than 18 or greater than 45 years of age; (8) presence of ulcer or mass on endoscopy; (9) reflux esophagitis; (10) Barrett's esophagus; (11) chronic gastric or duodenal ulceration; (12) duodenal or esophageal erosions; (13) cancer; (14) presence of alarm signs (anemia, gastrointestinal bleeding, recurrent vomiting, weight loss, and etc.); and (15) pregnant women. Physical examination and laboratory tests (complete blood cell count, renal and liver function, fasting blood glucose, and erythrocyte sedimentation rate) were carried out at baseline and showed normal results, excluding organic diseases. All studied patients signed an informed consent form and declared their willingness to allow the application of their anonymous data for research purposes.
Study design
All of the patients eligible for the study underwent gastroscopy with conscious sedation. Endoscopic samplings were performed with a Pentax EG-2990K Video Gastroscope (Pentax Medical Company, Tokyo, Japan) after overnight fasting, and biopsies were done at one antral site and one corpus site for histological examination (by modified Giemsa staining), and biopsy at another antral site was performed for RUT (Kimberly-Clark, CLO test, Rapid Urease Test Gel, GA 30076-2199 USA). Patients who were positive in both the RUT and histological examination were considered suitable candidates for the proposed H. pylori eradication therapy. Patients were blinded to this study and randomly assigned by an independent investigator using a computer-generated random number table to one of the two groups, the treatment group or control group. The allocation ratio was 1 : 1.
Given that the best results of H. pylori eradication in Iran are obtained with 2 weeks of furazolidone-based quadruple therapy or clarithromycin-based quadruple therapy [6], patients in the treatment group were administered with omeprazole, 20 mg twice daily; amoxicillin, 1000 mg twice daily; bismuth subcitrate, 240 mg twice daily and clarithromycin, 500 mg twice daily for 14 days. Patients in the control group were administered with placebo antibiotics for 2 week. The placebo consisted of similar-looking capsules as the treatment drugs. Neither the investigators nor the patients were aware of the treatment assignments. The patients returned 6 weeks and 3, 6, 9, and 12 months after the cessation of treatment. Helicobacter pylori eradication was evaluated by 13C-urea breath test (UBiT-IR300; Otsuka Electronics, Osaka, Japan), at least 4 weeks after the completion of treatment. The breath test, using 100 mg of 13C-urea, was performed. Follow-up gastroscopy was performed 1 year after H. pylori eradication.
Assessment of dyspepsia after Helicobacter pylori eradication
We assessed severity of abdominal symptoms using the Glasgow dyspepsia severity score (GDSS) [7], which is based on several aspects of dyspepsia: firstly, the frequency of dyspepsia symptoms and the effect that they have on normal activities and ability to work; secondly, the need for consultations with physicians for dyspepsia and the need for diagnostic investigations for dyspepsia; and thirdly, the need for over-the-counter and prescription medication for dyspepsia. A summary of the GDSS is given in Table I. GDSS can range from 0 to 20, with higher scores indicating more severe dyspepsia.
Table I.
Glasgow dyspepsia severity score scale
| Dyspeptic symptoms: | |
| Frequency | 0–5 |
| Effect on normal activities | 0–2 |
| Time off work | 0–2 |
| Consultation with physician: | |
| In physician's office | 0–2 |
| Home visits by general practitioner | 0–2 |
| Tests for dyspepsia | 0–2 |
| Medication for dyspepsia: | |
| Over the counter | 0–2 |
| On prescription | 0–3 |
| Total | 0–20 |
Statistical analysis
The statistical analysis of the data was done using SPSS software (Version 19, SPSS Inc, United States). The comparison of the continuous variables was performed with Student's t test, and for the comparison of the categorical variables the χ2 test was used. The Pearson correlation analysis was used in the evaluation of the correlation between scores of the different scales and other relevant variables. The effective rates of symptoms were analyzed according to intent-to-treat (ITT) methods. A p-value of < 0.05 was considered statistically significant. The results were expressed as means ± standard deviations.
Results
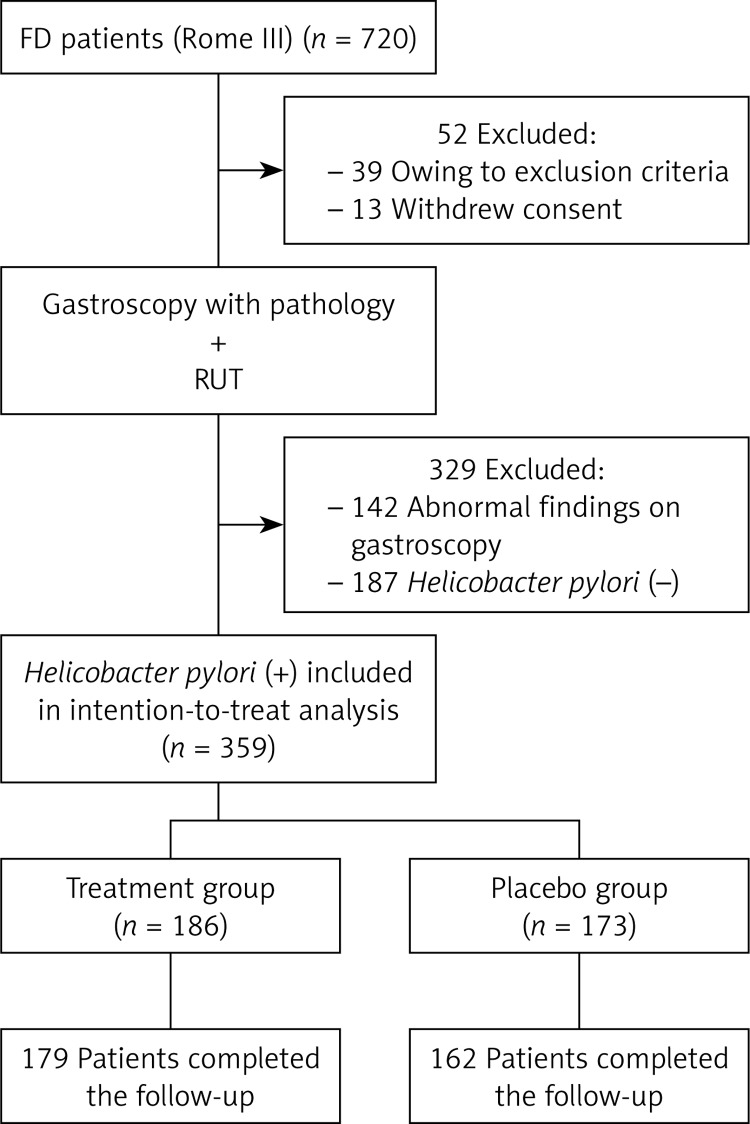
From June 2010 to October 2013, a total of 720 patients with FD were enrolled in the current study, and 359 FD patients with H. pylori infection (54.3% were women; mean age: 36.8 years) were randomized to the study. We excluded 381 patients with FD: 13 withdrew their the consent, 142 had abnormal findings on endoscopy, 187 were negative for H. pylori, and 39 had other exclusion criteria. The flow of patients through the trial and the reasons for exclusion are presented in Figure 1. A total of 186 patients were randomly assigned to receive omeprazole and antibiotics (treatment group), and 173 were assigned to receive placebo (control group). The two groups were well balanced with respect to demographic and clinical features. At 12 months, 96.2% of the patients in the treatment group (179 of 186) and 93.6% of the patients in the placebo group (162 of 173) completed the study.
Figure 1.
Flow of participants through the trial
Urea breath testing showed that 87.1% of the patients in the treatment group (162 of 186) were negative for H. pylori at 6 weeks, as compared with 2.9% of the patients in the placebo group (5 of 173) (p = 0.001). At 12 months, the eradication rates for H. pylori in the treatment and placebo group were 80.3% (139 of 179) and 4.9% (8 of 162), respectively, on the basis of urea breath testing (p = 0.001).
Adverse reactions documented during treatment included: darkening of the tongue and stool in 65 cases, nausea in 16 cases, diarrhea in 18 cases, headache in 9 cases, rash in 5 cases, coughing in 3 cases and blurred vision in 4 cases. The basic vital signs and routine blood, urine and stool tests were normal in all the participants.
The mean baseline GDSS was 12.1 ±2.4 for the treatment group and 11.9 ±2.2 for the placebo group. At 6 months, the score was 6.1 ±3.2 and 7.4 ±3.9 in the treatment group and placebo group, respectively (p = 0.081). The mean GDSS at 12 months was 4.9 ±2.8 in the treatment group, as compared with 5.2 ±3.4 in the placebo group (p = 0.064). The scores in both groups were lower than those at baseline. According to the intention-to-treat (ITT) analysis, at 12 months, treatment was successful (defined as the presence of no more than mild pain or discomfort) in 48.6% of the patients in the treatment group (87 of 179) and 51.2% of those in the placebo group (83 of 162) (p = 0.84). There was no significant difference in mean symptom scores between the two treatment groups at any point during follow-up.
Discussion
Although there are several hypotheses with regard to the role of H. pylori infection in the pathogenesis of FD, the mechanism remains unclear. Helicobacter pylori may cause altered smooth muscle dysfunction due to the induction of an inflammatory response or by the initiation of an antibody response [8]. However, studies have not found an association between H. pylori and abnormal gastric motor function in patients with FD [9].
Many studies have tried to show increased prevalence of H. pylori in patients with FD, but the results have been conflicting. Based on the hypothesis that H. pylori has a role in FD, the infection should be more frequent in patients with dyspepsia. Indeed, many epidemiological studies have tried to show such higher prevalence, but the results have been conflicting. In a population-based study of H. pylori infection, the results seem to support a role of H. pylori infection in dyspeptic symptoms [10, 11]. Dyspeptic symptoms were reported by 44% of the evaluated population. The prevalence of H. pylori infection, evaluated using the urea breath test (UBT), was 72% in individuals reporting dyspeptic symptoms and 64% in the asymptomatic population (p < 0.005). The prevalence of H. pylori infection was significantly higher in dyspeptic than in asymptomatic individuals [11].
The symptomatic benefit of eradicating H. pylori in patients with non-ulcer dyspepsia may be related to the background prevalence of H. pylori-related ulcer disease in the population being studied. Northern Ireland, southern Ireland, and Scotland have high prevalence of H. pylori-related ulcer disease. Studies in each of these countries have shown a symptomatic benefit of eradicating H. pylori in patients with normal endoscopic findings. Because of geographic and national differences in the causes of both ulcer disease and non-ulcer dyspepsia, treatment that is beneficial in one country may be ineffective in another [12]. A meta-analysis of randomized controlled studies with 12-month follow-up found that H. pylori eradication therapy is associated with improvement of dyspeptic symptoms in patients with FD, which is consistently demonstrated in the Asian (OR = 1.54; 95% CI: 1.07–2.21), European (OR = 1.49; 95% CI: 1.10–2.02), and American (OR = 1.43; 95% CI: 1.12–1.83) populations [13].
In 1998, Blum et al. [14] conducted a double-blind, multicenter trial of patients with H. pylori infection and dyspeptic symptoms. Patients were randomly assigned to seven days of treatment with omeprazole, amoxicillin, and clarithromycin or with omeprazole alone and then followed up for 1 year. After 12 months, gastritis had healed in 75% of the patients in the group given omeprazole and antibiotics and in 3% of the patients in the omeprazole group (p < 0.001); the respective rates of H. pylori eradication were 79% and 2%. In the group given omeprazole and antibiotics, the rate of treatment success among patients with persistent H. pylori infection was similar to that among patients in whom the infection was eradicated (26% vs. 31%). There were no significant differences between the groups in the quality of life after treatment. They concluded that in patients with non-ulcer dyspepsia, the eradication of H. pylori infection is not likely to relieve symptoms [14].
In 1999, Talley et al. [15] studied the effect of H. pylori eradication in patients with non-ulcer dyspepsia. They randomly assigned 170 H. pylori-infected patients with non-ulcer dyspepsia to receive twice-daily treatment with omeprazole, amoxicillin, and clarithromycin for 14 days and 167 such patients to receive identical-appearing placebos; all patients were then followed through regular visits for 12 months. At 12 months, there was no significant difference between groups in the rate of successful treatment (46% in the active-treatment group and 50% in the placebo group; p = 0.56). There was also no significant difference in the rate of successful treatment at 12 months between patients who were H. pylori-negative and those who were H. pylori-positive (48% vs. 49%). The rates of successful treatment were also similar when patients were analyzed according to the type of dyspepsia (ulcer-like, reflux-like, or dysmotility-like) and changes in the quality of life. There was no significant association between treatment success and histologic improvement in chronic gastritis at 12 months (p = 0.68). They found no evidence that curing H. pylori infection in patients with non-ulcer dyspepsia leads to relief of symptoms [15]. In the same year, Talley et al. [16] conducted a multicentre, randomized, double-blind, placebo-controlled trial (ORCHID study) of 278 patients infected with H. pylori who had FD. Helicobacter pylori was eradicated in 85% in the treatment group and 4% in the placebo group. At 12 months follow-up there was no significant difference between the proportion of patients treated successfully by intention to treat in the eradication arm (24%, 95% confidence interval 17% to 32%) and the proportion of patients treated successfully by intention to treat in the placebo group (22%, 15% to 30%). Changes in symptom scores and quality of life did not significantly differ between the treatment and placebo groups [16].
The inflammatory response induced by H. pylori may lower the discomfort threshold to gastric distension by causing alterations in the enteric or central nervous system [8]. However, in at least one study, H. pylori positive and negative patients with FD had no difference in the perception of mechanically induced gastric distension [17]. There is evidence from randomized controlled trials that eradication of H. pylori results in relief of dyspepsia in a minority of patients. However, studies have failed to establish a temporal relationship between H. pylori infection and FD, or the association of H. pylori with a specific symptom complex [8]. Therefore, relief of dyspepsia may reflect other factors such as cure of unrecognized peptic ulcer disease in patients misdiagnosed with FD.
Previous double-blind, randomized studies performed elsewhere in the world have shown a significant benefit in that direction. In 1998, McColl et al. [18] performed a randomized, placebo-controlled trial comparing the efficacy of treatment for 2 weeks with omeprazole, amoxicillin, and metronidazole (160 patients) with that of omeprazole alone (158 patients) for resolving symptoms of dyspepsia in patients with H. pylori infection but no evidence of ulcer disease on upper gastrointestinal endoscopy. One month after the completion of treatment, 88% of the group assigned to receive omeprazole and antibiotics had a negative test for H. pylori, as compared with 5% of the group assigned to receive omeprazole alone. One year later, dyspepsia had resolved in 21% of the group given omeprazole and antibiotics, as compared with 7% of the group given omeprazole alone (95% confidence interval for the difference, 7 to 22%; p < 0.001). Among the patients in the group given omeprazole and antibiotics, the symptoms resolved in 27% who had had symptoms for 5 years or less, as compared with 12% who had had symptoms for more than 5 years (p = 0.03). They concluded that in patients with H. pylori infection and non-ulcer, or functional, dyspepsia, treatment with omeprazole and antibiotics to eradicate the infection is more likely to resolve symptoms than treatment with omeprazole alone [18].
A systematic review of 17 randomized controlled trials included 3566 patients with functional dyspepsia. Eradication of H. pylori was associated with a small but significant benefit, with treatment of 14 patients needed to cure 1 case of functional dyspepsia (relative risk reduction (RRR) = 10%, 95% CI: 6–14) [19]. In a subsequent randomized control trial, 404 patients with functional dyspepsia and H. pylori were randomized to treatment of H. pylori or placebo [19]. At 12-month follow-up, patients treated with H. pylori eradication therapy were significantly more likely to have symptomatic improvement as compared with controls (49% vs. 36%) [19].
Another possibility for evaluating the role of H. pylori in FD is to consider symptom improvement resulting from cure of both the infection and associated inflammation of the gastric mucosa after eradication treatment [20]. Functional dyspepsia treatment trials, due to suboptimal design or unclear presentation of data, have rarely been able to provide unequivocal evidence of the efficacy of a given treatment. Based on a systematic overview of published studies, Veldhuyzen van Zanten et al. [21] evaluated drug treatment of patients with FD (including H. pylori positive individuals) and provided guidelines for future trials. Fifty-two eligible studies were evaluated. Many studies suffered from important weaknesses in study design and execution. Only 5 studies used previously validated outcome measures. They concluded that because of suboptimal design and/or unclear presentation of the data, none of the trials provided unequivocal evidence that there is efficacious therapy for the treatment of FD [21]. Of the 16 trials included in the analysis by Talley, eight indicated that H. pylori eradication treatment had a beneficial effect on FD, but the other eight studies did not show any important benefit [22].
In conclusion, in this study, we assessed the clinical benefits of the eradication of H. pylori infection in patients with FD. The results of our study provide no evidence that H. pylori eradication leads to relief of symptoms 12 months after treatment, and there is a need for further studies.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–79. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 2.Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol. 2006;12:2661–6. doi: 10.3748/wjg.v12.i17.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talley NJ. American Gastroenterological Association. American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterology. 2005;129:1753–5. doi: 10.1053/j.gastro.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Chey WD, Wong BC. Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–25. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 5.Moayyedi P, Soo S, Deeks JJ, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2011;2:CD002096. doi: 10.1002/14651858.CD002096. [DOI] [PubMed] [Google Scholar]
- 6.Malekzadeh R, Mohamadnejad M, Siavoshi F, Massarrat S. Treatment of Helicobacter pylori infection in Iran: low efficacy of recommended western regimens. Arch Iranian Med. 2004;7:1–8. [Google Scholar]
- 7.El-Omar EM, Banerjee S, Wirz A, McColl KE. The Glasgow Dyspepsia Severity Score – a tool for the global measurement of dyspepsia. Eur J Gastroenterol Hepatol. 1996;8:967–71. doi: 10.1097/00042737-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Talley NJ, Hunt RH. What role does Helicobacter pylori play in dyspepsia and nonulcer dyspepsia? Arguments for and against H. pylori being associated with dyspeptic symptoms. Gastroenterology. 1997;113:S67. doi: 10.1016/s0016-5085(97)80016-9. [DOI] [PubMed] [Google Scholar]
- 9.Minocha A, Mokshagundam S, Gallo SH, Rahal PS. Alterations in upper gastrointestinal motility in Helicobacter pylori-positive nonulcer dyspepsia. Am J Gastroenterol. 1994;89:1797–800. [PubMed] [Google Scholar]
- 10.Bazzoli F, Palli D, Zagari RM, et al. The Loiano-Monghidoro population-based study of Helicobacter pylori infection: prevalence by 13C-urea breath test and associated factors. Aliment Pharmacol Ther. 2001;15:1001–7. doi: 10.1046/j.1365-2036.2001.00972.x. [DOI] [PubMed] [Google Scholar]
- 11.Bazzoli F, De Luca L, Pozzato P, et al. Helicobacter pylori and functional dyspepsia: review of previous studies and commentary on new data. Gut. 2002;50(Suppl 4):iv33–5. doi: 10.1136/gut.50.suppl_4.iv33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McColl KE. Absence of benefit of eradicating Helicobacter pylori in patients with nonulcer dyspepsia. N Engl J Med. 2000;342:589–90. doi: 10.1056/NEJM200002243420814. [DOI] [PubMed] [Google Scholar]
- 13.Zhao B, Zhao J, Cheng WF, et al. Efficacy of Helicobacter pylori eradication therapy on functional dyspepsia: a meta-analysis of randomized controlled studies with 12-month follow-up. J Clin Gastroenterol. 2014;48:241–7. doi: 10.1097/MCG.0b013e31829f2e25. [DOI] [PubMed] [Google Scholar]
- 14.Blum AL, Talley NJ, O'Moráin C, et al. Lack of effect of treating Helicobacter pylori infection in patients with nonulcer dyspepsia. Omeprazole plus Clarithromycin and Amoxicillin Effect One Year after Treatment (OCAY) Study Group. N Engl J Med. 1998;339:1875–81. doi: 10.1056/NEJM199812243392602. [DOI] [PubMed] [Google Scholar]
- 15.Talley NJ, Vakil N, Ballard ED, 2nd, Fennerty MB. Absence of benefit of eradicating Helicobacter pylori in patients with nonulcer dyspepsia. N Engl J Med. 1999;341:1106–11. doi: 10.1056/NEJM199910073411502. [DOI] [PubMed] [Google Scholar]
- 16.Talley NJ, Janssens J, Lauritsen K, Rácz I, Bolling-Sternevald E. Eradication of Helicobacter pylori in functional dyspepsia: randomised double blind placebo controlled trial with 12 months’ follow up. The Optimal Regimen Cures Helicobacter Induced Dyspepsia (ORCHID) Study Group. BMJ. 1999;318:833–7. doi: 10.1136/bmj.318.7187.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mearin F, de Ribot X, Balboa A, et al. Does Helicobacter pylori infection increase gastric sensitivity in functional dyspepsia? Gut. 1995;37:47–51. doi: 10.1136/gut.37.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McColl K, Murray L, El-Omar E, et al. Symptomatic benefit from eradicating Helicobacter pylori infection in patients with nonulcer dyspepsia. N Engl J Med. 1998;339:1869–74. doi: 10.1056/NEJM199812243392601. [DOI] [PubMed] [Google Scholar]
- 19.Moayyedi P, Soo S, Deeks J, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2005;1:CD002096. doi: 10.1002/14651858.CD002096.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Mazzoleni LE, Sander GB, Francesconi CF, et al. Helicobacter pylori eradication in functional dyspepsia: HEROES trial. Arch Intern Med. 2011;171:1929–36. doi: 10.1001/archinternmed.2011.533. [DOI] [PubMed] [Google Scholar]
- 21.Veldhuyzen van Zanten SJ, Cleary C, Talley NJ, et al. Drug treatment of functional dyspepsia: a systematic analysis of trial methodology with recommendations for design of future trials. Am J Gastroenterol. 1996;91:660–73. [PubMed] [Google Scholar]
- 22.Talley NJ. A critique of therapeutic trials in Helicobacter pylori-positive functional dyspepsia. Gastroenterology. 1994;106:1174–83. doi: 10.1016/0016-5085(94)90007-8. [DOI] [PubMed] [Google Scholar]