Summary
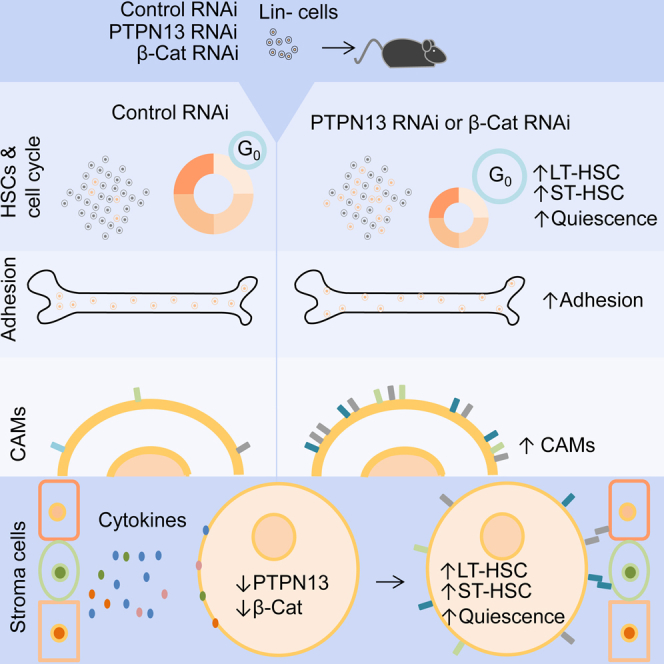
The regulation of hematopoietic stem cells (HSCs) depends on the integration of the multiple signals received from the bone marrow niche. We show the relevance of the protein tyrosine phosphatase PTPN13 and β-catenin as intracellular signaling molecules to control HSCs adhesiveness, cell cycling, and quiescence. Lethally irradiated mice transplanted with Lin– bone marrow cells in which PTPN13 or β-catenin had been silenced showed a significant increase of long-term (LT) and short-term (ST) HSCs. A decrease in cycling cells was also found, together with an increase in quiescence. The decreased expression of PTPN13 or β-catenin was linked to the upregulation of several genes coding for integrins and several cadherins, explaining the higher cell adhesiveness. Our data are consistent with the notion that the levels of PTPN13 and β-catenin must be strictly regulated by extracellular signaling to regulate HSC attachment to the niche and the balance between proliferation and quiescence.
Graphical Abstract
Highlights
-
•
PTPN13 or β-catenin silencing increases LT-HSCs and ST-HSCs frequency in vivo
-
•
The cell cycling of HSPCs was decreased by PTPN13 or β-catenin downregulation
-
•
LT-HSCs and ST-HSCs quiescence was increased by PTPN13 or β-catenin downregulation
-
•
PTPN13 and β-catenin levels modulate the interaction of HSPCs with the BM niche
In this article, Hernández-Hernández and colleagues show that in vivo silencing of PTPN13 and β-catenin increases the frequency of ST-HSCs and LT-HSCs, reduces cell cycling, and increases quiescence and the attachment to the niche. Accordingly, PTPN13 and β-catenin must be strictly regulated by extracellular signaling to regulate HSPCs attachment to the niche and the balance between proliferation and quiescence.
Introduction
Contrary to other processes that are mainly restricted to embryonic development, the differentiation of hematopoietic stem cells (HSCs) into the different blood lineages occurs along the life of the individual. For correct hematopoiesis, HSCs must maintain a fine balance between quiescence and proliferation, and between self-renewal and differentiation. The relevance of HSCs in regenerative medicine is remarkable (Mimeault et al., 2007), and the possibility of expanding HSCs in vitro, preserving their multipotency, would be a milestone in this regard. Therefore, understanding the orchestration of the multiple intercellular and intracellular signaling events that control HSCs quiescence and self-renewal in vivo should help to attain this goal.
Adult hematopoiesis occurs in the bone marrow (BM), and the importance of this niche in the regulation of HSCs was proposed many years ago (Schofield, 1978). The BM niche is a complex system formed by different cellular types that support HSCs (Ugarte and Forsberg, 2013). It is increasingly clear that the BM is not homogenous and that different kinds of niche can be found: osteoblastic, vascular, and perivascular. The influence of different types of environments could determine the fate of HSCs, depending on the body’s requirements (Kiel and Morrison, 2008). At the endosteal niche, HSCs establish direct contact with osteoblasts (Nakamura-Ishizu and Suda, 2013). This interaction seems to be important to maintain HSC quiescence (Zhang et al., 2003, Ellis et al., 2011). Moreover, osteoblasts produce soluble factors such as thrombopoietin (TPO) (Yoshihara et al., 2007) or osteopontin (OPN) (Nilsson et al., 2005), both of which contribute to the maintenance of HSC quiescence.
BM sinusoidal endothelial cells (BMSECs) define the vascular niche (Nakamura-Ishizu and Suda, 2013), and different authors have suggested that these cells contribute to regulating the balance between the self-renewal and differentiation of HSCs (Salter et al., 2009, Butler et al., 2010, Kobayashi et al., 2010).
Within the perivascular niche, two different types of cell seem to display niche functions: CXC chemokine ligand 12 (CXCL-12)-abundant reticular cells (CAR cells) and Nestin+ mesenchymal stem cells. CAR cells secrete stem cell factor (SCF) and CXCL12, also known as SDF-1 (stromal cell-derived factor-1) (Salter et al., 2009, Butler et al., 2010, Kobayashi et al., 2010). Nestin+ cells express high levels of genes involved in the regulation of HSCs, and acute depletion of these cells impairs HSC homing after irradiation (Méndez-Ferrer et al., 2010).
In order to understand how hematopoiesis is regulated, it is necessary not only to understand the different signals emanating from the niche (Anthony and Link, 2014), but also to comprehend the integration of these signals by HSCs.
Canonical Wnt signaling has been related to the regulation of HSCs homeostasis (Reya et al., 2003), and it has been reported that a switch toward a non-canonical Wnt signaling causes stem-cell aging (Florian et al., 2013). β-catenin is the nuclear effector of canonical Wnt signaling, and it also behaves as a cell adhesion molecule owing to its interaction with cadherins (Valenta et al., 2012). Although it has been shown that Wnt/β-catenin is required for hematopoiesis in Xenopus (Tran et al., 2010), the role of β-catenin in mammalian hematopoiesis remains highly controversial (Luis et al., 2012).
We have recently shown that the protein tyrosine phosphatase PTPN13 regulates β-catenin stability and function during in vitro megakaryopoiesis (Sardina et al., 2014). Our results also show that PTN13 is stabilized upon Wnt signaling activation, suggesting that PTPN13 is another important player in the context of canonical Wnt signaling (Sardina et al., 2014). The deficiency of PTPN13 in mice increases the in vitro differentiation of CD4+ T cells toward Th1 and Th2 (Nakahira et al., 2007), which together with our results (Sardina et al., 2014) suggests that PTPN13 may be an important regulator during hematopoiesis.
In the present work, we studied how the downregulation of PTPN13 or β-catenin affects in vivo hematopoiesis. We observed that reduced levels of PTPN13 or β-catenin increase the frequency of ST-HSCs and LT-HSCs, reduce cell cycling, and increase quiescence. Reduced levels of these two proteins are associated with an increased expression of several genes coding for cell adhesion molecules (CAMs), explaining the increased adhesiveness. Moreover, our data show that PTPN13 and β-catenin levels are modulated by mesenchymal cell conditioned medium and different cytokines involved in the control of HSCs, such as Wnt, TPO, CXCL-12, or SCF. Accordingly, we suggest that the regulation of PTPN13 and β-catenin levels in HSCs in response to extracellular signaling is of paramount importance in the regulation of HSC adhesion, cell cycling, and quiescence.
Results
Silencing of PTPN13 and β-Catenin in Murine Hematopoietic Progenitor Cells
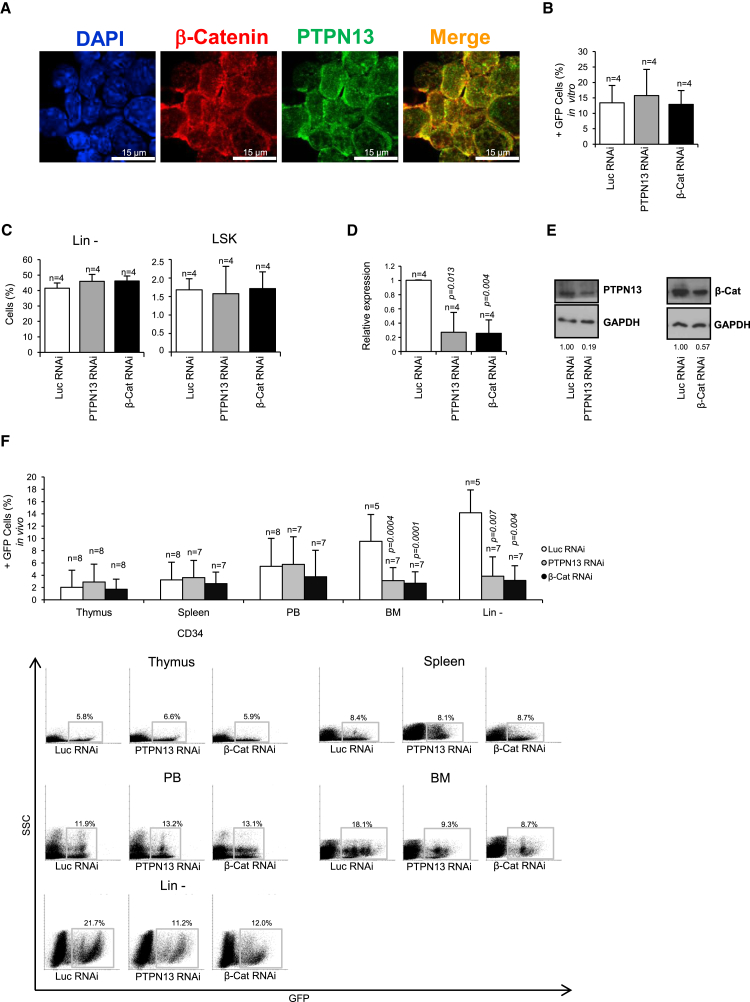
Here, we analyzed the effect of downregulating PTPN13 and β-catenin in mouse hematopoietic stem and progenitor cells (HSPCs) during in vivo hematopoiesis. We have previously shown that PTPN13 and β-catenin interact and colocalize in megakaryocytic cells (Sardina et al., 2014). In mouse BM progenitor cells (Lin– cells), we found a strong colocalization of the signals of both proteins (Figure 1A; Figure S1A). Small hairpin RNAs (shRNAs) against β-catenin or PTPN13 were introduced into mice Lin– cells by lentiviral infection, and an shRNA against firefly luciferase was used as a control (Sardina et al., 2014). Cells bearing the different shRNAs were identified by GFP expression. During in vitro culture, no differences were found in the percentage of total GFP+ cells (Figure 1B), GFP+ Lin– cells, or GFP+ LSK cells (Figure 1C; Figure S1C) between PTPN13 and β-catenin shRNAs and control cells. The cells were subsequently transplanted into lethally irradiated mice, and in vivo hematopoiesis was followed over 8 weeks after transplantation (Figure S1B). PTPN13 and β-catenin silencing in the mice was checked by quantitative qRT-PCR (Figure 1D) and by immunoblotting (Figure 1E) in GFP+ purified cells (Figure S1D). When we analyzed the percentage of GFP+ cells in the transplanted animals, no differences were found in peripheral blood (PB), thymus, or spleen (Figure 1F). However, in the BM the percentages of GFP+ and GFP+ Lin– cells were significantly reduced upon β-catenin and PTPN13 silencing (Figure 1F), suggesting that hematopoietic progenitor cells might be affected by PTPN13 and β-catenin downregulation.
Figure 1.
Silencing of PTPN13 or β-Catenin in Mouse Hematopoietic Progenitor Cells Decreases the Number GFP+ in the Bone Marrow
(A) PTPN13 and β-catenin localization in Lin– cells was analyzed by immunostaining with specific antibodies. Nuclei were identified by DAPI staining. A representative of three different experiments is shown.
(B) Lin– cells were modified with shRNA specific against PTPN13 or β-catenin, an shRNA against luciferase was used as a control. shRNA-carrying cells were identified by GFP expression (n = 4 independent experiments).
(C) No differences were found in the frequency of GFP+ Lin– or GFP+ LSK cells during in vitro culture (n = 4 independent experiments).
(D and E) Lethally irradiated mice were transplanted with Lin– modified cells. Spleen GFP+ cells were sorted and PTPN13 or β-catenin mRNA levels were analyzed in the corresponding downregulated cells by qRT-PCR (n = 4 different mice) (D) and immunoblotting (E) (n = 3 different mice).
(F) The frequency of GFP+ cells in different compartments in the mice was analyzed 8 weeks after transplantation (n = 5–8 different mice). Representative flow cytometry diagrams are shown. Numbers next to boxed areas indicate the percentage of cells in each region.
Means ± SD are shown. See also Figure S1.
PTPN13 and β-Catenin Silencing Increases the Number of HSCs
Hematopoietic stem cells (HSCs) are defined by their capacity to sustain the differentiation of all blood lineages (Osawa et al., 1996). In mice, it is possible to distinguish different HSC subpopulations on the basis of their surface markers: long-term HSCs (LT-HSCs), which would display an unlimited capacity for self-renewal; short-term HSCs (ST-HSCs), with a limited self-renewal capacity; and multipotent lympho-myeloid progenitors (LMPPs), which would not self-renew (Osawa et al., 1996, Morrison and Weissman, 1994, Morrison et al., 1997).
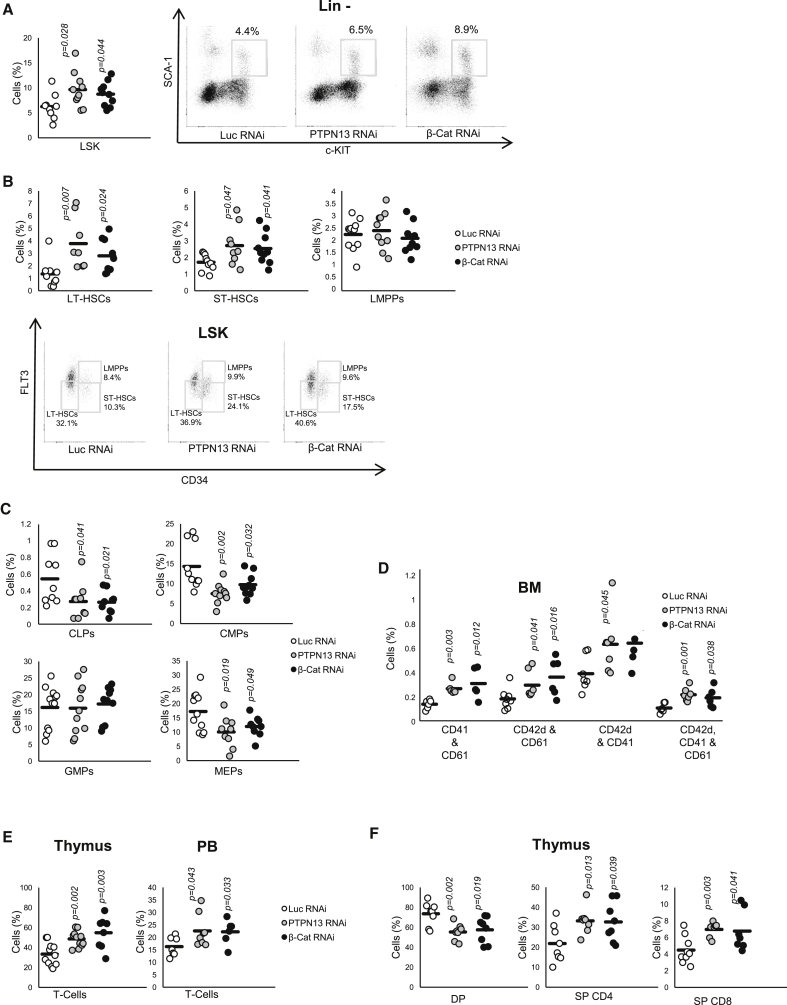
Lin– cells were purified from the BM of transplanted mice, GFP+ cells were gated, and different subpopulations of HSCs and progenitor cells were identified. Intriguingly, the frequency of LSKs (Figure 2A), LT-HSC, and ST-HSC (Figure 2B) subpopulations increased upon PTPN13 or β-catenin downregulation. When we analyzed more committed progenitors, no significant differences were found in LMPPs (Figure 2B), or granulocyte-monocyte progenitors (GMP) (Figure 2C). However, a significant decrease in common lymphoid progenitors (CLP), common myeloid progenitors (CMP), and megakaryocyte-erythrocyte progenitors (MEPs) was detected (Figure 2C). Thus, lower levels of PTPN13 or β-catenin led to a reduction in the total number of engrafted cells, and to a specific enrichment of the most immature populations (LSKs, LT-HSCs, and ST-HSCs).
Figure 2.
PTPN13 or β-Catenin Silencing Increases the Number of Hematopoietic Stem Cells
In vivo analysis of hematopoiesis in mice transplanted with Lin– control cells (Luc RNAi) or Lin– cells knocked down for PTPN13 or β-catenin.
(A) Identification of LSKs in BM (n = 9–10 different mice).
(B) LT-HSCs, ST-HSCs, LMPPs in BM (n = 8–10 different mice).
(C) Analysis of committed progenitors in BM: CLPs, CMPs, GMPs, and MEP (n = 9–12 different mice).
(D) Analysis of megakaryocytic markers (CD41, CD61, and CD42d) in BM (n = 4–8 different mice).
(E) Analysis of T cell (CD3+) in thymus and PB (n = 6–11 different mice).
(F) Thymocytes at late stages of maturation were analyzed by staining with CD4- and CD8-specific antibodies: DP (CD4+CD8+), CD4+ SP (CD4+CD8–), CD8+ SP (CD4–CD8+) (n = 7–9 different mice). Representative flow cytometry diagrams are shown. Numbers next to boxed areas indicate the percentage of cells in each region.
See also Figures S2 and S3.
Downregulation of PTPN13 or β-Catenin Increases Megakaryopoiesis and Lymphopoiesis
In agreement with our recently published in vitro data (Sardina et al., 2014), the silencing of PTPN13 and β-catenin increased the number of megakaryocytes in vivo (Figure 2D), which indeed suggests the involvement of these two proteins in megakarypoiesis. Erythroid cells were not modified in the BM or in PB, although their numbers were significantly reduced in spleen (Figure S2A). The percentage of granulocytes was reduced in peripheral blood (PB) and spleen, although no differences were found in bone marrow (BM) when β-catenin or PTPN13 were downregulated. No statistically significant differences were found in macrophages (Figure S2B).
Several reports suggest the involvement of β-catenin (Mulroy et al., 2003, Xu and Sen, 2003) and PTPN13 (Nakahira et al., 2007) in T cell differentiation. Our data pointed to a significant decrease in CLPs when PTPN13 or β-catenin was downregulated (Figure 2C), while the number of CD3+ T cells was significantly upregulated in the thymus and PB (Figure 2E; Figure S3A). In the thymus, we observed a significant increase in CD4 (CD4+ CD8–) and CD8 (CD4– CD8+) single-positive cells (Figure 2F; Figure S3B), which is consistent with the reduction in the DP and DN4 stages of differentiation (Figure S3C). Regarding the differentiation of B cells, we observed an upregulation in spleen when PTPN13 or β-catenin was downregulated, although no significant differences were found in PB or BM (Figure S3D). No differences were found in B cell maturation (Figure S3E).
In light of the above, our results support the notion of the involvement of PTPN13 and β-catenin in lymphopoiesis, especially in T cell maturation, in agreement with previous reports (Nakahira et al., 2007, Mulroy et al., 2003).
PTPN13 and β-Catenin Regulate HSC Proliferation and Quiescence
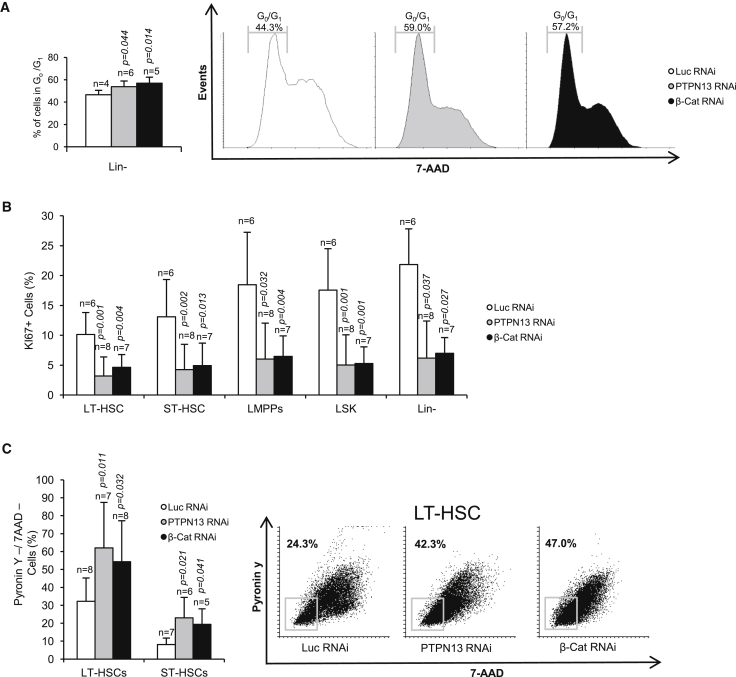
The reduced number of GFP+ cells within the Lin– fraction when β-catenin or PTPN13 were downregulated could be explained in terms of a lower proliferation rate. To test this possibility, the proportion of Lin– cells in G0/G1 was analyzed. Our results revealed a mild but significant increase in cells in G0/G1 phase when β-catenin or PTPN13 were downregulated (Figure 3A), suggesting an increase in the frequency of non-proliferating cells. To test this further, we studied the percentage of positive cells for the proliferation marker Ki67; the number of proliferating cells was significantly reduced in Lin– cells and in all the subpopulations of HSCs (LSKs, LMPPs, ST-HSCs, and LT-HSCs) upon PTPN13 or β-catenin silencing (Figure 3B). Cell-cycle activity must be strictly regulated in HSCs, and the balance between proliferation and quiescence is important to ensure hematopoiesis (Pietras et al., 2011). The lower number of proliferating cells suggested that PTPN13 or β-catenin silencing might increase quiescence within the HSC compartment. To test this, we analyzed the proportion of quiescent cells by pyronin Y staining experiments (Schmid et al., 2000). Our results showed that PTPN13 or β-catenin downregulation increased the proportion of quiescent cells within the LT-HSC and ST-HSC subpopulations (Figure 3C). Thus, our results suggested that PTPN13 and β-catenin would be involved in controlling the balance between the proliferation and quiescence of HSCs.
Figure 3.
PTPN13 or β-Catenin Silencing Reduces HSC Cycling and Increases Quiescence
In vivo analysis of BM progenitors in mice transplanted with Lin– control cells (Luc RNAi) or Lin– cells knocked down for PTPN13 or β-catenin.
(A) Lin– cells were stained with 7AAD and the frequency of cells in G0/G1 was analyzed by flow cytometry (n = 4–6 different mice). A representative diagram is shown.
(B) The percentage of Ki67+ proliferating cells was analyzed in different subpopulations of progenitor cells (n = 6–8 different mice).
(C) LT-HSCs and ST-HSCs quiescence was analyzed by PY/7AAD staining. The downregulation of PTPN13 or β-catenin increases the proportion of quiescent cells. A representative experiment is shown.
Means ± SD are shown.
PTPN13 and β-Catenin Regulate HSC Attachment to the Bone Marrow Niche
The balance between quiescence and proliferation is determined by intrinsic and extrinsic factors (Li and Bhatia, 2011), the anchorage of HSCs to the BM (Smith-Berdan et al., 2011) being especially important since it can contribute to the maintenance of LT-HSC quiescence (Yoshihara et al., 2007). We therefore wondered whether the capacity for attachment to the BM niche was affected by PTPN13 or β-catenin levels. BM cells were obtained by bone flushing. Subsequently, the bones were subjected to digestion with collagenase/dispase to release the cells that were strongly attached to the bone. The percentage of Lin– and LSKs within the GFP+ gated cells were analyzed in both fractions. The frequency of GFP+ Lin– and GFP+ LSKs adhered strongly to the bone was greater in the mice in which PTPN13 or β-catenin had been downregulated (Figure 4A). In control mice, the percentages of LSK cells in both fractions were very similar, but upon PTPN13 or β-catenin downregulation an increase of 1.7- and 2.35-fold occurred, respectively, in the percentage of LSKs strongly attached to the bone when compared to the BM flushed fraction. Histological analysis failed to reveal any obvious differences in the localization at the BM between cells downregulated for PTPN13 or β-catenin in comparison with control cells (Figure S4).
Figure 4.
PTPN13 or β-Catenin Silencing Increases Cell Adhesiveness and the Upregulation of Cell Membrane Adhesion Molecules
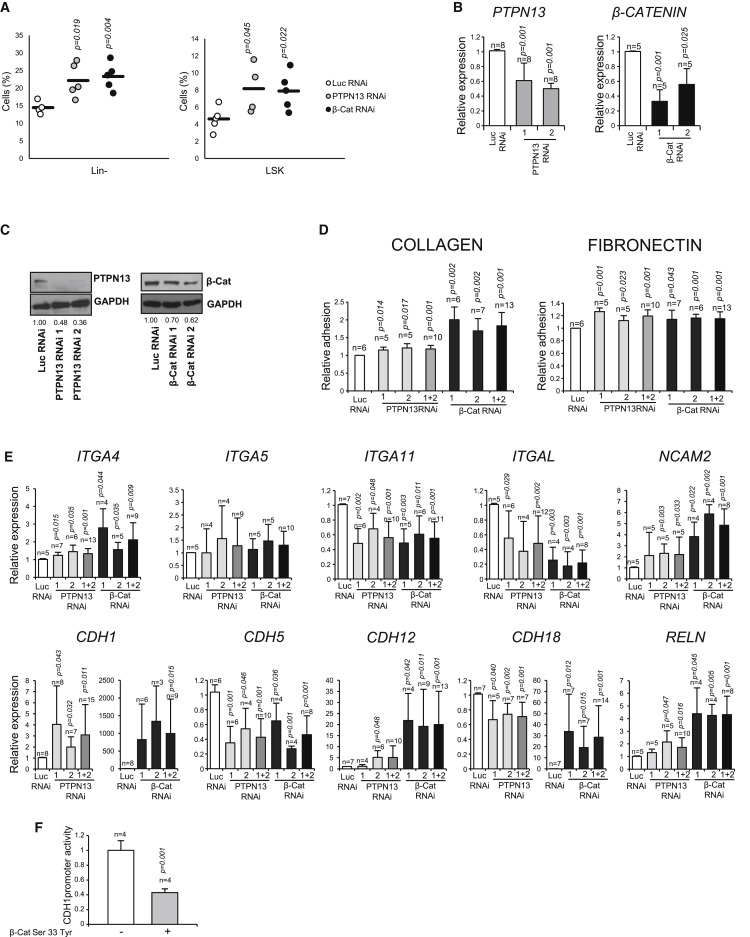
(A) The proportion of Lin–(CD45+Lin–) and LSKs (CD45+Lin–c-KIT+SCA1+) cells was analyzed in collagenase-/dispase-digested bones after BM had been flushed out (n = 4–5 different mice). The frequency of Lin– and LSKs strongly attached to the bone was higher in mice in which PTPN13 or β-catenin had been silenced.
(B and C) PTPN13 or β-catenin was silenced in HEL cells. The levels of expression were analyzed by qRT-PCR (n = 5 or 8 independent experiments) (B) and immunoblotting (n = 3 independent experiments) (C).
(D) Cell adhesiveness to collagen- or fibronectin-coated plates was analyzed in these cells (n = 5–7 independent experiments).
(E) The expression of several genes coding for CAMs was analyzed by qRT-PCR (n = 4–9 independent experiments).
(F) CDH1 promoter activity was analyzed by luciferase reporter assay in the presence or absence of a β-catenin stable mutant (β-catenin S33Y) (n = 4 independent experiments).
Means ± SD are shown. See also Figure S4.
These results suggested that the levels PTPN13 and β-catenin might regulate cell adhesiveness. To test this further, PTPN13 or β-catenin was silenced in HEL cells (Figures 4B and 4C). The capacity of adhesion to collagen- and fibronectin-coated plates was increased following the silencing of either protein (Figure 4D). The expression of ten different CAMs was analyzed by qRT-PCR. The expression of several of these genes (ITGA4, CDH1, CDH12, NCAM2, and RELN) was upregulated upon PTPN13 or β-catenin downregulation (Figure 4E), which could account for the higher adhesiveness of these cells. This change in expression could be explained whether these genes were β-catenin transcriptional targets. To test this hypothesis, the activity of the CDH1 gene promoter was analyzed (Figure 4F). β-catenin overexpression reduced CDH1 promoter activity, supporting the notion that β-catenin may control the transcription of the CAMs-coding genes referred above.
Regulation of β-Catenin and PTPN13 Levels by Cellular Signaling
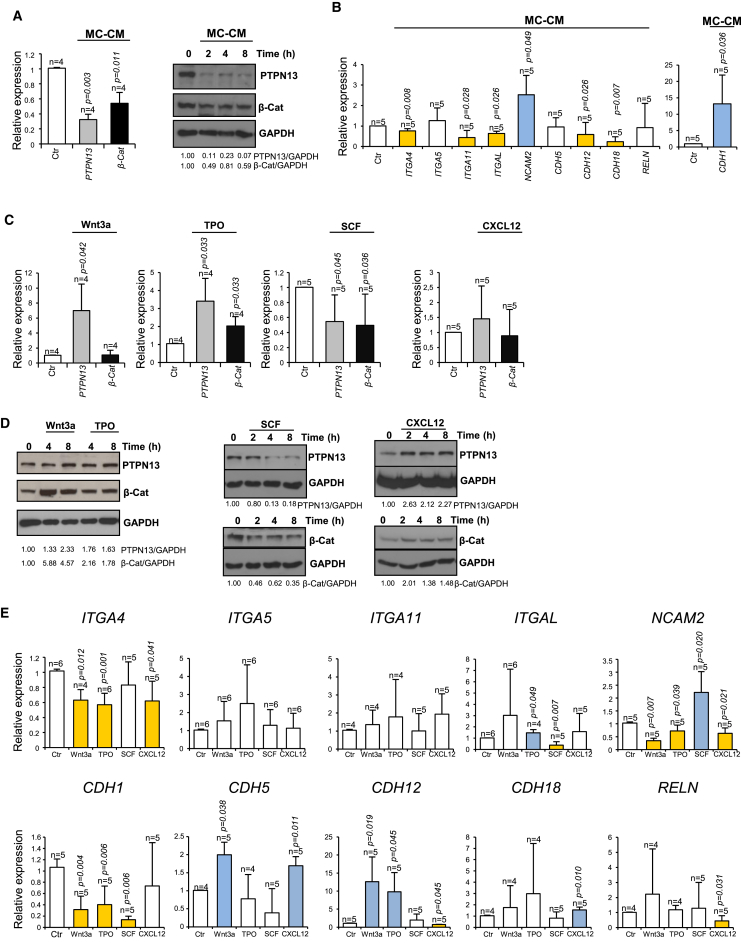
β-catenin levels are strictly regulated by extracellular signaling, and PTPN13 is involved in this regulation, preventing β-catenin degradation (Sardina et al., 2014). We therefore wondered whether the levels of these two proteins could be regulated by signals emanating from the BM niche. To test this idea, we first used a conditioned medium derived from immortalized bone marrow mesenchymal stroma cells (hMSC-TERTs) (Mihara et al., 2003). This conditioned medium induced a striking downregulation of PTPN13 and β-catenin in HEL cells (Figure 5A). With this treatment, the cells became adherent (Figure S5), which could be explained in terms of the observed altered expression of CAMs (Figure 5B). These results suggested that PTPN13 and β-catenin levels could be regulated by signals emanating from the niche, which in the long run would regulate cell adhesion capacity through control of CAMs expression.
Figure 5.
PTPN13 and β-Catenin Are Targets of Several Signaling Pathways
(A and B) HEL cells were treated with mesenchymal cell-conditioned medium (MC-CM). The levels of PTPN13 and β-catenin protein and messenger (A) and the levels of several genes coding for CAMs were analyzed (B) (n = 4 independent experiments). HEL cells were treated with 100 ng/ml Wnt3a, 100 ng/ml TPO, 100 ng/ml CXCL-12 or 100 ng/ml SCF, and the following parameters were analyzed.
(C) PTPN13 and β-catenin messenger levels (n = 4–5 independent experiments).
(D) PTPN13 and β-catenin proteins levels (n = 3–5 independent experiments).
(E) Expression of several genes coding for CAMs (n = 4–6 independent experiments). Messenger levels were analyzed after 8-hr treatment, and protein levels were monitored at different time points.
In (B) and (E), upregulated genes are shown in blue, and downregulated genes are in yellow. Means ± SD are shown. See also Figure S5.
To test this further, we next investigated the effect of several cytokines that have been implicated previously in the regulation of HSCs such as Wnt (Reya et al., 2003, Florian et al., 2013), TPO (Qian et al., 2007, Yoshihara et al., 2007, Sanjuan-Pla et al., 2013), CXCL-12 (Sugiyama et al., 2006), and SCF (Kent et al., 2008). Wnt, TPO, and CXCL12 increased PTPN13 and β-catenin levels, while SCF decreased their levels (Figures 5C and 5D). In sum, PTPN13 and β-catenin levels are modulated by different cytokines involved in the regulation of HSCs. Moreover, we observed that some of the CAMs that were upregulated by PTPN13 or β-catenin silencing were downregulated by Wnt, TPO, or CXCL12 signaling (ITGA4, NCAM2, or CDH1), while SCF signaling exerted the same effect as PTPN13 or β-catenin silencing on the expression of ITGAL and NCAM2 (Figure 5E).
β-Catenin and PTPN13 Downregulation Increases the Adhesiveness of Hematopoietic Progenitor Cells
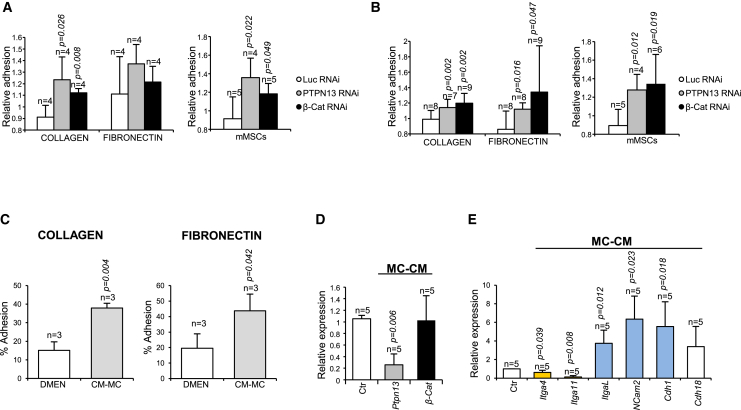
The increased adhesion of HSPCs to the bone in vivo (Figure 4A) could be explained by an increased adhesiveness of these cells upon PTPN13 and β-catenin downregulation, as we observed for HEL cells (Figure 4D; Figure S4). To test this further, we analyzed the adhesion capacity of BM mouse progenitor cells in vitro. Two different sources of HSPCs obtained from the transplanted animals were used: the Lin– fraction (Figure 6A), purified after the BM, and the hematopoietic cells that were more strongly attached to the bone, obtained after digestion with collagenase and dispase (Figure 6B). We observed increased adhesion to collagen with both sources of cells upon downregulation of PTPN13 or β-catenin. Similar results were found for adhesion to fibronectin. We then analyzed the capacity for adhesion to mouse mesenchymal stroma cells (mMSCs), and in these we also found that the ability HSPCs to adhere to these niche cells was also enhanced by PTPN13 or β-catenin downregulation (Figures 6A and 6B).
Figure 6.
Increased Adhesion of Mouse BM Progenitor Cells by PTPN13 or β-Catenin Silencing or by Treatment with Mesenchymal Cell-Conditioned Medium
(A) Lin– progenitor cells were purified after flushing the BM of the transplanted animals (n = 4–5 different mice).
(B) The hematopoietic cells that remained attached to the bone were obtained after collagenase/dispase digestion (n = 4–9 different mice). Both sources of cells were seeded in collagen- or fibronectin-coated plates, or in a monolayer of mouse mesenchymal stroma cells (mMSCs). 4 hr later, non-adherent cells were removed by gentle washing with PBS. The relative adhesion was monitored by comparing the percentage of GFP+ adhered cells to the initial % GFP+ cells.
(C) Adhesion to collagen- or fibronectin-coated plates of Lin– cells treated with mesenchymal cell-conditioned medium (MC-CM) or with control medium during 4 hr (n = 3 independent experiments).
(D) Messenger levels for PTPN13 and β-catenin in Lin– cells treated with MC-CM or control medium during 8 hr.
(E) Messenger levels for several genes coding for CAMs treated with MC-CM or control medium during 8 hr (n = 5 independent experiments). Upregulated genes are shown in blue, and downregulated genes are in yellow. Means ± SD are shown.
Given that our results suggest that PTPN13 and β-catenin levels might be regulated by niche signals, we considered that it was worth analyzing whether the adhesiveness of HSPCs might be regulated by niche signals. In fact, the adhesion of Lin– cells to collagen and fibronectin was significantly enhanced by treatment with mesenchymal cell-conditioned medium (Figure 6C). Moreover, we observed a significant decrease in PTPN13 mRNA (Figure 6D). Although there was no a clear reduction in β-catenin mRNA levels, it is likely that the downregulation of PTPN13 would cause a decrease in β-catenin protein levels, as we have shown previously (Sardina et al., 2014). Finally, we observed an upregulation of several CAMs (Itgal, Ncam2, and Cdh1) in the hematopoietic progenitors treated with mesenchymal cell-conditioned medium (Figure 6E), which could account for the increased adhesiveness (Figure 6C). In sum, these experiments show that, as was the case for HEL cells, the adhesion capacity of hematopoietic progenitors is altered by PTPN13 and β-catenin downregulation, a process that might be regulated by signaling from the BM niche.
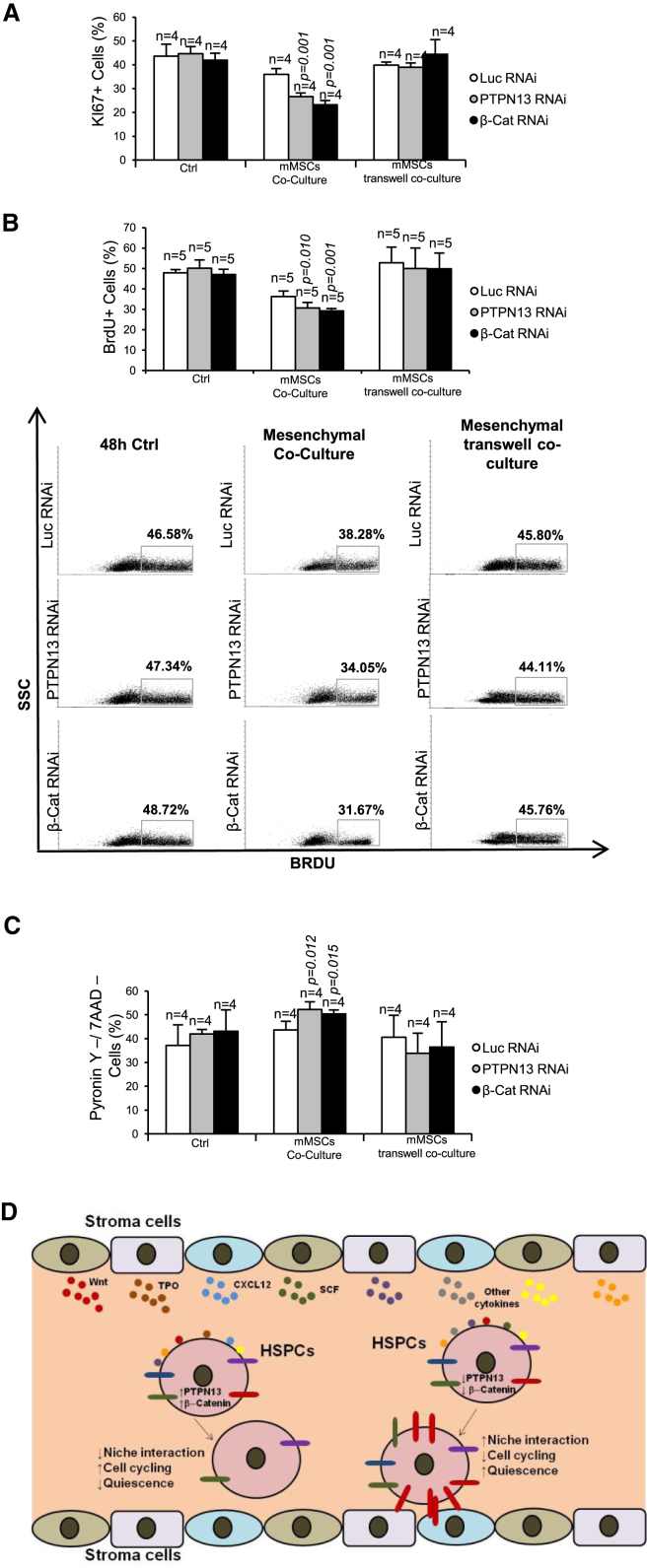
Functional Implications of PTPN13 or β-Catenin in the Regulation of Hematopoietic Progenitors by the BM Niche
Our in vivo analysis revealed that the downregulation of PTPN13 and β-catenin reduced cell cycling and increased the quiescence of HSCs (Figure 3). We surmised that this might be related to the increased adhesiveness of these cells to the niche. To test this hypothesis, the quiescence and cell cycling of Lin– progenitors were analyzed under in vitro culture conditions and in co-culture with mMSCs. During in vitro culture, no differences were observed between Lin– cells downregulated for PTPN13 or β-catenin and control cells regarding cell cycling (Figures 7A and 7B), or quiescence (Figure 7C). However, upon co-culture with mMSCs the cells downregulated for PTPN13 and β-catenin showed a significant decrease in cell cycling and an increase in quiescence in comparison with control cells, as seen from the decrease in Ki67 marker (Figure 7A) and the reduced incorporation of BrdU (Figure 7B), and by the increase in PY staining (Figure 7C), respectively. Strikingly, when the co-culture experiments were performed using transwell devices, no effects were found, suggesting that the above changes regarding cell cycling and quiescence would require direct cell-cell contact between HSPCs and mMSCs.
Figure 7.
PTPN13 or β-Catenin Silencing Reduces HSC Cycling and Increases Quiescence
Lin– cells were transduced in vitro with lentiviruses carrying shRNAs against PTPN13, β-catenin, or luciferase as a control, as depicted in Figure S1B. Cells were cultured in control medium or upon co-culture with mMSCs with or without a transwell device during 48 hr. The following parameters were analyzed in the three different culture conditions.
(A) Percentage of Ki67+ proliferating cells (n = 4 independent experiments).
(B) Bromodeoxyuridine (5-bromo-2-deoxyuridine, BrdU) incorporation to replicating cells after 16 hr of BrdU treatment (n = 5 independent experiments).
(C) Cell quiescence estimated by PY/7AAD staining (n = 4 independent experiments).
(D) Proposed model for PTPN13 and β-catenin as regulators of intracellular signaling in HSPCs. PTPN13 and β-catenin can be regulated by cytokine signaling. Low levels of these proteins would increase HSPCs attachment to the niche through the upregulation of CAMs. A stronger attachment to the niche would reduce cell cycling and increase the quiescence of HSPCs.
Means ± SD are shown.
Discussion
Understanding the mechanisms that govern the balance between self-renewal and differentiation or between cell-cycle progression and quiescence in HSCs is essential. These processes are regulated by the different signals received by HSCs from the BM niche. Accordingly, it is crucial to understand how these inputs are processed by HSC intracellular signaling. In this work, we show that the levels of PTPN13 and β-catenin are important for regulating HSCs quiescence and cell adhesiveness, and our results suggest that the regulation of the levels of these two proteins by different extracellular signaling pathways might be essential for the regulation of HSC biology. Moreover, in agreement with previous reports (Sardina et al., 2014, Nakahira et al., 2007, Mulroy et al., 2003, Xu et al., 2003), our results also suggest the involvement of these two proteins in megakaryocytic and lymphoid maturation. In future studies, we shall analyze this issue in greater depth.
The in vivo silencing of PTPN13 or β-catenin reduced the total number of engrafted GFP+ cells in BM. However, it led to an increased frequency of both GFP+ LT-HSCs and GFP+ ST-HSCs. HSCs have a low proliferation rate (Pietras et al., 2011), and hence the enrichment in HSCs would explain the reduced number of engrafted GFP+ cells upon PTPN13 or β-catenin downregulation. This was confirmed by analysis of the Ki67 proliferation marker, which was significantly reduced in all progenitor subpopulations when compared to control cells. This is in agreement with the increase in quiescence detected in the GFP+ LT-HSCs and GFP+ ST-HSCs.
Nevertheless, these observations did not explain why the frequency of HSCs and their quiescence was increased upon downregulation of PTPN13 or β-catenin. Considering the involvement of β-catenin in the cell adhesion process (Valenta et al., 2012), we thought it worthwhile to study whether attachment to the BM was altered. Surprisingly, our in vivo data showed a higher adhesion of mouse progenitor cells to the BM niche upon PTPN13 or β-catenin downregulation. These results are in agreement with many in vitro data that we report showing the alteration of cell adhesion when PTPN13 or β-catenin levels are modified, both in HEL cells and in also in murine hematopoietic progenitor cells. We therefore wondered whether the expression of genes coding for CAMs was altered. The expression of ten different genes, most of them expressed in HSCs (Prowse et al., 2011), was monitored in a cell line downregulated for PTPN13 or β-catenin. Interestingly five of these genes (ITGA4, CDH1, CDH12, NCAM2, and RELN) were upregulated; no differences were found in one of them (ITGA5), and only three of them were downregulated (ITGA11, ITGAL, CDH5). These dramatic changes at the cell surface could ultimately account for the increased adhesiveness. It has been reported previously that β-catenin suppresses the expression of cadherin genes (Jamora et al., 2003). Our analysis of CDH1 gene promoter activity allows us to propose that some of the CAMs coding genes may be β-catenin transcriptional targets, while PTPN13, preserving β-catenin stability (Sardina et al., 2014), would contribute to the transcriptional regulation of these genes. The biology of stem cells depends on their relationship with the niche, and it is clear that CAMs would play a leading role in this respect. For example, it is well known that CDH1 is required for the survival and self-renewal of hESCs (Li et al., 2012), and to maintain HSC multipotency in Drosophila (Gao et al., 2013). Recent reports show that CDH1 overexpression enhances the generation of induced pluripotent stem cells (iPS) (Chen et al., 2010), or the conversion from primed to naive-like pluripotent stem cells (PSCs) (Murayama et al., 2015). Moreover, it has recently been shown that a low expression of Itgal is linked to HSC long-term reconstitution capacity (Fathman et al., 2014).
We believe that the alteration of cell adhesion capacity may have important functional consequences, explaining the differences that we observed in the cell cycling and quiescence of hematopoietic progenitors. Supporting this notion, here we show that the downregulation of PTPN13 or β-catenin significantly reduces cell cycling and increases the quiescence of Lin– progenitor cells co-cultured with mMSCs. It is worth noting that this effect is attained by direct cell-cell contact, since no effects were seen during transwell co-culture experiments. In short, we propose that the greater adhesiveness would be responsible for the reduced proliferation and increased quiescence upon PTPN13 and β-catenin downregulation observed in the in vivo experiments, in agreement with previous studies showing that quiescent cells are tightly bound to the BM niche (Scott et al., 2003). Osteoblast seem to play a key role in the maintenance of HSC quiescence (Bowers et al., 2015), which may be related to a physical contact between osteoblast and HSCs (Zhang et al., 2003). Here, we show that the interaction with mesenchymal stroma cells might be also important for the regulation of HSC quiescence. Accordingly, we speculate that different types of niche cells might cooperate to maintain such quiescence.
We surmise that β-catenin and PTPN13 must be important for integrating the different signals received from the niche. Our results support this notion, since treatment with mesenchymal cell-conditioned medium led to the downregulation of PTPN13 and β-catenin, together with an increase in cell adhesiveness that could be explained by the change in CAM expression. The relevance of β-catenin in the context of cellular signaling has also been suggested for other types of stem cells (Murphy et al., 2014, Lindström et al., 2015). The fact that PTPN13 and β-catenin levels were modified by several cytokines (Wnt3a, TPO, CXCL12, and SCF) suggests to us that these two proteins could be targets of multiple signaling pathways emanating from the niche. CXCL12 and Wnt can synergize for the stabilization of β-catenin in cancer cells (Mo et al., 2013). SCF reinforces TPO signaling in megakaryocyte progenitors (Drayer et al., 2005). Regarding our results, it is feasible that different signaling pathways would converge at the level of PTPN13 and β-catenin, and hence these proteins would contribute to the integration of these signals by HSPCs.
The use of HSCs in regenerative medicine is of paramount importance (Felfly and Haddad, 2014). Bearing in mind our results, we suggest that PTPN13 and β-catenin might be interesting therapeutic targets, since the modification of their levels could facilitate the mobilization of HSCs from donors or favor engraftment in recipients.
In conclusion, we show that the modulation of PTPN13 and β-catenin levels must play an essential role in the regulation of HSC adhesiveness, quiescence, and proliferation. Our data are consistent with the notion that the levels of PTPN13 and β-catenin must be kept low in order to preserve HSC quiescence and attachment to the niche. We also show that the levels of these two proteins are modified by different signals that are important for HSC development, such as Wnt, TPO, CXCL-12, and SCF. We propose that the modulation of PTPN13 and β-catenin levels in HSC would be of paramount importance for the integration of such signals (Figure 7D).
Experimental Procedures
Cell Lines
HEL cells were grown in RPMI medium supplemented with 10% FBS, 100 U/ml penicillin, 100 U/ml streptomycin, and 2 mM L-glutamine. HEK293T cells and immortalized human bone marrow stroma cells (hMSC-TERTs) were grown in DMEM medium supplemented with 10% FBS, 100 U/ml penicillin, 100 U/ml streptomycin, and 2 mM L-glutamine (Mihara et al., 2003). Mesenchymal cell conditioned medium (MC-CM) was harvested after 72 hr, centrifuged, and filtered.
qRT-PCR
RNA was extracted with TRI reagent (Sigma) or with an RNAspin mini kit (GE Healthcare Life Sciences). The RevertAid M-MuLV retrotransciptase (Fermentas) or the GeneChip WT Terminal Labeling Kit (Affymetrix) was used to generate cDNA. qPCR was carried out using the Go-Taq qPCR master mix (Promega) in a StepOne Real-Time PCR System (Applied Biosystems). Analysis of PCR data was performed by the comparative CT method (ΔΔCT) (Schmittgen and Livak, 2008), using actin as an endogenous control. Oligonucleotide sequences are provided as Supplemental Information.
Animals
C57BL/6 mice were obtained from Charles River Laboratories and from the University of Salamanca Animal Facility Unit. All mice were kept at the pathogen-free animal facilities of University of Salamanca and were monitored daily. All experiments carried out on animals were approved by the Bioethics Committee of the University of Salamanca.
Lentiviral Production for RNA Interference
The expression of PTPN13 and β-catenin was reduced by lentiviral infection with specific small hairpin RNAs (shRNA). An oligonucleotide against firefly luciferase was used as a control, as previously described (Sardina et al., 2010, Sardina et al., 2014, Sánchez-Sánchez et al., 2014).
Bone Marrow Cell Purification and Lentiviral Transduction
BM cells were flushed with RPMI medium from femurs and tibias. Preparations were filtered to remove debris and washed twice in phosphate-buffered saline. Lin– cells were purified with the Lineage Cell Depletion Kit for mice (MiltenyiBiotec), according to the manufacturer’s instructions. Lin– cell lentiviral transduction was performed as described (Sardina et al., 2014).
Bone Marrow Transplantation
Syngenic C57BL/6 recipient mice received two doses of 5Gy, separated in time by 2 hr, from a Cs source (Gammacell 1000 Elite, Nucleus Facility, University of Salamanca), and were transplanted 6 hr after the second irradiation. 3 × 105 of unsorted lentiviral transduced Lin– cells were injected intravenously through a tail vein of recipients, 24 hr after spinoculation. shRNA-carrying cells were identified by GFP expression. In vivo hematopoiesis was followed over 8 weeks after transplantation. A schematic representation of the experimental setting can be seen in Figure S1B.
Flow Cytometry
The lineage-specific markers used were as follows: CD41, CD61, and CD42d for megakaryocytes; Ter119 for erythrocytes; Gr1 and CD11b for granulocytes and macrophages; CD19 and B220 for B cells, and CD3 for T cells. B cell maturation was analyzed in BM: ProB (B220+CD43+IgM–), PreB (B220+CD43–IgM–), and ImmBcells (B220+CD43–IgM+). T cell maturation was analyzed in the thymus: DN1 (CD44+CD25–), DN2 (CD44+CD25+), DN3 (CD44–CD25+), and DN4 (CD44–CD25–). DN (CD4–CD8–), DP (CD4+CD8+), CD4+ SP (CD4+CD8–), CD8+ SP (CD4–CD8+); BM Lin– cell subpopulations were identified as follows: LSKs (Lin–c-KIT+SCA1+), LT-HSCs (Lin–c-KIT+SCA1+CD34–FLT3–), ST-HSCs (Lin–c-KIT+SCA1+CD34+FLT3–), LMPPs (Lin–c-KIT+SCA1+CD34+FLT3+), CMPs (Lin–c-KIT+SCA1–CD34+CD16/32–), CLPs (Lin–c-KITlo, SCA1loIL-7-Rα–), MEPs (Lin–c-KIT+SCA1–CD34–IL-7-Rα–CD16/32–), GMPs (Lin–c-KIT+SCA1–CD34+ IL-7-Rα–CD16/32+). Fluorescence minus one (FMO) controls were used for fluorescence-activated cell sorting (FACS) gating optimization (Maecker and Trotter, 2006). The number of quiescent LT-HSCs was analyzed by staining of the Lin– fraction with specific surface markers, together with 1 μg/ml pyronin Y (PY), 10 μg/ml 7ADD and 10 μg/ml ADD, as previously described (Schmid et al., 2000). Bromodeoxyuridine (5-bromo-2-deoxyuridine, BrdU) incorporation was performed with the APC BrdU Kit (BD Biosciences), following the manufacturer’s instructions. The results were analyzed with the FACS Flowing 2 and Infinicyt Programs.
Isolation of Bone-Associated Cells
BM cells were fully flushed from the femurs and tibias of transplanted animals. The bones were then digested with 0.02 mg/ml collagenase/dispase (Roche) for 2 hr at 37°C with constant agitation. Flushed BM cells and collagenase-/dispase-digested bones were analyzed by flow cytometry to determine the percentage of LSK cells (CD45+Lin–c-KIT+SCA1+) in each fraction.
In Vitro Adhesion Assays
Cell adhesiveness was monitored in 100 μg/ml collagen- or 20 μg/ml fibronectin-coated plates. Cells were seeded for 3 hr at 37°C and washed twice with PBS, and the proportion of adhered cells was measured with the MTT assay (Sánchez-Sánchez et al., 2014) in the case of HEL cells, or by evaluating the % of GFP+ cells by flow cytometry in the case of BM primary cells.
CDH1 Promoter Luciferase Reporter Assay
CDH1 promoter activity was analyzed in 293T with the reporter plasmid pGL-E-Cad (−427+53) (Lee et al., 2011) in the absence or presence of a plasmid overexpressing a β-catenin stable mutant (pWPI-β-cateninS33Y). Luciferase activity was normalized with respect to β-galactosidase transfection control as described (Sardina et al., 2014).
Isolation of Mouse Mesenchymal Stromal Cell
BM was harvested from femurs of 8- to 10-week old C57BL/6 mice by flushing with RPMI medium. The cells were resuspended after washing in complete MesenCult medium (STEMCELL Technologies) and cultured at 37°C and 5% CO2. Non-adherent cells were removed after 72–96 hr in culture; adherent cells were harvested by trypsinization (0.05% trypsin-EDTA, GIBCO Invitrogen) when 90% confluence had been reached, after which they were replated. The medium was changed every 3–4 days. For co-culture assays, mMSC were used at passage 3–5.
Immunohistochemical Analysis
Immunohistochemical analysis on femurs sections was performed as described before (Carrancio et al., 2011). shRNAs modified cells were identified by GFP expression with a rabbit monoclonal anti-GFP antibody (AbCam). Primary antibody was revealed using a biotinylated secondary antibody and the ABC kit (Vector Laboratories) with Diaminobenzime (DAB, Sigma-Aldrich). Finally, slides were mounted and observed with an Olympus BX41TF microscope (Olympus Optical).
Statistical Analyses
Statistical analyses were performed using Student’s t test. Differences were considered statistically significant when p < 0.05.
Author Contributions
G.L.-R. performed experiments, analyzed data, created the figures, and edited the paper. R.P.-B., T.L.R, L.S.-S., and L.I.S.-A. performed experiments. F.S.-G, J.A.P.-S., J.S.-Y., and M.L. edited the paper. A.H.-H. designed and performed experiments and wrote the paper.
Acknowledgments
This work was supported by MCINN (BFU2006-10362, BFU2009-10568, CSD2007-00015, BFU2011-28467, and BFU2014-56490-R), by JCyL (SA010A10-2, SA126A07, BIO/SA59/13, and BIO/SA23/14) and FEDER-FIS (PS09/01075). G.L.-R. and R.P.-B. received pre-doctoral fellowships from the Regional Government of Castilla y León, Spain and European FEDER funds. The pGL-E-Cad (−427+53) vector was kindly provided by Dr. Seongman Kang (Korea University, Seoul, South Korea).
Published: September 3, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures and five figures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.08.003.
Supplemental Information
References
- Anthony B.A., Link D.C. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol. 2014;35:32–37. doi: 10.1016/j.it.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers M., Zhang B., Ho Y., Agarwal P., Chen C.C., Bhatia R. Osteoblast ablation reduces normal long-term hematopoietic stem cell self-renewal but accelerates leukemia development. Blood. 2015;125:2678–2688. doi: 10.1182/blood-2014-06-582924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J.M., Nolan D.J., Vertes E.L., Varnum-Finney B., Kobayashi H., Hooper A.T., Seandel M., Shido K., White I.A., Kobayashi M. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Yuan D., Wei B., Jiang J., Kang J., Ling K., Gu Y., Li J., Xiao L., Pei G. E-cadherin-mediated cell-cell contact is critical for induced pluripotent stem cell generation. Stem Cells. 2010;28:1315–1325. doi: 10.1002/stem.456. [DOI] [PubMed] [Google Scholar]
- Carrancio S., Blanco B., Romo C., Muntion S., Lopez-Holgado N., Blanco J.F., Briñon J.G., San Miguel J.F., Sanchez-Guijo F.M., del Cañizo M.C. Bone marrow mesenchymal stem cells for improving hematopoietic function: an in vitro and in vivo model. Part 2: Effect on bone marrow microenvironment. PLoS ONE. 2011;6:e26241. doi: 10.1371/journal.pone.0026241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayer A.L., Boer A.K., Los E.L., Esselink M.T., Vellenga E. Stem cell factor synergistically enhances thrombopoietin-induced STAT5 signaling in megakaryocyte progenitors through JAK2 and Src kinase. Stem Cells. 2005;23:240–251. doi: 10.1634/stemcells.2004-0153. [DOI] [PubMed] [Google Scholar]
- Ellis S.L., Grassinger J., Jones A., Borg J., Camenisch T., Haylock D., Bertoncello I., Nilsson S.K. The relationship between bone, hemopoietic stem cells, and vasculature. Blood. 2011;118:1516–1524. doi: 10.1182/blood-2010-08-303800. [DOI] [PubMed] [Google Scholar]
- Fathman J.W., Fernhoff N.B., Seita J., Chao C., Scarfone V.M., Weissman I.L., Inlay M.A. Upregulation of CD11A on hematopoietic stem cells denotes the loss of long-term reconstitution potential. Stem Cell Reports. 2014;3:707–715. doi: 10.1016/j.stemcr.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felfly H., Haddad G.G. Hematopoietic stem cells: potential new applications for translational medicine. J. Stem Cells. 2014;9:163–197. [PubMed] [Google Scholar]
- Florian M.C., Nattamai K.J., Dörr K., Marka G., Uberle B., Vas V., Eckl C., Andrä I., Schiemann M., Oostendorp R.A. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature. 2013;503:392–396. doi: 10.1038/nature12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Wu X., Fossett N. Drosophila E-cadherin functions in hematopoietic progenitors to maintain multipotency and block differentiation. PLoS ONE. 2013;8:e74684. doi: 10.1371/journal.pone.0074684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora C., DasGupta R., Kocieniewski P., Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent D., Copley M., Benz C., Dykstra B., Bowie M., Eaves C. Regulation of hematopoietic stem cells by the steel factor/KIT signaling pathway. Clin. Cancer Res. 2008;14:1926–1930. doi: 10.1158/1078-0432.CCR-07-5134. [DOI] [PubMed] [Google Scholar]
- Kiel M.J., Morrison S.J. Uncertainty in the niches that maintain haematopoietic stem cells. Nat. Rev. Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Butler J.M., O’Donnell R., Kobayashi M., Ding B.S., Bonner B., Chiu V.K., Nolan D.J., Shido K., Benjamin L., Rafii S. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat. Cell Biol. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Hong S., Kim S., Kang S. Ataxin-1 occupies the promoter region of E-cadherin in vivo and activates CtBP2-repressed promoter. Biochim. Biophys. Acta. 2011;1813:713–722. doi: 10.1016/j.bbamcr.2011.01.035. [DOI] [PubMed] [Google Scholar]
- Li L., Bhatia R. Stem cell quiescence. Clin. Cancer Res. 2011;17:4936–4941. doi: 10.1158/1078-0432.CCR-10-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Bennett S.A., Wang L. Role of E-cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cells. Cell Adhes. Migr. 2012;6:59–70. doi: 10.4161/cam.19583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström N.O., Carragher N.O., Hohenstein P. The PI3K pathway balances self-renewal and differentiation of nephron progenitor cells through β-catenin signaling. Stem Cell Reports. 2015;4:551–560. doi: 10.1016/j.stemcr.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis T.C., Ichii M., Brugman M.H., Kincade P., Staal F.J. Wnt signaling strength regulates normal hematopoiesis and its deregulation is involved in leukemia development. Leukemia. 2012;26:414–421. doi: 10.1038/leu.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maecker H.T., Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A. 2006;69:1037–1042. doi: 10.1002/cyto.a.20333. [DOI] [PubMed] [Google Scholar]
- Méndez-Ferrer S., Michurina T.V., Ferraro F., Mazloom A.R., Macarthur B.D., Lira S.A., Scadden D.T., Ma’ayan A., Enikolopov G.N., Frenette P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K., Imai C., Coustan-Smith E., Dome J.S., Dominici M., Vanin E., Campana D. Development and functional characterization of human bone marrow mesenchymal cells immortalized by enforced expression of telomerase. Br. J. Haematol. 2003;120:846–849. doi: 10.1046/j.1365-2141.2003.04217.x. [DOI] [PubMed] [Google Scholar]
- Mimeault M., Hauke R., Batra S.K. Stem cells: a revolution in therapeutics-recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin. Pharmacol. Ther. 2007;82:252–264. doi: 10.1038/sj.clpt.6100301. [DOI] [PubMed] [Google Scholar]
- Mo W., Chen J., Patel A., Zhang L., Chau V., Li Y., Cho W., Lim K., Xu J., Lazar A.J. CXCR4/CXCL12 mediate autocrine cell- cycle progression in NF1-associated malignant peripheral nerve sheath tumors. Cell. 2013;152:1077–1090. doi: 10.1016/j.cell.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S.J., Weissman I.L. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Morrison S.J., Wandycz A.M., Hemmati H.D., Wright D.E., Weissman I.L. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124:1929–1939. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- Mulroy T., Xu Y., Sen J.M. beta-Catenin expression enhances generation of mature thymocytes. Int. Immunol. 2003;15:1485–1494. doi: 10.1093/intimm/dxg146. [DOI] [PubMed] [Google Scholar]
- Murayama H., Masaki H., Sato H., Hayama T., Yamaguchi T., Nakauchi H. Successful reprogramming of epiblast stem cells by blocking nuclear localization of beta-catenin. Stem Cell Rep. 2015;4:103–113. doi: 10.1016/j.stemcr.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.M., Keefe A.C., Lawson J.A., Flygare S.D., Yandell M., Kardon G. Transiently active Wnt/β-catenin signaling is not required but must be silenced for stem cell function during muscle regeneration. Stem Cell Reports. 2014;3:475–488. doi: 10.1016/j.stemcr.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira M., Tanaka T., Robson B.E., Mizgerd J.P., Grusby M.J. Regulation of signal transducer and activator of transcription signaling by the tyrosine phosphatase PTP-BL. Immunity. 2007;26:163–176. doi: 10.1016/j.immuni.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Nakamura-Ishizu A., Suda T. Hematopoietic stem cell niche: an interplay among a repertoire of multiple functional niches. Biochim. Biophys. Acta. 2013;1830:2404–2409. doi: 10.1016/j.bbagen.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Nilsson S.K., Johnston H.M., Whitty G.A., Williams B., Webb R.J., Denhardt D.T., Bertoncello I., Bendall L.J., Simmons P.J., Haylock D.N. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–1239. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- Osawa M., Hanada K., Hamada H., Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- Pietras E.M., Warr M.R., Passegué E. Cell cycle regulation in hematopoietic stem cells. J. Cell Biol. 2011;195:709–720. doi: 10.1083/jcb.201102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prowse A.B., Chong F., Gray P.P., Munro T.P. Stem cell integrins: implications for ex-vivo culture and cellular therapies. Stem Cell Res. (Amst.) 2011;6:1–12. doi: 10.1016/j.scr.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Qian H., Buza-Vidas N., Hyland C.D., Jensen C.T., Antonchuk J., Månsson R., Thoren L.A., Ekblom M., Alexander W.S., Jacobsen S.E. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1:671–684. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Reya T., Duncan A.W., Ailles L., Domen J., Scherer D.C., Willert K., Hintz L., Nusse R., Weissman I.L. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Salter A.B., Meadows S.K., Muramoto G.G., Himburg H., Doan P., Daher P., Russell L., Chen B., Chao N.J., Chute J.P. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood. 2009;113:2104–2107. doi: 10.1182/blood-2008-06-162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Sánchez B., Gutiérrez-Herrero S., López-Ruano G., Prieto-Bermejo R., Romo-González M., Llanillo M., Pandiella A., Guerrero C., Miguel J.F., Sánchez-Guijo F. NADPH oxidases as therapeutic targets in chronic myelogenous leukemia. Clin. Cancer Res. 2014;20:4014–4025. doi: 10.1158/1078-0432.CCR-13-3044. [DOI] [PubMed] [Google Scholar]
- Sanjuan-Pla A., Macaulay I.C., Jensen C.T., Woll P.S., Luis T.C., Mead A., Moore S., Carella C., Matsuoka S., Bouriez Jones T. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature. 2013;502:232–236. doi: 10.1038/nature12495. [DOI] [PubMed] [Google Scholar]
- Sardina J.L., López-Ruano G., Sánchez-Abarca L.I., Pérez-Simón J.A., Gaztelumendi A., Trigueros C., Llanillo M., Sánchez-Yagüe J., Hernández-Hernández A. p22phox-dependent NADPH oxidase activity is required for megakaryocytic differentiation. Cell Death Differ. 2010;17:1842–1854. doi: 10.1038/cdd.2010.67. [DOI] [PubMed] [Google Scholar]
- Sardina J.L., López-Ruano G., Prieto-Bermejo R., Sánchez-Sánchez B., Pérez-Fernández A., Sánchez-Abarca L.I., Pérez-Simón J.A., Quintales L., Sánchez-Yagüe J., Llanillo M. PTPN13 regulates cellular signalling and β-catenin function during megakaryocytic differentiation. Biochim. Biophys. Acta. 2014;1843:2886–2899. doi: 10.1016/j.bbamcr.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Schmid I., Cole S.W., Korin Y.D., Zack J.A., Giorgi J.V. Detection of cell cycle subcompartments by flow cytometric estimation of DNA-RNA content in combination with dual-color immunofluorescence. Cytometry. 2000;39:108–116. doi: 10.1002/(sici)1097-0320(20000201)39:2<108::aid-cyto3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Scott L.M., Priestley G.V., Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol. Cell. Biol. 2003;23:9349–9360. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Berdan S., Nguyen A., Hassanein D., Zimmer M., Ugarte F., Ciriza J., Li D., García-Ojeda M.E., Hinck L., Forsberg E.C. Robo4 cooperates with CXCR4 to specify hematopoietic stem cell localization to bone marrow niches. Cell Stem Cell. 2011;8:72–83. doi: 10.1016/j.stem.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Kohara H., Noda M., Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Tran H.T., Sekkali B., Van Imschoot G., Janssens S., Vleminckx K. Wnt/beta-catenin signaling is involved in the induction and maintenance of primitive hematopoiesis in the vertebrate embryo. Proc. Natl. Acad. Sci. USA. 2010;107:16160–16165. doi: 10.1073/pnas.1007725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugarte F., Forsberg E.C. Haematopoietic stem cell niches: new insights inspire new questions. EMBO J. 2013;32:2535–2547. doi: 10.1038/emboj.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta T., Hausmann G., Basler K. The many faces and functions of β-catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Sen J. Beta-catenin expression in thymocytes accelerates thymic involution. Eur. J. Immunol. 2003;33:12–18. doi: 10.1002/immu.200390002. [DOI] [PubMed] [Google Scholar]
- Xu Y., Banerjee D., Huelsken J., Birchmeier W., Sen J.M. Deletion of beta-catenin impairs T cell development. Nat. Immunol. 2003;4:1177–1182. doi: 10.1038/ni1008. [DOI] [PubMed] [Google Scholar]
- Yoshihara H., Arai F., Hosokawa K., Hagiwara T., Takubo K., Nakamura Y., Gomei Y., Iwasaki H., Matsuoka S., Miyamoto K. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Zhang J., Niu C., Ye L., Huang H., He X., Tong W.G., Ross J., Haug J., Johnson T., Feng J.Q. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.