Abstract
Introduction
Autoimmune pemphigus diseases comprise several entities with serious prognoses, including the pemphigus vulgaris (PV) group and pemphigus foliaceus (PF) group. Antihypertensives are suspected to be one of the factors triggering/sustaining pemphigus. Here, the data of pemphigus patients regarding arterial hypertension (AH) and taking potentially noxious drugs were statistically analyzed in a setting of a Polish university dermatology department.
Material and methods
Medical histories of pemphigus patients (40 admissions of 24 female patients – 13 PV, 11 PF; and 102 admissions of 38 male patients – 24 PV, 14 PF), diagnosed at both immunopathological and biochemical-molecular levels, were studied.
Results
Ten of 16 (62.50%) AH-positive PV patients received known PV triggers/sustainers 11 times (1–3 per patient). Fourteen of 15 (93.33%) AH-positive PF patients received known PF triggers/sustainers 21 times (1–3 per patient). No differences in numbers of patients taking potentially culprit drugs were shown between PV and PF (Fisher's exact test: p = 0.0829; Yates’ χ2 test: p = 0.1048). The most frequently used culprit drugs were ramipril in PV and enalapril in PF. On average, each PV/PF AH-positive patient received 3.161 different antihypertensives in his/her history of admissions (2.155 antihypertensives per admission).
Conclusions
Drug triggering should be suspected in every case of newly diagnosed or exacerbated pemphigus, as eliminating possible PV/PF triggers/sustainers may alleviate the clinical symptoms and enable the decrease of dose/range of immunosuppressants regardless of pemphigus form. Eliminating possible drug PV/PF triggers/sustainers may alleviate the clinical symptoms and enable the decrease of dose/range of immunosuppressants regardless of pemphigus form.
Keywords: drug-induced, pemphigus, precipitating factors, hypertension, treatment
Introduction
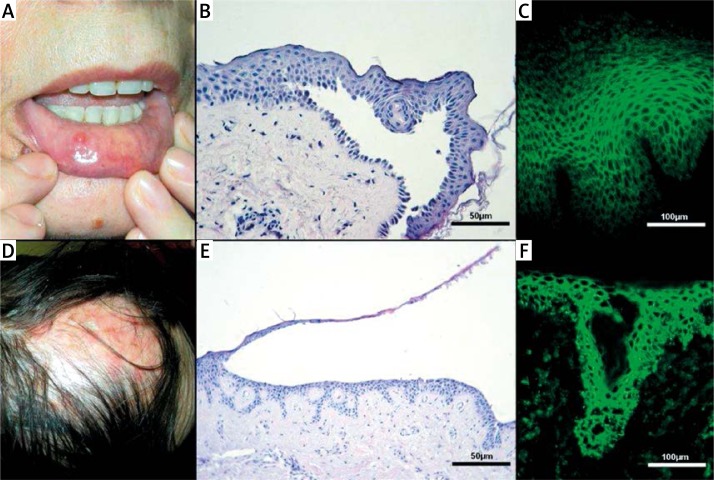
Pemphigus diseases comprise several entities with serious prognoses, including the pemphigus vulgaris (PV) (Figures 1 A–C) group and the pemphigus foliaceus (PF) (Figures 1 D–F) group. These diseases are characterized by autoimmunity to desmosomal cadherins: mucocutaneous PV is characterized by autoantibodies to desmoglein 3 (DSG3) and desmoglein 1 (DSG1), mucosal-dominant PV by autoantibodies to DSG3 and PF by autoantibodies to DSG1 [1]. The autoimmune response to these desmosomal structural proteins results in blistering by loss of cell adhesion in the epidermis and mucous membranes [2]. Numerous factors are thought to trigger/sustain PV/PF: drugs, malignancies, dietetic and environmental factors. The first case of drug-induced pemphigus (DIP), by penicillamine, was presented by Degos et al. in 1969 [3], whereas the first report on pemphigus induced by an antihypertensive (captopril) is dated 1980 [4]. Since that time a range of drugs has been reported to cause pemphigus, among them antihypertensives [3, 5–14]. It should be a matter of great concern, as this group of diseases affects mainly middle-aged and elderly people. According to the Framingham Heart Study, 60% of the population of sexagenarians develops arterial hypertension (AH), and the percentage increases to 65% of men and 75% of women aged 75 [15]. The HYVET study confirmed that AH treatment in the elderly reduces the risk of stroke and other cardiovascular events [16]. While internal medicine practitioners are somewhat conscious of the noxiousness of β-blockers in psoriasis, antihypertensive therapy in pemphigus poses a serious problem as the knowledge about relation of these dermatoses and antihypertensives is inadequate not only among hypertension specialists, but also among dermatology practitioners, who are not aware that recalcitrant or severe PV/PF, irresponsive to intensive treatment, may be caused by a harmful antihypertensive sustaining/triggering the disease. As many patients with DIP have tissue-bound as well as serum autoantibodies to DSG1 and/or DSG3 [17–19], the determination whether the disease is drug-induced or just drug-exacerbated is troublesome.
Figure 1.
A – An elderly woman taking ramipril with pemphigus vulgaris (PV) at active Th2 mediated stage. B – Suprabasal acantholysis of PV. “Row of tombstones” appearance of basal layer (H + E stain). C – Indirect immunofluorescence revealing serum IgG4 pemphigus antibodies. D – An elderly woman taking indapamide with pemphigus foliaceus (PF) relapse at active Th2 mediated stage. E – Subcorneal acantholytic separation of PF (H + E stain). F – Direct immunofluorescence of the perilesional skin revealing IgG4 pemphigus deposits in interfollicular and intrafollicular epithelium
Although the mechanism by which these agents trigger/sustain autoimmunity in DIP patients still remains a mystery, some theories propose a causative role of drug functional groups in DIP. There are some variations of subdivisions [3, 5, 20, 21], yet these chemicals can be assigned to four main groups: 1) containing a sulfhydryl (-SH) group – called thiols or mercaptans, 2) drugs with an active amide group, 3) phenolic drugs, 4) other non-thiol non-amide non-phenolic drugs. The authors often also distinguish “masked thiols” within the thiol group, as compounds that contain sulfur are potent in forming a -SH group during biotransformation.
Causal relations between PV/PF and drugs are based mainly on case reports and small-scale studies. Still, the knowledge of drug-disease associations arising from such literature may contribute to better care of patients by diminishing side-effects of the intensive treatment.
Here, the data of pemphigus patients regarding AH and taking potentially noxious drugs were statistically analyzed in the setting of a Polish university dermatology department.
Material and methods
Medical histories of pemphigus patients (40 admissions of 24 female patients – 13 PV, 11 PF; and 102 admissions of 38 male patients – 24 PV, 14 PF) diagnosed immunopathologically with direct immunofluorescence showing the pemphigus “fishing net” pattern of IgG deposition or “dew drops on spider web” pattern of IgG4 deposition in PV/PF and indirect immunofluorescence [2], and at the biochemical-molecular level (serum IgG anti-DSG1 and anti-DSG3 ELISA), admitted to the Department of Dermatology, Poznan University of Medical Sciences (Poznan, Poland) in the years 2005–2012, were analyzed.
Statistical analysis
Statistical analysis was performed with GraphPad software (Graph-pad InStat, USA).
Results
Of 142 admissions of PV/PF patients, 62 (42.96%) were patients with AH. Among 37 PV patients, 16 (43.24%) suffered from AH – 11 (29.73%) male, 5 (13.51%) female. Among 25 PF patients, 15 (60.00%) suffered from AH – 8 (32.00%) male, 7 (28.00%) female. The median number of admissions per patient was 2.708 (1–6 admissions) for PF and 1.947 (1–25 admissions) for PV. During PV/PF treatment, 3 (8.02%) PV and 3 (12.00%) PF male patients developed AH. Ten of 16 (62.50%) AH-positive PV patients received known PV-triggers/sustainers 11 times (1–3 per patient). Fourteen of 15 (93.33%) AH-positive PF-patients received known PF-triggers/sustainers 21 times (1–3 per patient). The differences in numbers of patients taking potentially culprit drugs in PV and PF were insignificant (GraphPad, USA) – Fisher's exact test: p = 0.0829; Yates’ χ2 test: p = 0.1048. The most frequently used culprit drugs were ramipril in PV and enalapril in PF (Table I). On average, each PV/PF AH-positive patient received 3.161 different antihypertensives in his/her history of admissions (2.155 antihypertensives per admission).
Table I.
Antihypertensives taken by pemphigus vulgaris/pemphigus foliaceus patients
| Antihypertensive/class of drug | Number of patients | |
|---|---|---|
| Pemphigus vulgaris | Pemphigus foliaceus | |
| β-Blockers | ||
| Bisoprolol | 4 | 5 |
| Angiotensin-converting enzyme inhibitors: | ||
| Captopril | 3* | 3* |
| Enalapril | 3* | 5* |
| Metoprolol | 5 | 2 |
| Perindopril | 3 | 1 |
| Ramipril | 5* | 2* |
| Angiotensin II receptor blockers: | ||
| Candesartan | 1 | 0* |
| Losartan | 4 | 1 |
| Valsartan | 1 | 1 |
| Calcium channel blockers: | ||
| Amlodipine | 4 | 5 |
| Thiazides and thiazide-like drugs: | ||
| Hydrochlorothiazide | 1 | 2* |
| Indapamide | 5 | 4* |
| Aldosterone antagonists: | ||
| Spironolactone | 4 | 2 |
| Epithelial sodium channel blockers: | ||
| Amiloride | 2 | 0 |
| Loop diuretics: | ||
| Furosemide | 1 | 3 |
| Torasemide | 1 | 1 |
DIP-associated drug according to literature.
Discussion
Pemphigus vulgaris and PF are severe autoimmune disease of mucous membranes and skin affecting predominantly middle-aged individuals and having numerous peculiarities of their clinical features [2, 6, 22–25]. Both diseases require immunosuppressive treatment, favorably with individualized drugs optimally tailored for each patient instead of non-selective glucocorticosteroids (GCSs) [26].
Many hypotheses could be coined how drugs could facilitate pemphigus. Interestingly, chemically and structurally diverse drugs may generally contribute to one pemphigus form. It was speculated that the clinical form and prognosis of DIP depend on mechanism of action of the drug [20]. Thiols usually induce PF, whereas non-thiols usually provoke PV, clinically indistinguishable from proper PV, which rarely remits after cessation of the drug [20]. Our study shows that the prevalence of drug-related PV and drug-related PF seems to be equal, yet the relation was based only on literature data and did not analyze possible diverse mechanisms of drug actions. For many years thiols were considered principal pemphigus-associated drugs, while in recent years the possibility of the offending role of amides has been raised [27, 28]. It is possible that drugs or their metabolites bind proteins, thus forming haptens stimulating T- and B-cell responses towards loss of self-tolerance or modify keratinocyte desmosomal autoproteins in a way that results in forming neoantigens. Inhibition of enzymes responsible for cell-cell adhesion (e.g. keratinocyte transglutaminase by thiols [29]), activation of acantholytic enzymes (e.g. acetylcholinesterase by thiol radical of angiotensin-converting enzyme inhibitors (ACEi) [7, 29, 30]) and cell adhesion disturbance by formation of thiol-cysteine bonds instead of cysteine-cysteine bonds could speculatively contribute to the loss of altered protein functionality [29, 31]. Also drug-associated autoimmunity may theoretically follow the shift in cytokine profile from Th1- to Th2-dependent [32, 33]. It was proposed that phenolic drugs could release tumor necrosis factor (TNF) and interleukin-1, indirectly influencing the plasminogen activator that participates in acantholysis [34, 35]. On the other hand, genetic factors must play a role in development of such autoimmunity, as not every patient treated with a suspected drug is prone to develop pemphigus, and not every drug with a suspected radical has been described as offensive (e.g. diltiazem, without any report on noxiousness in PV/PF, contains an amide group). The understanding of the impact of such drugs on the immune system in pemphigus is limited by the rarity of the disease itself. Thus, the biological model of drug-induced and drug-exacerbated pemphigus should be studied for further insight into this relationship. The fact that seems worth noting is that pemphigus does not develop naturally in mice. Furthermore, the set of mice desmosomal cadherins differs from the human set [36]. However, both diseases (PV/PF) naturally develop in dogs, and the relation of pemphigus to veterinary drugs seems not to be uncommon [37].
The active thiol group was the first chemical group suspected of inducing PV/PF. It is considered to bind DSGs, thus making them immunogenic [17–19, 38–40]. On the other hand, some researchers argue that thiol groups in vitro may alone cause acantholysis without raising an autoimmune response [41, 42]. Pemphigus-like autoantibodies may occur also in other conditions without PV/PF [8, 43–45], and such autoantibodies are sometimes absent in serum of DIP [8, 46]. In PV, autoantibodies to DSGs (mainly IgG1 and IgG4 subclasses of different tissue and antigenic specificity [47]) may be both pathogenic and nonpathogenic [48, 49]. It is an interesting issue as IgG4 autoantibodies, which are dominant in the active Th2-mediated stage of the disease, are heterobivalent – they possess the capacity of “Fab-arm exchange” with other IgG4 antibodies [47, 50]. This feature may contribute to the development of PV/PF due to the epitope spreading phenomenon [51], yet once the specific epitope is targeted, the epitope spreading seems not to influence the PV course [52].
ACEi, lowering intracapillary pressure by venodilatation [15], as confirmed in our study, seem to be the most often reported antihypertensive pemphigus-associated drugs in both PV (captopril [9, 19, 53], enalapril [10], fosinopril [9], cilazapril [11], quinapril [54], benazepril [55] and ramipril [6]) and PF (captopril [19, 56, 57], enalapril [58], fosinopril [31, 54], cilazapril [59], lisinopril [12], ramipril [60]). Some of the ACEi contain an active thiol group (captopril and zofenopril), some could possibly serve as masked thiols (e.g. spirapril), while all possess an active amide group in their compounds [27, 59, 61], which makes them potentially noxious for PV/PF patients. Interestingly, it has been shown that ACEi can induce circulating antibodies directed to antigens of the superficial epidermal cells in 52.38% of sera of non-pemphigus individuals [45]. These drug-induced PV/PF autoantibodies (e.g. with captopril) were shown to be characterized by the same antigenic specificity at a molecular level, as autoantibodies from PV/PF patients [19, 62].
Angiotensin II receptor blockers (ARBs, sartans), widely prescribed as a substitute for ACEi, were reported as possible triggers/sustainers in one case of PF in a woman taking candesartan and telmisartan [13]. It is speculated that these non-thiol, non-phenol drugs may induce loss of keratinocyte adhesion and autoantibody production via indirect immune mechanisms rather than via direct biochemical modifications of the antigens [13]. It may be advisable not to use these drugs for AH treatment in PF patients.
Calcium channel blockers (Ca-blockers) act by paralyzing the precapillary sphincter, thus causing dilatation of arterioles and increasing intracapillary pressure [15]. Among this group of antihypertensives, nifedipine was reported to be the culprit of both PV [63] and PF [64, 65]. It is debated whether individual predisposition to develop pemphigus or pemphigoid after nifedipine treatment depends on genetic factors [65, 66]. Ca-blockers may affect the desmoglein turnover, as they are desmosomal cadherins – calcium-dependent adhesive molecules [7]. There is also one case of PV in a pregnant woman treated with verapamil (non-dihydropyridine Ca-blocker) and methyldopa (aromatic-L-amino acid decarboxylase inhibitor, DOPA decarboxylase inhibitor), with an unconfirmed causative relation [67]. However, methyldopa has a phenol radical, which could place the drug in the non-thiol phenol drug category.
Thiazides, by inhibiting electrolyte transport at diluting sites of the cortex, decrease the glomerular filtration rate and increase the proximal water reabsorption. Indapamide, a thiazide-like diuretic antihypertensive drug, was reported as a possible cause of PF [14]. It is characterized by the presence of a sulfur atom and an amide radical [20]. There is only one report on PF with a possible role of a β-blocker-thiazide composite drug (bisoprolol-hydrochlorothiazide [68]).
The DIP issue can be complicated still further by increasingly frequent usage of combined antihypertensive medications which might contain DIP-associated drug/s and not DIP-associated drug/s. The fact that most patients use polypharmacotherapy and that the culprit drug often needs time to act makes tracking this peculiar medicament a challenge. The identification of DIP agents should be done ex vivo with a lymphocyte transformation test [69] or an interferon-γ (INF-γ) release test [55], while desmoglein immunolabeling [70] might be helpful for identifying DIP cases as such.
Oral GCSs and intravenous GCS pulse therapy, still the mainstay of pemphigus therapy in many centers, may lead to impaired glucose metabolism and may raise the blood pressure (BP). Some of the patients included in our retrospective study were repeatedly admitted to the ward with PV/PF aggravation until they had their antihypertensives changed and obtained temporary or permanent PV/PF remission. This study shows that AH was a serious problem in 42.96% of PV/PV patients, and about 10% of them developed AH at the time of treatment (due to the GCS or independently). Thus, the number of antihypertensives introduced per AH patient (on average, each PV/PF AH-positive patient received 3.161 different antihypertensives in his/her history of admissions) may interfere with the disease course. This may lead to a vicious circle, as high BP treated with a noxious antihypertensive aggravates PV/PF symptoms, and leads to enhancing the dose of GCSs to control the disease, yet raising the BP. Therefore, the clinician may be misled, expecting that a higher dose of antihypertensive or additional drug will lower the BP. The inadequate antihypertensive worsens the patient's state if the drug is a known PV/PF trigger/sustainer. Thus, it is worth emphasizing that elimination of possible triggers/sustainers in PV/PF patients may alleviate the clinical manifestations and enable reduction of the dose/range of medications [5] regardless of PV/PF form. To support this suggestion, the AH-positive PV patient included in this study, after cessation of ramipril, still remains in clinical and molecular long-lasting remission without any maintenance treatment [6]. Another patient drinking red wine, as it contains apparently beneficial natural red wine phenols, e.g. resveratrol, for years, who developed PV, also remains in long-lasting remission, after cessation of red wine intake, on just low dose oral GCS (alternate day 4 mg methylprednisolone) [71].
Importantly, drugs containing the same active substances as their brand name products vary in descriptions of side effects provided by the producers – some do list the PV/PF while others do not, which does not help the clinician to make a wise choice of the harmless antihypertensive. As every day new articles appear in biomedical databases, it is reasonable to check in each case of PV/PF possible triggers and replace all potentially offending drugs with those unreported in published DIP cases to optimize management of the patient according to accepted strategies [72].
Still, there might be multifactorial, e.g. malignancy and antihypertensives, causes of pemphigus triggering/exacerbation, as apparently in an elderly woman with a history of taking ramipril and indapamide with relapsing PF in whom low grade appendiceal mucinous neoplasm (pseudomyxoma peritonei) with metastasis to the right ovary and omentum was diagnosed [73].
We propose here that the still inadequately charted issue of DIP should be subdivided at least into the five categories: triggering of pemphigus autoimmunity by DIP-associated drugs without clinically overt pemphigus, DIP triggered exclusively by DIP-associated drugs, DIP triggered multifactorially including DIP-associated drugs, pemphigus triggered by non-DIP-associated drugs with the course modified by DIP-associated drugs, and idiopathic pemphigus with the course modified by DIP-associated drugs. Such a categorization might benefit patients, as they have variable severity and prognosis of their pemphigus, by individualizing their management strategies.
In conclusion, drug triggering, among other factors, should be suspected in every case of newly diagnosed or exacerbated pemphigus. PV-related antihypertensives constitute ACEi, Ca-blockers and methyldopa, while PF-related antihypertensives are ACEi, ARBs, Ca-blockers, thiazide-like diuretics and β-blocker-thiazide combinations. The AH is a serious problem in nearly half of PV/PF patients, and our data suggest that about 10% of them may develop AH during PV/PF treatment. The choice of proper antihypertensives in PV/PF patients in every case should be supported by up-to-date medical literature, as new data on pemphigus triggering constantly appear.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Amagai M. Autoimmunity against desmosomal cadherins in pemphigus. J Dermatol Sci. 1999;20:92–102. doi: 10.1016/s0923-1811(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 2.Dmochowski M. Wydawnictwo Naukowe Akademii Medycznej im. Poznan: Karola Marcinkowskiego; 2006. Autoimmune blistering dermatoses. [Google Scholar]
- 3.Wolf R, Tamir A, Brenner S. Drug-induced versus drug-triggered pemphigus. Dermatology. 1991;182:207–10. doi: 10.1159/000247795. [DOI] [PubMed] [Google Scholar]
- 4.Parfrey PS, Clement M, Vandenburg MJ, Wright P. Captopril-induced pemphigus. Br Med J. 1980;281:194. doi: 10.1136/bmj.281.6234.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner S, Goldberg I. Drug-induced pemphigus. Clin Dermatol. 2011;29:455–7. doi: 10.1016/j.clindermatol.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Pietkiewicz P, Gornowicz-Porowska J, Bowszyc-Dmochowska M, Dmochowski M. The chancre of pemphigus on the scalp as the first symptom of mucosal-dominant pemphigus vulgaris in an elderly man taking ramipril. Dermatol Klin. 2011;13:235–8. [Google Scholar]
- 7.Ruocco V, De Angelis E, Lombardi ML. Drug-induced pemphigus. II. Pathomechanisms and experimental investigations. Clin Dermatol. 1993;11:507–13. doi: 10.1016/0738-081x(93)90158-9. [DOI] [PubMed] [Google Scholar]
- 8.Ruocco V, Sacerdoti G. Pemphigus and bullous pemphigoid due to drugs. Int J Dermatol. 1991;30:307–12. doi: 10.1111/j.1365-4362.1991.tb03867.x. [DOI] [PubMed] [Google Scholar]
- 9.Parodi A, Cozzani E, Milesi G, Drosera M, Rebora A. Fosinopril as a possible pemphigus-inducing drug. Dermatology (Basel) 2002;204:139–41. doi: 10.1159/000051833. [DOI] [PubMed] [Google Scholar]
- 10.Adriano AR, Gomes Neto A, Hamester GR, Nunes DH, Di Giunta G. Pemphigus vegetans induced by use of enalapril. An Bras Dermatol. 2011;86:1197–200. doi: 10.1590/s0365-05962011000600023. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg I, Sasson A, Gat A, Srebrnik A, Brenner S. Pemphigus vulgaris triggered by glibenclamide and cilazapril. Acta Dermatovenerol Croat. 2005;13:153–5. [PubMed] [Google Scholar]
- 12.Patterson CRS, Davies MG. Pemphigus foliaceus: an adverse reaction to lisinopril. J Dermatolog Treat. 2004;15:60–2. doi: 10.1080/09546630310013379. [DOI] [PubMed] [Google Scholar]
- 13.Bae YI, Yun SJ, Lee SC, Park GT, Lee JB. Pemphigus foliaceus induced by an angiotensin II receptor blocker. Clin Exp Dermatol. 2008;33:721–3. doi: 10.1111/j.1365-2230.2008.02857.x. [DOI] [PubMed] [Google Scholar]
- 14.Bayramgürler D, Erçin C, Apaydin R, Unal G. Indapamide-induced pemphigus foliaceus. J Dermatolog Treat. 2001;12:175–7. doi: 10.1080/09546630152608320. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhry KN, Chavez P, Gasowski J, Grodzicki T, Messerli FH. Hypertension in the elderly: some practical considerations. Cleve Clin J Med. 2012;79:694–704. doi: 10.3949/ccjm.79a.12017. [DOI] [PubMed] [Google Scholar]
- 16.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–98. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 17.Bialy-Golan A, Brenner S. Penicillamine-induced bullous dermatoses. J Am Acad Dermatol. 1996;35:732–42. doi: 10.1016/s0190-9622(96)90729-x. [DOI] [PubMed] [Google Scholar]
- 18.Mutasim DF, Pelc NJ, Anhalt GJ. Drug-induced pemphigus. Dermatol Clin. 1993;11:463–71. [PubMed] [Google Scholar]
- 19.Korman NJ, Eyre RW, Zone J, Stanley JR. Drug-induced pemphigus: autoantibodies directed against the pemphigus antigen complexes are present in penicillamine and captopril-induced pemphigus. J Invest Dermatol. 1991;96:273–6. doi: 10.1111/1523-1747.ep12464471. [DOI] [PubMed] [Google Scholar]
- 20.Schmutz JL, Barbaud A, Trechot P. Indapamide-induced pemphigus foliaceus a sulfurous affair? [French] Ann Dermatol Venereol. 2002;129:1085. [PubMed] [Google Scholar]
- 21.Yamamoto T, Takata-Michigami M, Hisamatsu Y, et al. A prospective analysis of anti-desmoglein antibody profiles in patients with rheumatoid arthritis treated with thiol compounds. J Dermatol Sci. 2010;59:170–5. doi: 10.1016/j.jdermsci.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Wilson CL, Dean D, Wojnarowska F. Pemphigus and the terminal hair follicle. J Cutan Pathol. 1991;18:428–31. doi: 10.1111/j.1600-0560.1991.tb01379.x. [DOI] [PubMed] [Google Scholar]
- 23.Dańczak-Pazdrowska A, Bowszyc-Dmochowska M, Wolnik-Trzeciak G, Dmochowski M. IgG, IgG1 and IgG4 deposits in hair follicles in relation to IgG, IgG1 and IgG4 antibodies to desmogleins in pemphigus. Dermatol Klin. 2004;6:207–13. [Google Scholar]
- 24.Dmochowski M. Body orifices and mucous membranes in pemphigus vulgaris. Dermatol Klin. 2007;9:124–31. [Google Scholar]
- 25.James KA, Culton DA, Diaz LA. Diagnosis and clinical features of pemphigus foliaceus. Dermatol Clin. 2011;29:405–12. doi: 10.1016/j.det.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietkiewicz P, Torz M, Gornowicz-Porowska J, Bowszyc-Dmochowska M, Dmochowski M. Contemporary methods of treatment of pemphigus vulgaris from the point of view of a junior physician. Dermatol Klin. 2012;14:75–81. [Google Scholar]
- 27.Wolf R, Brenner S. An active amide group in the molecule of drugs that induce pemphigus: a casual or causal relationship? Dermatology (Basel) 1994;189:1–4. doi: 10.1159/000246749. [DOI] [PubMed] [Google Scholar]
- 28.Lo Schiavo A, Sangiuliano S, Puca R, Brunetti G, Ruocco E. Pemphigus relapse and acetazolamide, a drug with an active amide group: a casual or causal relationship? J Eur Acad Dermatol Venereol. 2009;23:716–7. doi: 10.1111/j.1468-3083.2009.03170.x. [DOI] [PubMed] [Google Scholar]
- 29.Scott MD, Davis D, Soderberg KI. Drug-induced pemphigus; http://emedicine.medscape.com/article/1063684-overview. [Google Scholar]
- 30.Baroni A, Buommino E, Ruocco E, et al. Captopril modulates acetylcholinesterase in human keratinocytes. Arch Dermatol Res. 2011;303:491–7. doi: 10.1007/s00403-011-1124-1. [DOI] [PubMed] [Google Scholar]
- 31.Dobrosavljević Vukojević D, Stojković Filipović J, Sjerobabin M, Vuković J, Vesić S. Lisinopril-induced pemphigus foliaceus in a patient with diabetes mellitus and Kaposi-Juliusberg varicelliform eruption. Serb J Dermatol Venereol. 2012;4:153–62. [Google Scholar]
- 32.Caproni M, Giomi B, Cardinali C, et al. Further support for a role for Th2-like cytokines in blister formation of pemphigus. Clin Immunol. 2001;98:264–71. doi: 10.1006/clim.2000.4974. [DOI] [PubMed] [Google Scholar]
- 33.Newby CS, Barr RM, Greaves MW, Mallet AI. Cytokine release and cytotoxicity in human keratinocytes and fibroblasts induced by phenols and sodium dodecyl sulfate. J Invest Dermatol. 2000;115:292–8. doi: 10.1046/j.1523-1747.2000.00056.x. [DOI] [PubMed] [Google Scholar]
- 34.Feliciani C, Toto P, Wang B, Sauder DN, Amerio P, Tulli A. Urokinase plasminogen activator mRNA is induced by IL-1alpha and TNF-alpha in in vitro acantholysis. Exp Dermatol. 2003;12:466–71. doi: 10.1034/j.1600-0625.2002.120415.x. [DOI] [PubMed] [Google Scholar]
- 35.Feliciani C, Toto P, Amerio P, et al. In vitro and in vivo expression of interleukin-1alpha and tumor necrosis factor-alpha mRNA in pemphigus vulgaris: interleukin-1alpha and tumor necrosis factor-alpha are involved in acantholysis. J Invest Dermatol. 2000;114:71–7. doi: 10.1046/j.1523-1747.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- 36.Whittock NV. Genomic sequence analysis of the mouse desmoglein cluster reveals evidence for six distinct genes: characterization of mouse DSG4, DSG5, and DSG6. J Invest Dermatol. 2003;120:970–80. doi: 10.1046/j.1523-1747.2003.12257.x. [DOI] [PubMed] [Google Scholar]
- 37.Olivry T. Drug-associated canine pemphigus vulgaris?, Who knows! Vet Dermatol. 2007;18:378–9. doi: 10.1111/j.1365-3164.2007.00621.x. [DOI] [PubMed] [Google Scholar]
- 38.Brenner S, Bialy-Golan A, Ruocco V. Drug-induced pemphigus. Clin Dermatol. 1998;16:393–7. doi: 10.1016/s0738-081x(98)00010-8. [DOI] [PubMed] [Google Scholar]
- 39.Patterson CRS, Davies MG. Carbamazepine-induced pemphigus. Clin Exp Dermatol. 2003;28:98–9. doi: 10.1046/j.1365-2230.2003.01156_6.x. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimura T, Seishima M, Nakashima K, et al. Increased antibody levels to desmogleins 1 and 3 after administration of carbamazepine. Clin Exp Dermatol. 2001;26:441–5. doi: 10.1046/j.1365-2230.2001.00854.x. [DOI] [PubMed] [Google Scholar]
- 41.Yokel BK, Hood AF, Anhalt GJ. Induction of acantholysis in organ explant culture by penicillamine and captopril. Arch Dermatol. 1989;125:1367–70. [PubMed] [Google Scholar]
- 42.Wolf R, Ruocco V. Gaining more insight into the pathomechanisms of thiol-induced acantholysis. Med Hypotheses. 1997;48:107–10. doi: 10.1016/s0306-9877(97)90277-2. [DOI] [PubMed] [Google Scholar]
- 43.Ueki H, Kohda M, Nobutoh T, et al. Antidesmoglein autoantibodies in silicosis patients with no bullous diseases. Dermatology (Basel) 2001;202:16–21. doi: 10.1159/000051578. [DOI] [PubMed] [Google Scholar]
- 44.Fleischmann M, Celerier P, Bernard P, Litoux P, Dreno B. Long-term interferon-alpha therapy induces autoantibodies against epidermis. Dermatology (Basel) 1996;192:50–5. doi: 10.1159/000246315. [DOI] [PubMed] [Google Scholar]
- 45.Cozzani E, Rosa GM, Drosera M, Intra C, Barsotti A, Parodi A. ACE inhibitors can induce circulating antibodies directed to antigens of the superficial epidermal cells. Arch Dermatol Res. 2011;303:327–32. doi: 10.1007/s00403-010-1060-5. [DOI] [PubMed] [Google Scholar]
- 46.Troy JL, Silvers DN, Grossman ME, Jaffe IA. Penicillamine-associated pemphigus: is it really pemphigus? J Am Acad Dermatol. 1981;4:547–55. doi: 10.1016/s0190-9622(81)70055-0. [DOI] [PubMed] [Google Scholar]
- 47.Gornowicz-Porowska J, Pietkiewicz P, Bowszyc-Dmochowska M, Dmochowski M. Immunoglobulin G4 is prevailing over immunoglobulin G1 in autoimmunity of pemphigus and bullous pemphigoid: analysis of tissue-bound antibodies in active diseases. Centr Eur J Immunol. 2013;38:80–91. [Google Scholar]
- 48.Bhol K, Natarajan K, Nagarwalla N, Mohimen A, Aoki V, Ahmed AR. Correlation of peptide specificity and IgG subclass with pathogenic and nonpathogenic autoantibodies in pemphigus vulgaris: a model for autoimmunity. Proc Natl Acad Sci USA. 1995;92:5239–43. doi: 10.1073/pnas.92.11.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hacker MK, Janson M, Fairley JA, Lin M-S. Isotypes and antigenic profiles of pemphigus foliaceus and pemphigus vulgaris autoantibodies. Clin Immunol. 2002;105:64–74. doi: 10.1006/clim.2002.5259. [DOI] [PubMed] [Google Scholar]
- 50.Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009;39:469–77. doi: 10.1111/j.1365-2222.2009.03207.x. [DOI] [PubMed] [Google Scholar]
- 51.Recke A, Rose C, Schmidt E, Bröcker EB, Zillikens D, Sitaru C. Transition from pemphigus foliaceus to bullous pemphigoid: intermolecular B-cell epitope spreading without IgG subclass shifting. J Am Acad Dermatol. 2009;61:333–6. doi: 10.1016/j.jaad.2008.10.061. [DOI] [PubMed] [Google Scholar]
- 52.Ohyama B, Nishifuji K, Chan PT, et al. Epitope spreading is rarely found in pemphigus vulgaris by large-scale longitudinal study using desmoglein 2-based swapped molecules. J Invest Dermatol. 2012;132:1158–68. doi: 10.1038/jid.2011.448. [DOI] [PubMed] [Google Scholar]
- 53.Butt A, Burge SM. Pemphigus vulgaris induced by captopril. Br J Dermatol. 1995;132:315–6. doi: 10.1111/j.1365-2133.1995.tb05038.x. [DOI] [PubMed] [Google Scholar]
- 54.Ong CS, Cook N, Lee S. Drug-related pemphigus and angiotensin converting enzyme inhibitors. Australas J Dermatol. 2000;41:242–6. doi: 10.1046/j.1440-0960.2000.00445.x. [DOI] [PubMed] [Google Scholar]
- 55.Goldberg I, Shirazi I, Brenner S. In vitro interferon-gamma release test in patients with drug-induced pemphigus. Isr Med Assoc J. 2008;10:424–7. [PubMed] [Google Scholar]
- 56.Blanken R, Doeglas HM, de Jong MC, Kardaun SH, van Leeuwen M. Pemphigus-like eruption induced by d-penicillamine and captopril, in the same patient. Acta Derm Venereol. 1988;68:456–7. [PubMed] [Google Scholar]
- 57.Christeler A, Waeber B, Brunner HR, Delacrétaz J. Superficial pemphigus caused by captopril. Schweiz Med Wochenschr. 1982;112:1483–6. [PubMed] [Google Scholar]
- 58.Stavropoulos PG, Kostakis PG, Papakonstantinou AMK, Panagiotopoulos A, Petridis AD. Coexistence of psoriasis and pemphigus after enalapril intake. Dermatology (Basel) 2003;207:336–7. doi: 10.1159/000073106. [DOI] [PubMed] [Google Scholar]
- 59.Buzón E, Pérez-Bernal AM, de la Pena F, Ríos JJ, Camacho F. Pemphigus foliaceus associated with cilazapril. Acta Derm Venereol. 1998;78:227. doi: 10.1080/000155598441639. [DOI] [PubMed] [Google Scholar]
- 60.Vignes S, Paul C, Flageul B, Dubertret L. Ramipril-induced superficial pemphigus. Br J Dermatol. 1996;135:657–8. doi: 10.1111/j.1365-2133.1996.tb03861.x. [DOI] [PubMed] [Google Scholar]
- 61.Menard PR, Suh JT, Jones H, et al. Angiotensin converting enzyme inhibitors. (Mercaptoaroyl)amino acids. J Med Chem. 1985;28:328–32. doi: 10.1021/jm00381a012. [DOI] [PubMed] [Google Scholar]
- 62.Brenner S, Bialy-Golan A, Anhalt GJ. Recognition of pemphigus antigens in drug-induced pemphigus vulgaris and pemphigus foliaceus. J Am Acad Dermatol. 1997;36:919–23. doi: 10.1016/s0190-9622(97)80273-3. [DOI] [PubMed] [Google Scholar]
- 63.Brenner S, Golan H, Bialy-Golan A, Ruocco V. Lesion topography in two cases of nifedipine-related pemphigus. J Eur Acad Dermatol Venereol. 1999;13:123–6. [PubMed] [Google Scholar]
- 64.Brenner S, Ruocco V. D-Penicillamine-induced pemphigus foliaceus with autoantibodies to desmoglein-1. J Am Acad Dermatol. 1998;39:137–8. doi: 10.1016/s0190-9622(98)70426-8. [DOI] [PubMed] [Google Scholar]
- 65.Brenner S, Ruocco V, Bialy-Golan A, et al. Pemphigus and pemphigoid-like effects of nifedipine on in vitro cultured normal human skin explants. Int J Dermatol. 1999;38:36–40. doi: 10.1046/j.1365-4362.1999.00601.x. [DOI] [PubMed] [Google Scholar]
- 66.Mobini N, Ahmed AR. Immunogenetics of drug-induced bullous diseases. Clin Dermatol. 1993;11:449–60. doi: 10.1016/0738-081x(93)90151-2. [DOI] [PubMed] [Google Scholar]
- 67.Danczak-Pazdrowska A, Bowszyc-Dmochowska M, Prokop J, Wolnik-Trzeciak G, Dmochowski M. A combination of exogenous and endogenous factors as a possible trigger of pemphigus vulgaris – case report and review of literature. Klin Dermatol. 2005;7:29–34. [Google Scholar]
- 68.Peterson JD, Worobec SM, Chan LS. An erythrodermic variant of pemphigus foliaceus with puzzling histologic and immunopathologic features. J Cutan Med Surg. 2007;11:179–84. doi: 10.2310/7750.2007.00021. [DOI] [PubMed] [Google Scholar]
- 69.Goldberg I, Gilburd B, Shovman O, Brenner S. Clinical and laboratory assays in the diagnosis of cutaneous adverse drug reactions. Isr Med Assoc J. 2004;6:50–1. [PubMed] [Google Scholar]
- 70.Maruani A, Machet MC, Carlotti A, Giraudeau B, Vaillant L, Machet L. Immunostaining with antibodies to desmoglein provides the diagnosis of drug-induced pemphigus and allows prediction of outcome. Am J Clin Pathol. 2008;130:369–74. doi: 10.1309/3CAKPEU8JXGWLEDC. [DOI] [PubMed] [Google Scholar]
- 71.Jakubowicz O, Bowszyc-Dmochowska M, Gaw(cki W, Gornowicz J, Dmochowski M. A case of mucosal-dominant variety of pemphigus vulgaris with laryngeal lesions and pemphigus deposits in hair plucked from clinically unchanged skin. Klin Dermatol. 2010;12:256–60. [Google Scholar]
- 72.Aronow WS. Commentary on recent guidelines for treating hypertension. Arch Med Sci. 2014;10:1069–72. doi: 10.5114/aoms.2014.47818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pietkiewicz P, Gornowicz-Porowska J, Bowszyc-Dmochowska M, Dmochowski M. Malignancy in relation to autoimmune blistering dermatoses: molecular and clinical aspects. In: Vereecken P, editor. Highlights in skin cancer. Rijeka, Croatia: InTech; 2013. pp. 159–210. [Google Scholar]