Abstract
Introduction
The aim of the study was to evaluate two methods of macroprolactin (MaPRL) detection – precipitation with polyethylene glycol (PEG) and ultrafiltration and to compare these techniques with “gold standard” – gel filtration chromatography (GFC).
Material and methods
The study was conducted on 245 patients – 45 with organic and 200 with functional hyperprolactinaemia. In all the subjects MaPRL was detected by precipitation with PEG and ultrafiltration. Additionally, gel filtration chromatography was performed in some of the serum samples.
Results
Macroprolactinaemia was detected in 27 patients – 8 with prolactinoma and 19 with functional hyperprolactinaemia. Assessing positive and negative results for MaPRL, we observed high diagnostic agreement (95.9%) and positive correlation (r = 0.506, p < 0.001) between the methods. The results of precipitation and ultrafiltration positive for MaPRL were concordant in 63%. The dominance of MaPRL detected with precipitation and/or ultrafiltration was confirmed by GFC in 76% of cases (all patients with functional hyperprolactinaemia). Among 6 examined patients with prolactinoma, GFC showed four false-positive results – 1 case of precipitation and 3 cases of ultrafiltration.
Conclusions
Efficacy of MaPRL detection with precipitation and ultrafiltration is comparable especially in cases of functional hyperprolactinaemia. In patients with prolactinoma, precipitation seems to be a more efficient separation method.
Keywords: prolactin, large forms, separation methods
Introduction
Prolactin (PRL) is a pituitary hormone which exists in human blood mainly in a monomeric form with molecular mass ∼23 kDa. Additionally, PRL occurs in complex forms which are big PRL (∼50 kDa) and big-big PRL, also called macroprolactin (MaPRL), when its molecular weight exceeds 100 kDa. In the majority of cases MaPRL consists of an antigen–antibody complex that is monomeric PRL and immunoglobulin G. However, it is not the biggest form of PRL in human serum – other forms having even a few times higher molecular mass (such as conglomerates of glycosylated PRL) have also been found. Macroprolactin has reduced biological activity because its large molecules make it difficult to cross through the capillary vessels and approach target cells and also connect with appropriate receptors [1–3]. Numerous studies have shown that in 10% to 45% of patients with hyperprolactinaemia the blood serum contains mainly MaPRL, and this clinical condition is termed macroprolactinaemia [4–8]. The diagnostic problem is that the assay systems used to measure PRL concentration recognize not only the monomeric form but also – to a variable extent – particles of MaPRL. Therefore, commercial immunoassays are classified as high-, medium- or low-reading tests towards MaPRL. Both macroprolactinaemia and relative sensitivity of the assays to macroprolactin may lead to the overestimation of laboratory results and to diagnostic mistakes [9–12].
The differentiation of PRL isoforms or the estimation of MaPRL molecules may be helpful in the proper evaluation of the hormone level. The method recognized as a gold standard for quantifying isoforms of PRL in serum is gel filtration chromatography (GFC), but the fact that it is time-consuming and the high cost of the procedure make this method useless in routine laboratory diagnostics [13]. The guidelines of the Pituitary Society and also the Polish Society of Endocrinology for the diagnosis and management of hyperprolactinaemia, which recommend taking MaPRL into consideration, indicate precipitation with polyethylene glycol (PEG) as a screening method for the detection of MaPRL [14, 15]. However, this relatively simple and cheap technique may interfere with some commercial PRL assays and lead to erroneous laboratory results [16, 17]. Because of this, alternative methods to separate MaPRL are still being sought. Recently, it has been shown that an ultrafiltration technique applying specific filtration membrane may be useful in MaPRL detection, but research concerning this issue is not common and its results are discordant [13, 16, 18, 19].
In the present study we performed the separation of MaPRL with an ultrafiltration and PEG precipitation technique. To verify the results of both methods we applied gel filtration chromatography for MaPRL detection.
Material and methods
Patients
The study was conducted on 245 patients (224 women and 21 men) hospitalized in the Department of Clinical Endocrinology, Medical University of Lodz. The inclusion criterion was the concentration of PRL exceeding 30 ng/ml measured in a fasting state. On the basis of clinical data and laboratory results, the following diagnoses were made: 45 cases of pituitary tumour (14 macroprolactinoma, 20 microprolactinoma and 11 non-functioning pituitary adenoma) and 200 patients with functional hyperprolactinaemia (idiopathic origin, a group of hyperandrogenic women with polycystic ovary morphology, primary hypothyroidism and drug-induced hyperprolactinaemia).
The study protocol was approved by the Bioethical Committee of the Medical University of Lodz (RNN/378/08/KB).
Prolactin immunoassay
Prolactin concentration was measured by enzyme-amplified chemiluminescent immunoassay (Immulite 1000, Siemens). The analytical sensitivity of the assay is 0.5 ng/ml. Intra-assay and inter-assay coefficients of variation (CV) are respectively 6.1% (PRL concentration: 6.3 ng/ml) and 9.6% (PRL concentration: 14.1 ng/ml). Reference ranges are 1.9–25.0 ng/ml for women and 2.5–17.0 ng/ml for men.
Precipitation with polyethylene glycol
Precipitation with PEG was performed similarly to the method proposed by Olukoga and Kane [6] and also followed the protocol recommended by Diagnostic Products Corporation (nowadays owned by Siemens) [20]. Equal volumes of PEG (Sigma) and serum were mixed. The solution was incubated at room temperature for 10 min and next centrifuged at 3000 rpm for 30 min. The supernatant obtained after this procedure was diluted 10-fold and checked for PRL concentration (PRLPEG). The result was compared with PRL concentration in 10-fold diluted, untreated serum (PRLtotal). In agreement with most literature data, we assumed that the recovery of monomeric prolactin in terms of the percentage ratio PRLPEG/PRLtotal equal to or below 40% means that MaPRL dominates in the serum samples (macroprolactinaemic subjects) [4, 6, 21, 22].
Ultrafiltration
The ultrafiltration process was performed according to the procedure described by Kavanagh-Wright [16, 23]. The serum samples (25 ml) were mixed with 475 ml of phosphate buffered saline (PBS) and placed in the upper part of a Microcon YM-100 unit equipped with filtrate membrane with a cut-off of 100 kDa (Millipore). The filtration unit with the serum sample was centrifuged at 3000 rpm for 45 min. Prolactin concentration was measured in the filterable fraction of serum. The recovery of monomeric hormone after ultrafiltration was calculated by the comparison of PRL concentration of ultrafiltrate (PRLUF) with PRL concentration of untreated serum (PRLtotal). On the basis of our statistical analysis (sensitivity, specificity and ROC curve), similar to our earlier study [24], we calculated that the cut-off point (recovery value) for the ultrafiltration method is the same as for the precipitation technique and is 40%.
Gel filtration chromatography
Gel filtration chromatography was performed on a Sephacryl 300HR column appropriate for proteins with a molecular mass of 10–1500 kDa. The separation column was previously calibrated with molecular weight markers (blue dextran – 2000 kDa, thyroglobulin – 669 kDa, apoferritin – 443 kDa, β-amylase – 200 kDa, alcohol dehydrogenase – 150 kDa, albumin – 66 kDa, carbonic anhydrase – 29 kDa). Using 50 mmol/l TRIS buffer (pH 7.40, 140 mmol/l NaCl, 1.25 mmol/l CaCl2, 0.50 mmol/l MgCl2) as an eluent, 1 ml fractions were collected at a flow rate of 0.5 ml/min. In the obtained fractions PRL concentrations were determined and the results were presented in the form of a curve. Macroprolactin and monomeric form of the hormone were quantified from the area under the peaks. Macroprolactinaemia (predominance of MaPRL in sample) was recognized if the serum contained more than 50% of MaPRL [5, 7, 25].
Repeatability
In order to determine the repeatability of precipitation and ultrafiltration methods, the separation of MaPRL was performed 15 times for each method in a pool of serum with the PRL concentration within (16.2 ng/ml) and above (57.8 ng/ml) the reference range. The within-run coefficients of the variation for precipitation and ultrafiltration were respectively 4.3 and 2.6% in normal serum and 4.9 and 5.3% in a sample with elevated PRL concentration.
Statistical analysis
The data obtained from the experiment were collected in Excel (MS Office 2007) worksheets. Basic descriptive statistics (mean, SE) were calculated. A statistical analysis was performed using one-way ANOVA followed by Fisher's test (LSD – least significant difference) according to the computer program Statistica 10 (licensed to the Medical University of Lodz). In the case of the analysis of data measured before and after PRL forms separation (precipitation and ultrafiltration methods) a pairwise test was applied. Additionally, the Pearson linear correlation coefficient (r) was determined. In the case of their statistical significance (p), the equation of regression was calculated (y = ax + b). Statistical differences between the tested values were at a significance level of p < 0.05.
Results
Comparison of results obtained with precipitation and ultrafiltration methods
The recovery values obtained after precipitation and/or ultrafiltration showed macroprolactinaemia (predominance of MaPRL in sample – MaPRL(+)) in 27 patients (11% – 21 women and 6 men) – in 8 persons with prolactinoma and in 19 subjects with functional hyperprolactinaemia. On the basis of precipitation results only, the dominance of MaPRL was shown in 21 (8.6%) persons and using the ultrafiltration method only, 23 (9.4%) cases of macroprolactinaemia were noted. In group of 27 subjects with predominance of MaPRL, results of both methods were in agreement in 17 (63%) subjects, mainly in the patients with functional hyperprolactinaemia. The characteristics of persons with macroprolactinaemia are presented in Tables I and II.
Table I.
Patients with organic hyperprolactinaemia – serum prolactin level and percentage of monomeric prolactin in sera after precipitation, ultrafiltration and gel filtration chromatography
| No. | Gender/age | PRL [ng/ml] | Recovery – PEG (%) | Recovery – UF (%) | mPRL-GFC (%) |
|---|---|---|---|---|---|
| 1 | M/22 | 6766 | 29 | 65 | 94 |
| 2 | M/52 | 939 | 79 | 29 | 65 |
| 3 | M/28 | 426 | 75 | 38 | 89 |
| 4 | F/28 | 233 | 65 | 38 | – |
| 5 | F/30 | 137 | 17 | 33 | 4.2 |
| 6 | F/27 | 110 | 40 | 34 | 18 |
| 7 | F/30 | 102 | 77 | 36 | 100 |
| 8 | F/31 | 69 | 18 | 38 | – |
PRL – Concentration of prolactin, mPRL – monomeric prolactin, PEG – precipitation with polyethylene glycol, UF – ultrafiltration, GFC – gel filtration chromatography.
Table II.
Patients with functional hyperprolactinaemia – serum prolactin level and percentage of monomeric prolactin in sera after precipitation, ultrafiltration and gel filtration chromatography
| No. | Gender/age | PRL [ng/ml] | Recovery – PEG (%) | Recovery – UF (%) | mPRL-GFC (%) |
|---|---|---|---|---|---|
| 1 | F/35 | 494 | 15 | 15 | 13 |
| 2 | F/34 | 313 | 15 | 26 | – |
| 3 | F/37 | 120 | 36 | 37 | 44 |
| 4 | F/45 | 113 | 74 | 23 | 19 |
| 5 | F/24 | 97 | 17 | 12 | – |
| 6 | F/25 | 83 | 6 | 62 | 32 |
| 7 | F/40 | 77 | 27 | 36 | – |
| 8 | F/18 | 77 | 18 | 16 | 14 |
| 9 | F/23 | 66 | 31 | 36 | 35 |
| 10 | F/41 | 61 | 20 | 27 | 20 |
| 11 | F/27 | 61 | 31 | 38 | 35 |
| 12 | F/20 | 55 | 28 | 40 | 29 |
| 13 | M/24 | 51 | 25 | 38 | – |
| 14 | M/25 | 48 | 33 | 43 | 44 |
| 15 | F/53 | 47 | 27 | 26 | – |
| 16 | F/22 | 47 | 39 | 37 | 26 |
| 17 | F/36 | 46 | 67 | 34 | – |
| 18 | M/34 | 34 | 27 | 56 | – |
| 19 | F/33 | 32 | 22 | 38 | – |
PRL – Concentration of prolactin, mPRL – monomeric prolactin, PEG – precipitation with polyethylene glycol, UF – ultrafiltration, GFC – gel filtration chromatography.
The recoveries of PRL after PEG precipitation and after ultrafiltration were compared by the test of linear regression. We noted a positive correlation between the two methods for the whole studied group (r = 0.5062, p < 0.001).
Performing a diagnostic concordance test (called also test effectiveness) between precipitation and ultrafiltration methods by comparison of positive [MaPRL(+)] and negative [MaPRL(–)] results for macroprolactinaemia, we found high agreement (∼96%) between the methods (Table III).
Table III.
Number of MaPRL “positive” or “negative” results based on 40% criterion for both methods
| Ultrafiltration | Precipitation | ||
|---|---|---|---|
| MaPRL(+) | MaPRL(–) | ||
| MaPRL(+) | 17 (6.9%) | 6 (2.5%) | |
| MaPRL(–) | 4 (1.6%) | 218 (89.0%) | |
| Diagnostic concordance | 235/245 (95.9%) | ||
MaPRL(+) – Macroprolactinaemia, prolactin recovery ≤ 40%, MaPRL(–) – prolactin recovery > 40%.
Verification of precipitation and ultrafiltration results using gel filtration chromatography
Gel filtration chromatography was performed in 17/27 serum samples with macroprolactinaemia – 6 from patients with organic and 11 from patients with functional hyperprolactinaemia. Macroprolactin predominance was confirmed by GFC in 13 sera – in all the persons with functional hyperprolactinaemia and only in 2/6 patients with prolactinoma. Negative GFC results for MaPRL (lack of MaPRL predominance) were observed in the patients with prolactinoma – the results of compared techniques turned out to be false-positive in 1 case of precipitation and in as many as 3 cases of ultrafiltration. Sample curves showing the distribution of MaPRL and monomeric PRL are shown in Figures 1 and 2. Summing up, the results of precipitation and GFC were compatible in 15 cases, and the results of ultrafiltration and GFC were compatible in 12 cases (precipitation vs. GFC – 88% of concordant results, ultrafiltration vs. GFC – 71% of concordant results) (Tables I and II). We observed a positive correlation between GFC and precipitation results (r = 0.5855, p = 0.0124) and also between GFC and ultrafiltration results (r = 0.5297, p = 0.0271).
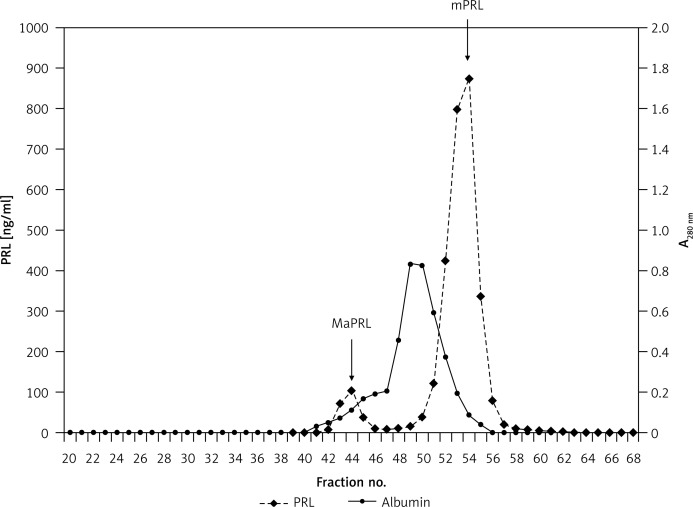
Figure 1.
Gel filtration chromatography of serum prolactin in a male patient – negative for significant macroprolactinaemia (patient 1, Table I)
PRL – Prolactin, MaPRL – macroprolactin, mPRL – monomeric prolactin.
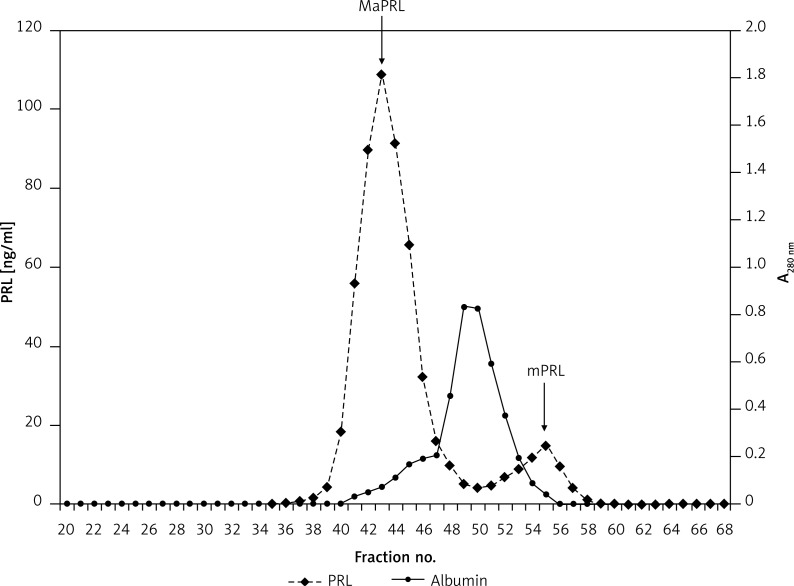
Figure 2.
Gel filtration chromatography of serum prolactin in a female patient with macroprolactinaemia (patient 1, Table II)
PRL – Prolactin, MaPRL – macroprolactin, mPRL – monomeric prolactin.
Discussion
Macroprolactinaemia occurs on average in 1/4 of patients with hyperprolactinaemia, and it is often one of the causes of elevated PRL concentration found by a laboratory test [5, 6, 25, 26]. Unfortunately, the antibodies that are components of PRL immunoassays recognize both hormone isoforms (monomeric PRL and MaPRL). An incorrect hormone value may lead to the implementation of treatment and/or doing studies which indeed are unnecessary [7, 27]. Therefore, a proper evaluation of MaPRL contents in serum is significant for the diagnosis of hyperprolactinaemia; however, it requires the use of an additional method. The most often applied separation technique is precipitation with PEG – but this method has the disadvantage of interfering with some immunoassays and precipitating MaPRL together with some amounts of monomeric PRL [6, 11, 17, 28]. A few authors suggest that ultrafiltration based on the physical separation of high weight molecules such as big-big PRL from the smaller isoforms of hormone may be useful in screening for MaPRL. In the literature there are only a few papers referring to the detection of MaPRL with the ultrafiltration method, and their data concerning efficacy of this technique in MaPRL separation are rather discordant [13, 16, 18, 19]. For that reason, we decided to compare both the above-mentioned MaPRL detection methods, and we evaluated one, described earlier by others, variant of each technique [6, 16].
In our study the frequency of macroprolactinaemia detected with both the methods is 11%, so it is rather low in comparison with other data [7, 27, 29]. But when we assumed that in, at least, every tenth patient with hyperprolactinaemia the treatment may be incorrect because of MaPRL dominance, it seems to be a significant diagnostic problem. Similarly to other studies, we found that the biggest group with macroprolactinaemia consists of patients with functional hyperprolactinaemia [25, 29]. Noteworthy is the fact that the great majority of these patients had previously diagnosed idiopathic origin of PRL elevation. It was confirmed also in other papers that some cases of idiopathic hyperprolactinaemia may be explained by the presence of large amounts of MaPRL in the blood [29, 30].
The comparison of recovery values obtained with precipitation and ultrafiltration showed a positive correlation between them, which was also noted in another study [19]. The diagnostic concordance of the results of both the methods was especially high in persons with functional hyperprolactinaemia even if patients’ PRL levels ranged widely. It may implicate that the compatibility of results does not depend on the concentration of PRL. On the other hand, we observed the majority of discordant results in patients with prolactinoma and very high PRL levels. When analyzing these results, we took into account the fact that huge amounts of hormone particles may impede proper separation of isoforms. On the basis of our own previous results and other data, we suppose that false-positive results for MaPRL, on the one hand, may possibly be the result of co-precipitation by PEG of large amounts of monomeric PRL with even small numbers of MaPRL particles. On the other hand, the cause may be the blockage of the filtrate membrane's pores by numerous molecules of the hormone crowded together, which can falsely indicate macroprolactinaemia [16, 19, 24, 31].
To compare the results of each method with gel filtration chromatography we took into consideration the classification of the results based on particular established criteria. We observed that the results of precipitation with PEG show slightly higher accordance with GFC than the results of ultrafiltration (respectively 88% and 71%). The lower concordance of ultrafiltration with GFC was caused mainly by recovery values in patients with prolactinoma – in this group we obtained 50% false-positive results using ultrafiltration. The above-mentioned process of mutual blockage of pores in the filtrate membrane by particles of PRL may be a possible explanation. Also, other authors have described false-positive results of ultrafiltration in sera with small amounts of MaPRL, which also occured in our study [13, 16]. Therefore, it seems that ultrafiltration may not be an adequate technique in patients with prolactinoma and a very high hormone concentration. However, both techniques are comparably effective in persons with functional disorders, who constitute a majority of patients with hyperprolactinaemia. Further studies will possibly confirm if ultrafiltration may be an alternative for precipitation, especially in laboratories equipped with immunoassay systems affected by PEG interference.
Irrespective of the method which should be used for separation of PRL isoforms, it is obvious that screening for MaPRL is necessary as a key element of laboratory assessment for hyperprolactinaemia.
In conclusion, the prevalence of macroprolactinaemia is rather high and is over 10%. Efficacy of MaPRL detection with precipitation and ultrafiltration is comparable, especially in cases of functional hyperprolactinaemia. In patients with organic hyperprolactinaemia (mainly prolactinoma) and usually with a very high PRL concentration, the results of PRL recovery after precipitation with polyethylene glycol show better diagnostic concordance with gel chromatography as compared to ultrafiltration results.
Acknowledgments
This study was supported by grant no. 503/5-020-02/503-01 of the Medical University of Lodz.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Leite V, Cosby H, Sobrinho LG, Fresnoza A, Santos AM, Friesen HG. Characterization of big, big prolactin in patients with hyperprolactinaemia. Clin Endocrin. 1992;37:365–72. doi: 10.1111/j.1365-2265.1992.tb02340.x. [DOI] [PubMed] [Google Scholar]
- 2.Hattori N, Inagaki C. Anti-prolactin (PRL) autoantibodies cause asymptomatic hyperprolactinemia: bioassay and clearance studies of PRL-immunoglobulin G complex. J Clin Endocrinol Metab. 1997;82:3107–3110. doi: 10.1210/jcem.82.9.4250. [DOI] [PubMed] [Google Scholar]
- 3.Hattori N, Nakayama Y, Kitagawa K, Li T, Inagaki C. Development of anti-PRL (prolactin) autoantibodies by homologous PRL in rats: a model for macroprolactinemia. Endocrinology. 2007;148:2465–70. doi: 10.1210/en.2006-1208. [DOI] [PubMed] [Google Scholar]
- 4.Fahie-Wilson MN, Soule SG. Macroprolactinaemia: contribution to hyperprolactinaemia in a district general hospital and evaluation of a screening test based on precipitation with polyethylene glycol. Ann Clin Biochem. 1997;34:252–8. doi: 10.1177/000456329703400305. [DOI] [PubMed] [Google Scholar]
- 5.Vieira JG, Tachibana TT, Obara LH, Maciel RM. Extensive experience and validation of polyethylene glycol precipitation as a screening method for macroprolactinemia. Clin Chem. 1998;44:1758–9. [PubMed] [Google Scholar]
- 6.Olukoga AO, Kane JW. Macroprolactinaemia: validation and application of the polyethylene glycol precipitation test and clinical characterization of the condition. Clin Endocrinol. 1999;51:119–26. doi: 10.1046/j.1365-2265.1999.00757.x. [DOI] [PubMed] [Google Scholar]
- 7.Vallette-Kasic S, Morange-Ramos I, Selim A, et al. Macroprolactinemia revisited: a study on 106 patients. J Clin Endocrinol Metab. 2002;87:581–8. doi: 10.1210/jcem.87.2.8272. [DOI] [PubMed] [Google Scholar]
- 8.Chawla R, Antonios T, Berhanu E, Ayana G. Detection of macroprolactinemia and molecular characterization of prolactin isoforms in blood samples of hyperprolactinemic women. J Med Biochem. 2012;31:19–26. [Google Scholar]
- 9.Seth J, Sturgeon CM, Ellis AR, Al-Sadie R, Logan M. UK NEQAS for peptide hormones and related substances. Annu Rev. 1998:A1–4. (Appendix) [Google Scholar]
- 10.Fahie-Wilson MN, Ellis AR, Seth J. Macroprolactin – a major problem in immunoassays for prolactin. Clin Chem. 1999;45:A83. [Google Scholar]
- 11.Smith TP, Suliman AM, Fahie-Wilson MN, McKenna TJ. Gross-variability in the detection of prolactin in sera containing big big prolactin (macroprolactin) by commercial immunoassays. J Clin Endocrinol Metab. 2002;87:5410–5. doi: 10.1210/jc.2001-011943. [DOI] [PubMed] [Google Scholar]
- 12.McKenna TJ, Smith T. Hyperprolactinemia due to macroprolactin: a commonly unrecognized phenomenon causing misdiagnosis and mismanagement. Endocrinologist. 2008;18:249–54. [Google Scholar]
- 13.Prazeres S, Santos MA, Ferreira HG, Sobrinho LG. A practical method for the detection of macroprolactinaemia using ultrafiltration. Clin Endocrinol. 2003;58:686–90. doi: 10.1046/j.1365-2265.2003.01721.x. [DOI] [PubMed] [Google Scholar]
- 14.Zgliczyński W, Zdunowski P. Hiperprolactinaemia – pitfalls in PRL assessment. Endokrynol Pol. 2005;6:980–5. [PubMed] [Google Scholar]
- 15.Casanueva FF, Molitch ME, Schlechte JA, et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol. 2006;65:265–73. doi: 10.1111/j.1365-2265.2006.02562.x. [DOI] [PubMed] [Google Scholar]
- 16.Kavanagh L, McKenna TJ, Fahie-Wilson MN, Gibney J, Smith TP. Specificity and clinical utility of methods for the detection of macroprolactin. Clin Chem. 2006;52:1366–72. doi: 10.1373/clinchem.2005.065854. [DOI] [PubMed] [Google Scholar]
- 17.Beltran L, Fahie-Wilson MN, McKenna TJ, Kavanagh L, Smith TP. Serum total prolactin and monomeric prolactin reference intervals determined by precipitation with polyethylene glycol: evaluation and validation on common immunoassay platforms. Clin Chem. 2008;54:1673–81. doi: 10.1373/clinchem.2008.105312. [DOI] [PubMed] [Google Scholar]
- 18.Quinn AM, Rubinas TC, Garbincius CJ, Holmes EW. Determination of ultrafilterable prolactin elimination of macroprolactin interference with a monomeric prolactin-selective sample pretreatment. Arch Pathol Lab Med. 2006;130:1807–12. doi: 10.5858/2006-130-1807-DOUPEO. [DOI] [PubMed] [Google Scholar]
- 19.Landberg E, Wahlberg J, Rydén I, Arvidsson BM, Ekman B. Detection of molecular variants of prolactin in human serum, evaluation of a method based on ultrafiltration. Clin Chim Acta. 2007;376:220–5. doi: 10.1016/j.cca.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 20.Biuletyn techniczny firmy DPC. Influence of macroprolactin on the results of Prolactin Immulite/Immulite 2000 assays; Methods of elimination. [Google Scholar]
- 21.Kostrzak A, Męczekalski B. Macroprolactinaemia. Pol Merk Lek. 2010;29:47–9. [PubMed] [Google Scholar]
- 22.Elenkova CA, Genov N, Abadzhieva Z, et al. Macroprolactinemia in patients with prolactinomas: prevalence and clinical significance. Exp Clin Endocrinol Diabetes. 2013;121:201–5. doi: 10.1055/s-0032-1333232. [DOI] [PubMed] [Google Scholar]
- 23.Kavanagh-Wright L, Smith TP, Gibney J, et al. Characterization of macroprolactin and assessment of markers of autoimmunity in macroprolactinaemic patients. Clin Endocrinol. 2009;70:599–605. doi: 10.1111/j.1365-2265.2008.03402.x. [DOI] [PubMed] [Google Scholar]
- 24.Beda-Maluga K, Pisarek H, Komorowski J, Pawlikowski M, Świętosławski J, Winczyk K. The detection of macroprolactin by precipitation and ultrafiltration methods. Pol J Endocrinol. 2011;62:537–44. [PubMed] [Google Scholar]
- 25.Cattaneo F, Kappeler D, Müller B. Macroprolactinaemia, the major unknown in the differential diagnosis of hyperprolactinaemia. Swiss Med Wkly. 2001;131:122–6. doi: 10.4414/smw.2001.06127. [DOI] [PubMed] [Google Scholar]
- 26.Hattori N, Nakayama Y, Kitagawa K, Ishihara T, Saiki Y, Inagaki C. Anti-prolactin (PRL) autoantibody-binding sites (epitopes) on PRL molecule in macroprolactinemia. J Endocrinol. 2006;190:287–93. doi: 10.1677/joe.1.06871. [DOI] [PubMed] [Google Scholar]
- 27.Beda K, Winczyk K. Macroprolactin – prevalence, diagnostic methods and clinical significance. Fol Med Lodz. 2009;36:87–110. [Google Scholar]
- 28.Gibney J, Smith TP, McKenna TJ. The impact on clinical practice of routine screening for macroprolactin. J Clin Endocrinol Metab. 2005;90:3927–32. doi: 10.1210/jc.2004-2234. [DOI] [PubMed] [Google Scholar]
- 29.Jeske W, Zdunowski P, Bartoszewicz Z, et al. Large molecular weight prolactin in patients with hyperprolactinaemia. Pol J Endocrinol. 2000;51:237–48. [Google Scholar]
- 30.Berinder K, Stackenäs I, Akre O, Hirschberg AL, Hulting AL. Hyperprolactinaemia in 271 women: up to three decades of clinical follow-up. Clin Endocrinol. 2005;63:450–5. doi: 10.1111/j.1365-2265.2005.02364.x. [DOI] [PubMed] [Google Scholar]
- 31.Fahie-Wilson M, Halsall D. Polyethylene glycol precipitation: proceed with care. Ann Clin Biochem. 2008;45:233–5. doi: 10.1258/acb.2008.007262. [DOI] [PubMed] [Google Scholar]