Abstract
In most animals that have X and Y sex chromosomes, chromosome-wide mechanisms are used to balance X-linked gene expression in males and females. In the fly Drosophila melanogaster, the dosage compensation mechanism also generally extends to X-linked transgenes. Over 70 transgenic lines of the Australian sheep blowfly Lucilia cuprina have been made as part of an effort to develop male-only strains for a genetic control program of this major pest of sheep. All lines carry a constitutively expressed fluorescent protein marker gene. In all 12 X-linked lines, female larvae show brighter fluorescence than male larvae, suggesting the marker gene is not dosage compensated. This has been confirmed by quantitative RT-PCR for selected lines. To determine if endogenous X-linked genes are dosage compensated, we isolated 8 genes that are orthologs of genes that are on the fourth chromosome in D. melanogaster. Recent evidence suggests that the D. melanogaster fourth chromosome, or Muller element F, is the ancestral X chromosome in Diptera that has reverted to an autosome in Drosophila species. We show by quantitative PCR of male and female DNA that 6 of the 8 linkage group F genes reside on the X chromosome in L. cuprina. The other two Muller element F genes were found to be autosomal in L. cuprina, whereas two Muller element B genes were found on the same region of the X chromosome as the L. cuprina orthologs of the D. melanogaster Ephrin and gawky genes. We find that the L. cuprina X chromosome genes are equally expressed in males and females (i.e., fully dosage compensated). Thus, unlike in Drosophila, it appears that the Lucilia dosage compensation system is specific for genes endogenous to the X chromosome and cannot be co-opted by recently arrived transgenes.
Introduction
Very different mechanisms are used in the fly Drosophila melanogaster, the nematode Caenorhabditis elegans and in mammals to achieve X chromosome dosage compensation [1–3]. In D. melanogaster the male-specific lethal or MSL complex is essential for dosage compensation [4, 5]. In males, the MSL complex binds to active genes and upregulates expression, thereby achieving dosage compensation [6, 7]. The MSL complex is thought to initially bind to 150–300 high affinity or chromatin entry sites on the X chromosome and then spread to actively transcribed genes [4]. Thus most genes on the X chromosome do not need to bind the MSL complex with high affinity in order to be compensated. Consistent with this model is the finding that, in general, X-linked transgenes are dosage compensated [8–10]. For example, Kuroda and colleagues studied X-linked insertions of the autosomal cg3702 and Rpl40 genes [9]. Neither gene attracted the MSL complex at their endogenous position on chromosome 2. However, at X chromosome locations both the cg3702 and Rpl40 genes were bound by the MSL complex in males and dosage compensated.
The Australian sheep blowfly, Lucilia cuprina, is a major pest of sheep in Australia and New Zealand [11]. Since it is a serious pest, there was a significant effort to produce detailed physical and genetic maps of the chromosomes. L. cuprina has five pairs of metacentric autosomes of approximately equal size [12, 13]. C-banding of L. cuprina chromosomes from late third instar larval neuroblasts produces procentric bands on all autosomes and deep staining over the Y chromosome and most of the X chromosome [13]. The X chromosome is the longest chromosome and the Y is the shortest chromosome, less than half the length of the X chromosome. The distal third of the L. cuprina X chromosome is lighter staining and one morphological marker (black body, b) maps to this region (Fig 1) [13, 14]. Genetic analysis of 72 loci facilitated linkage group-chromosome assignments in L. cuprina [15] (Fig 1). The six linkage groups or Muller elements (A-F) have been generally conserved in higher Diptera. For example, orthologs of genes encoded by the right arm of chromosome 3 in D. melanogaster (linkage group E) were mapped to chromosome 4 in L. cuprina (Fig 1). A major difference is that the genes that are X-linked in D. melanogaster (linkage group A), are autosomal in L. cuprina and map to chromosome 3 (Fig 1). It has been shown that orthologs of genes located on the fourth chromosome in D. melanogaster (linkage group F), are X-linked in some non-drosophilids (e.g. tephritids) [16, 17]. It appears that the Muller element F was an ancestral X chromosome in Diptera but that in the lineage leading to D. melanogaster it has reverted to an autosome [16].
Fig 1. Schematic illustration of male metaphase chromosomes in D. melanogaster and L. cuprina.
Muller elements (A-F) are indicated. The chromosomal location of linkage group F genes in L. cuprina was unclear from earlier genetic analysis but it has been suggested that these genes are on the X chromosome (this uncertainty is indicated by a question mark). In L. cuprina, C-banding produces dark staining of the sex chromosomes, except for a lighter staining region in the distal portion of the long arm of the X chromosome that is thought to contain active genes [13]. While the X chromosome is the largest chromosome, it is not drawn to scale so as to highlight the C-banding pattern.
Over the past decade we have made over 70 transgenic L. cuprina lines as part of our effort to make male-only strains for a genetic control program [11, 18]. In general, the lines either express the tetracycline transactivator (tTA) or carry a tTA-regulated proapoptotic gene such hid or reaper [18, 19]. Twelve of the lines showed X-linked inheritance [20–23]. That is, when transgenic males were mated with virgin females from the parental wild type strain, only female offspring inherited the transgene. We were interested in determining if X-linked genes and transgenes were dosage compensated in L. cuprina. Our initial aim was to determine if the marker gene was dosage compensated in transgenic L. cuprina lines that map to the X chromosome. If X chromosome gene dosage compensation is achieved by a similar mechanism as in D. melanogaster, then it would be predicted that X-linked transgenes would be expressed equally in males and females in L. cuprina. With the goal of obtaining endogenous genes that are X-linked, our second aim was to identify L. cuprina genes that are orthologs of genes encoded by the fourth chromosome in D. melanogaster and determine if these genes were X-linked. If so, our third aim was to determine if the X-linked genes are expressed at equal levels in males and females (i.e. dosage compensated). We identified 8 orthologs of Drosophila fourth chromosome genes and show that 6 are located on the X chromosome in L. cuprina and fully dosage compensated. We also show that, unlike Drosophila, X-linked transgenes are not fully compensated. We suggest that this is because L. cuprina employs a novel dosage compensation mechanism.
Results
X-linked transgenes are not fully dosage compensated
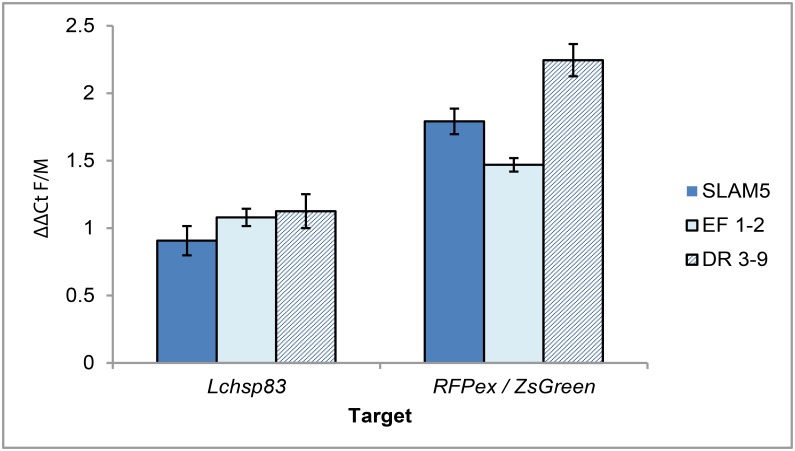
The transgenic L.cuprina lines carry either a ZsGreen or DsRed-express2 (RFPex) fluorescent protein marker gene driven by the L. cuprina hsp83 gene promoter that has a high basal activity [24]. Further details on the marker gene and any additional transgene in the X-linked lines are listed in S1 Table. Lines were bred to homozygosity by selecting for very brightly fluorescing larvae. In the X-linked lines, all of the brightly fluorescing larvae that are selected to make a homozygous line uniformly developed as females (Fig 2). With insertions on autosomes, approximately half of the most brightly fluorescent larvae develop as females and half as males (Fig 2). The concordance between fluorescence intensity and sex is strongly suggestive that X-linked marker gene expression is not fully dosage compensated. We next used quantitative RT-PCR (qRT-PCR) to confirm a lack of uniformly complete compensation. Four lines, SLAM5, DR3-9, EF1-2 and FL3-3 were selected for these experiments. In two lines, (SLAM5 and DR3-9) marker gene expression was twice as high in females compared to males (Fig 3). Thus, transgene expression is not dosage compensated. In a third line (EF1-2), females had 1.5 times as much transcript as males, suggesting partial compensation. In a fourth line (FL3-3) ZsGreen transcript levels were highly variable and much higher in females than males (5–14 times higher, not shown in Fig 3). In this line, the transgene has inserted into highly repetitive DNA and the marker shows variable and variegated patterns of expression (not shown).
Fig 2. L. cuprina larvae with X-linked or autosomal fluorescent protein transgenes.
The lines carry a constitutively expressed DsRed-express2 marker gene. Larvae from an X-linked line (SLAM5) and an autosomal line (EF3C) are shown. In the X-linked line, the most brightly fluorescent larvae develop as females (F). The more weakly fluorescent larvae develop as males (M). In contrast, larvae from the EF3C line show a uniform level of fluorescence. Two representative larvae from this line are shown, the sex of the larvae is unknown.
Fig 3. X-linked transgenes are not fully dosage compensated in L. cuprina.
qRT-PCR of marker gene (ZsGreen or DsRed-express2) RNA in hemisected adult males and females from the transgenic lines SLAM5, EF 1–2 or DR3-9. All lines carry a single copy of the marker gene on the X chromosome. Transcript levels were normalized to 28S rRNA. As expression of the marker genes was driven by the constitutive promoter from the autosomal Lchsp83 gene, Lchsp83 transcript levels are shown for comparison. Mean female/male ratio +/- standard error from three biologically independent replicate experiments are shown.
Orthologs of some D. melanogaster fourth chromosome genes are X-linked in L. cuprina
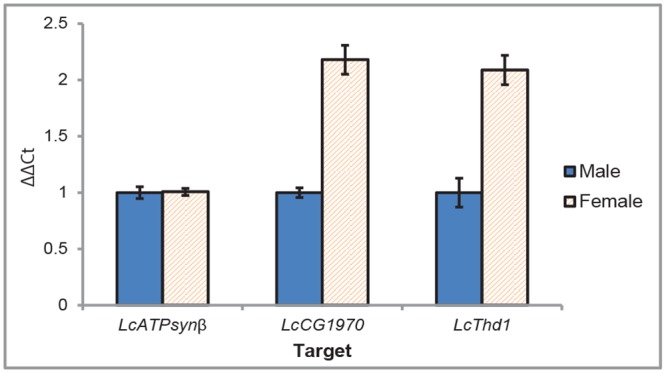
To investigate if linkage group F genes are X-linked in L. cuprina, we identified orthologs of Drosophila fourth chromosome genes in transcriptomes from the closely related blowfly, Lucilia sericata [25]. Most genes were identified from an embryo transcriptome, as this had the greatest depth of coverage. Using primers based on the L. sericata sequences, fragments of the homologous L. cuprina genes were amplified from genomic DNA, cloned, and sequenced. The genes isolated were the L. cuprina orthologs of, ATP synthase-β subunit (ATPsynβ), CG1970, Eph receptor tyrosine kinase (Eph), Ephrin, Slip1, Thd1 and Zn finger homeodomain 2 (zfh2). Quantitative PCR (qPCR) with male and female genomic DNA was used to determine X chromosome association. An X-linked gene would be twice as abundant in female relative to male DNA. In contrast, the copy number of an autosome gene would be the same in male and female DNA. Thus the expectation is that the male/female ratio of normalized Ct values would be either 0.5 or 1.0. For every gene, only one of the competing hypothetical ratios is contained by the calculated 95% confidence intervals (Table 1), so that there is little ambiguity as to whether or not the gene is sex-linked. We found that five of the L. cuprina (Lc) genes, LcCG1970, LcEph, LcSlip1, LcThd1 and Lczfh2 were associated with the X chromosome (Fig 4, Table 1). LcATPsynβ and LcEphrin are autosomal in L. cuprina. In addition, qPCR with male and female DNA confirmed the X-linked transgenes were associated with the X chromosome (Table 1). We also confirmed that the L. cuprina reference genes used in gene expression studies [26], LcGST-1 and Lchsp83 are autosomal (S2 Table). Further, males and females have equal levels of the 28S rRNA gene reference gene. As in Drosophila, rRNA genes are present on both the X and Y chromosomes in L. cuprina [27].
Table 1. Relative abundance of candidate X-linked genes and known X-linked transgenes in male and female genomic DNA.
| Gene | ΔΔCt Male d ± SEM | ΔΔCt Female± SEM | M/F Ratio (95% CI) | p-value M-F/2 = 0 e | p-value M-F = 0 e |
|---|---|---|---|---|---|
| Lcaru a | 1.0 ± 0.078 | 2.108 ± 0.129 | 0.475 (0.354–0.596) | 0.616 | 0.00073 |
| LcATPsynβ a | 1.0 ± 0.052 | 1.006 ± 0.031 | 0.994 (0.84–1.148) | 0.00026 | 0.925 |
| LcCG1970 a | 1.0 ± 0.043 | 2.179 ± 0.128 | 0.459 (0.373-.0545) | 0.298 | 0.00033 |
| LcEph b | 1.0 ± 0.048 | 1.986 ± 0.092 | 0.503 (0.417–0.589) | 0.92 | 0.00022 |
| LcEphrin b | 1.0 ± 0.028 | 1.076 ± 0.038 | 0.930 (0.822–1.037) | 0.00004 | 0.168 |
| Lcgw a | 1.0 ± 0.047 | 2.284 ± 0.111 | 0.438 (0.362–0.514) | 0.108 | 0.00013 |
| Lchsp83 c | 1.0 ± 0.017 | 0.953 ± 0.035 | 1.049 (0.94–1.158) | 0.00001 | 0.281 |
| LcJwa | 1.0 ± 0.0549 | 2.08 ± 0.0506 | 0.481 (0.406–0.555) | 0.5368 | 0.00003 |
| LcSlp1 b | 1.0 ± 0.066 | 2.127 ± 0.042 | 0.470 (0.386–0.553) | 0.401 | 0.00003 |
| LcThd1 b | 1.0 ± 0.128 | 2.088 ± 0.130 | 0.479 (0.304–0.654) | 0.771 | 0.0019 |
| Lczfh2 b | 1.0 ± 0.071 | 2.030 ± 0.053 | 0.493 (0.397–0.589) | 0.85 | 0.00008 |
| RFPex b | 1.0 ± 0.062 | 1.775 ± 0.036 | 0.563 (0.468–0.657) | 0.142 | 0.00012 |
| ZsGreen c | 1.0 ± 0.039 | 1.938 ± 0.111 | 0.516 (0.424–0.608) | 0.667 | 0.0005 |
a,b,cSuperscript indicates strain that was the source of genomic DNA for analysis.
a: wild type,
b: SLAM5, and
c: DR3-9.
d. For each gene the male sample was chosen as the control/ calibrator sample and set to equal 1.0.
e. Two p-values for each gene were calculated from the competing hypotheses that either the Male-Female/2 difference was zero or that the Male-female difference was zero. The latter would be expected for a M/F ratio of one.
Fig 4. Linkage group F genes are X-linked in L. cuprina.
qPCR of orthologs of Drosophila fourth chromosome genes with male and female L. cuprina genomic DNA. An X-linked gene is twice as abundant in female DNA relative to male DNA.
X-linked F-element genes are fully dosage compensated
qRT-PCR was used to determine if the X-linked genes were dosage compensated in L. cuprina. Young adults (1–4 day) were hemisected and RNA was isolated from heads and thoraces. Abdomens were discarded as they contain most of the sexually dimorphic tissues. If an X-linked gene is fully dosage compensated, the expectation is that males and females would express equal levels of mRNA transcripts. If, however, expression was not compensated then females would express twice the level of RNA than males. Student's t-tests were performed for each gene separately to investigate the hypothesis that male/female ratios were one. We found no evidence for departure from unity. That is, the five genes initially identified as on the X chromosome, LcCG1970, LcEph, LcSlip1, LcThd1 and Lczfh2 are all fully dosage compensated (Table 2).
Table 2. Dosage compensation of X chromosome genes in L. cuprina .
| Gene | Relative Expression Male:Female ± SEM a | p-value M/F ratio ≠ 1 b |
|---|---|---|
| Lcaru c | 1.179 ± 0.067 | 0.116 |
| LcCG1970 | 0.967 ± 0.060 | 0.799 |
| LcEph | 0.975 ± 0.058 | 0.866 |
| Lcgw | 1.164 ± 0.132 | 0.34 |
| LcSlip1 | 0.999 ± 0.068 | 0.992 |
| LcThd1 | 0.887 ± 0.120 | 0.297 |
| Lczfh2 | 0.998 ± 0.076 | 0.981 |
a. Mean of 3 independent experiments. Expression was normalized to the 28S rRNA reference gene.
b. Student's t-tests were performed for each gene separately
c. With the exception of Lcaru, all genes are orthologs of genes located on the fourth chromosome in D. melanogaster. The D. melanogaster aru gene is located on chromosome 2.
X chromosome genes do not appear to be highly clustered
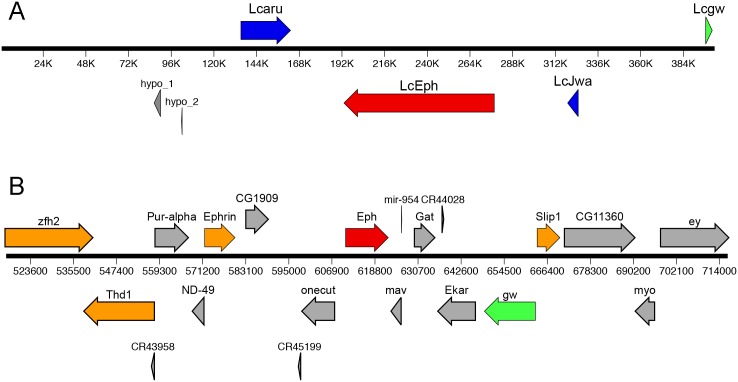
One explanation for our results is that the fully compensated genes are clustered within a small region of the large X chromosome and that by chance none of the transgenes are within this compensated chromatin domain. Subsequent to our isolation of L. cuprina linkage group F genes, a draft assembly of the L. cuprina genome became available as part of the i5k project [28, 29]. All five genes we identified as on the X chromosome mapped to different scaffolds of the L. cuprina genome. The scaffold sizes containing X-linked genes ranged from 30 to 904 kb, with an average of size of 357 kb. As the assembly is at a draft stage, a comprehensive analysis of X chromosome genomic regions is premature. Nevertheless, we have analyzed the scaffolds containing the five genes in greater detail. The L. cuprina orthologs of the D. melanogaster fourth chromosome genes CG1909 and maverick (mav) were found on the same scaffold (#114) that contained LcCG1970. Similarly part of the ortholog of the Drosophila gawky (gw) gene, Lcgw, was found on the same scaffold (#328) as LcEph. The other part of the Lcgw gene was found on a different scaffold (#1414). Orthologs of D. melanogaster fourth chromosome genes were not found on the scaffolds that contained the LcSlip1, LcThd1 or Lczfh2 genes. With the exception of the Lczfh2 gene, the Lucilia X-linked genes are larger than their corresponding Drosophila ortholog (at least twice the size). To compare the Drosophila and Lucilia Eph chromosomal regions, the 402kb scaffold 328 was examined in greater detail. Genes were identified by searching for homology to L. sericata transcriptomes from different life stages [25]. In addition to the LcEph and Lcgw genes, we found the L. cuprina orthologs of the arouser (aru) and Jwa genes in scaffold 328 (Fig 5A). The L. cuprina genome project identified two additional genes in scaffold 328 that code for novel small hypothetical proteins (Fig 5A). We did not examine these genes further as transcripts were not found in the L. sericata transcriptomes and the no orthologous genes were identified in the D. melanogaster genome. In contrast to the few genes present in the scaffold containing the LcEph gene, over 20 protein-coding genes are located in a 200 kb region that contains Eph in D. melanogaster [30] (Fig 5B). Interestingly, the D. melanogaster aru and Jwa genes are not located on the fourth chromosome but are found on the left arm of chromosome 2 (linkage group B). The LcEph, Lcaru and LcJwa genes are 3–5 times larger than their corresponding Drosophila orthologs, due to an increase in size and number of introns. We confirmed that the Lcgw, Lcaru and LcJwa genes were associated with the X chromosome by qPCR with male and female genomic DNA (Table 1). In addition, qRT-PCR of male and female RNA from hemisected adults showed that the Lcgw and Lcaru genes are fully compensated (Table 2).
Fig 5. A comparison of the Eph genetic region in L. cuprina and D. melanogaster.
The length and direction of arrows indicates the sizes of genes and direction of transcription. Genes in the L. cuprina 402 kb scaffold 328 (A) and 200 kb region of the D. melanogaster fourth chromosome containing the Eph gene (B) are shown. To facilitate comparison, LcEph and Eph are shown with red arrows whereas Lcgw and gw are in green. The D. melanogaster orthologs of L. cuprina genes that were part of this study are in orange. The Lcaru and LcJwa genes are in blue. The orthologous aru and Jwa genes are located on chromosome 2L in D. melanogaster. The approximate gene boundaries shown for the L. cuprina genes are based on alignments of transcripts with the genome sequence but remain to be experimentally confirmed.
Discussion
In the Australian sheep blowfly L. cuprina, the X chromosome is the largest chromosome and is mostly heterochromatic except for the lighter staining distal portion of the long arm [13]. Since L. cuprina is a major economic pest, a large effort was made to develop accurate genetic maps based on over 70 morphological and enzymatic markers [15]. Despite the relative ease of identifying X-linked mutations, only one gene was previously mapped to the X chromosome [14]. This suggested that the X chromosome had few genes compared to the autosomes. There are about 100 genes on the fourth chromosome in Drosophila species [31]. Thus, the apparent low number of X-linked genes in L. cuprina is consistent with the hypothesis that the X chromosome in non-drosophilid higher Diptera corresponds to the fourth chromosome in Drosophila [16]. Our study supports this hypothesis as we found that six of eight orthologs of Drosophila fourth chromosome genes were X-linked in L. cuprina. Consistent with our findings, it has recently been reported that the X chromosome in L. sericata contains linkage group F genes [32]. As the X chromosome accounts for 12.3% of the 570Mb male genome [33], this gives a size of 70Mb, much larger than the 4.4Mb D. melanogaster fourth chromosome. The distal 1.2Mb of the long arm of the D. melanogaster fourth chromosome is polytenized in salivary glands with 80 genes, a similar gene density as autosomes [31]. Similarly, it is possible that the active genes are located within the lighter staining region of the L. cuprina X chromosome. However, it would appear that gene density would be lower on the L. cuprina X chromosome than the Drosophila fourth chromosome. Consistent with this suggestion, the X-linked genes all mapped to a different scaffold in a draft assembly of the L. cuprina genome. Further, an analysis of a 400 kb scaffold containing the LcEph gene found far fewer protein-coding genes than are present in the region containing the Eph gene in D. melanogaster. Most of the Lucilia X-linked genes were larger than the corresponding orthologous Drosophila genes. For example, at approximately 85 kb, the LcEph gene is more than 7 times longer than the D. melanogaster Eph gene. However, the mean gene length for a protein-coding gene in L. cuprina is 12.197 kb [28], whereas the mean size is 6.935 kb in D. melanogaster [34]. Thus, it is not clear if the larger gene size of the few Lucilia X-linked genes we have studied is a general feature of the X chromosome or simply reflects that, on average, genes are longer in Lucilia than in Drosophila. Interestingly, two of the four protein-coding genes on the LcEph scaffold, Lcaru and LcJwa, are not orthologs of D. melanogaster fourth chromosome genes. Rather, the aru and Jwa genes are on 2L in D. melanogaster. So it is not only linkage group F genes that are on the X chromosome in L. cuprina. A more complete genome assembly will facilitate further analysis of X chromosome organization in L. cuprina.
An early study of radiation-induced deletion mutants suggested that X-linked genes were at least partially compensated in L. cuprina [14]. The deletion mutants contained the black body (b) gene, the one known X-linked morphological marker. Several of the X chromosome deficiencies uncovered vital genes linked to b. Further, females heterozygous for these deletions showed a characteristic phenotype of small body size, wing vein irregularities and variable fertility. Thus the lighter staining distal third of the X chromosome contains vital genes, which appear to be at least partially dosage compensated. We find that endogenous X-linked genes are fully compensated. In Drosophila, the male specific lethal (MSL) complex is required for X chromosome dosage compensation [4]. Somewhat surprisingly, Drosophila appears to employ an additional balancing mechanism to regulate gene expression of fourth chromosome genes [35]. The existence of such a mechanism was initially suggested to explain the viability of flies that are haploid for chromosome 4 (4/0) [36]. The Painting of fourth (POF) protein binds almost exclusively to the euchromatic portion of the fourth chromosome [37]. HP1a co-localizes with POF and in addition binds to the heterochromatic pericentromeric regions of chromosome 4 [38]. Loss of POF results in a significant decrease in fourth chromosome gene expression, suggesting POF has a stimulatory effect [35]. In contrast, HP1a appears to have a repressive effect on fourth chromosome gene expression. Thus the balancing mechanism somehow involves an interplay of POF and HP1a. The importance of pof for fourth chromosome dosage compensation is highlighted by the observation that 4/0 flies that are homozygous for a pof null allele are not viable [35]. It has been suggested that this dosage compensation mechanism is an evolutionary holdover from a Drosophila ancestor that employed pof to regulate gene expression of X-linked genes [16]. If so, this suggests that pof may play an important role in X chromosome dosage compensation in L. cuprina. This hypothesis could be tested by making a deletion mutation of the L. cuprina pof gene using CRISPR/Cas9 technology [39].
X chromosome dosage compensation can be achieved by increasing gene expression in males or decreasing expression in females. The majority of evidence shows that the Drosophila MSL complex acts by increasing expression of X-linked genes in males. There is some evidence that dosage compensation in L. cuprina may also be achieved by increasing expression in males. L. cuprina pupal trichogen cells contain large banded polytene chromosomes that have been used to develop detailed chromosome maps for all autosomes [40]. However, the X chromosomes in these preparations does not polytenize [41]. A portion of the X chromosome forms a loose granular structure, which is thought to correspond to the lightly stained C banded region of the long arm. The granular structure is observed in nuclei from male and female pupae. However, in XXY male pupae, one of the X chromosomes does not form the granular structure but instead is polytenized [41]. It was suggested that the polytene structure was less favorable for gene expression than the loose granular structure. If so, the formation of the polytene structure may have been in response to an increase in gene expression from both X chromosomes in XXY males.
In D. melanogaster, X-linked transgenes are often dosage compensated [8, 9]. This has been interpreted as a consequence of how the MSL complex regulates the expression of genes on the X chromosome. The MSL complex is thought to initially bind to high affinity or chromatin entry sites and then spread in cis to active genes, which could involve recognition of modified histone tails via the chromodomain of MSL3 [4]. In this way active X-linked transgenes would also be regulated by the MSL complex. In contrast, we find that X-linked transgenes are generally not dosage compensated in L. cuprina. We suggest three possible explanations for these results. Firstly, the X chromosome dosage compensation system in L. cuprina could be optimized for endogenous genes, which reside in a largely heterochromatic X chromosome environment. There is evidence that the POF/HP1a balancing system may be specific for fourth chromosome genes in D. melanogaster. A large number of transgenic lines have been obtained with fourth chromosome insertions of a white (w) transgene [31]. In many of these lines, eyes show variegated pigmentation, suggesting the w transgene has inserted in a relatively repressive chromatin environment. Indeed, the transgenes had often inserted into regions with high levels of bound HP1 in S2 cells [37]. However, the locations of the silenced transgenes did not correlate with regions where the chromosome 4 genes were silenced or weakly expressed. Given the stimulatory role of POF, it would be anticipated that in those lines that show whole eye pigmentation, the w transgene has inserted within or near a region with high levels of bound POF. However, in lines that show a wild type red eye phenotype, the w transgene has integrated into regions with low levels of POF. These results suggest that the fourth chromosome genes respond to POF/HP1a in a way that transgenes cannot [31, 37]. An alternative explanation for our observations is that the Lchsp83 promoter that is driving fluorescent protein gene expression in the transgenes, cannot respond to the X chromosome dosage compensation machinery. We previously showed that the nature of the gene promoter is important in determining if an X-linked transgene is dosage compensated in D. melanogaster [42]. A lacZ transgene driven by the constitutive armadillo (arm) promoter was fully dosage compensated at several locations on the X chromosome. In contrast, a lacZ transgene driven by the neuronal GMR-hsp70 enhancer/promoter was not dosage compensated when integrated at the same X chromosome locations as the arm-lacZ transgene. However, the D. pseudoobscura hsp83 gene was fully dosage compensated at X chromosome sites in D. melanogaster [43, 44]. Thus the Drosophila hsp83 promoter does respond to the MSL complex. A third explanation for the lack of compensation of X-linked transgenes is that the dosage compensation mechanism acts regionally rather than chromosome-wide. If the active genes are confined to the lighter staining distal portion of the long arm of the X chromosome, it would not be necessary to have a chromosome-wide dosage compensation mechanism. Given the large size of the X chromosome, it is possible than none of the twelve X-linked transgenes have integrated into a compensated chromatin domain. That is, none of the transgenes may have integrated into the lighter staining region of the X chromosome. A test of this model would be to insert a transgene directly adjacent to a compensated X-linked gene using CRISPR/Cas9 technology.
Studies on X chromosome gene expression in L. cuprina could have a practical application for control of this major pest of sheep. msl2 RNA is not translated in female D. melanogaster [45]. Expression of MSL2 protein in females delays development and reduces viability [46]. Thus it may be possible to employ the L. cuprina dosage compensation machinery to make a male-only strain, which is advantageous for a genetic control program [18].
Materials and Methods
Insect rearing
L. cuprina larvae were reared on ground meat (90% lean meat/ 10% fat) and adults fed water, sugar and protein-rich biscuit as described previously [20]. Typically, 50 g of meat was used for rearing 100 mg of first instar larvae. Tetracycline (100 μg/mL) was added to the larval and adult diet for the FL3#3 line [21]. The progeny of each cross was screened at the late embryo/early first instar larval for green or red fluorescence using a Leica M165FC microscope with the appropriate filter set (GFP2 filter [ex480/40, em LP510 nm], DsRed filter [ex545/25, em 595/50 nm]). Images were captured using a Leica DFC500 digital camera and images initially analyzed using the Leica LAS v4.0 application suite. For RNA isolation, adults were fed sugar and water after eclosion and collected at 1–4 days old. Flies were anesthesized with CO2, sorted by sex, and hemisected. Heads and thoraxes were snap frozen in liquid nitrogen and stored at -80°C until processing.
Genomic DNA isolation
Five to 6 frozen head/thoraxes were ground to powder with a mortar and pestle under liquid nitrogen. Powder was dissolved in 4 mL STE buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 10 mM EDTA, pH 8). Two hundred μL 10% SDS and 8μL RNase A (Cat# R4642 Sigma Aldrich St. Louis, Missouri) were added and samples were incubated at 56°C. After 30 min, Proteinase K (Cat# P2308 Sigma Aldrich) was added to 100 μg/mL and the sample was incubated overnight at 56°C. Three mL phenol:chloroform:isoamyl alcohol [25:24:1] (Cat#P2069, Sigma) was added and samples were rotated 10 min at room temperature (RT). Samples were then centrifuged 10 min at 3000 RPM at 4°C. The aqueous layer was transferred to a new tube. The extraction was repeated. One tenth volume 3M NaAcetate, pH 5.2 and 2 volumes cold 100% ethanol were added and the samples were inverted 2–3 times. The samples were incubated at -20°C for 1 h and centrifuged 30 min at 6000 RPM at 4°C. The supernatant was removed from the pellet and 1 mL cold 75% ethanol was added. The samples were centrifuged 10 min at 6000 RPM at 4°C. The supernatant was removed from the pellet and it was allowed to air dry 10 min. The last of the supernatant was removed and the pellet was allowed to air dry 10 additional minutes before being resuspended in 50–100 μL TE Buffer.
Primer design and efficiency testing
First, primers were designed for a 300–600bp region of the largest exon of candidate X-linked genes identified in L. sericata transcriptomes [25]. Five hundred ng of genomic DNA from the L. cuprina LA07 wild type strain (parental strain for transgenic lines) was used as template for PCR with MangoTaq (Cat# BIO-21083 Bioline, London, UK). Cycling conditions were: (95°C 3 min, [95°C 30 sec, 55°C 30 sec, 72°C 1 min] 30x, 72°C 8 min). For some primer sets, PCR was performed using OneTaq Hot Start 2X Master Mix (Cat#M0484S New England Biolabs) (94°C 3 min, [94°C 15 sec, 55°C 30 sec, 68°C 1 min] 30x, 68°C 5 min). PCR products were resolved on a 1% agarose gel and band slices were removed. Bands were gel purified, cloned into pGEMT-Easy (Promega, Fitchburg, WI, USA) and sequenced. The resulting sequence was used to design qPCR primers for each gene candidate. Primers were tested for efficiency by creating a dilution series from 0.00005ng- 500ng template genomic DNA per well. Thermo Maxima SYBR Green/ Rox qPCR Master Mix 2X (Cat#K0221 Thermo Fisher Scientific Waltham, Massachusetts) was used with the indicated primers to create a master mix. Template was added in quadruplicate wells of a 384 well optical plate (Cat#4309849 Applied Biosystems/ Life Technologies/ Thermo Fisher Scientific Waltham, Massachusetts), followed by master mix, which was added by a multi-channel pipet. The plate was sealed (Cat#4311971 Applied Biosystems/ Life Technologies/ Thermo Fisher Scientific Waltham, Massachusetts), vortexed, centrifuged 1 min at RT at 3400 RPM, and run. The run was performed on a BioRad CFX384 C1000 Touch Thermocycler (BioRad Hercules, CA) using the following program: 95°C 10 min, [95°C 15 s, 60°C 60 sec] 40x. Data acquisition was performed on the anneal/ extension step.
Primer efficiency was determined by plotting the log of the ng of template on the X-axis and the mean Ct of the quadruplicate replicates on the Y-axis. The slope of the best fit line was used in the following equation to calculate efficiency: [Efficiency = -1+10(-1/slope)]. Primers were accepted if efficiency was 90–105% and re-designed if efficiency fell outside this range. The oligonucleotide primers used in this study are listed in S3 Table.
RNA isolation
Two frozen head/thoraxes were homogenized in 500 μL of Trizol (Cat#15596026 Life Technologies/ Thermo Fisher Scientific Waltham, Massachusetts) in a 1 mL glass homogenizer that had previously been baked at 200°C overnight. 100 μL of chloroform was added, and samples were shaken for 15 s and allowed to incubate at RT for 15 min. Subsequently, samples were centrifuged at 13,000 RPM 15 minutes at 4°C. The aqueous layer was mixed with an equal volume cold RNase-free 70% ethanol, mixed vigorously, and loaded on onto a Qiagen RNeasy Mini Kit column (Cat#74104 Qiagen Venlo, Netherlands). DNAse treatment was performed on-column in accordance with the manufacturer’s recommended protocol using the RNase-free DNAse set (Cat#79254 Qiagen). To achieve complete elimination of DNA contamination, a second in-solution treatment was performed on 30 μg of total RNA using this set, followed by re-purification with the RNeasy mini kit according to manufacturer’s protocol.
qRT-PCR
To synthesize cDNA, 3.5 μg of DNAse treated RNA was utilized with the Superscript III First Strand Synthesis Supermix (Cat#18080–400 Invitrogen/ Life Technologies/ Thermo Fisher Scientific Waltham, Massachusetts) according to kit protocol. Random hexamers were used as primers. Negative control reactions containing water instead of enzyme mix were performed to confirm the absence of DNA contamination.
The cDNA template was diluted 1:4 with nuclease-free water. Thermo Maxima SYBR Green/ Rox qPCR Master Mix 2X (Cat#K0221 Thermo Fisher Scientific Waltham, Massachusetts) was used with the indicated primers to create a master mix. Template was added in quadruplicate wells of a 384 well optical plate (Cat#4309849 Applied Biosystems/ Life Technologies/ Thermo Fisher Scientific Waltham, Massachusetts), followed by master mix, which was added by a multi-channel pipet. The plate was sealed (Cat#4311971 Applied Biosystems/ Life Technologies/ Thermo Fisher Scientific Waltham, Massachusetts), vortexed, centrifuged 1 min at RT at 3400 RPM and run. The run was performed on a BioRad CFX384 C1000 Touch Thermocycler (BioRad Hercules, CA) using the following program: 95°C 10 min, [95°C 15s, 60°C 60 sec] 40x. Data acquisition was performed on the anneal/ extension step.
Data analysis of delta delta Ct was performed using BioRad CFX Manager. Mean Ct value was found for the 4 replicate wells. L. cuprina 28S rRNA was previously determined to be a suitable reference gene by geNORM and Normfinder [47], and was therefore chosen as the reference gene for this study. 28S rRNA was also confirmed in our hands to have a similar expression in L. cuprina EF1-2 males and females as measured by the mean of quadruplicate Ct values (S2 Table). Delta delta Ct was calculated and the male sample was chosen as the control/calibrator sample and set to equal 1.0. Replicate bar graphs represent delta delta Ct, with error bars delineating standard error of the mean. To calculate summary ratio, the male delta delta Ct was divided by the female delta delta Ct. The 3 biological replicates were then averaged and the standard error found.
qPCR
To confirm X-linkage, 5 ng of male or female genomic DNA was utilized as template and cycling conditions were as for qRT-PCR. L. cuprina glutathione S-transferase 1 (LcGST1) was used as the reference gene. LcGST1 was previously determined to be a suitable reference gene for qRT-PCR [47] and we found that the same primer pair efficiently amplified the correct DNA sequence from genomic DNA template. To confirm that LcGST1 was autosomal, we performed qPCR with male and female genomic DNA from line EF1-2, which carries a single copy of a marker gene on the X chromosome. Male/female ratio of mean Ct values with the LcGST1 primer pair from this line gave a ratio of 1.0, as expected (S2 Table). Analysis of delta delta Ct was performed using BioRad CFX Manager. Mean Ct value was found for the 4 replicate wells. The male sample was chosen as the control sample and set to 1. Samples with a male to female ratio close to 0.5 were determined to be X-linked.
L. cuprina gene identification and annotation
To identify orthologs of D. melanogaster fourth chromosome genes, we performed tblastn searches [48] of L. sericata transcriptomes [25] with the D. melanogaster protein sequence. To confirm the correct ortholog had been identified, the L. sericata transcript was translated and a blastp search of annotated D. melanogaster protein database was performed. Gene boundaries were identified by blastn alignments of L. sericata transcripts with the L. cuprina genome [28]. From these alignments, exon-intron boundaries were identified, which generally matched the consensus sequences for 5' and 3' splice sites. For some genes, such as those belonging to multi-gene families, it was uncertain if the Lucilia gene that had been identified was the ortholog of the D. melanogaster fourth chromosome gene. Such genes were not further investigated.
Statistical Analyses
qRT-PCR
To investigate the hypothesis that the Male/Female ratios of RNA are one, Student’s t-tests were performed for each gene separately based on the data from three or four independent replicates. No evidence of departure from unity was found. These tests suffer from low power, however, since they are based on only 3 or 4 replicates. As an alternative, we used a one-way factorial effects model (with PROC GLM in SAS) for the differences of the ratios from unity (ratio minus 1) and tested the hypothesis that all the underlying means for these differences were 0 for every gene. (H0: mu1 = mu2 =…= mu9 = 0) This enables us to pool information about variability in measurement of the ratios over all genes. The degrees of freedom for the tests for each gene go from 2 or 3 in the initial investigation up to 20 for the one-way ANOVA. There is very weak or no evidence of any departure of the ratios from 1. One gene, Lcaru, showed a p-value of less than 0.05 (0.0475) however, after Bonferroni adjustment for multiplicity of genes, the p-value was no longer significant.
qPCR
To determine whether the genes were autosomal or located on a sex chromosome, we used Fieller's method [49] to obtain approximate 95% confidence intervals for the gene-specific Male to Female ratios. In the construction of these intervals, we use multipliers from t-distributions with 5 degrees of freedom (2 for males, whose measurements have been normalized to unity and 3 for females.) Additionally, we computed two p-values for each gene from the competing hypotheses that either the Male-female difference was zero, or that the difference Male-Female/2 was zero based on corresponding t-statistics, again with 5 degrees of freedom.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We thank Jason Osborne for statistical analyses and Richard Meisel and Nadia Singh for comments on the manuscript. Amy Keeter and Jodie White for assistance with fly rearing.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
RJL was supported with start-up funds to MJS from NCSU.
References
- 1. Lucchesi JC. Dosage compensation in flies and worms: the ups and downs of X-chromosome regulation. Current Opinion in Genetics & Development. 1998;8(2):179–84. [DOI] [PubMed] [Google Scholar]
- 2. Akhtar A. Dosage compensation: an intertwined world of RNA and chromatin remodelling. Curr Opin Genet Dev. 2003;13(2):161–9. . [DOI] [PubMed] [Google Scholar]
- 3. Ferrari F, Alekseyenko AA, Park PJ, Kuroda MI. Transcriptional control of a whole chromosome: emerging models for dosage compensation. Nat Struct Mol Biol. 2014;21(2):118–25. 10.1038/nsmb.2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gelbart ME, Kuroda MI. Drosophila dosage compensation: a complex voyage to the X chromosome. Development. 2009;136(9):1399–410. 10.1242/dev.029645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Conrad T, Akhtar A. Dosage compensation in Drosophila melanogaster: epigenetic fine-tuning of chromosome-wide transcription. Nat Rev Genet. 2012;13(2):123–34. 10.1038/nrg3124 . [DOI] [PubMed] [Google Scholar]
- 6. Larschan E, Bishop EP, Kharchenko PV, Core LJ, Lis JT, Park PJ, et al. X chromosome dosage compensation via enhanced transcriptional elongation in Drosophila . Nature. 2011;471(7336):115–8. Epub 2011/03/04. nature09757 [pii] 10.1038/nature09757 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conrad T, Cavalli FM, Vaquerizas JM, Luscombe NM, Akhtar A. Drosophila dosage compensation involves enhanced Pol II recruitment to male X-linked promoters. Science. 2012;337(6095):742–6. 10.1126/science.1221428 . [DOI] [PubMed] [Google Scholar]
- 8. Fitzsimons HL, Henry RA, Scott MJ. Development of an insulated reporter system to search for cis-acting DNA sequences required for dosage compensation in Drosophila . Genetica. 1999;105(3):215–26. [DOI] [PubMed] [Google Scholar]
- 9. Gorchakov AA, Alekseyenko AA, Kharchenko P, Park PJ, Kuroda MI. Long-range spreading of dosage compensation in Drosophila captures transcribed autosomal genes inserted on X. Genes Dev. 2009;23(19):2266–71. Epub 2009/10/03. 23/19/2266 [pii] 10.1101/gad.1840409 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spradling AC, Rubin GM. The effect of chromosomal position on the expression of the Drosophila xanthine dehydrogenase gene. Cell. 1983;34(1):47–57. [DOI] [PubMed] [Google Scholar]
- 11. Sandeman RM, Levot GW, Heath AC, James PJ, Greeff JC, Scott MJ, et al. Control of the sheep blowfly in Australia and New Zealand—are we there yet? Int J Parasitol. 2014;44(12):879–91. 10.1016/j.ijpara.2014.08.009 . [DOI] [PubMed] [Google Scholar]
- 12. Ullerich F-H. Geschlechtschromosomen und Geschlechtsbestimmung bei einigen Calliphorinen (Calliphoridae, Diptera). Chromosoma. 1963;14:45–110. [Google Scholar]
- 13. Bedo DG. C, Q and H-banding in the analysis of Y chromosome rearrangements in Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae). Chromosoma. 1980;77(3):299–308. [DOI] [PubMed] [Google Scholar]
- 14. Maddern RH, Bedo DG. Properties of the sex chromosomes of Lucilia cuprina deduced from radiation studies. Genetica. 1984;63:203–12. [Google Scholar]
- 15. Weller GL, Foster GG. Genetic maps of the sheep blowfly Lucilia cuprina: linkage-group correlations with other dipteran genera. Genome. 1993;36(3):495–506. [DOI] [PubMed] [Google Scholar]
- 16. Vicoso B, Bachtrog D. Reversal of an ancient sex chromosome to an autosome in Drosophila . Nature. 2013;499(7458):332–5. 10.1038/nature12235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Landeen EL, Presgraves DC. Evolution: from autosomes to sex chromosomes—and back. Curr Biol. 2013;23(18):R848–50. 10.1016/j.cub.2013.08.021 . [DOI] [PubMed] [Google Scholar]
- 18. Scott MJ. Development and evaluation of male-only strains of the Australian sheep blowfly, Lucilia cuprina . BMC Genet. 2014;15 Suppl 2:S3 10.1186/1471-2156-15-S2-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heinrich JC, Scott MJ. A repressible female-specific lethal genetic system for making transgenic insect strains suitable for a sterile-release program. Proc Natl Acad Sci U S A. 2000;97(15):8229–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Concha C, Scott MJ. Sexual Development in Lucilia cuprina (Diptera, Calliphoridae) Is Controlled by the Transformer Gene. Genetics. 2009;182(3):785–98. Epub 2009/05/13. genetics.109.100982 [pii] 10.1534/genetics.109.100982 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li F, Wantuch HA, Linger RJ, Belikoff EJ, Scott MJ. Transgenic sexing system for genetic control of the Australian sheep blow fly Lucilia cuprina . Insect Biochem Mol Biol. 2014;51:80–8. 10.1016/j.ibmb.2014.06.001 . [DOI] [PubMed] [Google Scholar]
- 22. Edman RM, Linger RJ, Belikoff EJ, Li F, Sze SH, Tarone AM, et al. Functional characterization of calliphorid cell death genes and cellularization gene promoters for controlling gene expression and cell viability in early embryos. Insect Mol Biol. 2015;24(1):58–70. 10.1111/imb.12135 . [DOI] [PubMed] [Google Scholar]
- 23. Yan Y, Scott MJ. A transgenic embryonic sexing system for the Australian sheep blow fly Lucilia cuprina . Sci Rep. 2015;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Concha C, Belikoff EJ, Carey BL, Li F, Schiemann AH, Scott MJ. Efficient germ-line transformation of the economically important pest species Lucilia cuprina and Lucilia sericata (Diptera, Calliphoridae). Insect Biochemistry & Molecular Biology. 2011;41(1):70–5. Epub 2010/09/28. S0965-1748(10)00212-2 [pii] 10.1016/j.ibmb.2010.09.006 . [DOI] [PubMed] [Google Scholar]
- 25. Sze SH, Dunham JP, Carey B, Chang PL, Li F, Edman RM, et al. A de novo transcriptome assembly of Lucilia sericata (Diptera: Calliphoridae) with predicted alternative splices, single nucleotide polymorphisms and transcript expression estimates. Insect Molecular Biology. 2012;21(2):205–21. Epub 2012/01/31. 10.1111/j.1365-2583.2011.01127.x . [DOI] [PubMed] [Google Scholar]
- 26. Concha C, Edman RM, Belikoff EJ, Schiemann AH, Carey B, Scott MJ. Organization and expression of the Australian sheep blowfly (Lucilia cuprina) hsp23, hsp24, hsp70 and hsp83 genes. Insect Molecular Biology. 2012;21(2):169–80. Epub 2012/04/17. . [DOI] [PubMed] [Google Scholar]
- 27. Bedo DG. Nucleolar fragmentation in polytene trichogen cells of Lucilla cuprina and Chrysomya bezziana (Diptera: Calliphoridae). Genome. 1992;35(2):283–93. [DOI] [PubMed] [Google Scholar]
- 28. Anstead CA, Korhonen PK, Young ND, Hall RS, Jex AR, Murali SC, et al. Lucilia cuprina genome unlocks parasitic fly biology to underpin future interventions. Nat Commun. 2015;6:7344 10.1038/ncomms8344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poelchau M, Childers C, Moore G, Tsavatapalli V, Evans J, Lee CY, et al. The i5k Workspace@NAL—enabling genomic data access, visualization and curation of arthropod genomes. Nucleic Acids Res. 2015;43(Database issue):D714–9. 10.1093/nar/gku983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pierre SE, Ponting L, Stefancsik R, McQuilton P, FlyBase C. FlyBase 102—advanced approaches to interrogating FlyBase. Nucleic Acids Res. 2014;42(Database issue):D780–8. 10.1093/nar/gkt1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Riddle NC, Shaffer CD, Elgin SC. A lot about a little dot—lessons learned from Drosophila melanogaster chromosome 4. Biochem Cell Biol. 2009;87(1):229–41. 10.1139/O08-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vicoso B, Bachtrog D. Numerous transitions of sex chromosomes in Diptera. PLoS Biol. 2015;13(4):e1002078 10.1371/journal.pbio.1002078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ullerich FH, Schottke M. Karyotypes, constitutive heterochromatin, and genomic DNA values in the blowfly genera Chrysomya, Lucilia, and Protophormia (Diptera: Calliphoridae). Genome. 2006;49(6):584–97. Epub 2006/08/29. g06-013 [pii] 10.1139/g06-013 . [DOI] [PubMed] [Google Scholar]
- 34. Hoskins RA, Carlson JW, Wan KH, Park S, Mendez I, Galle SE, et al. The Release 6 reference sequence of the Drosophila melanogaster genome. Genome Res. 2015;25(3):445–58. 10.1101/gr.185579.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johansson AM, Stenberg P, Bernhardsson C, Larsson J. Painting of fourth and chromosome-wide regulation of the 4th chromosome in Drosophila melanogaster . Embo J. 2007. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hochman B. The fourth chromosome of Drosophila melanogaster In: Ashburner M, Novitski E, editors. The genetics and biology of Drosophila. 1b. New York: Academic Press; 1976. p. 903–28. [Google Scholar]
- 37. Johansson AM, Stenberg P, Pettersson F, Larsson J. POF and HP1 bind expressed exons, suggesting a balancing mechanism for gene regulation. PLoS Genet. 2007;3(11):e209 10.1371/journal.pgen.0030209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Riddle NC, Jung YL, Gu T, Alekseyenko AA, Asker D, Gui H, et al. Enrichment of HP1a on Drosophila chromosome 4 genes creates an alternate chromatin structure critical for regulation in this heterochromatic domain. PLoS Genet. 2012;8(9):e1002954 10.1371/journal.pgen.1002954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bassett A, Liu JL. CRISPR/Cas9 mediated genome engineering in Drosophila . Methods. 2014;69(2):128–36. 10.1016/j.ymeth.2014.02.019 . [DOI] [PubMed] [Google Scholar]
- 40. Foster GG, Whitten MJ, Konowalow C, Bedo DG, Maddern RH, Boon DJ. Cytogenetic studies of Lucilia cuprina dorsalis R.-D. (Diptera: Calliphoridae) Polytene chromosome maps of the autosomes and cytogenetic localization of visible genetic markers. Chromosoma. 1980;81:151–68. [Google Scholar]
- 41. Bedo DG. Differential sex chromosome replication and dosage compensation in polytene trichogen cells of Lucilia cuprina (Diptera: Calliphoridae). Chromosoma. 1982;87(1):21–32. [DOI] [PubMed] [Google Scholar]
- 42. Laverty C, Li F, Belikoff EJ, Scott MJ. Abnormal dosage compensation of reporter genes driven by the Drosophila glass multiple reporter (GMR) enhancer-promoter. PLoS ONE. 2011;6(5):e20455 Epub 2011/06/10. 10.1371/journal.pone.0020455 PONE-D-11-06710 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arkhipova I, Li J, Meselson M. On the mode of gene-dosage compensation in Drosophila . Genetics. 1997;145(3):729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sass H, Meselson M. Dosage compensation of the Drosophila pseudoobscura Hsp82 gene and the Drosophila melanogaster Adh gene at ectopic sites in D. melanogaster . Proceedings of the National Academy of Sciences of the United States of America. 1991;88(15):6795–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bashaw GJ, Baker BS. The msl-2 dosage compensation gene of Drosophila encodes a putative DNA-binding protein whose expression is sex specifically regulated by Sex-lethal . Development. 1995;121(10):3245–58. [DOI] [PubMed] [Google Scholar]
- 46. Kelley RL, Solovyeva I, Lyman LM, Richman R, Solovyev V, Kuroda MI. Expression of msl-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila . Cell. 1995;81(6):867–77. [DOI] [PubMed] [Google Scholar]
- 47. Bagnall NH, Kotze AC. Evaluation of reference genes for real-time PCR quantification of gene expression in the Australian sheep blowfly, Lucilia cuprina . Med Vet Entomol. 2010;24(2):176–81. 10.1111/j.1365-2915.2010.00866.x . [DOI] [PubMed] [Google Scholar]
- 48. Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fieller EC. Some problems in interval estimation. Journal of the Royal Statistical Society: Series B. 1954;16(2):175–85. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.