Abstract
Introduction
Colorectal cancer is common in developed countries. Polyunsaturated fatty acids (PUFAs) have been reported to possess tumoricidal action, but the exact mechanism of their action is not clear.
Material and methods
In the present study, we studied the effect of various n-6 and n-3 fatty acids on the survival of the colon cancer cells LoVo and RKO and evaluated the possible involvement of a mitochondrial pathway in their ability to induce apoptosis.
Results
It was observed that n-3 α-linolenic acid, eicosapentaenoic acid and docosahexaenoic acid (ALA, EPA and DHA respectively) and n-6 linoleic acid, gamma-linolenic acid and arachidonic acid (LA, GLA and AA respectively) induced apoptosis of the colon cancer cells LoVo and RKO at concentrations above 120 μM (p < 0.01 compared to control). The semi-differentiated colon cancer cell line RKO was more sensitive to the cytotoxic action of PUFAs compared to the undifferentiated colon cancer cell line LoVo. PUFA-treated cells showed an increased number of lipid droplets in their cytoplasm. PUFA-induced apoptosis of LoVo and RKO cells is mediated through a mitochondria-mediated pathway as evidenced by loss of mitochondrial membrane potential, generation of ROS, accumulation of intracellular Ca2+, activation of caspase-9 and caspase-3, decreased ATP level and increase in the Bax/Bcl2 expression ratio.
Conclusions
PUFAs induced apoptosis of colon cancer cells through a mitochondrial dependent pathway.
Keywords: polyunsaturated fatty acids, colon cancer, apoptosis, mitochondrial pathway, ATP, caspases, lipid droplets
Introduction
Colorectal cancer (CRC) is common in both men and women, accounting for approximately 9% of mortality due to cancer each year [1, 2]. The high incidence of colon cancer has been attributed to the replacement of the traditional, high-fiber, low-fat, and low-calorie diet by a high fat, low-fiber, and calorie dense diet [3]. This suggests that dietary intervention may form an innovative way to decrease the occurrence of colorectal cancer. Fish and vegetable oils such as flaxseed, mustard, and canola that are good sources of polyunsaturated fatty acids (PUFAs) have been reported to alter the incidence of cancer including colorectal cancer [4, 5]. Epidemiological studies showed that increased consumption of long chain PUFAs, especially n-3 fatty acids, is inversely correlated with colorectal cancer incidence [6]. Several in vitro studies have shown that PUFAs have growth-inhibitory and pro-apoptotic effects on colon cancer cells such as HT-29, HCT116, SW480, SW620, and Caco-2 [7–10].
Apoptosis is a key process of programmed cell death, which plays a crucial role in maintaining cellular homeostasis between cell division and cell death [11]. The Bcl-2 family members involved in apoptotic cell death play a vital role in regulating the outer mitochondrial membrane permeabilization [12]. Alterations in the mitochondrial membrane permeability could trigger release of cytochrome C into the cytoplasm, which activates caspases that, in turn, induce apoptosis. Despite extensive research conducted on the apoptotic mechanism(s) of PUFAs in colon cancer, relatively little is known about the effects of PUFAs on mitochondrial function that plays an important role in cellular energy metabolism and free radical generation. Previously, we showed that LA induced apoptosis of LoVo and RKO cancer cells by augmenting cellular oxidative stress and mitochondrial dysfunction [13].
In an extension of our previous study [13], in the present investigation we examined the effect of n-3 (ALA, EPA and DHA) and n-6 (LA, GLA and AA) PUFAs on LoVo and RKO colon cancer cells in vitro and evaluated whether the apoptotic process triggered by these fatty acids is mediated by changes in mitochondrial membrane potential, generation of ROS, intracellular Ca2+, ATP level, caspase-9, caspase-3 and expression of Bcl-2 and Bax.
Material and methods
Materials
α-Linolenic acid (ALA, 18 : 3 n-3), eicosapentaenoic acid (EPA, 20 : 5 n-3,) docosahexaenoic acid (DHA, 22 : 6 n-3), linoleic acid (LA, 18 : 2 n-6), γ-linolenic acid (GLA, 18 : 3 n-6), arachidonic acid (AA, 20 : 4 n-6,) and 5-FU (5-fluorouracil) were obtained from Sigma (St. Louis, MO, USA). ALA, EPA, DHA, LA, GLA, and AA were dissolved in anhydrous ethanol and stored as stock solutions (20 mM) at –20°C. Also, 50 mM of 5-FU was dissolved in high purity water as a stock solution. For all studies, fatty acids and 5-FU were freshly prepared from stock solutions with cell culture media.
Cell culture
For the present study, the undifferentiated colon cancer cell line LoVo and the semi-differentiated colon cancer cell line RKO were purchased from the Institute of Biochemistry and Cell Biology, Shanghai Institute for Biological Sciences, Chinese Academy of Sciences, and were cultured in RPMI Medium 1640 (Gibco) and grown in a humidified 37°C, 5% CO2 incubator. The medium was supplemented with penicillin (100 U/ml), streptomycin (100 U/ml) and 10% fetal bovine serum. The medium was replaced every 2 days.
MTT assay
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to evaluate the effect of unsaturated fatty acids on cell viability [14]. LoVo cells were plated in 96-well plates with a volume of 190 μl at a density of 1 × 105 cells/ml per well, while the density of RKO was 5 × 104 cells/ml. Twenty-four hours after seeding, both LoVo and RKO cells were exposed to different concentrations of various fatty acids and 5-FU in a volume of 10 μl for 24, 48, and 72 h. At the end of the incubation time, the medium was removed and 20 μl of 5 mg/ml MTT was added to each well, incubated at 37°C for an additional 4 h, at the end of which the colored formazan was dissolved in 150 μl of dimethyl sulfoxide (DMSO). The percentage of viable cells was calculated by measuring the absorbance of the colored formazan reaction product at 490 nm using a plate reader.
Apoptotic analysis
LoVo and RKO cells seeded in 6-well plates were supplemented with different doses of various fatty acids. At the end of the study period, cells were detached by treatment with trypsin followed by centrifugation at 1500 rpm for 5 min, and washing twice with cold PBS. Subsequently, cells were suspended gently in 400 μl of Annexin V-FITC binding buffer and then stained with 5 μl of Annexin V-FITC and 10 μl of PI according to the manufacturer's instructions (Bestbio, Shanghai, China). Cell apoptosis was performed immediately by flow cytometer after staining the cells with Annexin V-FITC/PI.
Oil red ‘O’ stain
Oil red ‘O’ stain was used to evaluate lipid droplets formed in the cytoplasm of LoVo and RKO cells supplemented with various fatty acids [15]. Oil red ‘O’ stock solution was prepared by dissolving 2.5 g of Oil red ‘O’ in 100 ml of isopropanol. A stock solution of oil red ‘O’ was diluted with double distilled water for staining fatty acid-treated cells. Twenty-four hours after seeding, LoVo and RKO cells in 24-well plates were supplemented with various PUFAs and 5-FU for 48 h. At the end of the treatment period, the medium was removed and cells were washed twice with PBS and fixed in 10% formalin for 10 min. Cells were washed three times with PBS, dried for 20 min at room temperature, and stained with 0.5 ml oil red ‘O’ working solution for 30 min. The cells were photographed after washing twice with 60% isopropanol and double distilled water.
Measurement of mitochondrial membrane potential, reactive oxygen species and Ca2+ concentration
Twenty-four hours after seeding, LoVo and RKO cells in 6-well culture plates were supplemented with various PUFAs and 5-FU for 48 h. At the end of the incubation time, cells were harvested and washed twice with PBS and then treated with the ROS indicator DCFH-DA [16] (10 μM), the mitochondrial membrane potential indicator Rhodamine 123 (1 μM) and the calcium probe Fluo-3 AM (3 μM) and incubated at 37°C for 30 min in the dark. Fluorescence intensity was measured by using a fluorescence microscope according to the manufacturer's instructions (Beyotime Institute of Biotechnology, Nangtong, China).
Lipid peroxides in PUFA-supplemented cells
LoVo and RKO cells supplemented with various PUFAs and 5-FU for 48 h were harvested using trypsin, and washed twice with PBS. Cells was disrupted using an ultrasonic cell disruption system followed by centrifugation at 1000×g for 5 min, and the pellet was re-suspended gently in 200 μl of PBS. The levels of malondialdehyde (MDA) (taken as a measure of total lipid peroxides formed) in the cells were determined by the commercial MDA assay reagent kit according to the manufacturer's instructions (Nanjing Kaiji Bioengineering Institute, Nanjing, China).
ATP assay
Cellular content of ATP was measured by liquid chromatography as described previously [14]. LoVo and RKO cells were seeded in 6-well plates and allowed to grow for 24 h. Subsequently, cells were supplemented with different concentrations of ALA, DHA, EPA, LA, GLA, DHA and 5-FU for 72 h. At the end of the incubation period of 72 h, cells were harvested and washed twice with cold PBS. Cells were subsequently homogenized with 1 ml of perchloric acid (0.5 M) in an ice bath for 10 min, centrifuged at 10,000 rpm for 5 min, then 450 μl of cold KOH (1 M) was added to the collected supernatant for 5 min in the ice bath, and the mixture was centrifuged at 10,000 rpm for 5 min. The samples were assessed for their ATP content by high-performance liquid chromatography as described previously [14].
Measurement of the activities of caspases-9 and -3
The activities of caspase-9 and caspase-3 were assessed by using the Caspase Activity Kit according to the manufacturer's instructions (Beyotime Institute of Biotechnology, Nangtong, China). In brief, LoVo and RKO cells were treated with various PUFAs and 5-FU for 48 h, washed twice with PBS, and resuspended in lysis buffer for 15 min in the ice bath. The mixture was centrifuged at 16,000 for 15 min at 4°C. The supernatants were collected and protein concentrations were adjusted to 1–3 mg/ml. The supernatants were mixed with caspase-3 and caspase-9 reaction substrates separately and incubated for 4 h at 37°C. The activities of caspase-3 and caspase-9 were identified by measuring the absorbance of the reaction product at 405 nm.
Total RNA isolation and quantitative real-time PCR analysis for expression of Bcl-2 and Bax
For total RNA isolation, LoVo and RKO cells were cultured in 6-well plates and treated with various PUFAs and 5-FU for 48 h. Total RNA was extracted from LoVo and RKO cells with TRIzol reagent (Haogene Biotech, Hangzhou, China) as per the manufacturer's instructions. The concentrations of RNA were determined by spectrophotometric assay (Eppendorf, Germany). The cDNA was synthesized from 500 ng of RNA using the 1st-Strand cDNA Synthesis Kit according to the manufacturer's instructions (Haogene Biotech, Hangzhou, China).
The measurement of relative gene expression of Bcl-2, Bax, and Human 18S in LoVo and RKO cells in vitro was done using primers as listed in Table I. Real-time quantitative PCR (RT-PCR) was performed with Power SYBR Master Mix (Haogene Biotech, Hangzhou, China). The total reaction volume was 25 μl: 10.5 μl of double distilled water, 12.5 μl of Power SYBR Master Mix (2×), 0.5 μl of PCR-F (10 μM), 0.5 μl of PCR-R (10 μM), and 1.0 μl of cDNA. Human 18S expression was used as an internal control. All experiments were conducted three times independently for reproducibility of the results obtained.
Table I.
Sequences of primers used in the real-time PCR amplifications
| Gene | Genbank accession | Primer sequences (5’ to 3’) | Size [bp] | Annealing (°C) |
|---|---|---|---|---|
| Human Bcl2 | BC027258 | CTGCACCTGACGCCCTTCACC | 119 | 63 |
| CACATGACCCCACCGAACTCAAAGA | ||||
| Human Bax | NM_004324 | CCCTTTTGCTTCAGGGTTTCATCCA | 122 | 62 |
| CTTGAGACACTCGCTCAGCTTCTTG | ||||
| Human 18S | NR_003286 | GACTCAACACGGGAAACCTCAC | 122 | 63 |
| CCAGACAAATCGCTCCACCAAC |
Statistical analysis
Each experiment was repeated at least three times and the data were analyzed with SAS 8.0 software. The results are expressed as means ± SD. All data were analyzed using the ANOVA procedure with significance analysis at the p < 0.05 level.
Results
Cell proliferation and viability
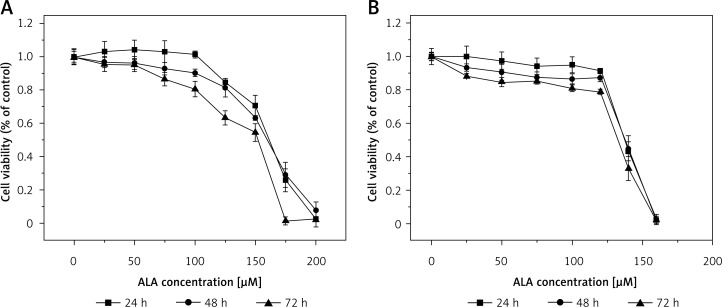
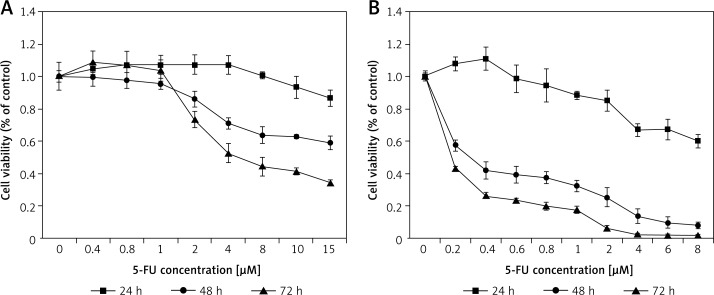
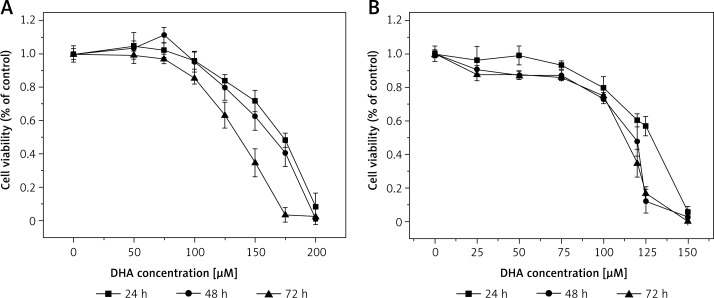
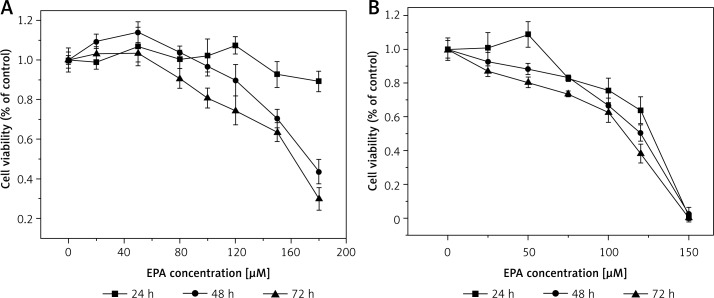
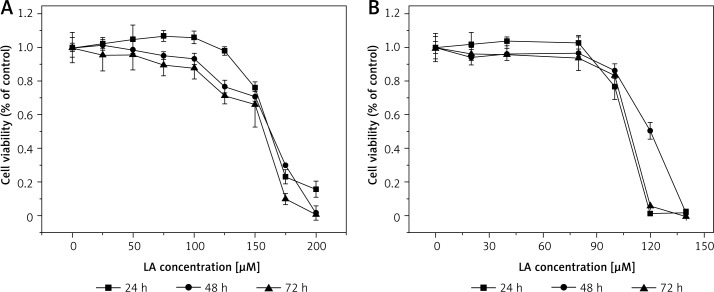
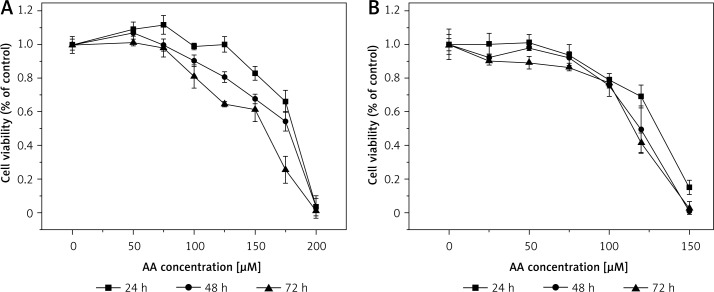
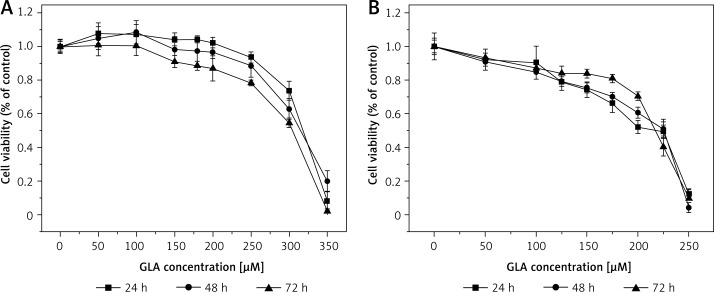
It is evident from the results shown in Figures 1–7 that the growth of LoVo and RKO cells was suppressed by all the fatty acids and 5-FU tested in a dose- and time-dependent manner. At low concentrations (0–50 μM), ALA, DHA and LA caused a slight increase in the growth of LoVo cells, while EPA produced a non-significant increase in the growth of RKO cells. It is obvious that both PUFAs and 5-FU were more effective in inhibiting the growth of RKO compared to LoVo cells, suggesting that semi-differentiated colon cancer cells (RKO) were more sensitive to the actions of PUFAs and 5-FU than the undifferentiated colon cancer cells LoVo. Based on these results (Figures 1–7), we selected the most effective concentrations of various fatty acids for further studies as depicted below: ALA (150 μM), EPA (150 μM), DHA (150 μM), LA (150 μM), GLA (300 μM), AA (150 μM), 5-FU (10 μM) to test on LoVo cells and ALA (140 μM), EPA (120 μM), DHA (120 μM), LA (120 μM), GLA (200 μM), AA (120 μM), 5-FU (0.4 μM) to test on RKO cells. In all subsequent studies, LoVo and RKO cells were incubated for 48 h at the above-mentioned doses of fatty acids and 5-FU.
Figure 1.
Effect of ALA on LoVo (A) and RKO (B) cell viability. LoVo and RKO cells were treated with ALA at the concentrations of 0, 25, 50, 75, 100, 120, 150, 150, 180, 200 μM for 24, 48, 72 h
Figure 7.
Effect of 5-FU on LoVo (A) and RKO (B) cell viability. LoVo and RKO cells were treated with 5-FU at the concentrations of 0, 0.2, 0.4, 0.8, 1, 2, 4, 8, 10, 15 μM for 24, 48, 72 h
Figure 2.
Effect of DHA on LoVo (A) and RKO (B) cell viability. LoVo and RKO cells were treated with DHA at the concentrations of 0, 50, 75, 100, 120, 150, 175, 200 μM for 24, 48, 72 h
Figure 3.
Effect of EPA on LoVo (A) and RKO (B) cell viability. LoVo and RKO cells were treated with EPA at the concentrations of 0, 20, 50, 80, 100, 120, 150, 180 μM for 24, 48, 72 h
Figure 4.
Effect of LA on LoVo (A) and RKO (B) cell viability. LoVo and RKO cells were treated with LA at the concentrations of 0, 20, 50, 80, 100, 120, 150, 180, 200 μM for 24, 48, 72 h
Figure 5.
Effect of AA on LoVo (A) and RKO (B) cell viability. LoVo and RKO cells were treated with AA at the concentrations of 0, 50, 75, 100, 120, 150, 175, 200 μM for 24, 48, 72 h
Figure 6.
Effect of GLA on LoVo (A) and RKO (B) cell viability. LoVo and RKO cells were treated with GLA at the concentrations of 0, 50, 100, 150, 180, 200, 250, 300, 350 μM for 24, 48, 72 h
PUFAs induce apoptosis in colon cancer cells
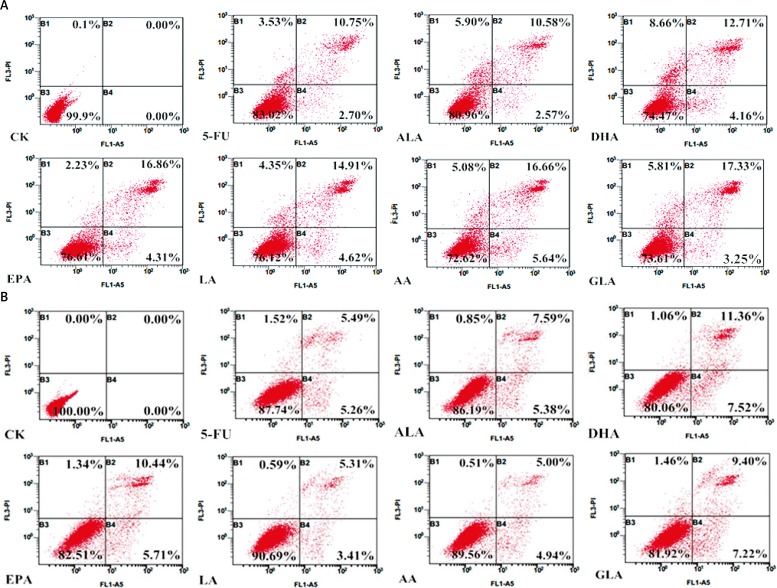
It is evident from the results shown in Figure 8 that all the fatty acids tested were capable of inducing apoptosis of both LoVo and RKO cells. Of all the fatty acids tested, n-3 fatty acids DHA and EPA and n-6 fatty acids AA and GLA were found to be more effective in inducing apoptosis of LoVo and RKO cells compared to other fatty acids.
Figure 8.
Apoptosis assay using flow cytometry after staining with Annexin V-FITC/PI (B1 represents necrotic cells, B2 + B4 represents apoptotic cells, B3 represents viable cells). A – LoVo cells were treated with ALA (150 μM), EPA (150 μM), DHA (150 μM), LA (150 μM), GLA (300 μM), AA (150 μM), 5-FU (10 μM) to LoVo cells for 48 h; B – RKO cells were exposed to ALA (140 μM), EPA (120 μM), DHA (120 μM), LA (120 μM), GLA (200 μM), AA (120 μM), 5-FU (0.4 μM) for 48 h
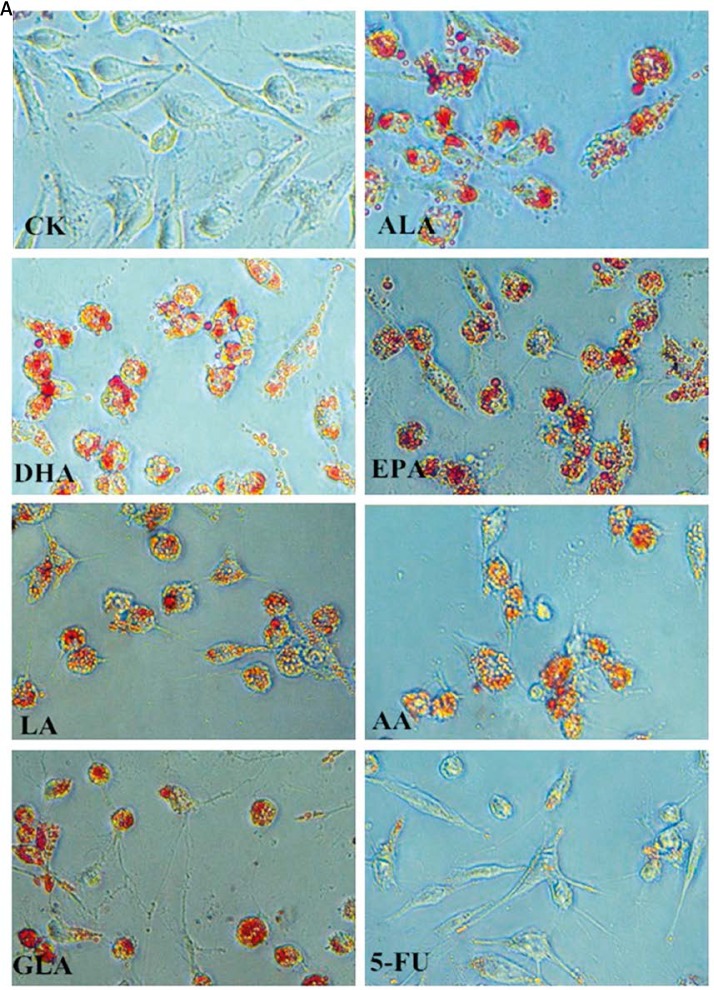
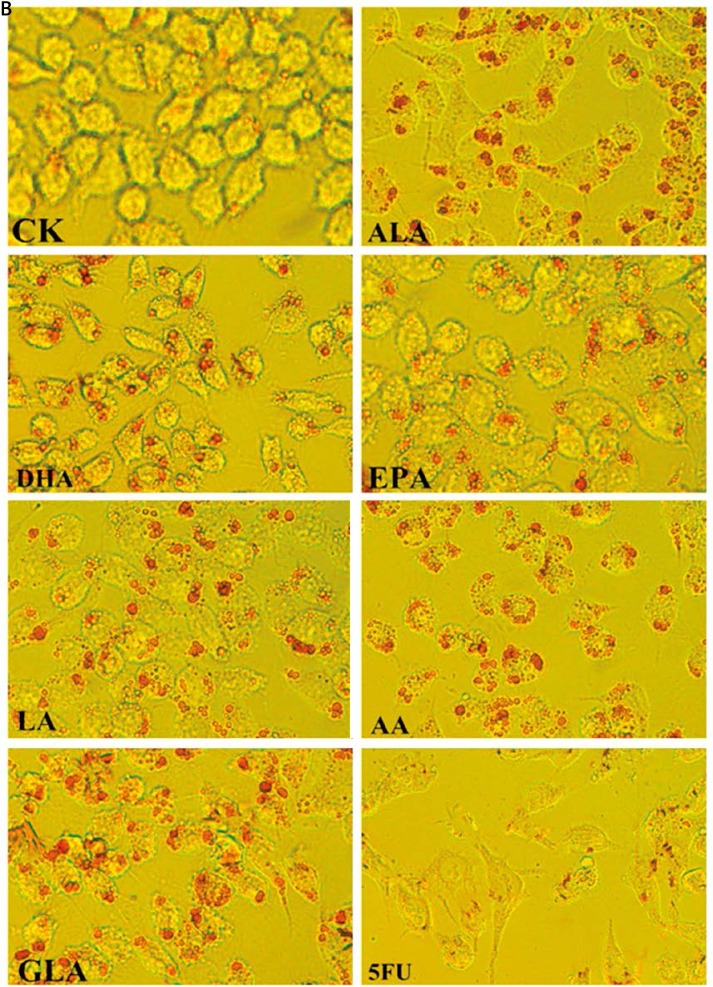
Lipid droplets in colon cancer cells supplemented with PUFAs
The accumulation of lipid droplets in the cytoplasm of LoVo and RKO cells in response to the supplementation of various PUFAs and 5-FU was evaluated by light microscopy. PUFA-supplemented LoVo and RKO cells showed an increased number and size of lipid droplets in their cytoplasm compared to the control (Figure 9). However, anticancer drug 5-FU supplemented cells displayed much fewer lipid droplets when compared to PUFA supplemented cells.
Figure 9.
Accumulation of lipid droplets in the cytoplasm of LoVo and RKO cells was assessed by oil red strain after being treated with ALA, EPA, DHA, LA, GLA, AA, 5-FU for 48 h, and the cells were observed by light microscopy (100×). A – LoVo cells
Figure 9.
Accumulation of lipid droplets in the cytoplasm of LoVo and RKO cells was assessed by oil red strain after being treated with ALA, EPA, DHA, LA, GLA, AA, 5-FU for 48 h, and the cells were observed by light microscopy (100×). B – RKO cells
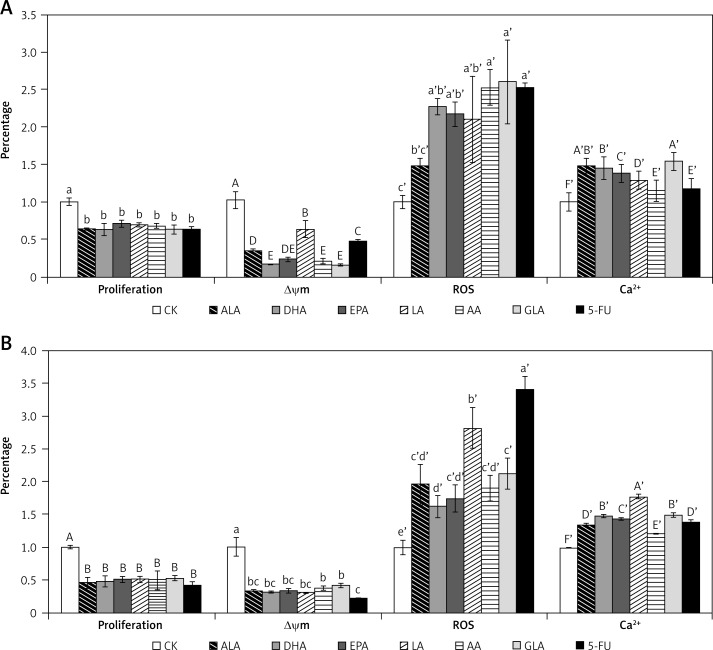
Effects of PUFAs on Δψm, ROS and Ca2+ in colon cancer cells
The spatial variation of Δψm in colon cancer cells treated with various fatty acids and 5-FU was estimated utilizing rhodamine-123, a fluorescent dye that accumulates rapidly and specifically within the mitochondria in varying amounts depending on the membrane potential [17]. As illustrated in Figure 10, after exposure to various fatty acids and 5-FU, significant loss of mitochondrial membrane potential was noted in comparison to the control in both LoVo and RKO cells, suggesting induction of cell apoptosis through dysfunction of mitochondria. All the fatty acids tested (ALA, DHA, EPA, LA, AA, GLA) showed a similar effect on Δψm in RKO cells, while AA and GLA were more effective in reducing mitochondrial potential in LoVo cells.
Figure 10.
Effect of ALA, DHA, EPA, LA, AA, GLA and 5-FU on the proliferation, the loss of Δψm, production of ROS and Ca2+ accumulation in LoVo and RKO cells in vitro. A – LoVo cells, B – RKO cells
Values with different letters are significantly different (p < 0.05).
PUFAs and 5-FU induced a significant increase in the production of ROS (reactive oxygen species) in LoVo and RKO cells (Figure 10). Similarly, PUFAs and 5-FU produced a significant increase in intracellular Ca2+, as shown in Figure 10. These results suggest that PUFAs and 5-FU induce mitochondrial dependent apoptosis of LoVo and RKO cells by augmenting intracellular Ca2+.
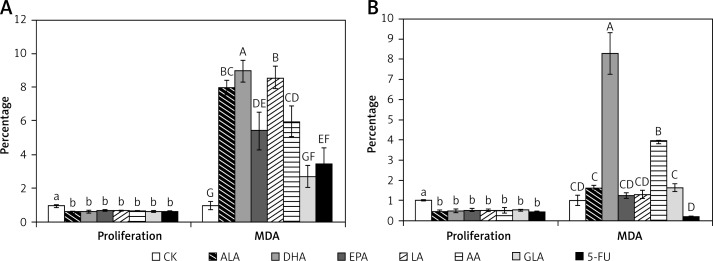
Levels of lipid peroxides
The results of this study shown in Figure 11 revealed that significantly higher amounts of lipid peroxides (measured as total MDA) were formed in LoVo cells that were supplemented with various PUFAs and 5-FU. On the other hand, only DHA and AA induced significant formation of lipid peroxides in RKO cells.
Figure 11.
Effect of ALA, DHA, EPA, LA, AA, GLA and 5-FU on the proliferation and corresponding MDA levels in LoVo and RKO cells in vitro. A – LoVo cells, B – RKO cells
Values with different letters are significantly different (p < 0.05).
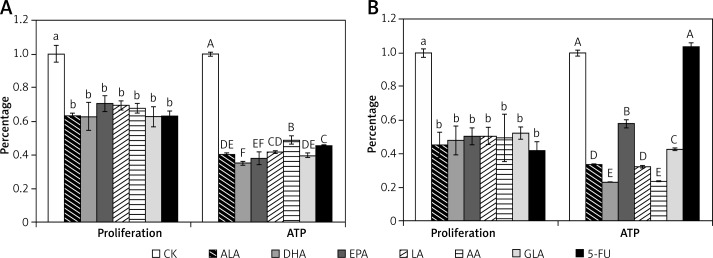
PUFAs decreased ATP content in colon cancer cells
ATP plays a crucial role in the mitochondria-dependent apoptosis program and can be used as a sensitive parameter to study mitochondrial dysfunction [18]. The results presented in Figure 12 show that supplementation with PUFAs produced a significant reduction in the generation of ATP in LoVo and RKO cells. In this context, it is interesting to note that 5-FU inhibited ATP synthesis in LoVo cells but not in RKO cells.
Figure 12.
Effect of ALA, DHA, EPA, LA, AA, GLA and 5-FU on the proliferation and ATP levels in LoVo and RKO cells in vitro. A – LoVo cells, B – RKO cells
Values with different letters are significantly different (p < 0.05).
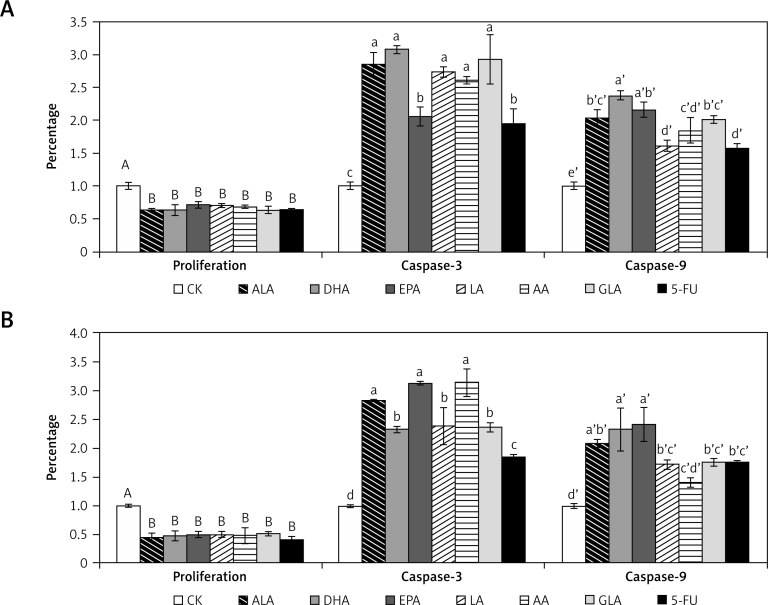
Effects of PUFAs on the caspase-3 and caspase-9 activity of colon cancer cells
Activation of caspases leads to the onset of apoptosis [19, 20]. Hence, we examined the effect of various PUFAs on the activities of caspase-3 and caspase-9. The results of this study presented in Figure 13 indicate that activities of both caspase-3 and caspase-9 were increased in LoVo and RKO cells when supplemented with various PUFAs and 5-FU, though certain fatty acids (ALA, DHA and EPA) produced a much higher increase in the activities of caspases compared to other fatty acids.
Figure 13.
Effect of ALA, DHA, EPA, LA, AA, GLA and 5-FU on the caspase-3 and caspase-9 activity in LoVo and RKO cells in vitro. A – LoVo cells, B – RKO cells
Values with different letters are significantly different (p < 0.05).
Effects of PUFAs on the expression of Bcl-2 and Bax
The Bcl-2 family plays a significant role in apoptosis and regulates mitochondrial outer membrane integrity [12]. In the present study, we measured the activities of the pro-apoptotic factor Bax and the anti-apoptotic factor Bcl-2 and the reference gene Human 18S RNA using real-time PCR following supplementation of PUFAs and 5-FU. The results, given in Table II, showed up-regulation of Bax and down-regulation of Bcl-2 following supplementation of PUFAs and 5-FU.
Table II.
Effect of ALA, DHA, EPA, LA, AA, GLA and 5-FU on relative gene expression of Bcl-2, Bax, and Human 18S in LoVo and RKO cells in vitro
| LoVo treatment | LoVo % survival | Bcl-2 (% of CK) | Bax (% of CK) | RKO treatment | RKO % survival | Bcl-2 (% of CK) | Bax (% of CK) |
|---|---|---|---|---|---|---|---|
| Control | 1.00 ±0.05A | 1.00 ±0.01a | 1.00 ±0.07d | Control | 1 ±0.03A | 1.00 ±0.02b | 1.00 ±0.06c’ |
| ALA 150 μm | 0.63 ±0.01B | 0.42 ±0.05c | 2.20 ±0.09b’ | ALA 140 μm | 0.45 ±0.08B | 0.52 ±0.04c | 1.37 ±0.12b’ |
| DHA 150 μm | 0.63 ±0.08B | 0.68 ±0.06b | 1.15 ±0.11d’ | DHA 120 μm | 0.48 ±0.09B | 1.30 ±0.14a | 1.65 ±0.15b’ |
| EPA 150 μm | 0.70 ±0.05B | 0.64 ±0.04b | 1.22 ±0.07d’ | EPA 120 μm | 0.50 ±0.05B | 0.70 ±0.08c | 1.43 ±0.06b’ |
| LA 150 μm | 0.69 ±0.03B | 0.70 ±0.02b | 1.59 ±0.14c’ | LA 120 μm | 0.50 ±0.05B | 0.65 ±0.07c | 1.49 ±0.09b’ |
| AA 150 μm | 0.68 ±0.03B | 0.37 ±0.02c | 1.17 ±0.13d’ | AA 120 μm | 0.49 ±0.14B | 0.72 ±0.05c | 1.65 ±0.15b’ |
| GLA 300 μm | 0.63 ±0.06B | 0.43 ±0.02c | 1.96 ±0.19b’ | GLA 200 μm | 0.52 ±0.04B | 0.57 ±0.09c | 0.81 ±0.11c’ |
| 5-FU 10 μm | 0.63 ±0.03B | 0.69 ±0.06b | 3.54 ±0.01a’ | 5-FU 0.4 μm | 0.42 ±0.05B | 0.74 ±0.10c | 3.95 ±0.15a’ |
Values with different letters are significantly different (p < 0.05).
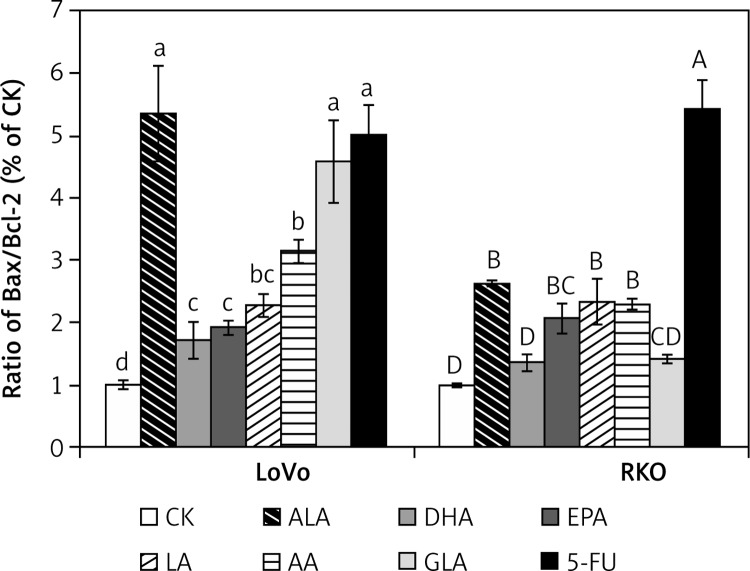
Since the ratio between Bax and Bcl-2 expression is a key factor in the regulation of apoptosis [21], we calculated the Bax/Bcl-2 ratio for various treatments employed and these results are given in Figure 14. It is observed that LoVo cells showed a much higher Bax/Bcl-2 ratio compared to RKO cells following supplementation with PUFAs and 5-FU, though the ratio was significantly higher compared to the control in both the cell types. The most pronounced effect among the PUFAs was recorded in the case of ALA and GLA in LoVo cells.
Figure 14.
Effect of ALA, DHA, EPA, LA, AA, GLA and 5-FU on the expression of Bax/Bcl2 ratio in LoVo and RKO cells in vitro. A – LoVo cells, B – RKO cells
Values with different letters are significantly different (p < 0.05).
Discussion
Many studies showed that n-3 and n-6 PUFAs possess growth inhibitory actions on normal and tumor cells, with more significant action on the latter [22–25]. The results of the present study are in support of this action of PUFAs. It is evident from the results shown in Figures 1–7 that under the conditions employed both n-3 (ALA, EPA, DHA) and n-6 (LA, GLA, AA) fatty acids induce apoptosis of the colorectal cancer cells LoVo (undifferentiated) and RKO (semi-differentiated). The effect of n-3 and n-6 fatty acids on LoVo and RKO cells seems to be dependent to some extent on the dose of the fatty acids employed, as evident from the results shown in Figures 1–7 wherein lower concentrations (< 50 μM) of ALA, EPA, DHA, LA and AA actually enhanced the growth (but not to a significant degree), though the effect was more evident on RKO cells compared to LoVo. It is interesting to note that the semi-differentiated colon cancer cell line RKO showed more sensitivity to the cytotoxic action of PUFAs compared to the undifferentiated colon cancer cell line LoVo. These results are in support of our previous study wherein we observed that RKO cells may metabolize PUFAs more efficiently to toxic metabolites (such as lipid peroxides) compared to LoVo cells [13]. Alternatively, LoVo cells may convert PUFAs to non-toxic metabolites or detoxify fatty acids more efficiently compared to RKO cells. But, paradoxically, formation of lipid peroxides measured as MDA-reactive substances was found to be much lower in RKO compared to LoVo cells on supplementation with various PUFAs. These results suggest that, possibly, PUFAs are converted into less toxic metabolites other than peroxides by LoVo cells.
Apoptosis plays a crucial part in maintaining cellular homeostasis [11]. The MTT assay detects necrotic and normal cells. To confirm that PUFAs induce apoptosis of LoVo and RKO cells, we performed flow cytometric analysis with Annexin V and PI double staining. These results showed that PUFAs (ALA, EPA, DHA, LA, GLA and AA) and 5-FU induced apoptosis of LoVo and RKO cells.As expected, PUFAs-treated cells showed a significant increase in the accumulation of lipid droplets compared to 5-FU supplemented and control cells. These results suggest that supplemented PUFAs accumulate in the form of triglycerides in the cells. This is probably in addition to their incorporation in the cell membrane lipid pool.
Mitochondria participate in cellular energy metabolism, free radical generation and apoptosis [12, 26–28]. Mitochondrial dysfunction leads to a decrease in cellular ATP content [29]. The results of the present study showed that supplementation of PUFAs leads to a loss of mitochondrial membrane potential and a decrease of ATP content in LoVo and RKO cells. It was also noted that PUFAs enhanced ROS generation and intracellular Ca2+ content and activated caspases-3 and 9 in LoVo and RKO cells, which resulted in the induction of apoptosis, which is supported by the observation that an increase in Bax/Bcl-2 ratio occurred in PUFA-treated cells.
In conclusion, the results of the present study showed that n-3 (ALA, EPA, DHA) and n-6 (LA, GLA, AA) fatty acids induce apoptosis of LoVo and RKO cells by a combination of mechanisms that include enhanced generation of ROS, accumulation of intracellular Ca2+, activation of caspases, and a decrease in mitochondrial membrane potential and ATP level, suggesting a major role for the mitochondria-mediated pathway. Based on the present findings, further studies are needed to know whether a combination of PUFAs and conventional anti-cancer drugs would form a rational approach in the management of colon cancer. This aspect of combination of conventional anti-cancer drugs and PUFAs for management of cancer is especially relevant since previously we and others [30–35] have shown that PUFAs enhance uptake and decrease efflux of anti-cancer drugs and thus enhance their (anti-cancer drugs) intracellular concentration, rendering tumor cells more susceptible to apoptosis. In an extension of this study, we also showed that PUFAs can reverse tumor cell drug resistance [36]. These studies support the non-cardiovascular benefits of PUFAs [37] in the clinic. Hence, more studies are needed to explore the possible use of combinations of conventional anti-cancer drugs and PUFAs in the prevention and management of cancer.
Acknowledgments
Undurti N. Das is in receipt of a Ramalingaswami Fellowship of the Department of Biotechnology, India, during the tenure of this study.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Granci V, Cai F, Lecumberri E, Clerc A, Dupertuis YM, Pichard C. Colon cancer cell chemosensitisation by fish oil emulsion involves apoptotic mitochondria pathway. Br J Nutr. 2013;109:1188–95. doi: 10.1017/S000711451200308X. [DOI] [PubMed] [Google Scholar]
- 4.Yashodhara BM, Umakanth S, Pappachan JM, Bhat SK, Kamath R, Choo BH. Omega-3 fatty acids: a comprehensive review of their role in health and disease. Postgrad Med J. 2009;85:84–90. doi: 10.1136/pgmj.2008.073338. [DOI] [PubMed] [Google Scholar]
- 5.Hofmanova J, Ciganek M, Slavik J, et al. Lipid alterations in human colon epithelial cells induced to differentiation and/or apoptosis by butyrate and polyunsaturated fatty acids. J Nutr Biochem. 2012;23:539–48. doi: 10.1016/j.jnutbio.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Fahy B, Bold RJ. Epidemiology and molecular genetics of colorectal cancer. Surg Oncol. 1998;7:115–23. doi: 10.1016/s0960-7404(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 7.Fasano E, Serini S, Piccioni E, et al. DHA induces apoptosis by altering the expression and cellular location of GRP78 in colon cancer cell lines. Biochim Biophys Acta. 2012;1822:1762–72. doi: 10.1016/j.bbadis.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Xia LJ, Li MG. Effect of n-3 polyunsaturated fatty acids on proliferation and apoptosis of human colon cancer cell. Zhonghua Wei Chang Wai Ke Za Zhi. 2012;15:490–4. [PubMed] [Google Scholar]
- 9.Bathen TF, Holmgren K, Lundemo AG, et al. Omega-3 fatty acids suppress growth of SW620 human colon cancer xenografts in nude mice. Anticancer Res. 2008;28:3717–23. [PubMed] [Google Scholar]
- 10.Kuan CY, Walker TH, Luo PG, Chen CF. Long-chain polyunsaturated fatty acids promote paclitaxel cytotoxicity via inhibition of the MDR1 gene in the human colon cancer Caco-2 cell line. J Am Coll Nutr. 2011;30:265–73. doi: 10.1080/07315724.2011.10719969. [DOI] [PubMed] [Google Scholar]
- 11.Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basile V, Belluti S, Ferrari E, et al. bis-Dehydroxy-curcumin triggers mitochondrial-associated cell death in human colon cancer cells through ER-stress induced autophagy. PLoS One. 2013;8:e53664. doi: 10.1371/journal.pone.0053664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu X, Yu H, Ma Q, Shen S, Das UN. Linoleic acid suppresses colorectal cancer cell growth by inducing oxidant stress and mitochondrial dysfunction. Lipids Health Dis. 2010;9:106. doi: 10.1186/1476-511X-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Yu H, Sun S, et al. Mechanism of free Zn(2+) enhancing inhibitory effects of EGCG on the growth of PC-3 cells: interactions with mitochondria. Biol Trace Elem Res. 2009;131:298–310. doi: 10.1007/s12011-009-8362-5. [DOI] [PubMed] [Google Scholar]
- 15.Dai J, Shen J, Pan W, Shen S, Das UN. Effects of polyunsaturated fatty acids on the growth of gastric cancer cells in vitro. Lipids Health Dis. 2013;12:71. doi: 10.1186/1476-511X-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–6. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 17.Yin H, Guo C, Wang Y, et al. Fengycin inhibits the growth of the human lung cancer cell line 95D through reactive oxygen species production and mitochondria-dependent apoptosis. Anticancer Drugs. 2013;24:587–98. doi: 10.1097/CAD.0b013e3283611395. [DOI] [PubMed] [Google Scholar]
- 18.Yu HN, Zhang LC, Yang JG, Das UN, Shen SR. Effect of laminin tyrosine-isoleucine-glycine-serine-arginine peptide on the growth of human prostate cancer (PC-3) cells in vitro. Eur J Pharmacol. 2009;616:251–5. doi: 10.1016/j.ejphar.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 19.Javadov S, Karmazyn M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell Physiol Biochem. 2007;20:1–22. doi: 10.1159/000103747. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong JS. The role of the mitochondrial permeability transition in cell death. Mitochondrion. 2006;6:225–34. doi: 10.1016/j.mito.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Yang HL, Chen CS, Chang WH, et al. Growth inhibition and induction of apoptosis in MCF-7 breast cancer cells by Antrodia camphorata. Cancer Lett. 2006;231:215–27. doi: 10.1016/j.canlet.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Begin ME, Das UN, Ells G, Horrobin DF. Selective killing of tumor cells by polyunsaturated fatty acids. Prostaglandins Leukotrienes Med. 1985;19:177–86. doi: 10.1016/0262-1746(85)90084-8. [DOI] [PubMed] [Google Scholar]
- 23.Begin ME, Ells G, Das UN, Horrobin DF. Differential killing of human carcinoma cells supplemented with n-3 and n-6 polyunsaturated fatty acids. J Natl Cancer Inst. 1986;77:1053–62. [PubMed] [Google Scholar]
- 24.Begin ME, Das UN, Ells G. Cytotoxic effects of essential fatty acids (EFA) in mixed cultures of normal and malignant human cells. Prog Lipid Res. 1986;25:573–5. [Google Scholar]
- 25.Geelen A, Schouten JM, Kamphuis C, et al. Fish consumption, n-3 fatty acids, and colorectal cancer: a meta-analysis of prospective cohort studies. Am J Epidemiol. 2007;166:1116–25. doi: 10.1093/aje/kwm197. [DOI] [PubMed] [Google Scholar]
- 26.Kroemer G. The mitochondrial permeability transition pore complex as a pharmacological target. An introduction. Curr Med Chem. 2003;10:1469–72. doi: 10.2174/0929867033457232. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki Y, Imai Y, Nakayama H, Takahashi K, Takio K, Takahashi R. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol Cell. 2001;8:613–21. doi: 10.1016/s1097-2765(01)00341-0. [DOI] [PubMed] [Google Scholar]
- 28.Zou X, Liu SL, Zhou JY, Wu J, Ling BF, Wang RP. Beta-asarone induces LoVo colon cancer cell apoptosis by up-regulation of caspases through a mitochondrial pathway in vitro and in vivo. Asian Pac J Cancer Prev. 2012;13:5291–8. doi: 10.7314/apjcp.2012.13.10.5291. [DOI] [PubMed] [Google Scholar]
- 29.Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria as targets for cancer chemotherapy. Semin Cancer Biol. 2009;19:57–66. doi: 10.1016/j.semcancer.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Sangeetha PS, Das UN. Gamma-linolenic acid and eicosapentaenoic acid potentiate the cytotoxicity of anti-cancer drugs on human cervical carcinoma (HeLa) cells in vitro. Med Sci Res. 1993;21:457–9. [Google Scholar]
- 31.Madhavi N, Das UN. Effect of n-6 and n-3 fatty acids on the survival of vincristine sensitive and resistant human cervical carcinoma cells in vitro. Cancer Lett. 1994;84:31–41. doi: 10.1016/0304-3835(94)90355-7. [DOI] [PubMed] [Google Scholar]
- 32.Madhavi N, Das UN. Reversal of KB-3-1 and KB-Ch-8-5 tumor cell drug-resistance by cis-unsaturated fatty acids in vitro. Med Sci Res. 1994;22:689–92. [Google Scholar]
- 33.Das UN, Madhavi N, Padma M, Sagar PS. Can tumor cell drug-resistance be reversed by essential fatty acids and their metabolites? Prostaglandins Leukot Essen Fatty Acids. 1998;58:39–54. doi: 10.1016/s0952-3278(98)90128-4. [DOI] [PubMed] [Google Scholar]
- 34.Kong X, Ge H, Chen L, et al. Gamma-linolenic acid modulates the response of multidrug-resistant K562 leukemic cells to anticancer drugs. Toxicol In Vitro. 2009;23:634–9. doi: 10.1016/j.tiv.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Mackie SJ, Sharma DM, Cooper AJ, Harris NM, Lwaleed BA. Meglumine eicosapentaenoic acid (MeEPA) a new soluble omega-3 fatty acid formulation: in vitro bladder cancer cytotoxicity tests in combination with epirubicin and mitomycin. Prostaglandins Leukot Essent Fatty Acids. 2006;75:367–73. doi: 10.1016/j.plefa.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Das UN, Madhavi N. Effect of polyunsaturated fatty acids on drug-sensitive and resistant tumor cells in vitro. Lipids Health Dis. 2011;10:159. doi: 10.1186/1476-511X-10-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cicero AFG, Reggi A, Parini A, Borghi C. Application of polyunsaturated fatty acids in internal medicine: beyond the established cardiovascular effects. Arch Med Sci. 2012;5:784–93. doi: 10.5114/aoms.2012.31613. [DOI] [PMC free article] [PubMed] [Google Scholar]