Abstract
Purpose
Short-term complications related to the perineal wound after abdominoperineal excision (APE) are a well-known problem. Perineal morbidity in the longer term is an almost unexplored area. The aim of this cross-sectional study was to investigate the prevalence of perineal symptoms 3 years after APE for rectal cancer, to identify potential risk factors and to explore the relationship between perineal morbidity and global quality of life.
Method
All patients who underwent APE in Sweden between 2007 and 2009 (n = 1373) were identified through the Swedish Colorectal Cancer Registry. Surviving patients were contacted 3 years after surgery and asked about participation. A total of 545 patients completed a detailed questionnaire. Clinical data was collected from the registry and surgical charts.
Results
Perineal symptoms occurred in 50 % of all patients 3 years after APE and more frequently in women (58 vs. 44 %; p = 0.001). Delayed healing of the perineal wound (>4 weeks) occurred in 25 % of all patients and more frequently after extralevator APE (ELAPE) than after conventional APE (32 vs. 11 %, p < 0.001). Delayed healing was associated with an increased risk of more severe perineal symptoms (relative risk (RR) 1.50, 95 % confidence interval (95 % CI) 1.09–2.05). Patients with more severe perineal symptoms (n = 129) had a significantly lower global quality of life as measured by EQ-5D visual analogue scale (VAS; median 75 vs. 83 points on the 100-point scale; p < 0.001).
Conclusion
Persistent perineal symptoms are common after APE and may have an impact on patients’ quality of life. Delayed wound healing may be a risk factor for persistent symptoms. Further studies are needed to identify avoidable clinical factors for the development of persistent perineal morbidity.
ClinicalTrials.gov Identifier
Keywords: Rectal cancer, Abdominoperineal excision, Perineal morbidity, Quality of life
Introduction
Treatment of patients with distal rectal cancer is a clinical challenge. Achieving an optimal oncologic result is paramount, but functional consequences of treatment must also be considered. Recently, several studies have focused on the proposed oncological advantage of extralevator abdominoperineal excision (ELAPE) compared to conventional abdominoperineal excision (conventional APE) [2, 7, 17, 18, 24, 26]. Other studies have investigated functional aspects of these procedures such as sexual and urological dysfunction [6, 9] and stoma-related problems [1, 14].
The occurrence and implications of perineal symptoms following abdominoperineal excision is a largely unexplored area. One study found persistent perineal pain in half of all patients 2 years after ELAPE [23]. Reports of other long-term perineal symptoms are scarce. In contrast, short-term perineal morbidity after abdominoperineal excision is well documented. Several studies have reported increased perineal wound complications after ELAPE compared to conventional APE [2, 7, 11, 13, 18, 24, 25]. Perineal wound complications frequently result in delayed healing, but whether this is associated with persistent perineal symptoms is not known.
We hypothesized that persistent perineal symptoms are frequent following any kind of abdominoperineal excision. In the present analysis, we explored the prevalence of perineal symptoms about 3 years after surgery in a national cohort. The primary objective was to determine the prevalence and severity of symptoms, and secondary objectives were to identify risk factors for long-term perineal morbidity and to investigate the impact of perineal morbidity on patients’ health-related quality of life. Oncologic results in this national cohort, including 3-year recurrence rates, have been reported previously [17–19].
Methods
In this registry-based, observational study, patients operated with abdominoperineal excision for rectal cancer in Sweden in the years 2007–2009 were identified through the Swedish Colorectal Cancer Registry. This national quality registry covers more than 97 % of all patients in Sweden and has a good internal validity [8, 15]. Data on sex, age, BMI, American Society of Anesthesiologists (ASA) classification, tumor height (distance from the lower edge of the tumor to the anal verge), neoadjuvant treatment, circumferential resection margin (CRM) involvement, and tumor pT and pN stage were retrieved from the registry. Data regarding perineal dissection and reconstruction techniques were not included in the registry but was extracted from operative notes [19]. This allowed for the classification of procedures as either conventional APE or ELAPE in a majority of cases, but surgical technique remained interminable in as many as 252 cases. Information regarding perineal symptoms after surgery was obtained through a study-specific questionnaire.
Questionnaire
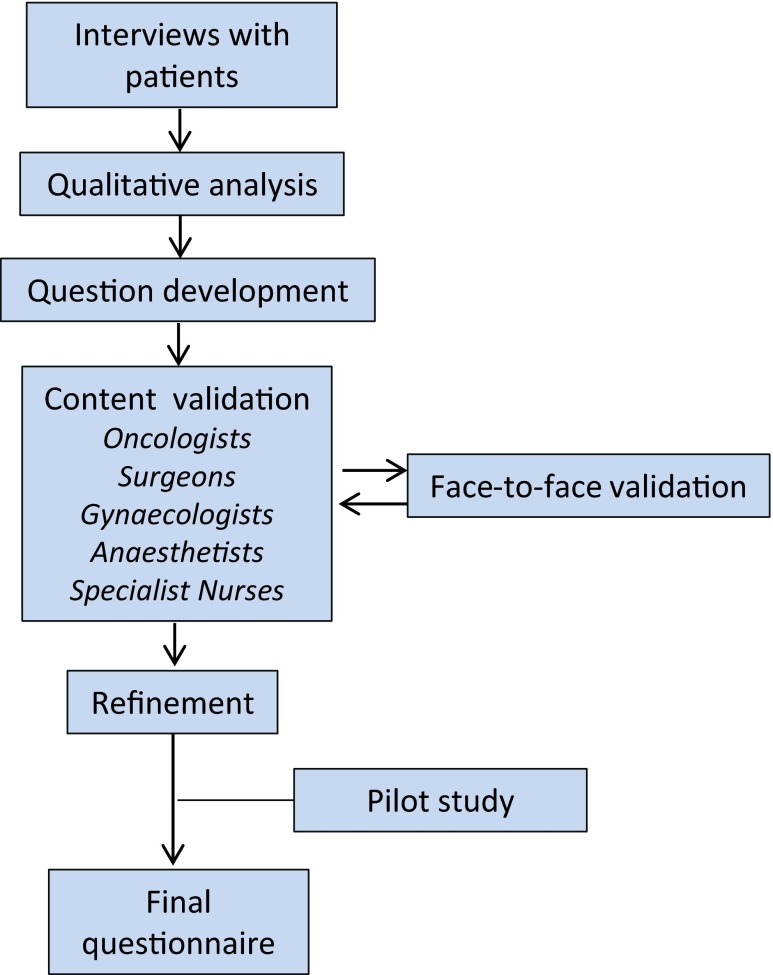
The questions on perineal symptoms (Table 1) analyzed in the present study were part of an extensive questionnaire that also covered many other aspects of functional outcome after abdominoperineal excision. The development and validation of this questionnaire is illustrated in Fig. 1 and has been described in detail elsewhere [3, 10, 12, 21, 22]. The process involved interviews with rectal cancer patients and subsequent analysis with qualitative methods, content validation in a multidisciplinary group of professionals with extensive clinical experience in the field, and face-to-face validation, where patients were asked to complete the questionnaire in the presence of a specialist nurse to detect any problems, misinterpretations, or concerns. Questions were revised accordingly, and the process continued until no uncertainties remained.
Table 1.
Questions on perineal symptoms and perineal wound healing
| Perineal symptoms |
| Have you had pain between the buttocks in the past month? |
| Have you had difficulties to sit in the past month? |
| Have you had loss of sensation/numbness in the buttocks in the past month? |
| Have you experienced tension in the buttocks in the past month? |
| Have you experienced a tingling/stinging sensation in the buttocks in the past month? |
| Have you experienced cramps/urgency that you perceived came from the previous location of your rectum in the past month? |
| Response options: Not at all/a little/quite a bit/very much |
| Perineal wound healing |
| For how long after your rectal surgery did you need to irrigate or change the dressing of the wound between the buttocks, at home or at a primary care centre/hospital? |
| Response options: 2 weeks/3–4 weeks/1–2 months/3–4 months/longer than 4 months |
| What was your experience of the wound healing process after your rectal surgery, when the wound between the buttocks was healing? |
| Response options: Not at all difficult/a little difficult/quite difficult/very difficult |
| Questions on symptom-associated distress (exemplified by the question regarding pain) |
| If you, for the rest of your life, would have as much pain between the buttocks as you did in the past month, would that distress you? |
| Response options: Not applicable, I have not had pain between the buttocks in the past month/not at all/a little/quite a bit/very much |
Fig. 1.
Development of a study-specific questionnaire
During the development of the questionnaire, six “core” perineal symptoms emerged and were included in this analysis: pain, sitting disability, paraesthesia, tension between the buttocks, sensation of tingling/stinging between the buttocks, and perineal cramps/sensation of urgency. The recall period was the past month. Response options were dichotomized as explained in the tables. Patients reporting at least one perineal symptom of severe intensity (response options quite a bit or very much, see Table 1) were defined by us to have a severe perineal morbidity.
Also included in this analysis were questions on post-operative perineal wound healing and the EQ-5D visual analogue scale (VAS) question on global health-related quality of life [4, 16, 20].
Statistical analyses
All data were collected in a database, and statistical analyses were performed using SPSS 21.0 (IBM SPSS Inc. Armonk, NY, USA) and SAS v. 9 (SAS institute). In the analysis of risk factors for severe perineal morbidity (the experience of one or more symptom of severe intensity, see above), variables chosen as potential predictors were radiotherapy, sex, age, tumor height, and delayed perineal wound healing. Because of the observed strong association between surgical technique and perineal wound healing (Table 2), surgical technique was not included in the regression model. In the subgroup of patients who underwent ELAPE, perineal repair and excision of the coccyx were included as potential predictors as well. A log-linear binomial regression model [5] was used to estimate the relative risk (RR) and 95 % confidence intervals (95 % CIs). In case the binomial model did not converge, a log-linear Poisson regression with a robust error variance was used [27]. Additional statistical analyses involved chi-square test and Mann–Whitney U test for categorical and continuous variables, respectively. No correction for multiple testing was made, and results should therefore be regarded as interesting findings rather than as conclusive evidence.
Table 2.
Time to healing of the perineal wound stratified on surgical technique
| Conventional APE | ELAPE | p value | Missing | |||
|---|---|---|---|---|---|---|
| Time to healing (%) | Normal | 0–4 weeks | 62 (88.6 %) | 146 (68.2 %) | 0.001a | 9 |
| Delayed | 1–4 months | 6 (8.6 %) | 48 (22.4 %) | |||
| >4 months | 2 (2.9 %) | 20 (9.3 %) |
Patients operated with an indeterminate surgical technique (n = 252) are omitted
APE abdominoperineal excision, ELAPE extralevator abdominoperineal excision
a normal versus delayed (>4 weeks) healing
Results
Out of the 1319 patients who were included in our previous analyses of oncological outcome [17, 18], 852 were alive 3 years post-operatively and 703 patients were eligible for inclusion in this study (Fig. 2). A total of 596 patients agreed to receive the questionnaire by mail and 545 returned the questionnaire and were included in the analysis. Reasons for non-inclusion (n = 774) are presented in Fig. 2. Clinical characteristics of included patients, non-responders, and deceased patients are presented in Table 3. Non-responders were older and had more comorbidity as reflected by the preoperative ASA grade and received less neoadjuvant radiotherapy than responders to the questionnaire. Conventional APE was more common among non-responders, as were involved resection margins.
Fig. 2.
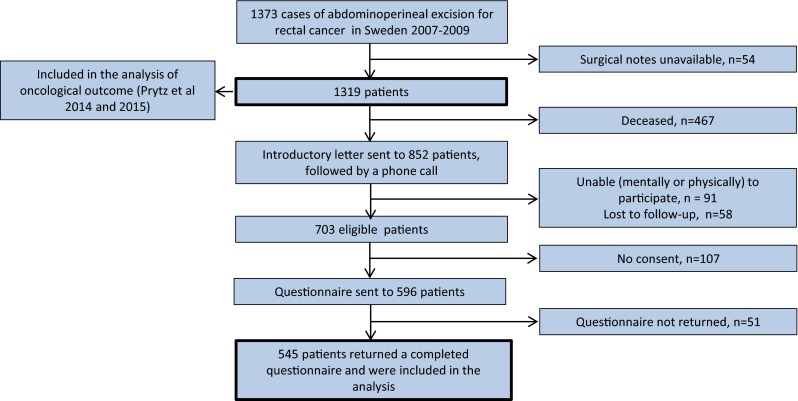
Flowchart of patients
Table 3.
Clinical characteristics of patients operated by abdominoperineal excision in Sweden 2007–2009
| Total cohort, n = 1319 | |||||
|---|---|---|---|---|---|
| Included in the analysis | Deceased at follow-up | Non-responders | p value | ||
| Number of patients | 545 | 467 | 307 | ||
| Sex | Female | 218 (40.0 %) | 170 (36.4 %) | 142 (46.3 %) | 0.076 |
| Male | 327 (60.0 %) | 297 (63.6 %) | 165 (53.7 %) | ||
| Age at operation | 66.0 | 71.9 | 69.3 | <0.001 | |
| BMI | 29.3 | 27.3 | 26.8 | 0.944 | |
| ASA classification | ASA 1 | 144 (27.0 %) | 69 (15.3 %) | 61 (20.5 %) | <0.001 |
| ASA 2 | 314 (58.9 %) | 235 52.2 %) | 165 (55.4 %) | ||
| ASA 3 | 73 (13.7 %) | 137 (30.4 %) | 72 (24.2 %) | ||
| ASA 4 | 2 (0.4 %) | 9 (2.0 %) | 0 | ||
| Radiation therapy | None | 64 (11.8 %) | 123 (26.7 %) | 64 (20.9 %) | <0.01 |
| Short (5 × 5 Gy) | 355 (65.6 %) | 222 (48.2 %) | 181 (59.2 %) | ||
| Long (25 × 1,8/2 Gy) | 122 (22.6 %) | 116 (25.2 %) | 61 (19.9 %) | ||
| Chemoradiotherapy | Yes | 110 (20.2 %) | 112 (24.0 %) | 57 (18.6 %) | 0.560 |
| No | 434 (79.8 %) | 354 (76 %) | 250 (81.4 %) | ||
| Tumor heighta | 4,1 | 4,3 | 4,3 | 0.811 | |
| pT stage | T0–T2 | 256 (47.9 %) | 98 (21.2 %) | 130 (42.9 %) | 0.385 |
| T3 | 252 (47.1 %) | 289 (62.4 %) | 156 (51.5 %) | ||
| T4 | 27 (5.0 %) | 76 (16.4 %) | 17 (5.6 %) | ||
| pN stage | N0 | 344 (63.9 %) | 187 (41.1 %) | 202 (68.2 %) | 0.354 |
| N1 | 130 (24.2 %) | 117 (25.7 %) | 67 (22.6 %) | ||
| N2 | 64 (11.9 %) | 151 (33.2 %) | 27 (9.1 %) | ||
| Microscopic radicalityb | Yes | 522 (96.0 %) | 386 (83.0 %) | 285 (92.8 %) | <0.05 |
| No/indeterminate | 22 (4.0 %) | 79 (17.0 %) | 22 (7.2 %) | ||
| Perineal dissection | Conventional APE | 71 (13.0 %) | 79 (16.9 %) | 59 (19.2 %) | <0.05 |
| ELAPE | 222 (40.7 %) | 172 (36.8 %) | 124 (40.45) | ||
| Indeterminate | 252 (46.2 %) | 216 (46.3 %) | 124 (40.4 %) | ||
| Coccyx resection | Yes | 124 (27.7 %) | 133 (35.6 %) | 73 (28.4 %) | 0.850 |
| No | 323 (72.3 %) | 241 (64.4 %) | 184 (71.6 %) | ||
| Perineal reconstruction | Suture | 430 (79.6 %) | 352 (76.4 %) | 238 (78.3 %) | 0.803 |
| Mesh | 64 (11.9 %) | 56 (12.1 %) | 36 (11.8 %) | ||
| Flap | 46 (8.5 %) | 53 (11.5 %) | 30 (9.9 %) | ||
p values refer to differences between included patients and non-responders
BMI body mass index, ASA American Society of Anesthesiologists, APE abdominoperineal excision, ELAPE extralevator abdominoperineal excision
aDistance in centimeter from the lower edge of the tumor to the anal verge
bCircumferential resection margin >1 mm
The conventional APE and ELAPE groups differed regarding sex and tumor height, with more male patients and more distal tumors in the ELAPE group. The frequency of coccyx resection and method of perineal repair also differed between groups with very few resections and no use of flap or mesh in the conventional APE group. pT and pN stage and ASA grade were no different, and there were no significant differences regarding neoadjuvant radiotherapy and chemoradiotherapy (Table 4).
Table 4.
Clinical characteristics of responders stratified on surgical technique
| Conventional APE | ELAPE | p value | ||
|---|---|---|---|---|
| Number of patients | 71 | 222 | ||
| Sex | Female | 38 (53.5 %) | 87 (39.2 %) | <0.05 |
| Male | 33 (46.5 %) | 135 (60.8 %) | ||
| Tumor heighta | 5,9 | 3.5 | <0.001 | |
| Coccyx resection | Yes | 2 (3.0 %) | 95 (46.6 %) | <0.001 |
| No | 64 (97.0 %) | 109 (53.4 %) | ||
| Perineal reconstruction: | Suture | 71 (100.0 %) | 120 (54.5 %) | <0.001 |
| Mesh | 0 | 54 (24.5 %) | ||
| Flap | 0 | 46 (20.9 %) |
Patients operated with an indeterminate surgical technique (n = 252) are omitted. Age, BMI, ASA grade, neoadjuvant treatment, pT and pN stage, and non-radical resections did not differ (data not shown)
APE abdominoperineal excision, ELAPE extralevator abdominoperineal excision
aDistance in centimeter from the lower edge of the tumor to the anal verge
Perineal symptoms and associated distress
Perineal symptoms were present in 50 % of all responding patients and more frequently in women (58 vs. 44 %; p = 0.001). In total, 129 patients (24 %) experienced one or more perineal symptom of severe intensity, i.e., severe perineal morbidity according to our definition.
Tension between buttocks, sitting disability, cramps/urgency, and perineal pain were most frequently experienced (Table 5). Sitting disability was particularly distressful to patients. There was a positive relationship between the symptom intensity and the level of associated distress for all symptoms (data not shown).
Table 5.
Perineal symptoms: prevalence, intensity, and associated distress
| Symptoms | Symptom-associated distress | ||||||
|---|---|---|---|---|---|---|---|
| None | Any intensity | Minor/severea | Missing | None or lowb | Highc | Missingd | |
| Pain | 419 (79 %) | 114 (21 %) | 76 (14 %)/38 (7 %) | 12 | 63 (62 %) | 38 (38 %) | 13 |
| Sitting disability | 410 (77 %) | 123 (23 %) | 81 (15 %)/42 (8 %) | 12 | 57 (55 %) | 47 (45 %) | 19 |
| Paraesthesia | 461 (86 %) | 76 (14 %) | 53 (10 %)/23(4 %) | 8 | 50 (68 %) | 23 (32 %) | 3 |
| Tension | 400 (75 %) | 132 (25 %) | 96 (18 %)/36 (7 %) | 13 | 98 (75 %) | 32 (25 %) | 2 |
| Tingling/stinging | 490 (93 %) | 40 (7 %) | 33 (6 %)/7 (1 %) | 15 | 28 (72 %) | 11 (28 %) | 1 |
| Cramps/urgency | 412 (77 %) | 123 (23 %) | 101 (19 %)/22 (4 %) | 10 | 100 (82 %) | 22 (18 %) | 1 |
aResponse options: a little/ quite a bit or very much
bResponse options: not at all or a little
cResponse options: quite a bit or very much
dNumber of symptomatic patients who did not respond to the distress question
Perineal wound healing
A total of 133 patients (25 %) reported delayed wound healing (>4 weeks) following their operation (Table 6). The wound healing process was associated with a high level of distress in 46 % of the patients. There was a positive relationship between the duration of the healing process and the level of associated distress (data not shown). Time to healing of the perineal wound was significantly related to surgical technique (Table 2). The relative risk of a delayed healing after ELAPE compared to conventional APE was 3.45 (95 % CI 1.46–8.15) after adjustment for smoking, diabetes, BMI, and preoperative radiotherapy. Radiotherapy alone was not associated with delayed healing in bivariate analysis.
Table 6.
Time to healing of the perineal wound and patients’ perception of the healing process
| All | Missing | |||
|---|---|---|---|---|
| Time to healing (%) | Normal | 0–4 weeks | 391 (75 %) | 21 |
| Delayed | 1–4 months | 91 (17 %) | ||
| >4 months | 42 (8 %) | |||
| Patients’ perception of the healing process | Not at all/a little difficult | 284 (54 %) | 21 | |
| Quite/very difficult | 240 (46 %) |
Risk factors for perineal symptoms
Delayed perineal wound healing and female gender were statistically significant risk factors for severe perineal morbidity whereas no significant contribution could be observed for the other variables including radiotherapy (Table 7). In a subgroup of ELAPE patients, plain suturing and flap reconstruction both increased the risk of severe perineal morbidity compared with mesh repair. Resection of the coccyx did not have a significant impact (Table 8).
Table 7.
Analysis of risk factors for severe perineal morbidity (corrected for age)
| Relative risk | 95 % CI | ||
|---|---|---|---|
| Radiotherapy | Yes/no | 0.816 | 0.455–1.463 |
| Sex | Female/male | 1.35 | 1.035–1.890 |
| Perineal wound healing | Delayed/normal | 1.546 | 1.132–2.111 |
| Tumor height | >4 cm/0–4 cm | 1.142 | 0.832–1.567 |
One or more perineal symptom of severe intensity (response options: quite a bit or very much)
CI confidence interval
Table 8.
Subgroup analysis of risk factors for severe perineal morbidity in ELAPE patients (corrected for age)
| Relative risk | 95 % CI | ||
|---|---|---|---|
| Perineal repair | Suture/mesh | 2.311 | 1.023–5.222 |
| Flap/mesh | 3.16 | 1.284–7.778 | |
| Suture/flap | 0.731 | 0.409–1.309 | |
| Excision of coccyx | Yes/no | 1.078 | 0.440–2.640 |
| Radiotherapy | Yes/no | 1.484 | 0.727–3.028 |
| Sex | Female/male | 0.968 | 0.603–1.554 |
| Perineal wound healing | Delayed/normal | 1.624 | 1.001–2.636 |
| Tumor height | >4 cm/0–4 cm | 1.149 | 0.684–1.931 |
One or more perineal symptom of severe intensity (response options: quite a bit or very much)
CI confidence interval
Perineal morbidity and quality of life
Patients who experienced one or more symptom of severe intensity (i.e., the group of 129 patients with severe perineal morbidity) had a significantly lower global quality of life as measured by EQ-5D VAS compared to those with less severe symptoms (median 75 vs. 83 points on the 100-point scale; p < 0.001).
Discussion
This study showed that 50 % of patients had perineal symptoms 3 years after surgery. Tension between buttocks, sitting disability, perineal cramps/sensation of urgency, and perineal pain were most frequently experienced, and sitting disability was associated with most distress. A delayed perineal wound healing emerged as a risk factor for persistent perineal symptoms in our analysis. Delayed healing was considerably more common after ELAPE compared to conventional APE with more than three times higher risk in ELAPE patients. Notably, this did not translate into more long-term perineal symptoms in ELAPE patients (data not shown). Finally, the experience of one or more perineal symptom of severe intensity was associated with a significantly lower global quality of life score in the EQ-5D visual analogue scale.
Several studies have reported high rates of short-term perineal wound complications after abdominoperineal excision in general and ELAPE in particular [11, 24], but few have explored long-term perineal morbidity in these patients. Musters et al. [11] found a perineal hernia or a persisting perineal sinus in 8–10 % of patients about 2 years after any kind of abdominoperineal excision. Welsch and colleagues [23] reported a frequency of persistent pain or other (unspecified) functional deficits of 50 % after ELAPE about 2 years after surgery. However, these studies are limited by small sample sizes, varying follow-up times, and lack of detail regarding perineal symptoms.
The present study is large in comparison to most other studies. It combines data from three different relevant sources. Included patients were collected from a prospective national registry with almost complete coverage, which strengthens the external validity. Original operative notes were retrieved and systematically analyzed, and finally, we used data on patients’ self-reported symptoms at a defined time point after surgery. In this way, we avoided the pitfalls of retrieving information on symptoms from hospital records, with their inherent bias and inconsistency of symptom documentation. This enables us to draw reliable conclusions.
We did not attempt to design an instrument to reflect perineal morbidity in a single score; instead, questions were analyzed individually. Patients were queried not only about the intensity of symptoms but also about the perceived significance (symptom-associated distress) of symptoms. Although not a new concept in oncologic and epidemiologic research [21, 22], it has seldom been studied in patients with rectal cancer before.
We have used the term severe perineal morbidity, defined as the experience of at least one symptom of severe intensity, in order to identify patients with more severe perineal symptoms. This definition is arguably arbitrary but it is clinically reasonable in our opinion.
Healing of the perineal wound was defined as delayed if the process exceeded 1 month. Wound healing is difficult to study; once patients have been discharged, outpatient visits are generally few and wound care is often carried out in a primary care setting. In our study, time to healing of the perineal wound was a patient-reported variable. We asked about the period of time during which the patient needed wound care after the operation, either at home, in a hospital, or at a primary care facility. This should correspond well with the time it took for the wound to heal. Although there is a risk of recall bias regarding this outcome, we believe that patients, even after 3 years, are able to give a fair estimate of the duration of the healing process, as it is often distressful. Notably, healing time did not differ significantly between patients receiving radiotherapy short course, long course, or not at all (data not shown).
The differences between responders and non-responders presented in Table 3 should be considered in relation to the generalizability of results. Non-responders were older with more comorbidity and fewer received radiotherapy compared with responders. However, whether these differences lead to overestimation or underestimation of perineal morbidity in the total population of 3-year survivors is unclear.
Although there are missing data for most variables and questions, numbers are low and should not disturb interpretation.
The retrospective non-randomized cross-sectional design has limitations. Baseline data on perineal symptoms are not available. However, this is probably not a major concern as perineal symptoms before treatment are likely to be infrequent. An alternative study design would be a prospective observational study. We are currently running such a study in 16 centers in Sweden and Denmark, but as inclusion is still ongoing, long-term follow-up results are not available [3].
For the long-term management of patients treated for distal rectal cancer, solid data on functional impairments are valuable. We present such data regarding perineal symptoms based on a national cancer registry for the first time. We conclude that the prevalence of persistent perineal symptoms is high following abdominoperineal excision. This should be conveyed to patients as part of the preoperative counselling along with information about other potential functional consequences of the procedure. Patients would probably benefit from closer surveillance and support during the wound healing process.
Acknowledgments
The authors gratefully acknowledge the support and excellent work performed by the research nurses at Scandinavian Surgical Outcomes Research Group: Anette Wedin and Jane Heath. We also acknowledge the avid assistance of the expert panel in the development of the questionnaire.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
The Regional Ethical Review Board in Gothenburg approved the study (Dnr 406–10, 407–10).
Funding
This study was supported by grants from the Swedish Research Council (2012–1768), the Swedish Cancer Society (2010/593; 2013/497), Sahlgrenska University Hospital (ALF grant, agreement concerning research and education of doctors), the Health and Medical Care Committee of the Regional Executive Board, Region Västra Götaland, the Gothenburg Medical Society, Assar Gabrielsson Foundation, Bengt Ihre Foundation, Ruth and Richard Julin’s Foundation, and Lion’s Cancer Research Foundation of Western Sweden.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author contribution
All authors have fulfilled the Uniform Requirements for Manuscripts Submitted to Biomedical Journals statement of the International Committee of Medical Journal Editors.
References
- 1.Angenete E, Correa-Marinez A, Heath J, et al. Ostomy function after abdominoperineal resection—a clinical and patient evaluation. Int J Colorectal Dis. 2012;27:1267–1274. doi: 10.1007/s00384-012-1463-1. [DOI] [PubMed] [Google Scholar]
- 2.Asplund D, Haglind E, Angenete E. Outcome of extralevator abdominoperineal excision compared with standard surgery: results from a single centre. Colorectal Dis. 2012;14:1191–1196. doi: 10.1111/j.1463-1318.2012.02930.x. [DOI] [PubMed] [Google Scholar]
- 3.Asplund D, Heath J, Gonzalez E, et al. Self-reported quality of life and functional outcome in patients with rectal cancer—QoLiRECT. Dan Med J. 2014;61:A4841. [PubMed] [Google Scholar]
- 4.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 5.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case–control studies. Am J Epidemiol. 2004;160:301–305. doi: 10.1093/aje/kwh221. [DOI] [PubMed] [Google Scholar]
- 6.Ho VP, Lee Y, Stein SL, et al. Sexual function after treatment for rectal cancer: a review. Dis Colon Rectum. 2011;54:113–125. doi: 10.1007/DCR.0b013e3181fb7b82. [DOI] [PubMed] [Google Scholar]
- 7.Holm T, Ljung A, Häggmark T, et al. Extended abdominoperineal resection with gluteus maximus flap reconstruction of the pelvic floor for rectal cancer. Br J Surg. 2007;94:232–238. doi: 10.1002/bjs.5489. [DOI] [PubMed] [Google Scholar]
- 8.Jorgren F, Johansson R, Damber L, et al. Validity of the Swedish Rectal Cancer Registry for patients treated with major abdominal surgery between 1995 and 1997. Acta Oncol. 2013;52:1707–1714. doi: 10.3109/0284186X.2013.805886. [DOI] [PubMed] [Google Scholar]
- 9.Kasparek MS, Hassan I, Cima RR, et al. Long-term quality of life and sexual and urinary function after abdominoperineal resection for distal rectal cancer. Dis Colon Rectum. 2012;55:147–154. doi: 10.1097/DCR.0b013e31823d2606. [DOI] [PubMed] [Google Scholar]
- 10.Kreicbergs U, Valdimarsdottir U, Onelov E, et al. Talking about death with children who have severe malignant disease. N Engl J Med. 2004;351:1175–1186. doi: 10.1056/NEJMoa040366. [DOI] [PubMed] [Google Scholar]
- 11.Musters GD, Sloothaak DA, Roodbeen S, et al. Perineal wound healing after abdominoperineal resection for rectal cancer: a two-centre experience in the era of intensified oncological treatment. Int J Colorectal Dis. 2014;29:1151–1157. doi: 10.1007/s00384-014-1967-y. [DOI] [PubMed] [Google Scholar]
- 12.Omerov P, Steineck G, Runeson B, et al. Preparatory studies to a population-based survey of suicide-bereaved parents in Sweden. Crisis. 2013;34:200–210. doi: 10.1027/0227-5910/a000175. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz H, Ciga MA, Armendariz P, et al. Multicentre propensity score-matched analysis of conventional versus extended abdominoperineal excision for low rectal cancer. Br J Surg. 2014;101:874–882. doi: 10.1002/bjs.9522. [DOI] [PubMed] [Google Scholar]
- 14.Pachler J, Wille-Jorgensen P. Quality of life after rectal resection for cancer, with or without permanent colostomy. Cochrane Database Syst Rev. 2012;12:CD004323. doi: 10.1002/14651858.CD004323.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pahlman L, Bohe M, Cedermark B, et al. The Swedish rectal cancer registry. Br J Surg. 2007;94:1285–1292. doi: 10.1002/bjs.5679. [DOI] [PubMed] [Google Scholar]
- 16.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prytz M, Angenete E, Bock D et al. (2015) Extralevator Abdominoperineal Excision for Low Rectal Cancer-Extensive Surgery to be Used With Discretion Based on 3-Year Local Recurrence Results: A Registry-based, Observational National Cohort Study. Annals of surgery [DOI] [PMC free article] [PubMed]
- 18.Prytz M, Angenete E, Ekelund J, et al. Extralevator abdominoperineal excision (ELAPE) for rectal cancer-short-term results from the Swedish Colorectal Cancer Registry. Selective use of ELAPE warranted. Int J Colorectal Dis. 2014;29:981–987. doi: 10.1007/s00384-014-1932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prytz M, Angenete E, Haglind E. Abdominoperineal extralevator resection. Dan Med J. 2012;59:A4366. [PubMed] [Google Scholar]
- 20.Rabin R, De Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 21.Steineck G, Bergmark K, Henningsohn L. Symptom documentation in cancer survivors as a basis for therapy modifications. Acta Oncol. 2002;41:244–252. doi: 10.1080/02841860260088782. [DOI] [PubMed] [Google Scholar]
- 22.Steineck G, Helgesen F, Adolfsson J, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002;347:790–796. doi: 10.1056/NEJMoa021483. [DOI] [PubMed] [Google Scholar]
- 23.Welsch T, Mategakis V, Contin P, et al. Results of extralevator abdominoperineal resection for low rectal cancer including quality of life and long-term wound complications. Int J Colorectal Dis. 2013;28:503–510. doi: 10.1007/s00384-012-1611-7. [DOI] [PubMed] [Google Scholar]
- 24.West N, Anderin C, Smith K, et al. Multicentre experience with extralevator abdominoperineal excision for low rectal cancer. Br J Surg. 2010;97:588–599. doi: 10.1002/bjs.6916. [DOI] [PubMed] [Google Scholar]
- 25.West NP, Finan PJ, Anderin C, et al. Evidence of the oncologic superiority of cylindrical abdominoperineal excision for low rectal cancer. J Clin Oncol. 2008;26:3517–3522. doi: 10.1200/JCO.2007.14.5961. [DOI] [PubMed] [Google Scholar]
- 26.Yu HC, Peng H, He XS, et al. Comparison of short- and long-term outcomes after extralevator abdominoperineal excision and standard abdominoperineal excision for rectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2014;29:183–191. doi: 10.1007/s00384-013-1793-7. [DOI] [PubMed] [Google Scholar]
- 27.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]