Abstract
Alpha 2-HS glycoprotein (AHSG), also designated as fetuin-A, exhibits polymorphism in population genetics consisting of two major alleles of AHSG ∗ 1 and AHSG ∗ 2. The serum level in the AHSG ∗ 1 homozygote is significantly higher than that of the AHSG ∗ 2 homozygote. This study examined the molecular mechanism for the cis-regulatory expression. To quantitate allele-specific mRNA in intra-assays of the heterozygote, RT-PCR method employing primers that were incorporated to the two closely located SNPs was developed. The respective magnitudes of AHSG ∗ 1 to AHSG ∗ 2 in the liver tissues and hepatic culture cells of PLC/PRF/5 were determined quantitatively as 2.5-fold and 6.2-fold. The mRNA expressional difference of two major alleles was observed, which is consistent with that in the serum level. The culture cells carried heterozygous genotypes in rs4917 and rs4918, but homozygous one in rs2248690. It was unlikely that the imbalance was derived from the SNP located in the promotor site. Furthermore, to investigate the effect of mRNA degradation, RNA synthesis in the cell culture was inhibited potently by the addition of actinomycin-D. No marked change was apparent between the two alleles. The results indicated that the cis-regulatory expressional difference is expected to occur at the level of transcription or splicing of mRNA.
1. Introduction
Alpha 2-HS glycoprotein (AHSG), also designated as fetuin-A, is a 46 kDa serum protein that is synthesized mainly in hepatic cells [1]. The protein is known to exhibit multiple functions [2]. AHSG acts as a natural inhibitor of insulin receptor tyrosine kinase [3]. Goustin and Abou-Samra [4] summarized its molecular mechanism and pathogenesis. AHSG was recently revealed to be an endogenous ligand for Toll-like receptor 4 playing a crucially important role in stimulating adipose tissue inflammation, resulting in insulin resistance [5]. These evidences show the close relation of AHSG to occurrence of type 2 diabetes [6]. Moreover, AHSG is bound to calcium phosphate with high affinity. It is a major component of the extracellular matrix of the bone [7]. The major physiological function is to prevent extracellular calcification [8]. The low plasma level of AHSG from hemodialysis accelerates the stiffening and calcification of arteries [9].
AHSG used to be a conventional genetic marker for use in isoelectric focusing in anthropology [10]. Two major alleles of AHSG ∗ 1 and AHSG ∗ 2 comprise double nonsynonymous substitutions of T230M (rs4917) in exon 6 and T238S (rs4918) in exon 7 [11]. In addition to the amino acid replacement, the plasma AHSG level in the AHSG ∗ 2 homozygote is approximately 20% lower than that of the AHSG ∗ 1 homozygote [12–14]. This phenomenon means that the linked nucleotide substitutions are directly attributable to biosynthesis or degradation of mRNA. In particular, SNP (rs2248690) of AP-1 binding site in the promotor region affects the transcriptional activity [15]. This SNP has been nominated as the most potent association with type 2 diabetes [16]. However, the mechanism of the cis-acting expressional differences has not been elucidated.
To assess the abundance of allele-specific mRNA, intraindividual assay of the heterozygote is preferred over interindividual assays, which are subject to several potential environmental errors such as variation in mRNA quality as a result from specimen degeneration [17]. For the intracellular assay, human hepatoma cell lines that have heterozygous AHSG genotype were searched [18]. In order to develop such intra-assays, the specific detection of coexisting alleles in the heterozygote is crucially important, relying on the ability to distinguish one allele from the other with no cross-reaction. Buckland [19] described several molecular procedures to assess allele-specific expressional differences.
In the present study, we developed a novel RT-PCT procedure to quantitate the amounts of two allelic transcripts of AHSG separately, in which primers were designed with incorporation to the two closely located SNPs of rs4917 and rs4918. The allelic expression differences in the heterozygote were examined in the liver tissue and the culture cells of hepatic carcinoma cell lines. Finally, to elucidate the effects of degradation, mRNA levels were examined after the addition of actinomycin-D synthesis as a potent inhibitor of RNA synthesis.
2. Materials and Methods
2.1. Subjects
To examine amounts of AHSG mRNA, four specimens of postmortem liver tissues obtained during autopsy at the Department of Forensic Medicine, Tokai University School of Medicine, were available for mRNA extraction. Then they were stored at −80°C until use [11]. All specimens were heterozygous in the AHSG genotype. After being approved by our institutional ethics committee, informed consent was obtained from the family members of deceased subjects. DNA from unrelated individuals was used to ensure the linkage of SNPs. Buccal swabs were obtained from 52 Japanese individuals for DNA extraction, as described previously [20].
2.2. Cell Culture
Cells of HepG2, PLC/PRF/5, and HeLa cell lines were cultured in DMEM medium supplemented with penicillin at 100 mg/L, streptomycin at 100,000 U/L and 10% fetal bovine serum (Biological Industries Ltd., Israel) in a humidified atmosphere containing 5% CO2 at 37°C. The cells for culture were dispersed in 60 mm dishes with 7.5 × 106 cells in 5 mL of the medium at overnight and were collected for DNA and total RNA extraction. After washing with phosphate buffered saline, the confluent cells were treated in 0.25% w/v trypsin and 1 mM EDTA at 37°C for 5 min, followed by adjustment to 1 × 105 cells in 1 mL of DMEM medium.
To arrest RNA synthesis, the medium was replaced with fresh medium containing 5 μM actinomycin-D (Wako Pure Chemical Industries Ltd., Osaka, Japan). Cells were harvested at 0, 2.5, 5, 7.5, and 10 h after addition of the transcriptional inhibitor. Then they were examined using real-time PCR.
2.3. Extraction of DNA and RNA
Homogenized liver tissue of 1 g and the suspended culture cells were dissolved in Trizol solution (Invitrogen Life Technologies, Carlsbad, CA, USA). From 500 μL of the cell lysates, RNA was extracted using as Rneasy Lipid Tissue Mini Kit (Qiagen Inc., Hilden, Germany) according to instructions provided by the manufacturer. Total RNA was reverse-transcribed to cDNA using Superscript III (Invitrogen) priming with oligo-dT combined with T7 RNA polymerase promoter.
DNA was extracted using spin columns of the QIAamp mini kit (Qiagen). Genotyping was performed using PCR-restriction fragment length polymorphism (RFLP), as described previously [11].
2.4. Allele-Specific PCR
To detect mRNA expression levels of AHSG ∗ 1 and AHSG ∗ 2 separately in the intra-assays, allele-specific amplification was performed in the real-time PCR procedure. We designed specific oligonucleotide primer sets of 5′-AGGTTGCAGTGACCTGCAC-3′ and 5′-TCTGGTTGGGGCTGTGAGG-3′ for AHSG ∗ 1, and 5′-AGGTTGCAGTGACCTGCAT-3′ and 5′-TCTGGTTGGGGCTGTGAGC-3′ for AHSG ∗ 2, in which substitutions of rs4917 and rs4918 are incorporated into the 3′-terminal of both forward and reverse oligonucleotide primers. The amplified fragments were detected using Power SYBER Green PCR Master Mix (Applied Biosystems, Life Technologies) in optical 96-well MicroAmp plates using 7300 real-time PCR system (Applied Biosystems). To generate standard curves, the plasmid vectors recombined by the AHSG ∗ 1 and AHSG ∗ 2 cDNA fragments were used as the template [11]. Fivefold serial dilutions at 0.4, 0.08, 0.016, 0.0032, and 0.00064 ng were added to the reaction mixture of 25 μL, in which 1 ng of the template corresponded to 4.18 × 10−16 mol and 2.52 × 108 copies. To confirm the cross-reactivity to the other allele, the allele-specific primer pairs operated against the other allelic vector as the template. For the control, β-actin amplification using a primer set of 5′-GGACTTCGAGCAAGAGATGG-3′ and 5′-AAGGAAGGCTGGAAGAGTGC-3′ was employed using pGEMT-Easy plasmid recombined by the PCR fragment.
The critical threshold (Ct) values for each PCR reaction were calculated automatically using the ABI prism sequence detection system (ver. 1.6). The starting concentration of DNA fragment present in each reaction was calculated by comparing Ct values of unknown samples to those of standards with known amounts of target DNA fragment; Ct values were plotted against the log of the initial concentration of DNA to produce a standard curve. The Ct value that is dependent upon the starting copy number of the target is defined as the cycle number at which a statistically significant increase of the reporter fluorescence can first be detected.
The mean of triplicates was selected for each measurement. To compare two groups statistically, data from the experiments were analyzed using Student's t-test. Results for which P < 0.01 were inferred as significant.
2.5. Linkage Analysis
PCR amplification was performed with extracted DNA from each of 52 individuals. The three SNPs examined here were presented in Figure 1. Linkage analysis was conducted using software (SNPAlyze ver. 7 standard; Dynacom Co. Ltd., Yokohama, Japan); r square (r 2) and the linkage disequilibrium (D′) were calculated.
Figure 1.
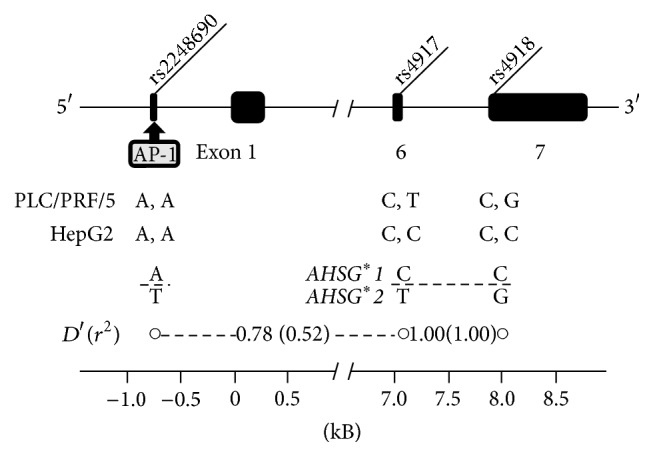
Gene structure of AHSG indicating three major SNPs introduced into this study. Genotypes in the culture cell lines of HepG2 and PLC/PRF/5 and their linkage disequilibrium of D′ and r 2 are indicated.
3. Results
3.1. Allele-Specific Detection of mRNA
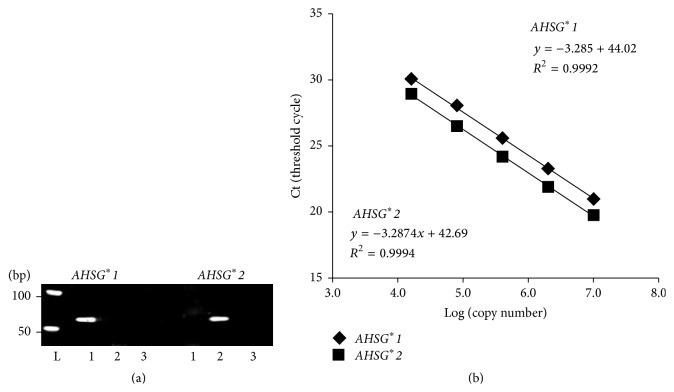
The first objective was comparison of the relative expression levels of two AHSG alleles within the same individual and the single culture cell line. The comparison in these intra-assays of the heterozygote depends on specific detection of the alleles. As Figure 1 shows, the highly linked nucleotide substitutions of rs4917 and rs4918 are closely located in adjacent exons 6 and 7. In the present attempt, these two substitutions are incorporated into the 3′ end of both forward and reverse PCR primers, which yields 61 bp DNA products in length from cDNA. As presented in Figure 2(a), no cross-reactivity was observed using the other template like the AHSG ∗ 1 amplification against AHSG ∗ 2 allelic fragment as the template.
Figure 2.
Allele-specific PCR amplification. (a) Nondenaturing polyacrylamide gel electrophoresis of allele-specific RT-PCR products; left: primer set for AHSG ∗ 1 and right: that for AHSG ∗ 2. Lane 1: recombinant AHSG ∗ 1 fragment for template; lane 2: recombinant AHSG ∗ 2 one for template; lane 3: no template for the negative control. (b) Standard plot for allele-specific real-time PCR amplification. The least-squares regression model was calculated from values of cycle threshold (Ct) versus addition of the recombinant DNA fragment in triplicate.
The efficiencies of amplification might be mutually distinct. Therefore, the standard curve was constructed separately to obtain the absolute copy number. However, no apparent difference of fluorescence intensity in the amplification curves was observed in the subcloned DNA fragments of AHSG ∗ 1 and AHSG ∗ 2 as the template. The standard curve was generated by regression in which amplification of the template of recombinant plasmid at various concentrations showed linearity over a range of four orders of magnitude (Figure 2(b)).
3.2. Intraindividual Assay
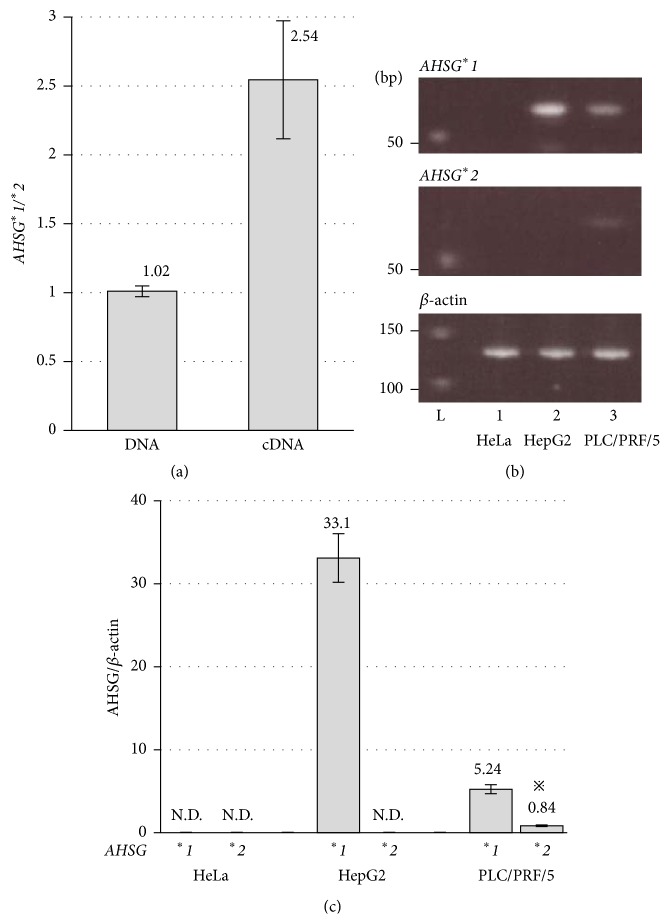
The real-time PCR procedure quantitated the abundance of transcript amounts of AHSG ∗ 1 and AHSG ∗ 2 mRNA in the hepatic tissues from four heterozygous individuals. As depicted in Figure 3(a), a relative amount of ∗ 1 mRNA was significantly greater from 2.2-fold to 3.2-fold than that of ∗ 2 with a mean and SD of 2.5 ± 0.4. As the control, the allele-specific PCR amplification was applied to genomic DNA, which caused equal amplification of ∗ 1 and ∗ 2.
Figure 3.
Two intra-assays of intraindividual assay in the human liver tissues (a) and intracellular assay in three culture cell lines (b and c). (a) Relative amount of AHSG ∗ 1 mRNA to that of AHSG ∗ 2 was obtained in genomic DNA (left) and mRNA from the liver tissue (right). Bars represent the mean ± SD of four specimens. (b) Nondenaturing polyacrylamide gel electrophoresis of allele-specific RT-PCR for ∗ 1, ∗ 2, and β-actin products. Lane 1: HeLa; lane 2: HepG2; and lane 3: PLC/PRF/5. (c) Comparison of allelic mRNA amount of AHSG ∗ 1 and AHSG ∗ 2 to β-actin. N.D. denotes not detected. Bars represent the mean ± SD in triplicate; ※ P < 0.01 significant versus the amount of AHSG ∗ 1.
3.3. Intracellular Assay
The allelic imbalance was evaluated in cells of culture cell lines, as well. Cells of the HepG2 cell line, of which genotyping from the genome sequence revealed AHSG ∗ 1 homozygote, expressed only AHSG ∗ 1 mRNA abundantly but not AHSG ∗ 2 (Figure 3(b)). The HeLa cells did not express AHSG mRNA, which served as the negative control. Another hepatic PLC/PRF/5 cell line was genotyped to the heterozygote. Allele-specific amplification revealed that the AHSG ∗ 1 mRNA count was approximately 6.2-fold greater than that of AHSG ∗ 2 (P < 0.01) in the PLC/PRF/5 cells (Figure 3(c)).
3.4. Effect of Actinomycin-D
To assess the cause of allelic imbalance of AHSG transcripts further, actinomycin-D, which arrests RNA synthesis, was added to the culture medium for the PLC/PRF/5 cells. Cells were harvested at 2.5, 5, 7.5, and 10 h after addition of the transcription inhibitor. Then the mRNA level was quantitated using real-time PCR analysis. Figure 4 demonstrates the degradation of mRNA as the ratio to the amount at time zero. No significant change was evident in the degradation curves between AHSG ∗ 1 and AHSG ∗ 2 mRNA transcripts, indicating that a major factor that affects the allelic imbalance may be attributable to transcription, but not to degradation.
Figure 4.
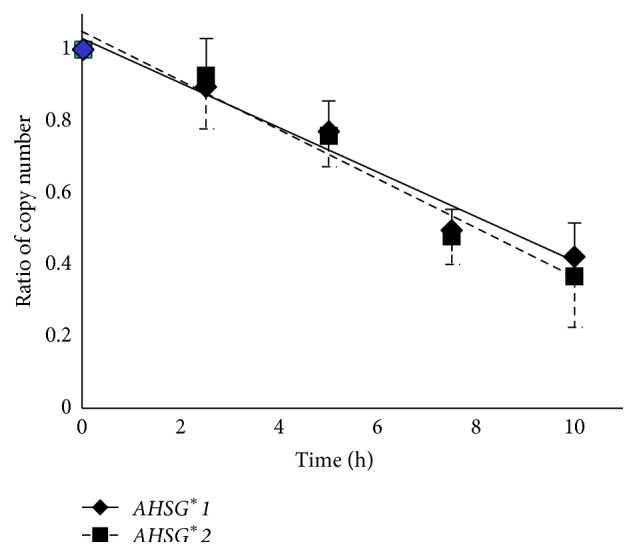
Inhibition of mRNA synthesis by the addition of actinomycin-D. After addition of actinomycin-D, the cells of PLC/PRF/5 were harvested to find mRNA numbers using the allele-specific RT-PCR. Solid and broken lines indicate AHSG ∗ 1 and AHSG ∗ 2, respectively.
3.5. Linkage Analysis from Genotyping
The A/T SNP at rs2248690 in the promoter regulatory region potentially affects transcriptional activity of AHSG [15]. The genotype of rs2248690 in cells of the PLC/PRF/5 cell line was a homozygote of (A, A), although the PLC/PRF/5 cells exhibited the AHSG heterozygote. This result indicated no apparent interaction of the allelic imbalance of AHSG with the variety of AP-1 binding in the promoter region. Therefore, the linkage between the SNP and the phenotypic determinants of two SNPs (rs4917 and rs4918) was confirmed in the unrelated Japanese individuals. Incomplete linkage was found: the linkage disequilibrium values were 0.78 in D′ and 0.52 in r 2 (Figure 1). Moreover, in the four heterozygous specimens using the intraindividual assay included three homozygotes of (A, A) at rs2248690.
4. Discussion
Our previous work demonstrated that the AHSG serum level in AHSG ∗ 1 homozygote is approximately 20% higher than that of the AHSG ∗ 2 homozygote [12]. For many years, association studies in a population group showed that this polymorphism is related to various physical statures such as bone mineral density [21, 22] and leanness [13]. Moreover, a number of association studies with major diseases such as type 2 diabetes [6, 16, 23], lipid levels [24, 25], and ischemic heart disease [26–28] have been reported extensively.
In the present study, to examine the origin of the major phenotypic differences, we developed a quantitative real-time PCR assay for the sensitive and specific detection of the two AHSG alleles, where two separated titrations for absolute quantification were necessary to analyze the respective alleles. This series of experiments confirmed a significant difference in the mRNA transcription level of two major alleles.
In the intraindividual assay using liver tissues of the heterozygote, the AHSG ∗ 1 mRNA level was significantly, over two times, higher than that of AHSG ∗ 2 mRNA in the real-time PCR detection. In the intracellular assay using PLC/PRF/5 cells of the heterozygote, ∗ 1 mRNA level was approximately six times higher than that of ∗ 2 mRNA as well. It was readily apparent that the AHSG protein level in serum was correlated with mRNA level in the hepatocytes. This result means that some cis-acting mechanism operates in the process of protein biosynthesis. A number of other functional cis-regulatory variations are known. Among them, allelic differences of twofold or greater in the rate of transcription are common, and even more than 20-fold differences are not uncommon [29, 30].
In the experiment using actinomycin-D that inhibits mRNA synthesis, no apparent difference was observed in the ratio of ∗ 1/∗ 2 allele numbers, suggesting that the allelic imbalance appeared not to be derived from the difference in the degeneration process of mRNA.
In terms of the allelic imbalance of mRNA level, the molecular cause was unknown. However, the possibility exists of linkage of the ∗ 1 and ∗ 2 alleles with SNP in the promoter region, in which transcriptional factor AP-1 binding site is present. Inoue et al. [15] demonstrated that SNP at −768 nucleotide position in the AP-1 binding site affects the transcription of AHSG in the cis-acting manner. The AP-1 binding type (TGTGTCA) is bound to AP-1 more tightly than the other AP-1 nonbinding type (AGTGTCA). Their luciferase assay showed that tight binding of T-type reduces the transcriptional activity. This SNP (rs2248690) in the promoter region potentially links to AHSG ∗ 1 and AHSG ∗ 2 polymorphism and affects the allelic imbalance of mRNA by changing the transcription. The homozygote of (A, A) at −768 n.p. was observed in the heterozygous cells of PLC/PRF/5, indicating that the promoter SNP associated with AP-1 binding is unlikely to be the main cause for allele-specific imbalance of transcription. We deduce that the allelic imbalance originated from the two SNPs themselves of exons 6 and 7 or other closely linked SNPs within the haploblock.
Because the two critical SNPs of rs4917 and rs4918 are present in the vicinities of exon-intron junctions, the linked substitutions potentially affect splicing of transcripts [31]. To examine another mRNA species caused by alternative splicing, RT-PCR using several primer pairs was performed extensively, but no evidence was obtained (data not shown). Moreover, another extensive search of the Expression Sequence Tag (EST) database at the NCBI site (http://www.ncbi.nlm.nih.gov/nucest/) failed to reveal any potential cDNA fragment to infer the occurrence of alternative splicing. These preliminary results suggest that the allelic imbalance was not caused by alternative splicing.
We have speculated that the imbalance originated from biosynthesis of mRNA or inhibited splicing activity in AHSG ∗ 2, which reduces the amount of mature mRNA, but the exact mechanism has not been elucidated through this series of experiments. It seems certain that the imbalance derived from the relation with the two major SNPs and other highly linked SNPs, at least.
Although AHSG ∗ 2 has been derived from the older ∗ 1 allele lately, this ∗ 2 allele distributes rapidly to all examined population group, as shown previously [32]. This polymorphism is potentially related to natural selection in the human evolution, but its true physiological significance remains unclear.
Supplementary Material
Genotypes of three SNPs in 52 unrelated individuals.
Acknowledgments
The authors thank Professor Yutaka Inagaki, Department of Regenerative Medicine, Tokai University School of Medicine, for his kind provision of the cells of the three culture cell lines. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (no. 15K08884).
Conflict of Interests
All authors declared no conflict of interests.
References
- 1.Lee C.-C., Bowman B. H., Yang F. M. Human alpha 2-HS-glycoprotein: the A and B chains with a connecting sequence are encoded by a single mRNA transcript. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(13):4403–4407. doi: 10.1073/pnas.84.13.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mori K., Emoto M., Inaba M. Fetuin-A: a multifunctional protein. Recent Patents on Endocrine, Metabolic and Immune Drug Discovery. 2011;5(2):124–146. doi: 10.2174/187221411799015372. [DOI] [PubMed] [Google Scholar]
- 3.Auberger P., Falquerho L., Contreres J. O., et al. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell. 1989;58(4):631–640. doi: 10.1016/0092-8674(89)90098-6. [DOI] [PubMed] [Google Scholar]
- 4.Goustin A.-S., Abou-Samra A. B. The ‘thrifty’ gene encoding Ahsg/Fetuin-A meets the insulin receptor: insights into the mechanism of insulin resistance. Cellular Signalling. 2011;23(6):980–990. doi: 10.1016/j.cellsig.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Pal D., Dasgupta S., Kundu R., et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nature Medicine. 2012;18(8):1279–1285. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 6.Dahlman I., Eriksson P., Kaaman M., et al. α2-Heremans-Schmid glycoprotein gene polymorphisms are associated with adipocyte insulin action. Diabetologia. 2004;47(11):1974–1979. doi: 10.1007/s00125-004-1556-7. [DOI] [PubMed] [Google Scholar]
- 7.Vetter U., Fisher L. W., Mintz K. P., et al. Osteogenesis imperfecta: changes in noncollagenous proteins in bone. Journal of Bone and Mineral Research. 1991;6(5):501–505. doi: 10.1002/jbmr.5650060512. [DOI] [PubMed] [Google Scholar]
- 8.Heiss A., DuChesne A., Denecke B., et al. Structural basis of calcification inhibition by α2-HS glycoprotein/fetuin-A: formation of colloidal calciprotein particles. The Journal of Biological Chemistry. 2003;278(15):13333–13341. doi: 10.1074/jbc.m210868200. [DOI] [PubMed] [Google Scholar]
- 9.Ketteler M., Bongartz P., Westenfeld R., et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. The Lancet. 2003;361(9360):827–833. doi: 10.1016/s0140-6736(03)12710-9. [DOI] [PubMed] [Google Scholar]
- 10.Cox D. W., Andrews B. J., Wills D. E. Genetic polymorphism of α2HS-glycoprotein. American Journal of Human Genetics. 1986;38(5):699–706. [PMC free article] [PubMed] [Google Scholar]
- 11.Osawa M., Umetsu K., Ohki T., Nagasawa T., Suzuki T., Takeichi S. Molecular evidence for human alpha2-HS glycoprotein (AHSG) polymorphism. Human Genetics. 1997;99(1):18–21. doi: 10.1007/s004390050302. [DOI] [PubMed] [Google Scholar]
- 12.Osawa M., Tian W., Horiuchi H., Kaneko M., Umetsu K. Association of alpha2-HS glycoprotein (AHSG, fetuin-A) polymorphism with AHSG and phosphate serum levels. Human Genetics. 2005;116(3):146–151. doi: 10.1007/s00439-004-1222-7. [DOI] [PubMed] [Google Scholar]
- 13.Lavebratt C., Wahlqvist S., Nordfors L., Hoffstedt J., Arner P. AHSG gene variant is associated with leanness among Swedish men. Human Genetics. 2005;117(1):54–60. doi: 10.1007/s00439-005-1286-z. [DOI] [PubMed] [Google Scholar]
- 14.Jensen M. K., Bartz T. M., Djousse L., et al. Genetically elevated fetuin- A levels, fasting glucose levels, and risk of type 2 diabetes: the cardiovascular health study. Diabetes Care. 2013;36(10):3121–3127. doi: 10.2337/dc12-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue M., Takata H., Ikeda Y., et al. A promoter polymorphism of the α2-HS glycoprotein gene is associated with its transcriptional activity. Diabetes Research and Clinical Practice. 2008;79(1):164–170. doi: 10.1016/j.diabres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Siddiq A., Lepretre F., Hercberg S., Froguel P., Gibson F. A synonymous coding polymorphism in the alpha2-Heremans-schmid glycoprotein gene is associated with type 2 diabetes in French Caucasians. Diabetes. 2005;54(8):2477–2481. doi: 10.2337/diabetes.54.8.2477. [DOI] [PubMed] [Google Scholar]
- 17.MacKenzie A., Hing B., Davidson S. Exploring the effects of polymorphisms on cis-regulatory signal transduction response. Trends in Molecular Medicine. 2013;19(2):99–107. doi: 10.1016/j.molmed.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jahnen-Dechent W., Trindl A., Godovac-Zimmermann J., Muller-Esterl W. Posttranslational processing of human α2-HS glycoprotein (human fetuin). Evidence for the production of a phosphorylated single-chain form by hepatoma cells. European Journal of Biochemistry. 1994;226(1):59–69. doi: 10.1111/j.1432-1033.1994.tb20026.x. [DOI] [PubMed] [Google Scholar]
- 19.Buckland P. R. Allele-specific gene expression differences in humans. Human Molecular Genetics. 2004;13(2):R255–R260. doi: 10.1093/hmg/ddh227. [DOI] [PubMed] [Google Scholar]
- 20.Tamura T., Osawa M., Kimura R., et al. Evaluation of the allele-sharing approach, known as the IBS method, in kinship analysis. Journal of Forensic and Legal Medicine. 2013;20(2):112–116. doi: 10.1016/j.jflm.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Eichner J. E., Friedrich C. A., Cauley J. A., et al. Alpha2-HS glycoprotein phenotypes and quantitative hormone and bone measures in postmenopausal women. Calcified Tissue International. 1990;47(6):345–349. doi: 10.1007/BF02555885. [DOI] [PubMed] [Google Scholar]
- 22.Sritara C., Thakkinstian A., Ongphiphadhanakul B., et al. Causal relationship between the AHSG gene and BMD through fetuin-A and BMI: multiple mediation analysis. Osteoporosis International. 2014;25(5):1555–1562. doi: 10.1007/s00198-014-2634-4. [DOI] [PubMed] [Google Scholar]
- 23.Mori K., Emoto M., Araki T., et al. Effects of pioglitazone on serum fetuin-A levels in patients with type 2 diabetes mellitus. Metabolism: Clinical and Experimental. 2008;57(9):1248–1252. doi: 10.1016/j.metabol.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Andersen G., Burgdorf K. S., Sparsø T., et al. AHSG tag single nucleotide polymorphisms associate with type 2 diabetes and dyslipidemia: studies of metabolic traits in 7,683 white danish subjects. Diabetes. 2008;57(5):1427–1432. doi: 10.2337/db07-0558. [DOI] [PubMed] [Google Scholar]
- 25.Temesszentandrási G., Vörös K., Böröcz Z., et al. Association of human fetuin-A rs4917 polymorphism with obesity in 2 cohorts. Journal of Investigative Medicine. 2015;63(3):548–553. doi: 10.1097/JIM.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 26.Stenvinkel P., Wang K., Qureshi A. R., et al. Low fetuin-A levels are associated with cardiovascular death: impact of variations in the gene encoding fetuin. Kidney International. 2005;67(6):2383–2392. doi: 10.1111/j.1523-1755.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 27.Fisher E., Stefan N., Saar K., et al. Association of AHSG gene polymorphisms with fetuin-A plasma levels and cardiovascular diseases in the EPIC-potsdam study. Circulation: Cardiovascular Genetics. 2009;2(6):607–613. doi: 10.1161/CIRCGENETICS.109.870410. [DOI] [PubMed] [Google Scholar]
- 28.Ma S., He Z., Zhao J., et al. Association of AHSG gene polymorphisms with ischemic stroke in a Han Chinese population. Biochemical Genetics. 2013;51(11-12):916–926. doi: 10.1007/s10528-013-9625-6. [DOI] [PubMed] [Google Scholar]
- 29.Rockman M. V., Wray G. A. Abundant raw material for cis-regulatory evolution in humans. Molecular Biology and Evolution. 2002;19(11):1991–2004. doi: 10.1093/oxfordjournals.molbev.a004023. [DOI] [PubMed] [Google Scholar]
- 30.Tollervey J. R., Curk T., Rogelj B., et al. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nature Neuroscience. 2011;14(4):452–458. doi: 10.1038/nn.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kameyama T., Suzuki H., Mayeda A. Re-splicing of mature mRNA in cancer cells promotes activation of distant weak alternative splice sites. Nucleic Acids Research. 2012;40(16):7896–7906. doi: 10.1093/nar/gks520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osawa M., Yuasa I., Kitano T., et al. Haplotype analysis of the human alpha2-HS glycoprotein (fetuin) gene. Annals of Human Genetics. 2001;65(part 1):27–34. doi: 10.1046/j.1469-1809.2001.6510027.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genotypes of three SNPs in 52 unrelated individuals.