Abstract
Objective
Treatment in the ultra-high risk stage for a psychotic episode is critical to the course of symptoms. Markers for the development of psychosis have been studied, to optimize the detection of people at risk of psychosis. One possible marker for the transition to psychosis is social cognition. To estimate effect sizes for social cognition based on a quantitative integration of the published evidence, we conducted a meta-analysis of social cognitive performance in people at ultra high risk (UHR).
Methods
A literature search (1970-July 2015) was performed in PubMed, PsychINFO, Medline, Embase, and ISI Web of Science, using the search terms ‘social cognition’, ‘theory of mind’, ‘emotion recognition’, ‘attributional style’, ‘social knowledge’, ‘social perception’, ‘empathy’, ‘at risk mental state’, ‘clinical high risk’, ‘psychosis prodrome’, and ‘ultra high risk’. The pooled effect size (Cohen’s D) and the effect sizes for each domain of social cognition were calculated. A random effects model with 95% confidence intervals was used.
Results
Seventeen studies were included in the analysis. The overall significant effect was of medium magnitude (d = 0.52, 95% Cl = 0.38–0.65). No moderator effects were found for age, gender and sample size. Sub-analyses demonstrated that individuals in the UHR phase show significant moderate deficits in affect recognition and affect discrimination in faces as well as in voices and in verbal Theory of Mind (TOM). Due to an insufficient amount of studies, we did not calculate an effect size for attributional bias and social perception/ knowledge. A majority of studies did not find a correlation between social cognition deficits and transition to psychosis, which may suggest that social cognition in general is not a useful marker for the development of psychosis. However some studies suggest the possible predictive value of verbal TOM and the recognition of specific emotions in faces for the transition into psychosis. More research is needed on these subjects.
Conclusion
The published literature indicates consistent general impairments in social cognition in people in the UHR phase, but only very specific impairments seem to predict transition to psychosis.
Introduction
Early signs of psychosis can be present several years before the actual onset of illness. Many individuals show a decline in social functioning before onset [1]. Other signs that may occur prior to onset are mild positive symptoms, negative symptoms such as social withdrawal, depressive symptoms and basic symptoms such as subclinical self-experienced disturbances in thought, speech and perception processes [2].
People at heightened risk for psychosis, usually referred to as Clinical high risk (CHR), Ultra high risk (UHR), psychosis prodrome or At Risk Mental State (ARMS), meet the following criteria: presence of attenuated psychotic symptoms (APS) and/or a family history of schizophrenia combined with problems in functioning [3, 4] and/or presence of one or more brief limited intermittent psychotic symptoms (BLIPS) such as delusions or hallucinations [5]. A recent meta-analysis shows that the average 1-year transition rate to psychosis in this UHR group was 22%, increasing to 36% after three years follow-up [6].
Basic symptoms (BS) where suggested as an alternative set of criteria to detect at risk patients [7]. Basic symptoms consist of subclinical subtle disturbances in stress tolerance, affect, thinking, speech, drive, perception and motor action [8, 9] appearing before the appearance of APS or BLIPS, thus allowing for detection of at risk patients at an earlier stage. There is accumulating evidence that the onset of psychosis can be prevented by intervening in this risk phase [10–12] and treatment in the early stages of schizophrenia is critical to the progression of the disease [13–15].
Given the high number of false positives, it is difficult to predict which individuals in the UHR group will develop psychosis on the basis of their clinical features [16]. Therefore there is a clear need for better prediction models that can be used to help clinicians identify a subgroup of subjects that will benefit most from preventive interventions [17]. In the past decade markers for the development of psychosis have been studied, to optimize the detection of people in the prodromal phase. Several clinical models have been proposed to further increase the validity of prediction of transition to psychosis in the UHR group [18, 19].
One possible marker of a liability for psychosis is social cognition [20], which is defined as the perception, interpretation and processing of social information [21]. Impaired social cognition is considered to result in poor social functioning, a well-established risk factor for transition to psychosis [22]. Expert surveys identified four core domains of social cognition in schizophrenia research [23]: emotional perception and processing, in particular the recognition of facial and vocal affect [24, 25], social perception and knowledge, such as decoding of non-verbal communication and social cue recognition [26], theory of mind (TOM), the mental capacity to infer ones own and other mental states, with subdomains verbal and visual TOM [27, 28] and attributional style, the tendency to attribute the cause of events to the self, others or the environment [29, 30].
Impairments in all of these domains have been consistently found in the acute phase of psychosis as well as in the remission phase [31] and in patients with chronic schizophrenia as well as patients with first onset psychosis [32]. Furthermore, a recent meta-analysis found that adult first degree relatives of people with schizophrenia show moderate difficulties in social cognition [33] and a recent meta-analysis on theory of mind in participants in the UHR phase showed significant, moderate effect sizes [34].
In the current meta-analysis we aimed to investigate whether performance on measures of social cognition is already impaired in the at risk phase. We limited our analyses to studies that used the UHR criteria for inclusion, as most studies on social cognition in the at risk phase use these criteria and inclusion of both UHR and BS may yield an overly heterogeneous sample and hence complicate interpretation.
Two previous reviews on social cognition and at risk participants were found. Thompson et al. [20] used a qualitative approach and reviewed seven studies (published before 2011) on social cognition in people at clinical ultra high risk of psychosis, concluding that deficits in social cognitive abilities do appear to be present in the UHR population. Lee et al. [35] performed a meta-analysis on social cognition in UHR patients and BS patients taken together as one group. They investigated the four general domains of social cognition as stated by Pinkham et al. [23], and found that deficits in social cognitive abilities are present in the at risk population.
Our meta-analysis adds to the previous one by selecting a more homogeneous sample and by conducting additional, more fine-grained analyses to the four domains of social cognition by examining whether specific impairments occur for prosodic or facial affect recognition and verbal or visual TOM. We also include three additional studies that appeared after the meta-analysis by Lee et al. [36–38] and we conduct a comprehensive qualitative evaluation of social cognition and transition to psychosis. Finally, we investigate whether potential moderators (age, sex, IQ, sample size) influence general social cognition in UHR participants.
Methods
Selection of studies
A literature search was performed by three independent researchers (RD, RN, MP) in PubMed, PsychINFO, Medline, Embase, Picarta and ISI Web of Science published between January 1970 and July 2015, using the following search terms in all possible combinations: ‘social cognition’, ‘theory of mind’, ‘emotion recognition’, ‘attributional style’, ‘social knowledge’, ‘social perception’, ‘empathy’, ‘at risk mental state’, ‘clinical high risk’, ‘psychosis prodrome’, and ‘ultra high risk’. A first selection was made based on the title of the studies followed by a second selection based on the abstract of the remaining articles. Lastly the full articles were examined to determine the final selection to be included into the meta-analysis. A cross-reference search of eligible articles was conducted to identify additional studies not found in the electronic search.
Inclusion criteria
Inclusion criteria were: a) UHR was defined in line with the criteria of Yung and McGorry [3], b) individuals in the UHR phase were help-seeking, c) a healthy control group was included, d) at least one social cognitive test was administered. For the research question regarding transition into psychosis, we used the same criteria, but we did not exclude studies without a healthy control group.
Statistical analysis
An effect size was computed for each social cognitive test result, using software developed by Wilson [39]. For each study, one effect size was calculated for each cognitive domain: when multiple tests for the same social cognitive domain were used, the results were combined into one mean effect to ensure all studies contributed equally to the total effect size of the social cognitive domain. The analyses where performed with the program ‘Review Manager 5.0’, developed by the Cochrane Collaboration. Effect sizes where weighted by their standard error to ensure that larger studies had more weight in the mean effect size across studies. Because of the variance in measurements and because the underlying concepts are not unequivocal, a random effects model was used. Overall effect size was calculated and represented in a forest plot, as well as the effect size of each study. Mean effect size, standard error, 95% confidence interval, corresponding z-value and significance level are reported. Variability in outcome of studies caused by clinical and methodological diversity of the studies was tested with Chi-square tests. We performed an Egger’s test [40] to investigate whether publication bias was present, using a regression analysis with the standard normal deviate (Cohen’s D/SE) as a dependent variable and the precision (1/SE) as an independent variable.
We planned to evaluate the potential moderating influence on effect size of age and gender using categorical models (Chi square). These factors were chosen given that social cognition was associated with age, gender an IQ in previous studies [41–43]. Unfortunately, the moderating effect of IQ could not be calculated as not enough data were provided in the articles. We investigated sample size as an additional moderator as smaller samples provide a greater risk of finding a false positive result. We separated the included studies in two groups based on each factor, calculated the mean effect size of each group and tested the significance of the difference between the mean effect sizes using ANOVA-analysis.
Results
Literature search
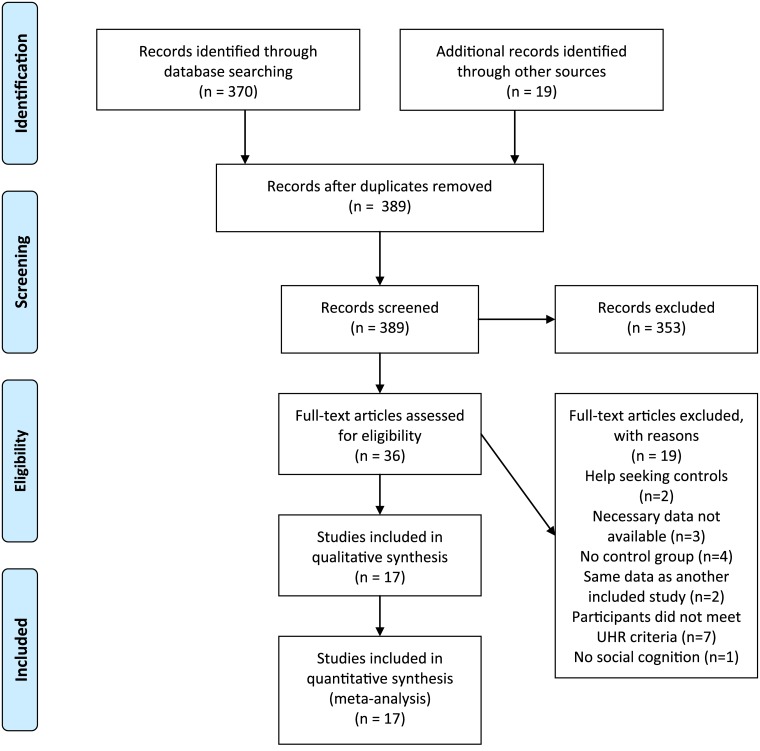
The literature search based on title resulted in 389 abstracts. See Fig 1 for a flow diagram of the search. After examination, most of the selected studies where excluded because they did not report on social cognition in individuals in the UHR phase. Thirty-six studies met the inclusion criteria based on the abstract. With further examination of the full text, two more studies were excluded because the control group consisted of help seeking controls instead of healthy controls [44, 45]. Three studies were omitted because necessary data for meta-analysis (mean and standard deviation of the separate groups or change scores) were not provided in the paper, and could not be obtained upon request [46–48]. Four studies were excluded for the first research questions due to a research design without a control group [49–52]. One of these studies [49] reported on conversion to psychosis and was therefore included in the analysis of the possible prediction of psychosis with social cognition. Two studies [53, 54] were excluded because they reported on the same data as another included study [55, 56], we included the most recent studies, since they provided the necessary data and included more participants. Seven more studies were omitted because the included participants did not meet the UHR criteria for this analysis: two studies selected the UHR participants based on cognitive disturbances [57, 58]; another study included participants with familial high risk without functional decline [59]; one study included all help-seeking individuals without selection based on symptoms [60]; one study included schizophrenia patients and no at risk group [61]; one study included participants with a history of psychosis in the at risk group [62] and one study included non-help seeking persons in the UHR group [63]. Finally, one more article was excluded because it examined the processing of facial features (the ability of participants to discriminate between different faces) instead of the recognition of affect in faces [64]. Eventually, seventeen studies were selected for this analysis. All studies were published between 2006 and 2015 and a total of 793 individuals in the UHR phase and 630 in the control condition were included. For an overview of the included studies, see Table 1.
Fig 1. Flow diagram of the literature search.
PRISMA flow diagram for meta-analysis of social cognition in individuals at ultra-high risk of psychosis. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:10.1371/journal.pmed1000097. For more information, visit www.prisma-statement.org.
Table 1. Overview of included studies.
| Reference | n(UHR) | Criteria | Other groups | Assessments | Subgroup |
|---|---|---|---|---|---|
| Addington et al., 2008 | 86 | SIPS | Chron, FEP | FEIT, FEDT | AR |
| Amminger et al., 2012 | 79 | CAARMS | FEP | Facial, Prosodic | AR |
| An et al., 2010 | 24 | SIPS | FEP | Hostility, Blame | AS |
| Comparelli et al., 2013 | 43 | SIPS | Chron, FEP | Identification, Recognition | AR |
| Corcoran et al., 2015 | 49 | SIPS | None | EMODIFF, Audio recordings | AR |
| Corigliano et al., 2014 | 36 | SIPS | Chron, FEP | Facial affect recognition task | AR |
| Couture et al., 2008 | 88 | SIPS | Early sz < 5jr | Eyes, ATT | TOM, SP |
| DeVylder et al., 2013 | 33 | SIPS | None | IPSAQ | AS |
| Gill et al., 2014 | 60 | SIPS | None | Video Social Inference task | TOM |
| Green et al., 2011 | 50 | SIPS | Chron, FEP | MSCEIT. TASIT, RAD | AR, TOM, SP |
| Hur et al., 2013 | 55 | CAARMS | None | False Belief, Strange Story, Cartoon | TOM |
| Lee et al., 2015 | 40 | SIPS | FEP | Facial emotion recognition task | AR |
| Pinkham et al., 2007 | 19 | SIPS | Chron, FEP | FEIT, FEDT | AR |
| Seifert et al., 2008 | 12 | SIPS | None | Discrimination task | AR |
| Stanford et al., 2011 | 63 | SIPS | Chron | Strange Story, Eyes | TOM |
| Szily et al., 2009 | 26 | CAARMS | Depression | Eyes | TOM |
| Thompson et al., 2012/13 | 30 | CAARMS | FEP | Hinting task, Visual jokes, DANVA-2, MSCEIT, ANSIE | AR, TOM, AS, SP |
SIPS = Structured interview for Prodromal Syndromes, SOPS = Scale of Prodromal Symptoms, CAARMS = Comprehensive Assessment of At Risk Mental States, Chron = chronic schizophrenia patients, FEP = first episode patients, FEIT = Facial Emotion Identification Test, FEDT = Facial Emotion Discrimination Test, EMODIFF = Penn Emotion Discrimination Task ATT = Abbreviated Trustworthiness Task, IPSAQ = Internal, Personal and Situational Attributions Questionnaire, MSCEIT = Mayer-Salovey-Caruso Emotional Intelligence Test, TASIT = The Awareness of Social Inference Test, RAD = Relationships Across Domains Test, DANVA-2 = Diagnostic Assessment of Non Verbal Accuracy, ANSIE = Adult Nowicki Strickland Internal External Scale, AR = affect recognition, AS = attributional style, TOM = theory of mind, SP = social perception.
Social cognition and transition to psychosis
Nine studies reported longitudinal data on the transition of UHR individuals to psychosis and the possible predictive value of social cognition for transition. Four studies examined Theory of Mind, four articles examined affect recognition, one study investigated attributional style and one study examined social perception (trustworthiness task). Not enough data were available to perform a meta-analysis to compare social cognitive performance between converters and non-converters across studies. We evaluated the studies qualitatively.
Three out of the four studies on Theory of Mind [45, 65, 66] did not find significant differences on baseline data between converters and non-converters after controlling for symptoms, IQ and age. This was the case for verbal (Strange Stories task, VSIT) and visual (Cartoon task, Eyes task) TOM tasks. This indicates that TOM does not predict transition into psychosis and is not useful as marker for the prediction of onset. One study on TOM [54] did find that a verbal TOM task (False Belief task) predicted conversion, however converters in this study had a significant lower IQ score than nonconverters and the control group was better educated than the UHR group.
Two [44, 49] out of three studies that investigated the difference in prosodic affect recognition between converters and non-converters at baseline did not find a significant effect. The third study [37] did not include enough participants that fulfilled the prosodic affect recognition task (2 converters) to be able to analyze the effect. Two studies [44, 67] did not find a significant difference on facial affect recognition and discrimination at baseline between converters and non-converters. However, one study [37] did find a clear difference for affect recognition as well as affect discrimination. Whereas there was no difference between the total at risk group and the control group, the authors did find a significant difference between converters and non-converters/controls. Converters where less accurate in the recognition of fear and anger. They labeled more emotional faces as ‘neutral’ in comparison to the non-converter group. A second study [49] found a significant difference between converters and non-converters in identifying neutral and fearful faces. In this study, converters mislabeled neutral faces as fearful, after controlling for symptoms, functioning and age.
A study on social perception [45] and attributional style [68] did not find any difference between converters and non-converters at baseline.
Overall difference social cognition in UHR versus healthy controls
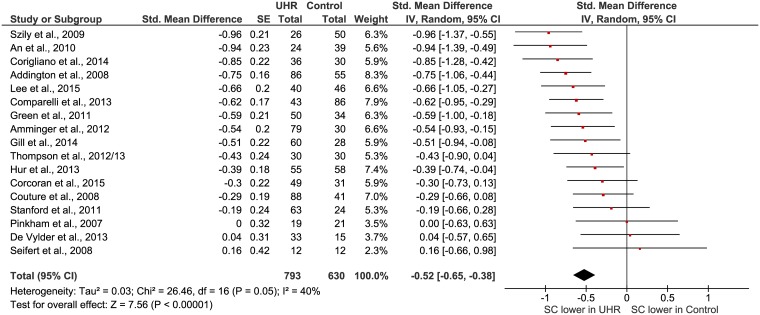
The significant mean weighted effect size of all seventeen studies on the difference in social cognition in UHR versus healthy controls is 0.52 (95% Cl = 0.38–0.65). This is a medium effect size, according to Cohen’s nomenclature and this effect size is significant (Z = 7.56, p < 0.00001). The forest plot of this analysis is represented in Fig 2. Publication bias is not likely, as revealed by the Eggers test (F = 2.26; P>0.1). The Chi-square test was statistically significant and indicates heterogeneity; results must be interpreted with caution. The heterogeneity may be due to moderating factors, we intended to identify these possible factors using a moderator analysis (next paragraph). Another possible explanation for the heterogeneity may be the inclusion of several different constructs of social cognition in this meta-analysis. We therefore proceeded by splitting the studies into four subgroups based on previous literature: Affect recognition/discrimination, Theory of Mind, Attribution and Social perception/knowledge.
Fig 2. Forest plot of social cognition in UHR versus healthy controls.
Effect of moderators
The results of the moderator analyses are shown in Table 2. All effect sizes remained significant. The median split for age was 20 years and for number of participants the split was set on 50 participants. No significant moderator effects were found for age, number of subjects and gender. Unfortunately the moderating effect of IQ could not be studied due to the small number of studies reporting sufficient data.
Table 2. Moderator analyses.
| Variabele | k | N (UHR) | Cohen’s D | 95% CI | Chi2 within | Chi2 between |
|---|---|---|---|---|---|---|
| Age (mean years) | ||||||
| <20 | 9 | 529 | 0.49 sign | 0.34–0.64 | 9.31 ns | 0.21 ns |
| >20 | 8 | 264 | 0.55 sign | 0.31–0.80 | 16.40 sign | |
| Gender | ||||||
| > 50% women | 6 | 233 | 0.61 sign | 0.39–0.84 | 8.25 ns | 1.11 ns |
| > 50% men | 11 | 560 | 0.46 sign | 0.30–0.63 | 26.46 ns | |
| Number subjects | ||||||
| <50 | 10 | 312 | 0.53 sign | 0.31–0.75 | 19.85 sign | 0.08 ns |
| >50 | 7 | 481 | 0.49 sign | 0.35–0.64 | 5.92 ns |
Affect recognition and discrimination
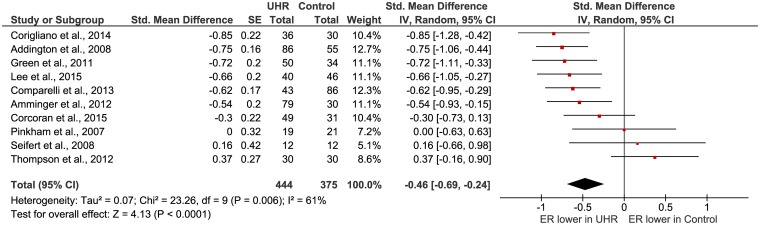
Ten studies reported on affect recognition in UHR participants and healthy controls (Table 1). All ten studies investigated the recognition of emotion in faces; three articles also examined the ability to recognize emotions in voices [37, 55, 69]. Affect recognition was the primary outcome in all articles except for Seifert et al. [70] who used the task in an fMRI study. All studies investigated affect recognition (to be able to name the appropriate emotion when a facial expression is shown), three studies also investigated affect discrimination (the ability to see if two facial expressions show the same or a different emotion) [37, 67, 71]. For the calculation of the total effect size, subtests where combined into one mean effect size before they were entered into the analysis to ensure independent observations [37, 55, 67, 69–72]. The significant (Z = 4.13; P < 0.0001) mean effect size of the studies was medium according to Cohen’s nomenclature (d = 0.46, 95% Cl = 0.24, 0.69) (Fig 3), with lower affect recognition in the at risk group. The Chi-square test was significant, which indicates that substantial heterogeneity is present. When we omitted the discrimination tasks and only included the ten recognition tasks, the significant mean effect size (Z = 4.21; P<0.0001) was approximately the same (d = 0.47, 95% Cl = 0.25, 0.69), with lower affect recognition in the at risk group. The Chi-square test was still significant. Only three studies investigated affect discrimination, therefore we did not calculate the effect size of this subgroup. Two out of three of the studies investigated if patients could see if two faces showed the same or a different emotion, and both studies did not find a significant difference between UHR patients and healthy controls [67, 71]. The third study [37] took a slightly different approach and investigated if participants could discriminate which of two faces showed a more intense expression of the same emotion. This study did find a significant difference between the healthy controls and the UHR patients. More research into emotion discrimination is needed.
Fig 3. Forest plot of affect recognition in UHR versus healthy controls.
When we looked at the facial affect recognition tasks only, the significant effect size (Z = 4.47; P<0.00001) stayed approximately the same (d = 0.48, 95% Cl = 0.27, 0.69), with lower facial affect recognition in the UHR group. The Chi-square test was still significant. Only three studies [37, 55, 69] reported on prosodic emotion recognition, which is not enough to calculate a mean effect size. All three articles found a significant lower score on prosodic emotion recognition in UHR participants in comparison to healthy controls. More research is needed on this subject.
Theory of mind
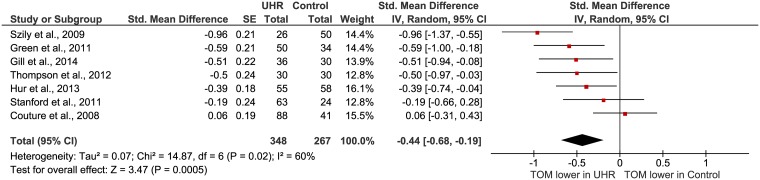
Seven studies investigated Theory of Mind (TOM) in participants in the UHR phase (Table 1). TOM was the primary outcome for all studies. Verbal tasks (False Belief task, Strange Story task, the Awareness of Social Inference task and Hinting task) as well as visual tasks (Cartoon task, Reading the Eyes task, Visual Jokes task) were used in the studies. Multiple tests within one study where combined into one mean effect size before entering into the analysis to ensure independent observations [56, 66, 69]. The significant (Z = 3.47; P = 0.0005) mean effect size of the studies was medium according to Cohen’s nomenclature (d = 0.44, 0.19, 0.68) (Fig 4). The Chi-square test for homogeneity was significant, which indicates the presence of considerable variability in outcome. This may be caused by clinical and methodological diversity of the studies. The results must therefore be interpreted with caution. We performed a meta-analysis on the subgroups verbal TOM and visual TOM. The medium (d = 0.52; 95% Cl = 0.30, 0.74) total effect size of the five verbal TOM tasks was significant (Z = 4.61, P = 0.00001) and the Chi-square test was not significant. The mean effect size of the five visual TOM tasks was medium (d = 0.33, 95% Cl = 0.03, 0.68) and not significant (Z = 1.83; P = 0.07). The Chi square test was significant for this subsample.
Fig 4. Forest plot of Theory of Mind in UHR versus healthy controls.
Attribution style
Only three studies where found on attribution style and individuals in the UHR phase (Table 1). Because of the low amount of studies on this subject, we did not calculate a pooled effect size but investigated the studies separately. Thompson et al. [73] and DeVylder et al. [68] investigated the extent in which individuals tended to attribute the cause of an event to external causes (externalizing bias). An et al. [74] and DeVylder et al. [68] investigated the extent in which individuals tended to attribute the cause of a negative event to other people (personalizing bias). For both the externalizing and the personalizing bias, one study found a significant effect and the other study did not find any effect. Clearly, more research is needed on this subject before any conclusions can be made.
Social perception / social knowledge
Three studies investigated social perception/knowledge in UHR participants (Table 1). All studies used very different measurement instruments and examined different elements of social perception/knowledge: managing emotions and the emotions of others in social situations [69], how to estimate the trustworthiness of strangers [75] and how to understand social relationships, based on relational models theory [76]. Because of the low amount of studies on this subject and the diversity of the used measurement scales, we did not calculate a pooled effect size but investigated the studies separately.
Discussion
In addition to previous work [20, 35] we performed a meta-analysis of published studies to examine the evidence regarding impaired social cognition in individuals at risk of psychosis. We chose to only include studies that defined at risk patients using UHR criteria of Yung and McGorry [3], too ensure a homogeneous sample. In agreement with previous studies [20, 35] we found evidence for significant impairment of social cognition in the UHR group, with effect sizes in the moderate range, which is comparable to the severity of other cognitive deficits in participants in the UHR phase [4, 17, 77]. There was substantial heterogeneity of effect sizes. No moderator effect was found for age, gender or sample size. Not enough data were available to calculate the possible moderator effect of IQ.
Sub analyses for affect recognition showed that UHR participants demonstrate significant moderate deficits in affect recognition in faces. Not enough studies were published to perform an analysis of prosodic affect recognition tasks, but the three studies that were found all reported problems in recognizing emotions in voices for UHR participants in comparison to controls. More research on this subject is needed. Furthermore, sub analyses showed that UHR patients have problems with recognizing emotions, whereas it is not clear if they have the same difficulties in discriminating between twee different emotions. The two studies that were found on this subject did not find a significant difference between the UHR group and controls. A possible explanation for this result can be that the emotion discrimination task is less difficult than the emotion identification task [78]. It is possible that people at risk of psychosis do not experience difficulties in discriminating between two different emotions, however they possibly do have difficulties in differentiating between intense or less intense versions of the same emotions, as one study found (Corcoran et al. 2015). More research is needed on this subject.
Sub analyses of TOM showed significant problems with verbal TOM tasks in UHR participants in comparison to healthy controls. A moderate effect was found for visual TOM, but this effect was not significant. This is not in line with a previous meta-analysis on TOM in participants in the UHR phase [34], where significant moderate effect sizes for visual TOM where found (d = 0.40, 95% Cl = 0.14–0.70; Z = 2.94, P = 0.003). The same articles as used in this meta-analysis were used in their study, except for Kim et al. [54]. This article is the precursor of Hur et al. [56] that we included. The samples were drawn from the same participant pool in both articles, but Hur included more participants resulting in a higher weight of the results of this study. Bora et al. also used data from Ohmuro et al. [79] that were presented at a conference, while conference data did not meet our inclusion criteria. A lack of power might be accountable for the absence of a significant effect for visual TOM in our study, given that the pooled ES approached significance (P = 0.07). Another possible explanation for the difference in outcome in verbal and visual TOM is that verbal TOM tasks require more cognitive effort in comparison to visual TOM tasks. Verbal tasks ask for the ability to process a lot of verbal information such as large amounts of written texts. These tasks also correlate highly with general cognitive functioning [56]. Thus verbal TOM tasks might be challenging for people in the UHR phase due to problems in general cognitive functioning. More research is needed on visual TOM tasks to determine if only verbal TOM is affected.
Three studies where found regarding attributional style in participants in the UHR phase, with different results. For both the externalizing and the personalizing bias, one study found a significant effect and the other study did not find any effect. A possible explanation for these different outcomes is that the studies used different measurement instruments and measured different constructs of attributional style. Clearly, more research is needed on this subject before any conclusions can be made.
All three included studies on social perception used very different measurement instruments and examined different elements of social perception/knowledge. Therefore we decided not to calculate a pooled effect size for this subdomain. As both social perception and social knowledge are severely impaired in patients with schizophrenia [80], further research is needed on this subject to investigate the extent and nature of impairments in this domain for individuals in the UHR phase.
A recent meta-analysis in social cognition in people with schizophrenia [80] revealed large effects on emotion perception and TOM, among others. This may suggest that UHR groups perform better than schizophrenia patients, but worse than healthy controls as would be expected on the basis of previous findings regarding cognitive functioning and UHR participants [81].
Not enough data were available to perform a meta-analysis to compare social cognitive performance between converters and non-converters across studies. We evaluated the studies qualitatively. Verbal as well as visual Theory of Mind did not seem to predict transition into psychosis, as three out of four studies did not find any differences between converters and non-converters at baseline after controlling for symptoms, IQ and age. The one study that did find a verbal TOM task to predict conversion [54] did not control for IQ and converters had a significant lower IQ score than non-converters at baseline. The lower performance of the converters in this study may therefore be caused by lower general cognitive function instead of specific TOM problems, as the verbal information processing that is required for verbal TOM tasks may be challenging for participants with lower IQ scores.
First studies on prosodic affect recognition as a predictor of transition found no significant differences between converters and non-converters at baseline. Results on facial affect recognition are less clear. Most studies did not find any difference between converters and non-converters on overall affect recognition in faces. However, two studies showed significant problems of converters with recognizing specific emotions, in particular fear and anger, in comparison to non-converters at baseline. More studies are needed on this subject and the possible problems with the recognition of specific emotions need to be taken into account.
The two studies on attributional style and social perception did not find any significant differences between converters and non-converters at baseline. More longitudinal studies are needed to clarify the possible predictive value of these domains of social cognition for transition.
With the current quantitative integration of published findings, we aimed to investigate whether the four social cognitive subdomains as identified by Pinkham et al. [23] are impaired in the UHR phase. Pinkham et at al. additionally highlighted ‘empathy’ as an area of importance within the field of social cognition and psychosis, but did not nominate this construct as a separate domain of social cognition, due to the possible overlap of the cognitive elements of empathy with Theory of Mind. As many authors present empathy as a separated construct [82] and as several instruments measure empathy specifically [83, 84], additional research is needed at a fundamental level to identify the possible overlap and unique elements of these domains of social cognition.
A number of limitations of our study should be noted. Since only three studies on attributional style and four very different studies on social perception were found, more research is needed on these subjects before any conclusions can be drawn on impairment in the UHR phase. More research on the association of impaired social cognition in the UHR phase with actual transition into psychosis is needed to determine the clinical consequences of impaired social cognition in the UHR phase.
There is some evidence for social cognition to be significantly influenced by IQ [56, 66]. It would therefore be useful to compare studies that include IQ or education level into the analysis as a covariate with studies that do not include these factors as covariates. Unfortunately, the necessary data to perform this analysis (F value or d after correction for IQ) was not provided in the articles included in this study. The studies that did include IQ as a covariate did not find a different effect before and after correction for IQ. Further research is needed on this subject.
A general limitation of meta-analysis is the dependence on methodology of published studies. The diversity in measurements used in the studies was large and the psychometric properties of most of the used instruments are unknown. As Pinkham et al. [23] have stated; there is a clear need for studies designed to assess the psychometric properties of social cognition measurement instruments.
Conclusion
This meta-analysis reveals consistent impairments in social cognition in people in the UHR phase, in comparison to healthy controls. Significant deficits are detected in affect recognition and verbal TOM. We did not calculate the effect sizes for social cognition/knowledge and attributional style as more studies are needed for these subdomains. No moderator effects were found for age, gender and sample size. A majority of studies did not find a correlation between social cognition deficits and transition to psychosis, after controlling for IQ, education and baseline symptoms, which may suggest that social cognition in general is not a useful marker for the development of psychosis. However some studies suggest the possible predictive value of more specific forms of social cognition, in particular verbal TOM and the recognition of specific emotions in faces for the transition into psychosis.
Supporting Information
(DOC)
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. MacBeth A, Gumley A. Premorbid adjustment, symptom development and quality of life in first episode psychosis: a systematic review and critical reappraisal. Acta Psychiatr Scand. 2008; 117(2): 85–99. [DOI] [PubMed] [Google Scholar]
- 2. Schultze-Lutter F, Ruhrmann S, Berning J, Maier W, Klosterkötter J. Basic symptoms and ultrahigh risk criteria: symptom development in the initial prodromal state. Schizophr bull. 2010; 36(1): 182–91. 10.1093/schbul/sbn072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yung AR, McGorry PD. Prediction of psychosis: setting the stage. Br J Psychiatry Suppl. 2007; 51: s1–8. 10.1192/bjp.191.51.s1 [DOI] [PubMed] [Google Scholar]
- 4. Bora E, Lin A, Wood SJ, Yung AR, McGorry PD, Pantelis C. Cognitive deficits in youth with familial and clinical high risk to psychosis: a systematic review and meta-analysis. Acta Psychiatr Scand. 2014; 130(1): 1–15. 10.1111/acps.12261 [DOI] [PubMed] [Google Scholar]
- 5. McGlashan TH, Miller TJ, Woods SW. Pre-onset detection and intervention research in schizophrenia psychoses: current estimates of benefit and risk. Schizophr Bull. 2001; 27(4): 563–70. [DOI] [PubMed] [Google Scholar]
- 6. Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012; 69(3): 220–9. 10.1001/archgenpsychiatry.2011.1472 [DOI] [PubMed] [Google Scholar]
- 7. Ruhrmann S, Schultze-Lutter F, Klosterkötter J. Early detection and intervention in the initial prodromal phase of schizophrenia. Pharmacopsychiatry. 2003; 36(3): 162–7. [DOI] [PubMed] [Google Scholar]
- 8. Gross G. The ‘basic’ symptoms of schizophrenia. Br J Psychiatry. 1989; 155(7): 21–25. [PubMed] [Google Scholar]
- 9. Klosterkötter J, Schultze-Lutter F, Gross G, Huber G, Steinmeyer EM. Early self-experienced neuropsychological deficits and subsequent schizophrenic diseases: an 8-year average follow-up prospective study. Acta Psychiatr Scand. 1997; 95(5): 396–404. [DOI] [PubMed] [Google Scholar]
- 10. Morrison AP, French P, Walford L, Lewis SW, Kilcommons A, Green J, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. Br J Psychiatry. 2004; 185: 291–7. [DOI] [PubMed] [Google Scholar]
- 11. Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010; 67(2): 146–154. 10.1001/archgenpsychiatry.2009.192 [DOI] [PubMed] [Google Scholar]
- 12. Ising HK, Smit F, Veling W, Rietdijk J, Dragt S, Klaassen RM, et al. Cost-effectiveness of preventing first-episode psychosis in ultra-high-risk subjects: multi-centre randomized controlled trial. Psychol Med. 2014; 21: 1–12. [DOI] [PubMed] [Google Scholar]
- 13. Chiliza B, Asmal L, Emsley R. Early intervention in schizophrenia in developing countries: focus on duration of untreated psychosis and remission as a treatment goal. Int Rev Psychiatry. 2012; 24(5): 483–8. 10.3109/09540261.2012.704873 [DOI] [PubMed] [Google Scholar]
- 14. Marshall M, Lewis S, Lockwood A, Drake R, Jones P, Croudace T. Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: a systematic review. Arch Gen Psychiatry. 2005; 62(9): 975–83. [DOI] [PubMed] [Google Scholar]
- 15. Perkins DO, Gu H, Boteva K, Lieverman JA. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005; 162(10): 1785–804. [DOI] [PubMed] [Google Scholar]
- 16. Fusar-Poli P, Borgwardt S. Predictive power of attenuated psychosis syndrome: is it really low? The case of mild cognitive impairment. Schizophr Res. 2012; 135(1–3): 192–3. 10.1016/j.schres.2011.11.023 [DOI] [PubMed] [Google Scholar]
- 17. Fusar-Poli P, Deste G, Smieskova R, Barlati S, Yung AR, Howes O, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012; 69(6): 562–71. 10.1001/archgenpsychiatry.2011.1592 [DOI] [PubMed] [Google Scholar]
- 18. Ruhrmann S, Schultze-Lutter F, Salokangas RK, Heinimaa M, Linszen D, Dingemans P, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010; 67(3): 241–51. 10.1001/archgenpsychiatry.2009.206 [DOI] [PubMed] [Google Scholar]
- 19. Nieman DH, Ruhrmann S, Dragt S, Soen F, Van Tricht MJ, Koelman JH, et al. Psychosis prediction: stratification of risk estimation with information-processing and premorbid functioning variables. Schizophr Bull. 2014; 40(6): 1482–90. 10.1093/schbul/sbt145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thompson AD, Bartholomeusz C, Yung AR. Social cognition deficits and the 'ultra high risk' for psychosis population: a review of literature. Early Interv Psychiatry. 2011; 5(3): 192–202. 10.1111/j.1751-7893.2011.00275.x [DOI] [PubMed] [Google Scholar]
- 21. Ostrom J. The sovereignty of social cognition In Wyer R. S. and Srull T. K., Handbook of Social Cognition, vol. 1 Hillsdale: Erlbaum; 1984. pp. 1–38. [Google Scholar]
- 22. Gee DG, Cannon TD. Prediction of conversion to psychosis: review and future directions. Rev Bras Psiquiatr. 2011; 33(2): s129–s142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pinkham AE, Penn DL, Green MF, Buck B, Healy K, Harvey PD. The Social Cognition Psychometric Evaluation Study: Results of the Expert Survey and RAND Panel. Schizophren Bull. 2014; 40(40): 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Edwards J, Pattison PE, Jackson HJ, Wales RJ. Facial affect and affective prosody recognition in first-episode schizophrenia. Schizophr Res. 2001; 48(2–3): 235–53. [DOI] [PubMed] [Google Scholar]
- 25. Hoekert M, Kahn RS, Pijnenborg M, Aleman A. Impaired recognition and expression of emotional prosody in schizophrenia: review and meta-analysis. Schizophr Res. 2007; 96(1–3): 135–45. [DOI] [PubMed] [Google Scholar]
- 26. Ihnen GH, Penn DL, Corrigan PW, Martin J. Social perception and social skill in schizophrenia. Psychiatry Res. 1998; 80(3): 275–86. [DOI] [PubMed] [Google Scholar]
- 27. Brüne M. “Theory of mind” in schizophrenia: a review of the literature. Schizophr Bull. 2005; 31(1): 21–42. [DOI] [PubMed] [Google Scholar]
- 28. Sprong M, Schothorst P, Vos E, Hox J, Van Engeland H. Theory of mind in schizophrenia: meta-analysis. Br J Psychiatry. 2007; 191: 5–13. [DOI] [PubMed] [Google Scholar]
- 29. Lee DA, Randall F, Beattie G, Bentall RP. Delusional discourse: an investigation comparing the spontaneous causal attributions of paranoid and non-paranoid individuals. Psychol Psychother. 2004; 77(4): 525–40. [DOI] [PubMed] [Google Scholar]
- 30. Langdon R, Ward PB, Coltheart M. Reasoning anomalies associated with delusions in schizophrenia. Schizophr Bull. 2010; 36(2): 321–30. 10.1093/schbul/sbn069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mehta UM, Thirthalli J, Naveen Kumar C, Keshav Kumar J, Keshavan MS, Gangadhar BN. Schizophrenia patients experience substantial social cognition deficits across multiple domains in remission. Asian J Psychiatr. 2013; 6(4): 324–9. 10.1016/j.ajp.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 32. Mazza M, Pollice R, Pacitti F, Pino MC, Mariano M, Tripaldi S, et al. New evidence in theory of mind deficits in subjects with chronic schizophrenia and first episode: correlation with symptoms, neurocognition and social function. Riv Psychiatr. 2012; 47(4): 327–36. [DOI] [PubMed] [Google Scholar]
- 33. Lavoie MA, Plana I, Bédard Lacroix J, Godmaire-Duhaime F, Jackson PL, Achim AM. Social cognition in first-degree relatives of people with schizophrenia: a meta-analysis. Psychiatry Res. 2013; 209(2): 129–35. 10.1016/j.psychres.2012.11.037 [DOI] [PubMed] [Google Scholar]
- 34. Bora E, Pantelis C. Theory of mind impairments in first-episode psychosis, individuals at ultra-high risk for psychosis and in first-degree relatives of schizophrenia: systematic review and meta-analysis. Schizophren Res. 2013; 144(1–3): 31–6. [DOI] [PubMed] [Google Scholar]
- 35. Lee TY, Hong SB, Shin NY, Kwon JS. Social cognitive functioning in prodromal psychosis: a meta-analysis. Schizophr Res. 2015; 164(1–3): 28–34. 10.1016/j.schres.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 36. Corigliano V, De Carolis A, Trovini G, Dehning J, Di Pietro S, Curto M, et al. Neurocognition in schizophrenia: from prodrome to multi-episode illness. Psychiatry Res. 2014; 220(1–2): 129–34. 10.1016/j.psychres.2014.07.067 [DOI] [PubMed] [Google Scholar]
- 37. Corcoran CM, Keilp JG, Kayser J, Klim C, Butler PD, Bruder GE, et al. Emotion recognition deficits as predictors of transition in individuals at clinical high risk for schizophrenia: a neurodevelopmental perspective. Psychol Med. 2015; 4: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee SY, Bang M, Kim KR, Lee MK, Park JY, Song YY, et al. Impaired facial emotion recognition in individuals at ultra-high risk for psychosis and with first-episode schizophrenia and their associations with neurocognitive deficits and self-reported schizotypy. Schizophr Res. 2015; 165(1): 60–5. 10.1016/j.schres.2015.03.026 [DOI] [PubMed] [Google Scholar]
- 39.Wilson DB. Professional Development Course on Meta-analysis. 2010. Available: http://mason.gmu.edu/~dwilsonb/ma.html.
- 40. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Brit Med J. 1997; 315: 639–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008; 9(4): 267–77. 10.1038/nrn2353 [DOI] [PubMed] [Google Scholar]
- 42. Lawrence K, Campbell R, Skuse D. Age, gender and puberty influence the development of facial emotion recognition. Front Psychol. 2015; 6: 761 10.3389/fpsyg.2015.00761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Corcoran R., Cahill C., Frith C.D., 1997. The appreciation of visual jokes in people with schizophrenia: a study of mentalizing ability. Schizophr. Res. 24 (3), 319–327. [DOI] [PubMed] [Google Scholar]
- 44. Addington J, Piskulic D, Perkins D, Woods SW, Liu L, Penn DL. Affect recognition in people at clinical high risk of psychosis. Schizophren Res. 2012; 1040: 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Healey KM, Penn DL, Perkins D, Woods SW, Addington J. Theory of mind and social judgements in people at clinical high risk of psychosis. Schizohren Res. 2013; 150: 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chung YS, Kang D, Shin NY, Yoo SY, Kwon JS. Deficit of theory of mind in individuals at ultra-high-risk for schizophrenia. Schizophren Res. 2008; 99: 111–118. [DOI] [PubMed] [Google Scholar]
- 47. Gee DG, Karlsgodt KH, Van Erp TG, Bearden CE, Lieberman MD, Belger A, et al. Altered age-related trajectories of amygdala-prefrontal circuitry in adolescents at clinical high risk for psychosis: a preliminary study. Schizophr Res. 2012; 134(1): 1–9. 10.1016/j.schres.2011.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kohler CG, Richard JA, Brensinger CM, Borgmann-Winter KE, Conroy CG, Moberg PJ, et al. Facial emotion perception differs in young persons at genetic and clinical high-risk for psychosis. Psychiatry Res. 2014; 216(2): 206–12. 10.1016/j.psychres.2014.01.023 [DOI] [PubMed] [Google Scholar]
- 49. Allott KA, Schäfer MR, Thompson A, Nelson B, Bendall S, Bartholomeusz CF, et al. Emotion recognition as a predictor of transition to a psychotic disorder in ultra-high risk participants. Schizophren Res. 2014; 153: 25–31. [DOI] [PubMed] [Google Scholar]
- 50. Barbato M, Liu L, Penn DL, Keefe RS, Perkins DO, Woods SW, et al. Social cognition as a mediator between neurocognition and functional outcome in individuals at clinical high risk for psychosis. Schizophr Res. 2013; 150(2–3): 542–6. 10.1016/j.schres.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bartholomeusz CF, Whittle SL, Pilioussis E, Allott K, Rice S, Schäfer MR, et al. Relationship between amygdala volume and emotion recognition in adolescents at ultra-high risk for psychosis. Psychiatry Research: Neuroimaging. 2014; 224: 159–167. 10.1016/j.pscychresns.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 52. Yong E, Barbato M, Penn DL, Keefe RS, Woods SW, Perkins DO, et al. Exploratory analysis of social cognition and neurocognition in individuals at clinical high risk for psychosis. Psychiatry Res. 2014; 218(1–2): 39–43. 10.1016/j.psychres.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Schlögelhofer M, Mossaheb N, et al. Emotion recognition in individuals at clinical high-risk for schizophrenia. Schizophren Bull. 2012; 38(5): 1030–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim HS, Shin NY, Jang JH, Kim E, Shim G, Park HY, et al. Social cognition and neurocognition as predictors of conversion to psychosis in individuals at ultra-high risk. Schizophren Res. 2011; 130(1–3): 170–5. [DOI] [PubMed] [Google Scholar]
- 55. Amminger GP, Schäfer MR, Klier CM, Schlögelhofer M, Mossaheb N, Thompson A, et al. Facial and vocal affect perception in people at ultra-high risk of psychosis, first-episode schizophrenia and healthy controls. Early Interv Psychiatry. 2012; 6(4): 450–4. 10.1111/j.1751-7893.2012.00362.x [DOI] [PubMed] [Google Scholar]
- 56. Hur JW, Byun MS, Shin NY, Shin YS, Kim SN, Jang JH, et al. General intellectual functioning as a buffer against theory-of-mind deficits in individuals at ultra-high risk for psychosis. Schizophren Res. 2013; 149(1–3): 83–7. [DOI] [PubMed] [Google Scholar]
- 57. Brüne M, Ozgürdal S, Ansorge N, Von Reventlow HG, Peters S, Nicolas V, et al. An fMRI study of "theory of mind" in at-risk states of psychosis: comparison with manifest schizophrenia and healthy controls. Neuroimage. 2011; 55(1): 329–37. 10.1016/j.neuroimage.2010.12.018 [DOI] [PubMed] [Google Scholar]
- 58. Van Rijn S, Aleman A, de Sonneville L, Sprong M, Ziermans T, Schothorst P, et al. Misattribution of facial expressions of emotion in adolescents at increased risk of psychosis: the role of inhibitory control. Psychol Med. 2011; 41(3): 499–508. 10.1017/S0033291710000929 [DOI] [PubMed] [Google Scholar]
- 59. Eack SM, Mermon DE, Montrose DM, Miewald J, Gur RE, Gur RC, et al. Social cognition deficits among individuals at familial high risk for schizophrenia. Schizophr Bull 2010; 36(6): 1081–8. 10.1093/schbul/sbp026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Guastella AJ, Hermens DF, Van Zwieten A, Naismith SL, Lee RS, Cacciotti-Saija C, et al. Social cognitive performance as a marker of positive psychotic symptoms in young people seeking help for mental health problems. Schizophr Res. 2013; 149(1–3): 77–82. 10.1016/j.schres.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 61. Schenkel LS, Spaulding WD, Silverstein SM. Poor premorbid social functioning and theory of mind deficit in schizophrenia: evidence of reduced context processing? J Psychiatr Res. 2005; 39(5): 499–508. [DOI] [PubMed] [Google Scholar]
- 62. Szily E, Kéri S. Delusion Proneness and emotion appraisal in individuals with high psychosis vulnerability. Clin Psychol Psychother. 2013; 20: 166–170. 10.1002/cpp.1763 [DOI] [PubMed] [Google Scholar]
- 63. Marjoram D, Job DE, Whalley HC, Gountouna VE, McIntosh AM, Simonotto E, et al. A visual joke fMRI investigation into Theory of Mind and enhanced risk of schizophrenia. Neuroimage. 2006; 31(4): 1850–8. [DOI] [PubMed] [Google Scholar]
- 64. Kim HS, Shin NY, Choi JS, Jung MH, Jang JH, Kang DH, et al. Processing of facial configuration in individuals at ultra-high risk for schizophrenia. Schizophr Res. 2010; 118(1–3): 81–7. 10.1016/j.schres.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 65. Gill KE, Cressman V, Poe SL, Steinfeld S, Ben-David S, Keilp JG, et al. Social inference in individuals at clinical high risk for psychosis. Early Intervention in Psychiatry. 2014; September 9: Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 66. Stanford AD, Messinger J, Malaspina D, Corcoran CM. Theory of Mind in patients at clinical high risk for psychosis. Schizophr Res. 2011; 131(1–3): 11–7. 10.1016/j.schres.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pinkham AE, Penn DL, Perkins DO, Graham KA, Siegel M. Emotion perception and social skill over the course of psychosis: a comparison of individuals "at-risk" for psychosis and individuals with early and chronic schizophrenia spectrum illness. Cogn Neuropsychiatry. 2007; 12(3): 198–212. [DOI] [PubMed] [Google Scholar]
- 68. DeVylder JE, Ben-David S, Kimhy D, Corcoran CM. Attributional style among youth at clinical risk for psychosis. Early Interv Psychiatry. 2013; 7(1): 84–8. 10.1111/j.1751-7893.2012.00347.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Thompson A, Papas A, Bartholomeusz C, Allott K, Amminger GP, Nelson B, et al. Social cognition in clinical "at risk" for psychosis and first episode psychosis populations. Schizophr Res. 2012; 141(2–3): 204–9. 10.1016/j.schres.2012.08.007 [DOI] [PubMed] [Google Scholar]
- 70. Seiferth NY, Pauly K, Habel U, Kellermann T, Shah NJ, Ruhrmann S, et al. Increased neural response related to neutral faces in individuals at risk for psychosis. Neuroimage. 2008; 40(1): 289–97. 10.1016/j.neuroimage.2007.11.020 [DOI] [PubMed] [Google Scholar]
- 71. Addington J, Penn D, Woods SW, Addington D, Perkins DO. Facial affect recognition in individuals at clinical high risk for psychosis. Br J Psychiatry. 2008; 192(1): 67–8. 10.1192/bjp.bp.107.039784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Comparelli A, Corigliano V, De Carolis A, Mancinelli I, Trovini G, Ottavi G, et al. Emotion recognition impairment is present early and is stable throughout the course of schizophrenia. Schizophr Res. 2013; 143(1): 65–9. 10.1016/j.schres.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 73. Thompson A, Papas A, Bartholomeusz C, Nelson B, Yung A. Externalized attributional bias in the Ultra High Risk (UHR) for psychosis population. Psychiatry Res. 2013; 206(2–3): 200–5. 10.1016/j.psychres.2012.10.017 [DOI] [PubMed] [Google Scholar]
- 74. An SK, Kang JI, Park JY, Kim KR, Lee SY, Lee E. Attribution bias in ultra-high risk for psychosis and first-episode schizophrenia. Schizophr Res. 2010; 118(1–3): 54–61. 10.1016/j.schres.2010.01.025 [DOI] [PubMed] [Google Scholar]
- 75. Couture SM, Penn DL, Addington J, Woods SW, Perkins DO. Assessment of social judgments and complex mental states in the early phases of psychosis. Schizophr Res. 2008; 100(1–3): 237–41. 10.1016/j.schres.2007.12.484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Green MF, Bearden CE, Cannon TD, Fiske AP, Hellemann GS, Horan WP, et al. Social cognition in schizophrenia, Part 1: performance across phase of illness. Schizophr Bull. 2012; 38(4): 854–64. 10.1093/schbul/sbq171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Giuliano AJ, Li H, Mesholam-Gately RI, Sorenson SM, Woodberry KA, Seidman LJ. Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Curr Pharm Des. 2012; 18(4): 399–415. [DOI] [PubMed] [Google Scholar]
- 78. Addington J, Saeedi H, Addington D. Facial affect recognition: a mediator between cognitive and social functioning in psychosis? Schizophr Res. 2006; 85(1–3): 142–50. [DOI] [PubMed] [Google Scholar]
- 79. Ohmuro N, Matsumoto K, Katsura M, Sakuma A, Izuka K, Kikuchi T, et al. Association of deficits in theory of mind and functioning in at-risk mental states and first-episode psychosis IEPA conference, San Fransisco, 2012. [Google Scholar]
- 80. Savla GN, Vella L, Armstrong CC, Penn DL, Twamley EW. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr Bull. 2013; 39(5): 979–92. 10.1093/schbul/sbs080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, et al. North American Prodrome Longitudinal Study (NAPLS) Group. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry. 2010; 67(6): 578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shamay-Tsoory SG. The neural bases for empathy. Neuroscientist. 2011; 17(1): 18–24. 10.1177/1073858410379268 [DOI] [PubMed] [Google Scholar]
- 83. Zaki J, Bolger N, Ochsner K: It takes two: the interpersonal nature of empathic accuracy. Psychol Sci 2008, 19:399–404. 10.1111/j.1467-9280.2008.02099.x [DOI] [PubMed] [Google Scholar]
- 84. Davis MH: A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychol 1980, 10:85. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper.