Summary
Neural stem cells in different locations of the postnatal mouse ventricular-subventricular zone (V-SVZ) generate different subtypes of olfactory bulb (OB) interneurons. High Sonic hedgehog (SHH) signaling in the ventral V-SVZ regulates the production of specific subtypes of neurons destined for the OB. Here we found a transient territory of high SHH signaling in the dorsal V-SVZ beneath the corpus callosum (CC). Using intersectional lineage tracing in neonates to label dorsal radial glial cells (RGCs) expressing the SHH target gene Gli1, we demonstrate that this region produces many CC cells in the oligodendroglial lineage and specific subtypes of neurons in the OB. The number of oligodendroglial cells generated correlated with the levels of SHH signaling. This work identifies a dorsal domain of SHH signaling, which is an important source of oligodendroglial cells for the postnatal mammalian forebrain.
Highlights
-
•
High Gli1 expression appears transiently in the dorsal V-SVZ during early postnatal ages
-
•
Gli1-expressing RGCs in the subcallosal V-SVZ generate many oligodendroglial cells
-
•
Oligodendrogenesis in the subcallosal V-SVZ is SHH dependent
-
•
Ectopic activation of SMO increases oligodendrogenesis in the neonatal and adult mice
In this article, Alvarez-Buylla and colleagues show that high Gli1 expression in the dorsal V-SVZ appears transiently during early postnatal development. Intersectional lineage tracing demonstrates that Gli1-expressing radial glia in the subcallosal V-SVZ generate many oligodendroglial cells that migrate to the corpus callosum. This postnatal wave of oligodendrogenesis is SHH dependent. These studies highlight the fine spatial organization of progenitors in the V-SVZ.
Introduction
Oligodendrocyte progenitor cells (OPCs) in the mammalian cerebral cortex have been suggested to originate from the ventral telencephalon (He et al., 2001, Marshall and Goldman, 2002). However, cell fate mapping using Emx1::Cre transgenic mice show that many oligodendrocytes in the cortex are generated dorsally (Gorski et al., 2002). These seemingly contradictory observations are reconciled in a study using Nkx2.1::Cre; Gsh2::Cre and Emx1::Cre transgenic mice to lineage trace progenitor cells of the medial and lateral ganglionic eminences (MGE and LGE) and the cortex, respectively. These analyses demonstrate three sequential waves of oligodendrocyte production in the forebrain (Kessaris et al., 2006). The first OPCs arise from the MGE and AEP in the ventral telencephalon at embryonic day 11.5 (E11.5); the second wave of OPCs originates from the LGE around E15, and the third wave comes from the cortex during the early postnatal period (Kessaris et al., 2006). The MGE and AEP are enriched with Sonic hedgehog (Shh), its receptor Patched (Ptc), and oligodendrocyte transcription factor 2 (Olig2) (Spassky et al., 2001, Tekki-Kessaris et al., 2001, Fuccillo et al., 2004). However, the region of origin of this third wave and whether these progenitors are regulated by SHH signaling remain unknown.
Interestingly, in rodents, the last wave of OPCs appears to be generated postnatally coinciding with the production of many neurons destined for the olfactory bulb (OB). These neurons and oligodendrocytes are derived from persistent germinal activity in the postnatal ventricular-subventricular zone (V-SVZ) (Levison and Goldman, 1993, Luskin and McDermott, 1994, Menn et al., 2006). The V-SVZ is an extensive germinal layer on the walls of the lateral ventricles that includes components derived from the MGE, LGE, septum, and cortex (Merkle et al., 2007, Alvarez-Buylla et al., 2008, Fuentealba et al., 2015). Different subregions of the V-SVZ are specialized for the production of different subtypes of OB interneurons, but the origin of OPCs from this postnatal germinal niche remains unknown. Here we identify a dorsal SHH-dependent domain in the postnatal V-SVZ, where many oligodendroglial lineage cells are generated. This domain may be critical for oligodendrogenesis and myelination in the postnatal forebrain.
Results
Transient Expression of Gli1 in the Dorsal V-SVZ
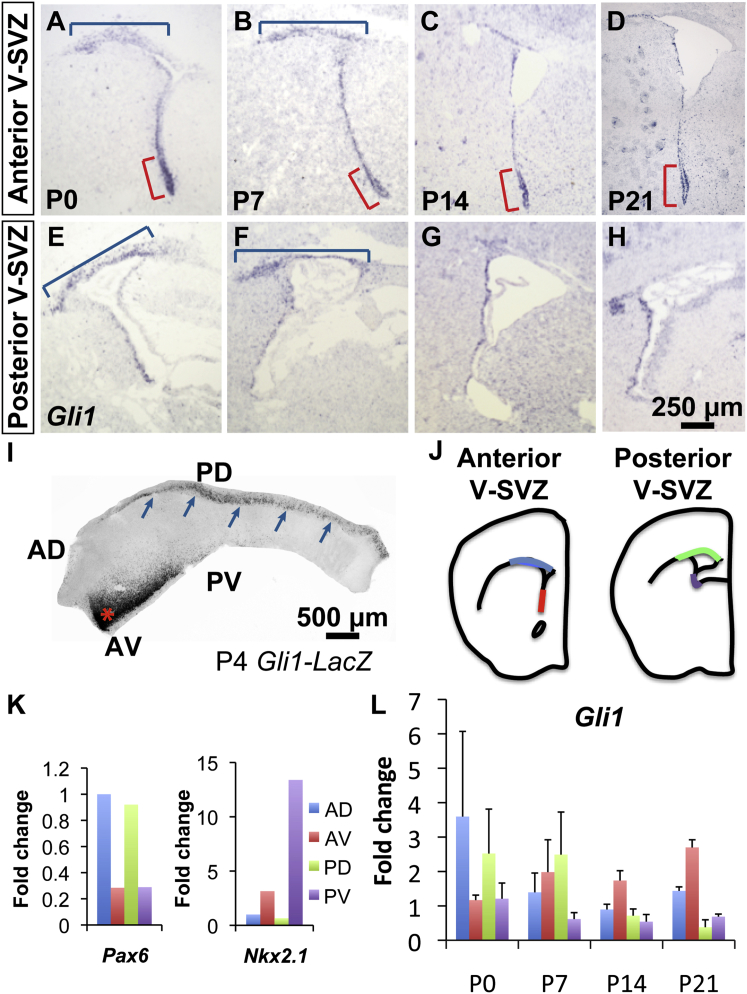
In the postnatal V-SVZ, SHH is important for the generation and proliferation of neural stem cells (Lai et al., 2003, Balordi and Fishell, 2007) and for the specification of regional identity (Ihrie et al., 2011, Merkle et al., 2014). Consistently, expression of Gli1 is higher in the ventral V-SVZ compared with the dorsal V-SVZ (Fuccillo et al., 2004, Palma et al., 2005, Ihrie et al., 2011, Bai et al., 2002, Petrova et al., 2013). However, these studies were performed mainly on adult brains. In situ hybridization (ISH) for Gli1 in mice at postnatal age (P)0, P7, P14, and P21 showed that Gli1 expression at P0 and P7 was enriched not only at the anterior ventral (AV) V-SVZ (Figures 1A–1H), but also dorsally in a region between the lateral ventricle and the CC (the subcallosal V-SVZ) (Figures 1A, 1B, 1E, and 1F). Expression of Gli1 in the subcallosal V-SVZ was reduced by P14 and mostly absent at P21 (Figures 1C, 1D, 1G, and 1H). In contrast, the ventral domain of high Gli1 expression was maintained into adult life (Ihrie et al., 2011).
Figure 1.
Gli1 Expression Is Transiently Enriched in the Subcallosal Region
(A–H) ISH shows high expression of Gli1 at the AV tip of the V-SVZ (red brackets). Low expression of Gli1 is observed throughout the lateral walls of the lateral ventricle: anterior (A–D) and posterior (E–H) views are shown. At P0 and P7 (A, B, E, and F), Gli1 expression is enriched in the subcallosal region (blue brackets); Gli1 expression is reduced at P14 and P21 (C, D, G, and H).
(I) V-SVZ whole mount from P4 Gli1-LacZ mouse showing strong X-gal staining in the AV (red asterisk) and subcallosal domains (blue arrows).
(J) Schematic showing the AD (blue), AV (red), PD (green), and PV (purple) regions of the V-SVZ that were microdissected at P0, P7, P14, and P21.
(K and L) RT-PCR data from three animals per age group. A representative RT-PCR result confirming that Pax6 is highly expressed in the dorsal regions whereas Nkx2.1 is highly enriched in the ventral regions is shown (K). Gli1 expression is enriched in both the dorsal regions and the AV V-SVZ at P0 and P7 but is decreased in the dorsal regions relative to the ventral regions at P14 and P21 (L).
The scale bar in (H) applies to (A)–(H) and represents 250 μm. The scale bar in (I) represents 500 μm.
V-SVZ whole mounts (Mirzadeh et al., 2010) dissected from Gli1::LacZ reporter mice (Bai et al., 2002) at P4 clearly showed the two domains of Gli1 expression (Figure 1I): in the AV region and at the dorsal edge of the whole mount corresponding to the subcallosal V-SVZ. qRT-PCR analysis of tissue dissected from the anterior dorsal (AD), AV, posterior dorsal (PD), and posterior ventral (PV) regions (Figure 1J) confirmed this temporal and spatial pattern of expression. Expression of the transcription factors Pax6 and Nkx2.1, which are highly expressed in the dorsal and ventral forebrain, respectively (Toresson et al., 2000, Xu et al., 2008), were used as controls for the microdissection (Figures 1J and 1K). At P0 and P7, elevated levels of Gli1 were observed in the subcallosal V-SVZ (Figure 1L). However, at P14 and P21, the highest expression of Gli1 was observed in the AV V-SVZ (Figure 1L). These results reveal a dorsal domain of elevated Gli1 expression, which appears transiently during early postnatal development.
Radial Glia in the Subcallosal V-SVZ Produce Neurons and Oligodendroglial Cells
To determine the types of cells derived from the Gli1-expressing subcallosal V-SVZ, we injected adenovirus-expressing Cre (Ad::Cre) next to the dorsal pia of neonatal Ai14 reporter mice. This results in permanent labeling of radial glial cells (RGCs) through their basal processes in the cortex (Figures 2A and 2B) (Merkle et al., 2007). Analysis of the brains at P28 showed that, in addition to neurons in the OB, many tdTomato+ cells were present in the CC. These tdTomato+ cells expressed oligodendroglial lineage markers: OLIG2 (Figures 2C–2F), sex determining region Y-box 10 (SOX10), and adenomatous polyposis coli (APC) (Figures 2G–2J). Some tdTomato+ cells in the CC also had processes that contained the mature oligodendrocyte marker myelin basic protein (MBP) (Figures 2K–2N); however, these cells were rare, and it was difficult to colocalize the tdTomato signal, which was stronger in the cell body, with MBP staining, which were mainly in the processes. This suggests that subcallosal V-SVZ RGCs give rise to oligodendroglial cells that migrate to the CC postnatally.
Figure 2.
Inactivation and Ectopic Activation of SMO at the Cortical V-SVZ Lead to Decreased and Increased Oligodendrogenesis in the CC, Respectively
(A and B) Neonatal Ai14 reporter mice were injected with Ad::Cre to infect RGCs in the cortical V-SVZ. In Ai14 mice, tdTomato is expressed upon CRE recombination (Madisen et al., 2010).
(C–N) At P28, tdTomato+ cells in the CC expressed the oligodendroglial markers OLIG2 (C–F; arrows), SOX10, APC (G–J; arrows), and MBP (K–N; arrowheads, tdTomato+ cell body; arrows, MBP+ process).
(O–Q) Data from four animals per group. Compared with their Smo+/+; Ai14 littermates, Smofl/fl; Ai14 mice have decreased tdTomato+ OLIG2+ (O), tdTomato+, SOX10+ (P), and tdTomato+ APC+ (Q) cells in the CC.
(R–T) Data from five animals per group. Ectopic activation of SmoM2 results in increased cells expressing oligodendroglial markers in the CC.
(U–W) Neurolucida tracings. Compared to tdTomato+ cells in Smo+/+; Ai14 mice (V) and Smofl/fl; Ai14 mice (U), SmoM2; Smo+/+; Ai14 mice have increased tdTomato+ cells in the CC (W).
Scale bars in (F), (J), and (N) apply to (C)–(N) and represent 20 μm. Scale bars in the large and small panels of (U), (V), and (W) represent 1 mm and 500 μm, respectively.
Oligodendrogenesis at the Subcallosal V-SVZ Is SHH Dependent
Since the subcallosal V-SVZ transiently expressed the SHH target gene Gli1 during early postnatal life (Figure 1), we next asked whether oligodendrogenesis in the subcallosal V-SVZ required SHH signaling. We injected Ad::Cre into Smofl/fl; Ai14 mice (Long et al., 2001) at P0, to infect the RGCs at their cortical V-SVZ (Figure 2A). This allowed the labeling of cells derived from these RGCs, by the conditional activation of the Ai14 reporter, and at the same time the conditional inactivation of Smoothened (Smo), which is essential for SHH signaling. Analysis of the brains at P28 showed more than 50% decrease in the tdTomato+ cells expressing OLIG2 (61.7%) (Figure 2O), SOX10 (59.5%) (Figure 2P), or APC (58.4%) (Figure 2Q) in the CC of the Smofl/fl; Ai14 mice (Figure 2U) compared with the Smo+/+; Ai14 controls (Figure 2V). The opposite result was obtained when we injected Ad::Cre into SmoM2; Smo+/+; Ai14 mice (Figure 2B), which expressed a constitutively active form of the SMO receptor upon CRE recombination (Jeong et al., 2004). An approximately 10-fold increase in the numbers of tdTomato+ OLIG2+ (Figure 2R), tdTomato+ SOX10+ (Figure 2S), and tdTomato+ APC+ (Figure 2T) cells was observed in the CC of SmoM2; Smo+/+; Ai14 mice (Figure 2W). These results indicate that oligodendrocyte production by subcallosal V-SVZ RGCs at P0 is regulated by SHH signaling; inactivation of Smo decreased, while constitutive activation of SMO (using SmoM2) increased the production of oligodendroglial cells.
Subcallosal V-SVZ Gli1-Expressing RGCs Produce Cells in the Oligodendrocyte Lineage
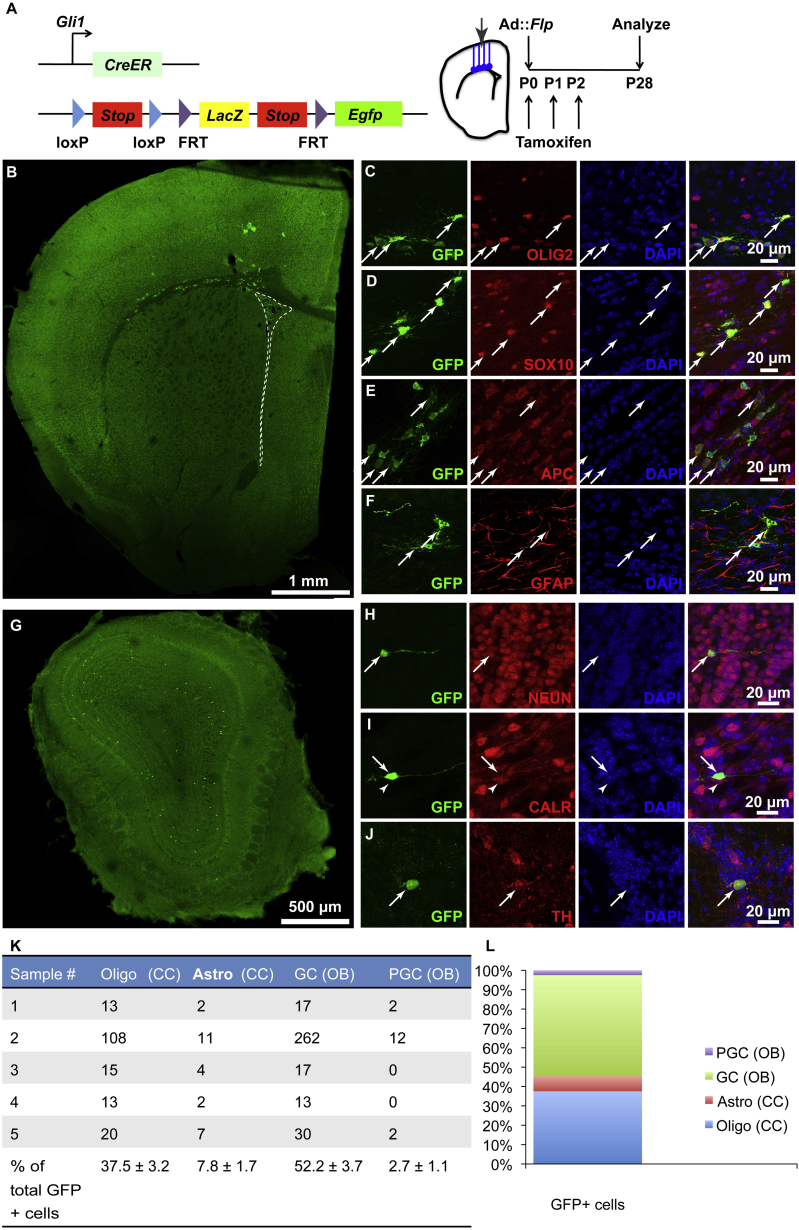
The above lineage tracing experiments show that large numbers of oligodendroglial cells are derived from subcallosal V-SVZ RGCs. To directly show that these oligodendrocytes are derived from those RGCs that express Gli1, we made use of LacZ/EGFP double reporter mice (Yamamoto et al., 2009), which express EGFP after dual CRE and FLP recombination (Figure 3A). By crossing these reporter mice with Gli1::CreER mice and administering tamoxifen to their offspring at P0–P2, we induced CRE recombination in the Gli1-expressing cells (Figure 3A). Then we stereotaxically injected adenovirus-expressing Flp (Ad::Flp) to target the RGCs at the cortical V-SVZ (Figure 3A). In this way, only the Gli1-expressing RGCs in the subcallosal V-SVZ and their progeny were labeled with EGFP.
Figure 3.
Gli1-Expressing RGCs at the Cortical V-SVZ Give Rise to Oligodendroglial Cells in the CC
(A) Mice carrying Gli1::CreER and the LacZ/EGFP double reporter were given tamoxifen from P0–P2 and were injected with Ad::Flp at P0 to infect dorsal RGCs.
(B) At P28, GFP+ cells in the CC.
(C–F) Confocal micrographs of GFP+ cells in the CC expressing OLIG2 (C), SOX10 (D), APC (E), and GFAP (F).
(G) GFP+ sGCs and PGCs in the OB.
(H–J) Confocal micrographs showing a GFP+ NEUN+ GC (H), a GFP+ CALR− GC (arrow in I), an adjacent GFP− CALR+ GC (arrowhead in I) and a GFP+ TH+ PGC (J).
(K) Table showing numbers of GFP+ cells in the CC identified as oligodendroglia [Oligo(CC)] and astrocytes [astro(CC)] and cells in the OB identified as GCs [GC(OB)] and PGCs [PGC(OB)]. The last row shows the number of each cell type as a percentage of the total number of GFP+ cells.
(L) Graph showing percentage of each cell type in the GFP+ cells.
Data in (K) and (L) are from five animals. Scale bars represent 1 mm (B), 500 μm (G), and 20 μm (C–F and H–J).
As with the single dorsal targeting methods, the intersectional (CRE-FLP) genetic labeling resulted in EGFP+ neurons in the OB (Figure 3G) and many labeled cells in the CC (Figure 3B) at P28. The majority of EGFP+ cells in the CC corresponded to cells in the oligodendroglial lineage (Figure 3B). These cells expressed OLIG2 (Figure 3C), SOX10 (Figure 3D), and APC (Figure 3E). Some of the EGFP+ cells in the CC were fibrous astrocytes that expressed the glial fibrillary acidic protein (GFAP) (Figure 3F). Consistent with previous findings that RGCs in the dorsal V-SVZ generate superficial granule cells (sGCs) (Merkle et al., 2007), the majority of EGFP+ neurons in the OB corresponded to sGCs (Figure 3G). These neurons expressed the pan-neuronal marker NEUN (Figure 3H) but did not express the calcium-binding protein calretinin (CALR) (Figure 3I). A small number of EGFP+ periglomerular cells (PGCs) expressed the dopaminergic neuronal marker tyrosine hydroxylase (TH) (Figure 3J). Of the EGFP+ cells, 37.5% ± 3.2% were oligodendroglial cells in the CC, 7.8% ± 1.7% were astrocytes in the CC, 52.2% ± 3.7% were OB GCs, and 2.7% ± 1.1% were PGCs (Figures 3K and 3L). A few EGFP+ cells were also present in the cortex (Figure 3B). These included SOX10+ oligodendroglial cells and protoplasmic astrocytes that were GFAP− (data not shown). However, it is not clear whether these cortical cells were the progeny of Ad::Flp-infected RGCs or were directly infected by Ad::Flp along the needle track. These results show that Gli1-expressing, dorsally projecting RGCs give rise to a subpopulation of sGCs, PGCs, many CC oligodendroglial cells and a subpopulation of CC fibrous astrocytes.
Ectopic Activation of SMO Increases Oligodendrogenesis at the Dorso-lateral V-SVZ
The above experiments demonstrate that subcallosal V-SVZ RGCs produce many CC oligodendroglial cells and that this process is SHH dependent. In contrast, the dorso-lateral wall facing the striatum normally gives rise to very few oligodendrocytes in the CC (Menn et al., 2006). We were therefore curious whether activation of SHH signaling in the dorso-lateral V-SVZ had any effect on the production of oligodendrocytes. We performed SmoM2 activation by injecting Ad::Cre laterally into SmoM2; Smo+/+; Ai14 mice (Figure 4A). This resulted in a dramatic (10- to 20-fold) increase in the production of tdTomato+ cells (Figures 4E and 4F) that expressed OLIG2 (Figure 4B), SOX10 (Figure 4C), and APC (Figure 4D) in the CC. These observations suggest that as in the subcallosal V-SVZ, ectopic SHH activity in the dorso-lateral V-SVZ promotes the generation of oligodendroglial cells that migrate into the CC.
Figure 4.
Activation of SMO in the Neonatal and Adult Dorso-lateral V-SVZ Results in Increased Oligodendroglial Cells in the CC
(A) Ad::Cre injected into the lateral striatum of neonatal pups infected RGCs at the dorso-lateral V-SVZ.
(B–D) At P28, the CC of SmoM2; Smo+/+; Ai14 mice have increased numbers of tdTomato+ cells that expressed OLIG2 (B), SOX10 (C), and APC (D).
(E and F) Compared with the Smo+/+; Ai14 controls (E), SmoM2; Smo+/+; Ai14 mice (F) have increased tdTomato+ cells in the CC; (Neurolucida tracings).
(G–J) Similarly, in the adult (P30) mice, activation of SmoM2 in the dorsal V-SVZ B1 cells results in increased tdTomato+ cells in the CC (G and I–J). Co-immunostaining shows higher number of tdTomato+ OLIG2+ cells in the SmoM2; Smo+/+; Ai14 mice (H–J). White arrows point to tdTomato+ OLIG2+ cells (I and J).
(K–R) Some tdTomato+ cells in the CC also expressed SOX10 and/or APC. White arrows indicate tdTomato+ SOX10+ cells. Blue arrows indicate tdTomato+ SOX10+ APC+ cells.
Data in (B–D) and (H) are from at least three animals per group. Scale bars in the large and small panels of (E) and (F) represent 1 mm and 500 μm, respectively. Scale bars in (I)–(R) represent 200 μm (I and J) and 200 μm (K–R).
SHH Activity Increases Oligodendrogenesis at the Dorsal V-SVZ in the Adult
Our ISH (Figures 1C, 1D, 1G, and 1H) and RT-PCR (Figure 1L) data indicate that Gli1 expression in the subcallosal V-SVZ diminished after the first postnatal week. If SHH activity were reinstated in the adult, would dorsal V-SVZ progenitor cells be stimulated to produce oligodendrocytes? We injected Ad::GFAPpCre into the dorsal V-SVZ of P30 SmoM2; Smo+/+; Ai14 mice (Figure 4G). Analysis at 1 month post-injection showed a significantly (41.2%) higher number of tdTomato+ OLIG2+ cells in the CC of SmoM2; Smo+/+; Ai14 mice compared with Smo+/+; Ai14 mice (Figures 4H–4J). We also observed more tdTomato+ cells that expressed SOX10 and APC in the SmoM2; Smo+/+; Ai14 mice (Figures 4K–4R). This result suggests that ectopic activation of SMO in the dorsal V-SVZ promotes oligodendrogenesis in the adult. This is consistent with a previous study showing increased OPCs in the adult mouse CC upon SHH delivery in the lateral ventricle (Loulier et al., 2006).
Discussion
We identified a dorsal V-SVZ domain that transiently expresses high levels of Gli1 during the first postnatal week in mice. We lineage traced Gli1-expressing RGCs in this dorsal domain and showed that a subpopulation of OB interneurons, CC astrocytes, and oligodendroglial cells are derived from this region. Genetic ablation of Smo in these dorsal V-SVZ RGCs resulted in a reduction in their production of oligodendroglial cells in the CC. In contrast, expression of constitutively active SmoM2 significantly increased the number of oligodendroglial cells produced. Moreover, this effect of SmoM2 was also observed in adult mice, when Gli1 expression was greatly diminished in the dorsal V-SVZ.
In the developing neural tube, levels of SHH ligand vary along the dorsoventral axis to create a morphogen gradient (Ahn and Joyner, 2004, Ribes and Briscoe, 2009), which results in graded levels of GLI. In the adult V-SVZ, we have previously reported that ablation of Shh decreases production of ventrally derived OB neuronal subtypes (Ihrie et al., 2011). In the present study, we identified a dorsal V-SVZ domain, where ectopic activation of the SHH pathway led not only to the respecification from superficial to deep interneurons (Ihrie et al., 2011), but also to a dramatic increase in oligodendrogenesis. In contrast, deletion of Smo had the opposite result (i.e., decreased oligodendrogenesis). Interestingly, dorsal-targeted inactivation of Smo also decreased production of dorsally derived neurons in the OB (data not shown). This observation indicates that SHH is not only controlling the types of neurons produced but also their numbers.
It is interesting that OB neurons produced from the transient Gli1-enriched subcallosal domain in juvenile mice mostly corresponded to sGCs, when they were derived from a region with active SHH signaling. It is possible that the relative levels of SHH signaling are adjusted in different domains. The intensity of Gli1 expression (Figure 1) suggests higher levels ventrally than dorsally, but further quantitative analysis of SHH signaling would be required to understand how neuronal cell types are specified. Location in the V-SVZ might be associated not only with differences in SHH signaling levels, but also other signaling pathways. Similarly, high SHH signaling in V-SVZ progenitors is not always associated with high oligodendrogenesis; the ventral V-SVZ has high Gli1 expression, but produces few oligodendrocytes postnatally (Kessaris et al., 2006, Menn et al., 2006).
These results reveal a dorsal domain in the juvenile telencephalon with high levels of SHH signaling, where many oligodendrocytes are produced. While our study provides evidence that this transient and restricted domain is an important source of OPCs, we cannot rule out that other regions within the Emx1+ dorsal forebrain (Kessaris et al., 2006) could also contribute to oligodendrogenesis. Together with other studies (Merkle et al., 2007, Merkle et al., 2014), this work illustrates the high level of regionalization in the postnatal V-SVZ. It will be interesting to determine whether the developing human brain contains a similar Gli1-expressing territory. The production of large numbers of OPCs from a similar subcallosal territory could help explain the developmental origin of the many oligodendrocytes required for the greatly expanded white matter in the human brain. Gliomas can originate from OPCs (Alcantara Llaguno et al., 2009, Jacques et al., 2010, Liu et al., 2011); the territory of heightened OPC production we describe here could also help better understand the origin of some brain tumors.
Experimental Procedures
Mice
All experiments were performed with adherence to the guidelines from the University of California, San Francisco Institutional Animal Care and Use Committee. Additional details are described in the Supplemental Experimental Procedures.
V-SVZ Whole-Mount Preparation
V-SVZ whole-mount dissection was carried out as previously described (Mirzadeh et al., 2010). X-gal staining was performed after 30-min fixation in 4% paraformaldehyde (PFA).
ISH
ISH was performed in 12-μm sections using standard procedures (Han et al., 2008, Ihrie et al., 2011) and antisense riboprobe from Gli1 cDNA (from A. Ruiz I Altaba, University of Geneva Medical School).
qRT-PCR
Different regions of the V-SVZ (Figure 1J) were microdissected from 300-μm vibratome sections of unfixed brains for RT-PCR analysis. Additional details are described in the Supplemental Experimental Procedures.
Immunohistochemistry
Fifty-μm tissue sections were incubated with primary antibodies (Abs) at 4°C overnight, followed by secondary Abs at 4°C overnight. Additional details are described in the Supplemental Experimental Procedures.
Virus Injections
Neonatal pups were injected with 20 nl adenovirus in a region-specific manner as previously described (Merkle et al., 2007). Adult (P30) mice were injected with 20 nl Ad::GFAPpCre as previously described (Merkle et al., 2007). Additional details are described in the Supplemental Experimental Procedures.
Author Contributions
C.K.T. and L.C.F. designed and performed the experiments and analyzed the data. J.K.S., C.D.G., and J.L.R.-R. performed the experiments. R.A.L. and R.A.I. designed the experiments. A.A.-B. designed the experiments and analyzed the data.
Acknowledgments
We are grateful to Dr. A. Ruiz I Altaba for the ISH probe for Gli1. This work is sponsored by grants from the NIH (HD032116 and NS028478) and a generous gift from the JG Bowes Foundation. A.A.-B. is the Heather and Melanie Muss Endowed Chair. L.C.F. is a HHMI fellow of the Helen Hay Whitney Foundation. R.A.L. is supported by the UCSF Medical Scientist Training Program, Neuroscience Graduate Program, and Discovery Fellows Program.
Published: September 24, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.08.013.
Supplemental Information
References
- Ahn S., Joyner A.L. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. doi: 10.1016/j.cell.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Alcantara Llaguno S., Chen J., Kwon C.H., Jackson E.L., Li Y., Burns D.K., Alvarez-Buylla A., Parada L.F. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15:45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Kohwi M., Nguyen T.M., Merkle F.T. The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harb. Symp. Quant. Biol. 2008;73:357–365. doi: 10.1101/sqb.2008.73.019. [DOI] [PubMed] [Google Scholar]
- Bai C.B., Auerbach W., Lee J.S., Stephen D., Joyner A.L. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- Balordi F., Fishell G. Mosaic removal of hedgehog signaling in the adult SVZ reveals that the residual wild-type stem cells have a limited capacity for self-renewal. J. Neurosci. 2007;27:14248–14259. doi: 10.1523/JNEUROSCI.4531-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuccillo M., Rallu M., McMahon A.P., Fishell G. Temporal requirement for hedgehog signaling in ventral telencephalic patterning. Development. 2004;131:5031–5040. doi: 10.1242/dev.01349. [DOI] [PubMed] [Google Scholar]
- Fuentealba L.C., Rompani S.B., Parraguez J.I., Obernier K., Romero R., Cepko C.L., Alvarez-Buylla A. Embryonic origin of postnatal neural stem cells. Cell. 2015;161:1644–1655. doi: 10.1016/j.cell.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J.A., Talley T., Qiu M., Puelles L., Rubenstein J.L., Jones K.R. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.G., Spassky N., Romaguera-Ros M., Garcia-Verdugo J.M., Aguilar A., Schneider-Maunoury S., Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat. Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- He W., Ingraham C., Rising L., Goderie S., Temple S. Multipotent stem cells from the mouse basal forebrain contribute GABAergic neurons and oligodendrocytes to the cerebral cortex during embryogenesis. J. Neurosci. 2001;21:8854–8862. doi: 10.1523/JNEUROSCI.21-22-08854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrie R.A., Shah J.K., Harwell C.C., Levine J.H., Guinto C.D., Lezameta M., Kriegstein A.R., Alvarez-Buylla A. Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron. 2011;71:250–262. doi: 10.1016/j.neuron.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques T.S., Swales A., Brzozowski M.J., Henriquez N.V., Linehan J.M., Mirzadeh Z., O’ Malley C., Naumann H., Alvarez-Buylla A., Brandner S. Combinations of genetic mutations in the adult neural stem cell compartment determine brain tumour phenotypes. EMBO J. 2010;29:222–235. doi: 10.1038/emboj.2009.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J., Mao J., Tenzen T., Kottmann A.H., McMahon A.P. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N., Fogarty M., Iannarelli P., Grist M., Wegner M., Richardson W.D. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K., Kaspar B.K., Gage F.H., Schaffer D.V. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- Levison S.W., Goldman J.E. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Liu C., Sage J.C., Miller M.R., Verhaak R.G., Hippenmeyer S., Vogel H., Foreman O., Bronson R.T., Nishiyama A., Luo L., Zong H. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146:209–221. doi: 10.1016/j.cell.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long F., Zhang X.M., Karp S., Yang Y., McMahon A.P. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- Loulier K., Ruat M., Traiffort E. Increase of proliferating oligodendroglial progenitors in the adult mouse brain upon Sonic hedgehog delivery in the lateral ventricle. J. Neurochem. 2006;98:530–542. doi: 10.1111/j.1471-4159.2006.03896.x. [DOI] [PubMed] [Google Scholar]
- Luskin M.B., McDermott K. Divergent lineages for oligodendrocytes and astrocytes originating in the neonatal forebrain subventricular zone. Glia. 1994;11:211–226. doi: 10.1002/glia.440110302. [DOI] [PubMed] [Google Scholar]
- Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C.A., Goldman J.E. Subpallial dlx2-expressing cells give rise to astrocytes and oligodendrocytes in the cerebral cortex and white matter. J. Neurosci. 2002;22:9821–9830. doi: 10.1523/JNEUROSCI.22-22-09821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B., García-Verdugo J.M., Yaschine C., Gonzalez-Perez O., Rowitch D., Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle F.T., Mirzadeh Z., Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Merkle F.T., Fuentealba L.C., Sanders T.A., Magno L., Kessaris N., Alvarez-Buylla A. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat. Neurosci. 2014;17:207–214. doi: 10.1038/nn.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z., Doetsch F., Sawamoto K., Wichterle H., Alvarez-Buylla A. The subventricular zone en-face: wholemount staining and ependymal flow. J. Vis. Exp. 2010;39:1938. doi: 10.3791/1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma V., Lim D.A., Dahmane N., Sánchez P., Brionne T.C., Herzberg C.D., Gitton Y., Carleton A., Alvarez-Buylla A., Ruiz i Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova R., Garcia A.D., Joyner A.L. Titration of GLI3 repressor activity by sonic hedgehog signaling is critical for maintaining multiple adult neural stem cell and astrocyte functions. J. Neurosci. 2013;33:17490–17505. doi: 10.1523/JNEUROSCI.2042-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes V., Briscoe J. Establishing and interpreting graded Sonic Hedgehog signaling during vertebrate neural tube patterning: the role of negative feedback. Cold Spring Harb. Perspect. Biol. 2009;1:a002014. doi: 10.1101/cshperspect.a002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N., Heydon K., Mangatal A., Jankovski A., Olivier C., Queraud-Lesaux F., Goujet-Zalc C., Thomas J.L., Zalc B. Sonic hedgehog-dependent emergence of oligodendrocytes in the telencephalon: evidence for a source of oligodendrocytes in the olfactory bulb that is independent of PDGFRalpha signaling. Development. 2001;128:4993–5004. doi: 10.1242/dev.128.24.4993. [DOI] [PubMed] [Google Scholar]
- Tekki-Kessaris N., Woodruff R., Hall A.C., Gaffield W., Kimura S., Stiles C.D., Rowitch D.H., Richardson W.D. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development. 2001;128:2545–2554. doi: 10.1242/dev.128.13.2545. [DOI] [PubMed] [Google Scholar]
- Toresson H., Potter S.S., Campbell K. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development. 2000;127:4361–4371. doi: 10.1242/dev.127.20.4361. [DOI] [PubMed] [Google Scholar]
- Xu Q., Tam M., Anderson S.A. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J. Comp. Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Shook N.A., Kanisicak O., Yamamoto S., Wosczyna M.N., Camp J.R., Goldhamer D.J. A multifunctional reporter mouse line for Cre- and FLP-dependent lineage analysis. Genesis. 2009;47:107–114. doi: 10.1002/dvg.20474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.