Abstract
Purpose of Review
Arterial tortuosity is emerging as a common feature in genetically mediated thoracic aortic disease that may be prognostic. This review will summarize recent literature on arterial tortuosity in the setting of genetic arteriopathies.
Recent Findings
While arterial tortuosity has been primarily described in Loeys-Dietz syndrome due to TGFBR1 and TGFBR2 mutations and in arterial tortuosity syndrome due to SLC210A mutations, recent studies that use quantitative measures of tortuosity suggest that tortuosity is present in many other genetic conditions associated with aortic dilation and dissection. The mechanisms of the development of tortuosity in these disorders are not fully understood, but are founded in the concept that there is abnormal, pathologic arterial lengthening in a fixed space, resulting in more tortuous vessels. Further studies suggest that patients with increased arterial tortuosity are at increased risk of adverse cardiovascular events, including aortic surgery, aortic dissection, and death.
Summary
Arterial tortuosity in commonly present in genetically mediated aortic disease. Given the suboptimal performance of aortic dimension alone in predicting aortic dissection, quantification of tortuosity may augment the current algorithms for determining risk in patients with aortic disease.
Keywords: tortuosity, Marfan, Loeys-Dietz, aortic aneurysm, aortic dissection
Introduction
Arterial tortuosity, defined as the property of the artery having many turns, is becoming more recognized as a common feature in genetic conditions associated with aortic disease. While arterial tortuosity has been most commonly described in Loeys-Dietz syndrome (TGFBR1/2)1 and arterial tortuosity syndrome (SLC2A10)2, it has been observed in multiple other genetic disorders associated with aortic dilation and dissection, including Marfan syndrome (FBN1)3, aneurysms-osteoarthritis syndrome (SMAD3)4,5, and familial thoracic aneurysm and aortic dissection (FTAAD) due to TGFB26 and PRKG1 mutations7. A limitation of the majority of these reports is the qualitative assessment of tortuosity, which likely varies significantly between those interpreting radiologic studies, unless severe tortuosity is present. Recent measures to quantify tortuosity have allowed more detailed and rigorous study of tortuosity3,8. A study in 2011 demonstrated that increased tortuosity, as measured by the vertebral artery tortuosity index on magnetic resonance angiography (MRA), is associated with earlier adverse cardiovascular outcomes in children and young adults with Marfan syndrome and Loeys-Dietz syndrome (Figures 1 and 2). These findings, along with studies suggesting that aortic dimension alone is a poor predictor of aortic dissection9,10, have highlighted tortuosity as a potential prognostic indicator to aid in determining risk and appropriate surgical timing.
Figure 1.
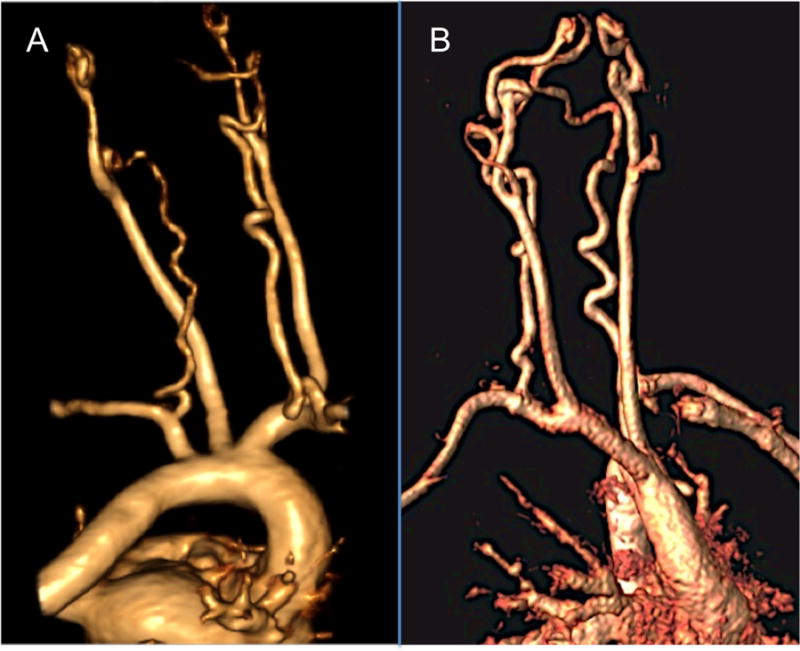
Examples of vertebral artery tortuosity in Marfan syndrome with FBN1 mutation (A) and Loeys-Dietz syndrome with a TGFBR2 mutation (B).
Figure 2.
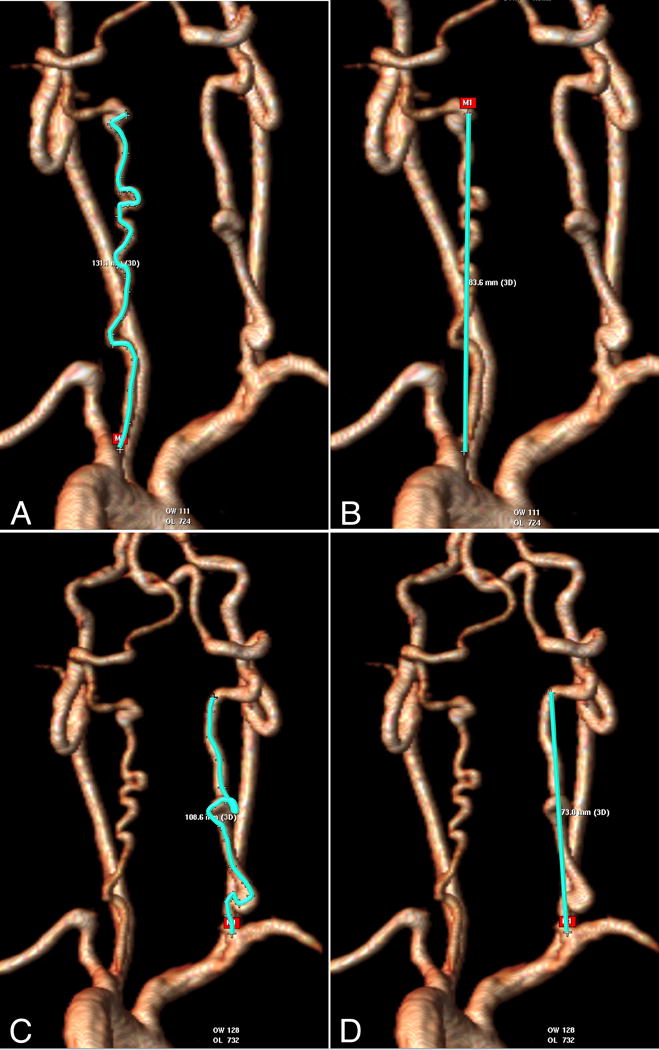
Calculating the vertebral artery tortuosity index (VTI)3. This example uses a volume-rendered 3D magnetic resonance angiogram. Actual length (A and C) and straight length (B and D) of each vertebral artery are measured from the origin of vessel to the vertebral level C2 before the normal posteriolateral bend of the vessel. For both vertebral arteries, the distance factor is calculated: (actual/straight length−1)*100. The maximum distance factor of the two vessels is the VTI. In this example of a patient with Loeys-Dietz syndrome, the left vertebral artery distance factor is ((131.1/83.6)−1)*100=57. This can be thought of as 57% excess length of the vessel. The right distance factor is ((108.6/73.0)−1)*100=49, or 49% excess length. Therefore this patient’s VTI is 57%. Images complements of Alex Dodd, Texas Children’s Hospital.
This review will highlight the recent literature regarding arterial tortuosity, including proposed mechanisms, new descriptions of tortuosity in different genotypes and phenotypes, and the association between arterial tortuosity and cardiovascular outcomes.
Text of Review
Mechanisms of tortuosity
The exact cause and timing of arterial tortuosity in these conditions are unclear, although several mechanisms are postulated. An overarching hypothesis is that there is abnormal gradual lengthening of the arteries in a fixed space, resulting in forced curving and bending of the vessels. This lengthening may be a maladaptation to axial stress along the vessel, as an intrinsic effort to reduce stress along the vessel11. Another possibility is that the genetically abnormal vessel wall actually has lower manifest axial tension, which has been shown in manipulated rabbit carotid arteries to result in increased tortuosity12. Increased TGFβ activity has also been postulated to affect the degree of arterial tortuosity13.
Most studies to date have centered on the lengthening and tortuosity observed in with aging, hypertension, and atherosclerosis14. Indeed, data on vertebral artery tortuosity by Alicioglu et al suggest again that increased tortuosity is seen in aging15,16. It remains unclear if the mechanisms in genetically mediated aortic disease are the same. The study by Franken et al (discussed in more detail below) suggested a small but gradual increase in aortic tortuosity in patients with Marfan syndrome over a three-year period, supporting this hypothesis. Morris et al., however, demonstrated the presence of significant vertebral artery tortuosity at a very young age in some patients with aortopathy, with a decrease and then plateau in tortuosity during adolescence17. The cause of the decrease in tortuosity was postulated to be secondary increases in height (resulting in ‘stretching’ of the artery), medical therapy, or changes in vascular signaling with age. Some investigators have proposed that the presence of an aneurysm may be actually reduce a “critical buckling pressure” of the artery, and that buckling may be a mechanism of tortuosity in genetically mediated aortopathy18. While there are multiple proposed mechanisms, further work is needed to better delineate etiologies and timing of tortuosity.
Conditions with associated arterial tortuosity
Arterial tortuosity has been previously described in several genetically mediated conditions associated with aortopathy (Table 1). For most of the conditions listed in the table, tortuosity has only been described qualitatively. Reports usually describe tortuosity in the head and neck vessels, but affected aortas are also described. Conditions in which tortuosity has been described in detail or only recently are detailed below, including recently evaluated conditions: x-linked dominant periventricular heterotopia, type 1b autosomal recessive cutis laxa, occipital horn syndrome, and Turner syndrome.
Table 1.
Genetic disorders associated with aortic disease and arterial tortuosity
| TGFBR11,19 | Loeys-Dietz syndrome or FTAAD |
| TGFBR21,19 | Loeys-Dietz syndrome or FTAAD |
| FBN13 | Marfan syndrome |
| SMAD34,5 | Osteoarthritis-aneurysm syndrome or FTAAD |
| SLC2A102 | Arterial tortuosity syndrome |
| TGFB26 | FTAAD |
| PRKG17 | FTAAD |
| FBLN4/EFEMP220,21 | Cutis laxa |
| ATP7A22–24 | Occipital horn syndrome/Menkes disease |
| Monosomy X/mosaic monosomy X25 | Turner syndrome |
Loeys-Dietz syndrome due to TGFBR1 and TGFBR2 mutations
The first report of Loeys-Dietz syndrome described diffuse arterial tortuosity, and images included examples of aortic and carotid tortuosity19. Rodrigues et al qualitatively evaluated the neurovascular system26. All 25 patients evaluated in the study qualitatively had arterial tortuosity present in the neurovascular system, with specific examples of the common and internal carotid arteries given. Kono et all compared the vascular systems in 10 patients Loeys-Dietz syndrome compared to 20 patients with Marfan syndrome27. Tortuosity of the vertebral arteries and carotid arteries was graded using a semi-quantitative score ranging from 0–3. The results of the study demonstrated that the vertebral arteries showed the most tortuosity in both conditions, and that vertebral artery tortuosity was greater in Loeys-Dietz syndrome than in Marfan syndrome. Morris et al quantified vertebral artery tortuosity in 13 patients with Loeys-Dietz syndrome and 57 patients with Marfan syndrome, as well as 20 other patients with either Ehlers-Danlos syndrome or a diagnosis of non-specific connective tissue disorder3. Using the quantitative measure, the vertebral artery tortuosity index (VTI), they demonstrated significantly increased vertebral artery tortuosity in Loeys-Dietz syndrome and Marfan syndrome compared to controls. Diedrich et al described increased basilar artery and vertebral artery tortuosity in Loeys-Dietz syndrome compared to normal controls using a variation of the quantitative measure distance factor28.
Marfan syndrome
Tortuosity was only rarely described in confirmed Marfan syndrome until recently. The papers discussed above by Kono and Morris both demonstrated vertebral artery tortuosity in Marfan syndrome3,27. Franken et al evaluated aortic tortuosity in 211 patients with Marfan syndrome compared to 20 controls using the aortic tortuosity index (ATI, Figure 3). The ATI was derived from a magnetic resonance angiogram (MRA), defined as the ratio of the length of a centerline through the entire aorta (from annulus to aortic bifurcation) divided by the Cartesian (geometric) distance between aortic valve annulus and aortic bifurcation. The study demonstrated that patients with Marfan syndrome had significantly increased tortuosity compared to controls29. Further investigated associations with outcomes are discussed below.
Figure 3.
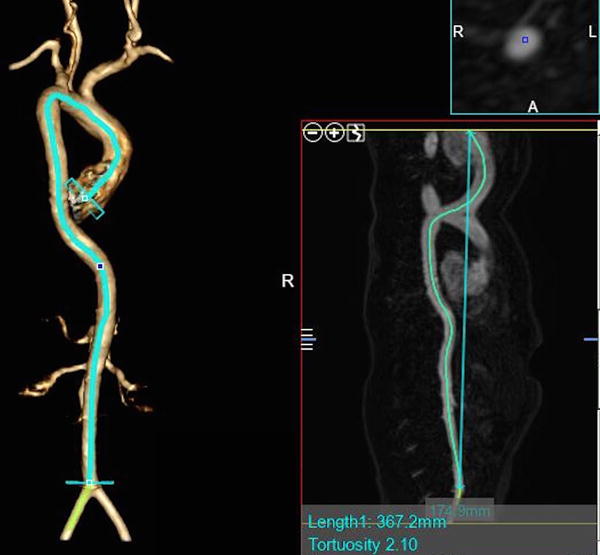
Calculating the aortic tortuosity index (ATI), per Franken et al., International Journal of Cardiology 201529 from a 3D computed tomography angiogram. ATI is calculated as the ratio of aortic length (actual length) to geometric length (straight length). The aortic length (left side of image) was defined as the length of a centerline through the entire aorta (from annulus to aortic bifurcation) created by manually placed seeding points through the lumen of the aorta using post-processing software. Geometric length is the Cartesian distance between its 2 endpoints (added in smaller image on right, overlying the centerline). Aortic tortuosity index (ATI) is measured by dividing aortic length by geometric length. In this example in a patient with significant aortic tortuosity, aortic length = 367.2 mm, geometric length = 174.9 mm, ATI = 2.10. Images complements of Alex Dodd, Texas Children’s Hospital.
Arterial Tortuosity Syndrome
Given that the predominant feature of arterial tortuosity syndrome (due to a SLC2A10, mutation encoding Glucose Transporter 10) is arterial tortuosity, significant tortuosity has been described since the first description of the syndrome2. All reports to date have been qualitative.
X-linked dominant periventricular heterotopia
X-linked dominant periventricular heterotopia is caused by mutations in filamin-A (FLNA)30. In addition to periventricular nodular heterotopia and epilepsy, vascular features are common, including patent ductus arteriosus, bicuspid aortic valve, and dilation of the sinuses of Valsalva or the thoracic aorta30. Reinstein at al. evaluated the vertebral artery tortuosity in a mother and daughter with periventricular heterotopia caused by a Filamin A mutation (8-bp deletion in exon 6, c.883_890 that constitutes a null FLNA allele). The mother was a 38-year-old female with joint laxity who ultimately had a 5.7 cm thoracic aortic aneurysm. Her 19-year old daughter had diffuse heterotopia and was loose jointed, bruised easily, and had soft skin. The daughter had a 2.7 cm right subclavian aneurysm. The vertebral artery tortuosity index (VTI) was calculated from MRA in both patients, and tortuosity was not increased, despite significant arterial dilation.
Cutis laxa due to FBLN4/EFEMP2 mutations
Severe tortuosity has previously been described in a rare form of cutis laxa caused by a mutation in the gene that encodes fibulin-4 (FBLN4 also known as EFEMP2)20,21,31. Rajeshkannan at al described in detail the imaging findings in large cohort (n=31) of related patients with a novel fibulin-4 (FBLN4) mutation that results in a severe lethal inherited arteriopathy syndrome (homozygous c.608A>C, p.Asp203Ala mutation in exon 7, chromosome 11q13)32. Twenty-seven affected children died by age 3 years. Cardiovascular features included aneurysmal dilatation, elongation, tortuosity and narrowing of the aorta, pulmonary artery and their branches. The phenotype included a variable combination of cutis laxa (52%), long philtrum with a thin vermillion (90%), micrognathia (43%), hypertelorism (57%), prominent eyes (43%), sagging cheeks (43%), long slender digits (48%), and visible arterial pulsations (38%). In addition to a high prevalence of both aortic dilation and stenosis, diffuse arterial tortuosity was noted, including in the cerebral vessels, neck vessels including the vertebral arteries, and the aorta. Quantitative measures of tortuosity were not performed.
Aneurysms-osteoarthritis syndrome due to SMAD3 mutations
Arterial tortuosity was discussed in the first paper describing aneurysms-osteoarthritis syndrome4. The cardiovascular findings were reviewed in greater detail by van der Lindle et al5. They qualitatively describe arterial tortuosity throughout the great vessels of the abdomen and thorax 11 of 23 affected patients with cardiovascular imaging, with specific examples given of splenic artery and internal carotid tortuosity.
Menkes disease/Occipital horn syndrome
Mutations in ATP7A cause Menkes disease and occipital horn syndrome, and have been associated with arterial aneurysms22,33,34. Two recent papers highlight qualitatively marked cerebrovascular tortuosity seen in this condition23,24.
Turner syndrome
In a brief report of 26 children and young women with Turner syndrome undergoing cardiac magnetic resonance angiography, mildly increased vertebral artery tortuosity was noted in a subset of patients using VTI, and this was associated with larger aortic dimensions25.
Tortuosity and outcomes
In addition to the prior study by Morris et al. that demonstrated an association between vertebral artery tortuosity and adverse cardiovascular outcomes, three recent studies support the hypothesis that increased tortuosity is an indicator of poorer prognosis in aortic disease, as previously proposed3. Shirali et al investigated whether anatomic measurements from a CT angiogram could predict Type B aortic dissection in hypertensive adults8. Of note, patients with known genetically mediated disease were specifically excluded. While this study did not address genetically mediated aneurysms, it is one of the first to investigate the predictive value of tortuosity. In addition to length, diameter, and volume of the aorta, ascending aortic and thoracic aortic tortuosity were examined, as well as arch vessel angulation in hypertensive adults with and without Type B aortic dissection. Tortuosity of the aorta was defined as the length of the midline within the aorta divided by the linear distance between the aortic root and the iliac bifurcation, and tortuosity of the ascending aorta was defined as the measured length of the ascending aorta divided by the linear distance between the aortic root and left subclavian artery.
Patients with aortic dissection were found to have longer aortas, greater aortic dimension and volume, and greater arch tortuosity. When all the variables were included in a multivariable model, arch tortuosity only added a small amount of predictive power when larger arch diameter, longer arch length, and decreased brachiocephalic artery angle were included. While this study continues to support tortuosity as a prognostic indicator, other anatomic factors may be stronger. In addition, images analyzed in those with dissection were after the dissection, so the anatomic variables studies could have been influenced by presence of the dissection.
Franken et al also examined the tortuosity of the aorta, this time in 211 patients with Marfan syndrome who were enrolled in the Dutch COMPARE trial13. For this, they utilized the aortic tortuosity index (ATI, discussed above). The authors demonstrated that there were significant but weak correlations between both baseline larger aortic volume and larger aortic root diameter and higher ATI (r = 0.280, p = 0.001 and r = 0.223, p = 0.006, respectively).
In this adult cohort, they demonstrated a slow but nonsignificant increase in ATI over 3 years of follow up. Higher ATI was not associated with faster aortic root growth over the course of the study (r = 0.043, p = 0.63), but was strongly associated with occurrence of Type B aortic dissection (p=0.002) and reaching the combined clinical endpoint (prophylactic aortic surgery, aortic dissection and death, p=0.015). In multivariable Cox regression analysis, higher ATI was the only variable associated with earlier aortic dissection, and higher ATI and larger aortic root dimension were independently associated with earlier occurrence of the combined endpoint. The authors found that most discriminating value of ATI for aortic dissection over the three year period was >1.95. Interestingly, Losartan had no effect on ATI over the study, and use of Losartan was not associated with lower occurrence of dissection or the combined endpoint.
In the small study regarding Turner syndrome and vertebral artery tortuosity discussed above, the authors investigated the association between tortuosity (using VTI) and presence of BAV, aortic root z-score, and ascending aortic z-score. No patients in the study of children and young women had aortic dissection. Higher VTI was associated with larger aortic root z-score and larger ascending aortic z-score. VTI did not differ by BAV status or age. In multivariable analysis controlling for age, both BAV and higher VTI remained independently associated with larger ascending aortic z-scores. Of note, patients who had received growth hormone had lower VTIs and poorer correlation between VTI and aortic dimensions than in patients not receiving growth hormone.
The relationship between dilation, tortuosity, and dissection in terms of cause and effect is still unclear. Authors in the papers by Franken et al and Hatakeyama propose that an altered flow profile through a tortuous aorta may independently lead to a more severe distal aortic phenotype and enhanced susceptibility to aortic dissections. This may be true, although no study investigating causation has been performed. It may be that aortic tortuosity and susceptibility to dissection coexist, and these are secondary to a yet unknown more primary etiology.
In the papers by both Franken and Morris et al., increased tortuosity was associated with adverse events completely independently of aortic dimension3,13. This was also previously demonstrated in abdominal aortic aneurysms35. Apart from aortic dimensions, quantitative measures to guide prophylactic aortic surgery are rare. Degree of tortuosity may ultimately prove to be a helpful component of the medical or surgical management algorithm.
Conclusions
Arterial tortuosity continues to be recognized as a common feature in genetically mediated aortopathies. The genetic disorders in which tortuosity is seen are not limited to TGF-b signaling pathway genes, but also include genes responsible for extracellular matrix proteins, vascular smooth muscle relaxation, and copper transport. Mild tortuosity is also apparent in Turner syndrome, and was associated with more significant aortopathy. Studies continue to suggest that increased vertebral artery and arch tortuosity are markers for more severe disease and are associated with earlier or more severe outcomes, most recently Type B aortic dissection. Continued work to standardize quantification and study outcomes in larger populations is indicated to determine if tortuosity should be included in decision-making about patients with genetic aortopathies.
Key points.
Arterial tortuosity is present in multiple genetic conditions associated with aortopathy.
Quantitative measures of arterial tortuosity of the aortic arch and vertebral arteries have been developed, and allow investigation of tortuosity in research studies.
The mechanisms of development of tortuosity in genetically mediated aortopathy are not known, but biomechanical studies suggest tortuosity may be secondary to pathologic lengthening of the vessel as an attempt to modify axial stress.
Increased arterial tortuosity is associated with earlier and more severe adverse cardiovascular events in Marfan syndrome and Loeys Dietz syndrome.
Acknowledgments
None
Financial support and sponsorship: This work was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health awards R21HL121630 and K23HL127266
Footnotes
Conflicts of Interest: None
References
- 1.Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006 Aug 24;355(8):788–98. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 2.Coucke PJ, Willaert A, Wessels MW, Callewaert B, Zoppi N, De Backer J, et al. Nature Genetics. 4. Vol. 38. Nature Publishing Group; 2006. Apr, Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome; pp. 452–7. [DOI] [PubMed] [Google Scholar]
- 3.Morris SA, Orbach DB, Geva T, Singh MN, Gauvreau K, Lacro RV. Circulation. 4. Vol. 124. Lippincott Williams & Wilkins; 2011. Jul 26, Increased vertebral artery tortuosity index is associated with adverse outcomes in children and young adults with connective tissue disorders; pp. 388–96. [DOI] [PubMed] [Google Scholar]
- 4.van de Laar IMBH, Oldenburg RA, Pals G, Roos-Hesselink JW, de Graaf BM, Verhagen JMA, et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nature Genetics. 2011 Feb;43(2):121–6. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- 5.van der Linde D, van de Laar IMBH, Bertoli-Avella AM, Oldenburg RA, Bekkers JA, Mattace-Raso FUS, et al. Aggressive cardiovascular phenotype of aneurysms-osteoarthritis syndrome caused by pathogenic SMAD3 variants. Journal of the American College of Cardiology. 2012 Jul 31;60(5):397–403. doi: 10.1016/j.jacc.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 6.Lindsay ME, Schepers D, Bolar NA, Doyle JJ, Gallo E, Fert-Bober J, et al. Nature Genetics. 8. Vol. 44. Nature Publishing Group; 2012. Aug, Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm; pp. 922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo D-C, Regalado E, Casteel DE, Santos-Cortez RL, Gong L, Kim JJ, et al. Recurrent Gain-of-Function Mutation in PRKG1 Causes Thoracic Aortic Aneurysms and Acute Aortic Dissections. 2013 Aug 8;93(2):398–404. doi: 10.1016/j.ajhg.2013.06.019. Available from: http://dx.doi.org/10.1016/j.ajhg.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **8.Shirali AS, Bischoff MS, Lin H-M, Oyfe I, Lookstein R, Griepp RB, et al. Predicting the risk for acute type B aortic dissection in hypertensive patients using anatomic variables. JACC: Cardiovascular Imaging. 2013 Mar;6(3):349–57. doi: 10.1016/j.jcmg.2012.07.018. This paper is one of the few to elegantly evaluate the correlation between arterial tortuosity and outcomes. [DOI] [PubMed] [Google Scholar]
- 9.Pape LA, Tsai TT, Isselbacher EM, Oh JK, O’gara PT, Evangelista A, et al. Aortic diameter >or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD) 2007 Sep 4;116(10):1120–7. doi: 10.1161/CIRCULATIONAHA.107.702720. Available from: http://circ.ahajournals.org/cgi/doi/10.1161/CIRCULATIONAHA.107.702720. [DOI] [PubMed] [Google Scholar]
- 10.Parish LM, Gorman JH, III, Kahn S, Plappert T, St John-Sutton MG, Bavaria JE, et al. Aortic size in acute type A dissection: implications for preventive ascending aortic replacement. 2009 Jun;35(6):941–6. doi: 10.1016/j.ejcts.2008.12.047. Available from: http://ejcts.oxfordjournals.org/cgi/doi/10.1016/j.ejcts.2008.12.047. [DOI] [PubMed] [Google Scholar]
- 11.Humphrey JD, Eberth JF, Dye WW, Gleason RL. Fundamental role of axial stress in compensatory adaptations by arteries. 2009 Jan 5;42(1):1–8. doi: 10.1016/j.jbiomech.2008.11.011. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0021929008005952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson ZS, Dajnowiec D, Gotlieb AI, Langille BL. Partial off-loading of longitudinal tension induces arterial tortuosity. 2005 May;25(5):957–62. doi: 10.1161/01.ATV.0000161277.46464.11. Available from: http://atvb.ahajournals.org/cgi/doi/10.1161/01.ATV.0000161277.46464.11. [DOI] [PubMed] [Google Scholar]
- **13.Franken R, Morabit El A, de Waard V, Timmermans J, Scholte AJ, van den Berg MP, et al. Increased aortic tortuosity indicates a more severe aortic phenotype in adults with Marfan syndrome. International Journal of Cardiology. 2015 May 15;194:7–12. doi: 10.1016/j.ijcard.2015.05.072. This is the most rigorous study to date evaluating tortuosity and its relationship to adverse cardiovascular outcomes. The population study was drawn from a larger randomized controlled trial. [DOI] [PubMed] [Google Scholar]
- 14.Del Corso L, Moruzzo D, Conte B, Agelli M, Romanelli AM, Pastine F, et al. Tortuosity, kinking, and coiling of the carotid artery: expression of atherosclerosis or aging? Angiology. 1998 May;49(5):361–71. doi: 10.1177/000331979804900505. [DOI] [PubMed] [Google Scholar]
- *15.Alicioglu B, Gulekon N, Akpinar S. Age-related morphologic changes of the vertebral artery in the transverse process. Analysis by multidetector computed tomography angiography. Spine J. 2015 Apr 27; doi: 10.1016/j.spinee.2015.04.031. This paper supports the findings that arterial tortuosity increases with age. [DOI] [PubMed] [Google Scholar]
- 16.Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age-associated elongation of the ascending aorta in adults. JACC: Cardiovascular Imaging. 2008 Nov;1(6):739–48. doi: 10.1016/j.jcmg.2008.06.010. [DOI] [PubMed] [Google Scholar]
- *17.Morris SA, Payne WA, Sami S, Wang Y, LeMaire SA, Tyson JE, et al. Arterial tortuosity and change with age in young patients with aortopathy. Journal of Cardiovascular Magnetic Resonance. 2015;17(S1):P403. This paper is the first to suggest that in individual patients, tortuosity may decrease with age. [Google Scholar]
- *18.Lee AY, Sanyal A, Xiao Y, Shadfan R, Han H-C. J Biomech. 16. Vol. 47. Elsevier; 2014. Dec 18, Mechanical instability of normal and aneurysmal arteries; pp. 3868–75. This paper suggests that presence of vascular aneurysms may actually change the vascular environment by decreasing a buckling threshold, and may foster arterial tortuos. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, et al. Nature Genetics [Internet] 3. Vol. 37. Nature Publishing Group; 2005. Mar 1, A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2; pp. 275–81. Available from: http://www.nature.com/doifinder/10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 20.McLaughlin PJ, Chen Q, Horiguchi M, Starcher BC, Stanton JB, Broekelmann TJ, et al. Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. 2006 Mar;26(5):1700–9. doi: 10.1128/MCB.26.5.1700-1709.2006. Available from: http://mcb.asm.org/cgi/doi/10.1128/MCB.26.5.1700-1709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kappanayil M, Nampoothiri S, Kannan R, Renard M, Coucke P, Malfait F, et al. Orphanet J Rare Dis. 1. Vol. 7. BioMed Central Ltd; 2012. Characterization of a distinct lethal arteriopathy syndrome in twenty-two infants associated with an identical, novel mutation in FBLN4 gene, confirms fibulin-4 as a critical determinant of human vascular elastogenesis; p. 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grange DK, Kaler SG, Albers GM, Petterchak JA, Thorpe CM, DeMello DE. Am J Med Genet. 2. 139A. Wiley Subscription Services, Inc A Wiley Company; 2005. Dec 1, Severe bilateral panlobular emphysema and pulmonary arterial hypoplasia: unusual manifestations of Menkes disease; pp. 151–5. [DOI] [PubMed] [Google Scholar]
- *23.Kim JH, Lee BH, Kim Y-M, Choi J-H, Kim G-H, Cheon CK, et al. Metab Brain Dis. 1. Vol. 30. Springer US; 2015. Feb, Novel mutations and clinical outcomes of copper-histidine therapy in Menkes disease patients; pp. 75–81. This paper provides clinical description and images of dilated and tortuous head and neck vessels in patients with ATP7A mutations. [DOI] [PubMed] [Google Scholar]
- *24.Smpokou P, Samanta M, Berry GT, Hecht L, Engle EC, Lichter-Konecki U. Menkes disease in affected females: the clinical disease spectrum. Am J Med Genet. 2015 Feb;167A(2):417–20. doi: 10.1002/ajmg.a.36853. This paper focuses on the often overlooked female phenotype with ATP7A mutations, which includes marked cerebrovascular tortuosity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *25.Morris SA, Payne WA, Lacro RV, Maskatia SA, Masand P, Noel CV, et al. Vertebral artery tortuosity in Turner syndrome: is tortuosity a component of the aortopathy phenotype? Journal of Cardiovascular Magnetic Resonance. BioMed Central Ltd. 2014;16(Suppl 1):P115. This paper is the first to evaluate tortuosity in Turner syndrome, and shows a correlation between increased tortuosity and a larger ascending aortic z-score. [Google Scholar]
- 26.Rodrigues VJ, Elsayed S, Loeys BL, Dietz HC, Yousem DM. Neuroradiologic manifestations of Loeys-Dietz syndrome type 1. AJNR Am J Neuroradiol. 2009 Sep;30(8):1614–9. doi: 10.3174/ajnr.A1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kono AK, Higashi M, Morisaki H, Morisaki T, Tsutsumi Y, Akutsu K, et al. High prevalence of vertebral artery tortuosity of Loeys-Dietz syndrome in comparison with Marfan syndrome. Jpn J Radiol. 2010 May;28(4):273–7. doi: 10.1007/s11604-010-0420-6. [DOI] [PubMed] [Google Scholar]
- 28.Diedrich KT, Roberts JA, Schmidt RH, Albright LAC, Yetman AT, Parker DL. AMIA Annu Symp Proc. 10. Vol. 2011. American Medical Informatics Association; 2011. Medical record and imaging evaluation to identify arterial tortuosity phenotype in populations at risk for intracranial aneurysms; pp. 295–304. [PMC free article] [PubMed] [Google Scholar]
- 29.Franken R, Morabit El A, de Waard V, Timmermans J, Scholte AJ, van den Berg MP, et al. International Journal of Cardiology. C. Vol. 194. Elsevier Ireland Ltd; 2015. Sep 1, Increased aortic tortuosity indicates a more severe aortic phenotype in adults with Marfan syndrome; pp. 7–12. [DOI] [PubMed] [Google Scholar]
- 30.Fox JW, Lamperti ED, Ekşioğlu YZ, Hong SE, Feng Y, Graham DA, et al. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron. 1998 Dec;21(6):1315–25. doi: 10.1016/s0896-6273(00)80651-0. [DOI] [PubMed] [Google Scholar]
- 31.Renard M, Holm T, Veith R, Callewaert BL, Adès LC, Baspinar O, et al. Eur J Hum Genet. 8. Vol. 18. Nature Publishing Group; 2010. Aug, Altered TGFbeta signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency; pp. 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **32.Rajeshkannan R, kulkarni C, Kappanayil M, Nampoothiri S, Malfait F, De Paepe A, et al. Eur Radiol. 8. Vol. 24. Springer Berlin Heidelberg; 2014. May 17, Imaging findings in a distinct lethal inherited arteriopathy syndrome associated with a novel mutation in the FBLN4 gene; pp. 1742–8. This paper describes in detail cardiovascular imaging findings in a large cohort with a severe familial mutation in the FBLN4 gene. [DOI] [PubMed] [Google Scholar]
- 33.de Figueiredo Borges L, Martelli H, Fabre M, Touat Z, Jondeau G, Michel J-B. Pediatr Dev Pathol. 3. Vol. 13. Springer New York; 233 Spring Street, New York, NY 10013: 2010. May, Histopathology of an iliac aneurysm in a case of Menkes disease; pp. 247–51. [DOI] [PubMed] [Google Scholar]
- 34.Adaletli I, Omeroglu A, Kurugoglu S, Elicevik M, Cantasdemir M, Numan F. Pediatr Radiol. 10. Vol. 35. Springer-Verlag; 2005. Oct, Lumbar and iliac artery aneurysms in Menkes’ disease: endovascular cover stent treatment of the lumbar artery aneurysm; pp. 1006–9. [DOI] [PubMed] [Google Scholar]
- 35.Hatakeyama T, Shigematsu H, Muto T. Risk factors for rupture of abdominal aortic aneurysm based on three-dimensional study. J Vasc Surg. 2001 Mar;33(3):453–61. doi: 10.1067/mva.2001.111731. [DOI] [PubMed] [Google Scholar]


