Summary
We demonstrate that the pluripotency gene OCT4 has a role in regulating differentiation via Wnt signaling. OCT4 expression levels in human embryonic stem cells increases transiently during the first 24 hr of in vitro differentiation, with OCT4 occupancy increasing at endoderm regulators such as SOX17 and FOXA2. This increased occupancy correlates with loss of the PRC2 complex and the inhibitory histone mark H3K27me3. Knockdown of OCT4 during differentiation inhibits mesendoderm formation and removal of the H3K27me3 mark from the SOX17 promoter, suggesting that OCT4 acts to induce removal of the PRC2 complex. Furthermore, OCT4 and β-catenin can be co-immunoprecipitated upon differentiation, and Wnt stimulation is required for the enhanced OCT4 occupancy and loss of the PRC2 complex from the SOX17 promoter. In conclusion, our study reveals that OCT4, a master regulator of pluripotency, may also collaborate with Wnt signaling to drive endoderm induction by pre-patterning epigenetic markers on endodermal promoters.
Highlights
-
•
OCT4 occupancy increases at endoderm genes early in ES cell differentiation
-
•
The PRC2 complex is lost from OCT4 sites on endoderm genes during differentiation
-
•
OCT4 associates with β-catenin during ES cell differentiation
-
•
OCT4 and Wnt are both required for mesendoderm induction
In this article, Gadue and colleagues show that OCT4 in collaboration with WNT signaling acts to modulate epigenetic marks at definitive endoderm genes as little as 24 hr after differentiation initiation, priming them for eventual expression. This study demonstrates that a well-described regulator of pluripotency, OCT4, can also help direct downstream differentiation.
Introduction
In mammals, cells from the blastocyst stage can be used to form embryonic stem (ES) cell lines that can self-renew in culture while maintaining the ability to differentiate into cells of the three germ layers (Reubinoff et al., 2000). Directed in vitro differentiation protocols generally attempt to utilize our knowledge of the normal signaling pathways guiding embryogenesis and mimic this process in vitro, theoretically providing an unlimited supply of any desired cell type (Murry and Keller, 2008). The molecular mechanisms underlying pluripotent stem cell differentiation are of great interest for understanding development, disease, and regenerative medicine.
A growing body of evidence underscores the importance of pluripotency factors during differentiation. Human and mouse ES cell studies have demonstrated that the core pluripotency transcription factors, SOX2, OCT4, and NANOG, play distinct roles in coordinating ES cell lineage commitment (Lu et al., 2009, Thomson et al., 2011, Wang et al., 2012). NANOG promotes definitive endoderm (DE) formation by coordinating with the activation of the TGF-β signaling pathway through the induction of EOMES (Teo et al., 2011). SOX2 is important in ectoderm formation by limiting mesendoderm formation (Wang et al., 2012).
The role of OCT4 in lineage commitment is somewhat less clear. In zebrafish, the OCT4 homolog spg is required for endoderm induction (Lunde et al., 2004). The role of OCT4 during human ES (hES) cell differentiation and lineage commitment is controversial because knockdown studies performed by different labs have resulted in the induction of trophectoderm, primitive endoderm, or neuroectoderm cell fates (Niwa et al., 2000, Wang et al., 2012). Another study suggested that OCT4 knockdown induced DE formation, even though gene expression was low (Teo et al., 2011). These studies also demonstrated a critical role for OCT4 in maintaining the undifferentiated state.
Several studies have demonstrated that OCT4 may play a role in regulating the epigenetic landscape in ES cells and during differentiation. Pull-down assays indicate that major Oct4 partners in mouse ES cells are related to chromatin remodeling (Pardo et al., 2010, van den Berg et al., 2010). Bernstein et al. (2006) defined a specific chromatin modification pattern called the “bivalent domain,” which harbors both the inhibitory, H3K27me3, and activating, H3K4me3, histone modifications at genes important in regulating early development in ES cells. Genes present in bivalent domains are typically silent in ES cells, but are poised for activation. Genome-wide studies have shown that OCT4 co-localizes with Polycomb2 (PRC2) components, which are responsible for laying down the inhibitory H3K27me3 mark and generating bivalent domains in hES cells (Boyer et al., 2005, Boyer et al., 2006). Based on these findings, OCT4 may play an important role in chromatin remodeling during differentiation in response to external signals.
The Wnt signaling pathway is important for both maintaining pluripotency (Sokol, 2011, Wang and Wynshaw-Boris, 2004) and inducing differentiation to primitive streak and mesendoderm (Cheng et al., 2008, Gadue et al., 2006, Lyashenko et al., 2011). In mouse ES cells, Oct4 has been shown to play a role in the Wnt signaling pathway by physically interacting with β-catenin to reinforce pluripotency (Kelly et al., 2011).
In this study, we define a role for OCT4 and Wnt signaling in establishing an appropriate chromatin signature during hES cell specification into endoderm. By utilizing a directed differentiation approach coupled with siRNA knockdown of OCT4, we show that OCT4 and Wnt signaling play a role in the chromatin pre-patterning of endoderm genes such as SOX17 and FOXA2. During mesendoderm commitment, OCT4 physically associates with β-catenin while the knockdown of OCT4 eliminates the mesendoderm differentiation capacity of hES cells.
OCT4 knockdown also led to a failure to remove the PRC2 complex from primitive streak and endodermal genes. In the absence of Wnt pathway activation during endoderm induction, hES cells maintain high levels of OCT4 protein but fail to evict the PRC2 complex and downregulate H3K27me3 on primitive streak and endodermal genes. In summary, OCT4 plays a key role in the pluripotency core network and is indispensable for lineage commitment by coordinating with WNT signaling to target bivalent genes for activation. These data underscore the importance of pluripotent transcription factors in differentiation as well as for maintenance of the pluripotent state.
Results
Dynamic Changes of Pluripotency and Primitive Streak Genes during DE Differentiation of hES Cells
H9 hES cells were differentiated toward DE using a previously described protocol (Nostro et al., 2008) that utilizes high levels of activin A and Wnt pathway activation with the small molecule CHIR99021 (CHIR) (Figure 1A). Confirming previous findings (Teo et al., 2011), we see that the level of two pluripotency genes, OCT4 and NANOG, is transiently increased at day 1 of differentiation before subsequently declining (Figures S1A and S1B). In contrast, SOX2 is progressively lost through all days of the differentiation protocol (Figures S1A and S1B). Primitive streak genes such as T, GSC, and EOMES were quickly induced by D1 of differentiation (Figure S1B). By days 2 and 3 of differentiation, DE genes, such as FOXA1, FOXA2, and SOX17 were expressed which represents commitment to DE (Figures 1B and S1B).
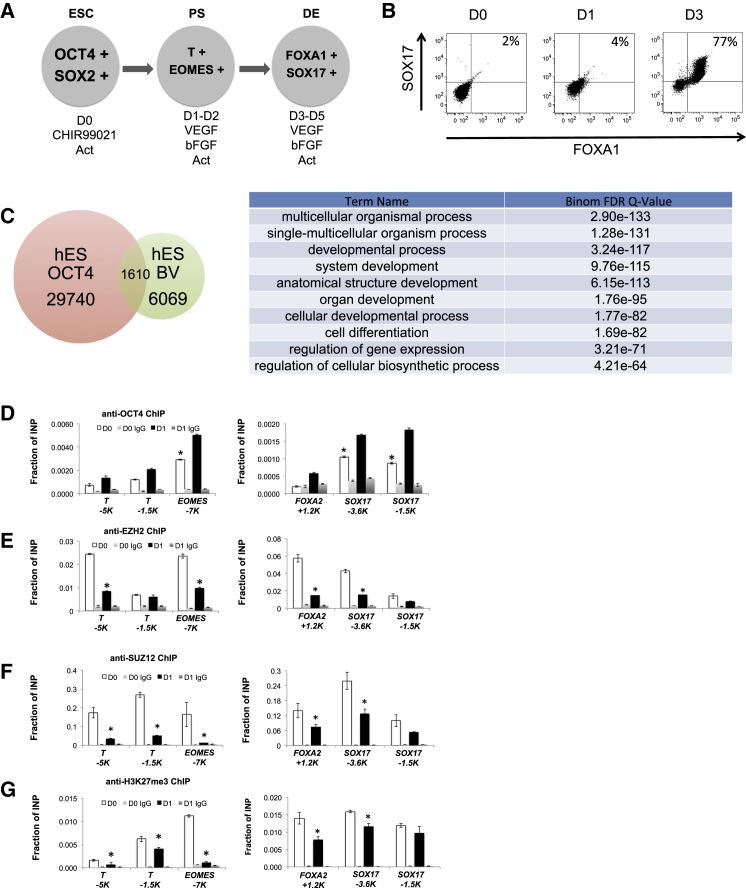
Figure 1.
Efficient Differentiation of hES Cells into DE
(A) Scheme of endodermal differentiation.
(B) Flow cytometry analysis of hES cells and D0, D1, and D3 of endoderm differentiation cultures with anti-SOX17 and anti-FOXA1 antibodies.
(C) (Left) Venn diagram of OCT4 and bivalent domain occupancy in hES cells. (Right) GO terms from the gene set overlapping OCT4 and bivalent domains.
(D) Anti-OCT4.
(E) Anti-EZH2.
(F) Anti-SUZ12.
(G) Anti-H3K27me3 ChIP on H9 D0 and D1 cells. Sites used for ChIP-qPCR are indicated in kilobases (K) from the transcription start site. The IgG control for each ChIP is also shown. Data shown are an average from three independent experiments. Statistical significance indicated as ∗p < 0.05. Activin A, Act; input, INP.
To study the mechanism of OCT4 involvement during endoderm induction, we took advantage of existing genome-wide ChIP-seq (Guenther et al., 2010, Zhao et al., 2007) and transcription profile datasets (Cheng et al., 2012) to analyze the co-localization of OCT4 binding and histone modification sites. The analysis of previously published data revealed that >25% of defined “bivalent domains” co-localize with OCT4 binding sites (Zhao et al., 2007) (Figure 1C). Gene Ontology (GO) analysis of these sites showed an enrichment of terms related to development and differentiation (Figure 1C). We hypothesized that OCT4 may play a role in the regulation of these bivalent domains to affect early differentiation. ChIP analyses were performed on ES cell cultures at D0 and D1 of differentiation, a time point when early primitive streak markers are induced but the majority of endodermal genes are not yet expressed. The ChIP primers were designed to detect OCT4 binding sites within bivalent domains of primitive streak (T and EOMES) and DE genes (FOXA2 and SOX17). OCT4 binding increased on both primitive streak and endoderm gene sites at D1 of differentiation (Figure 1D). We examined occupancy of EZH2 and SUZ12, two components of the PRC2 complex, and the H3K27me3 inhibitory histone mark at these same OCT4 binding sites. On the primitive streak gene loci, the binding of EZH2, SUZ12, and the H3K27me3 mark decreased at day 1 of differentiation (Figures 1E–1G), correlating well with the induction of these genes as shown in Figure S1B. We found similar decreases in EZH2 and SUZ12 occupancy and the H3K27me3 mark on DE genes at D1 of differentiation, prior to SOX17 gene expression (Figures 1E–1G). We also examined a second WT iPS cell line, CHOPWT2.1, for the H3K27me3 mark and see similar results (Figure S1C). The expression of EZH2 and SUZ12 does not significantly decline at D1 of differentiation at the mRNA or protein level (Figure S1E), and SUZ12 and H3K27me3 were not depleted on neuroectodermal genes at D1, such as PAX6 and OLIGO1 (Figure S1D), suggesting that the PRC2 complex is not globally decreased at this time point. These data support the idea that OCT4 may play a role in evicting the PRC2 complex specifically from mesendodermal and definitive endodermal genes, priming them for expression.
OCT4 Interacts with β-Catenin to Pre-pattern the Epigenetic Profile of hES Cells during DE Differentiation
Wnt signaling has a well-established role in primitive streak and mesendoderm formation in the mammalian embryo and in ES cell differentiation cultures (Gadue et al., 2005). Therefore, we examined this signaling pathway and its interactions with OCT4 during endoderm induction in the hES cell differentiation system. Activated β-catenin (ABC) increased in response to Wnt signaling induced by the GSK3 inhibitor, CHIR, during ES cell differentiation (Kunisada et al., 2012) (Figure S1A), and β-catenin was shown to translocate from the cytoplasm to the nucleus (Figure 2A).
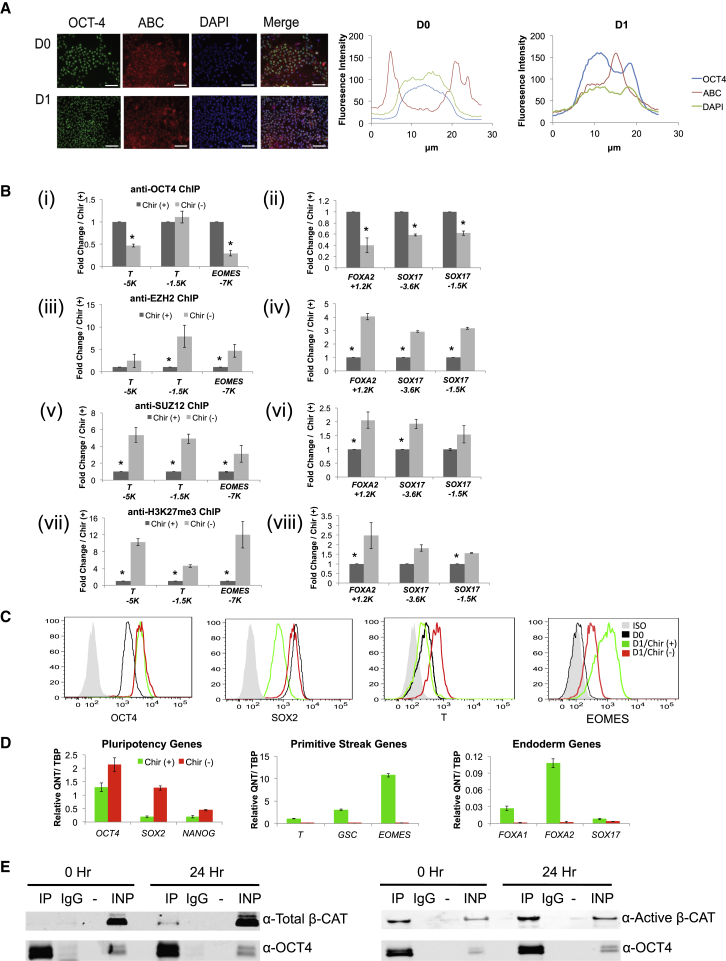
Figure 2.
WNT Pathway Activation Is Required for Primitive Streak Commitment and Endoderm Differentiation
(A) Immunofluorescence staining of anti-OCT4 (green) and anti-ABC (red) on D0 and D1 differentiation cultures performed as described in Figure 1, counterstained with DAPI (blue) for nuclei. Distribution of fluorescence intensity across a single representative cells was measured and plotted, demonstrating nuclear localization of ABC at D1. Scale bar represents for 100 μm.
(B) ChIP-qPCR analysis comparing D1 differentiation cultures with and without the Wnt agonist CHIR99021 (Chir) on primitive streak genes (T and EOMES) and endodermal genes (FOXA2 and SOX17). (i) and (ii) anti-OCT4, (iii) and (iv) anti-EZH2, (v) and (vi) anti-SUZ12, (vii) and (viii) anti-H3K27me3. Sites used for ChIP-qPCR are indicated in kilobases (K) from the transcription start site. Data shown are an average from three independent experiments. Statistical significance represented as ∗p < 0.05.
(C) Flow cytometry analysis on D0 and D1 differentiation cultures with or without Chir addition, examining SOX2, OCT4, EOMES, and T expression.
(D) QRT-PCR analysis of D1 differentiation cultures with or without Chir addition.
(E) Immunoprecipitation of nuclear extracts from hES cells and D1 differentiation cultures with anti-OCT4 antibody and blotted with (left) anti-β-CATENIN and anti-OCT4 antibodies or (right) anti-active β-Catenin and anti-OCT4 antibodies; 1% of total lysate was run in the input lanes. Immunoprecipitation, IP; skipped lane, −; input, INP.
To determine whether β-catenin activity is important for epigenetic changes during differentiation, we performed ChIP assays on hES cells at D1 of differentiation in the absence or presence of CHIR. Without CHIR, OCT4 recruitment to primitive streak and DE genes (Figure 2B, i and ii) is impaired. The decreased OCT4 binding on primitive streak and DE genes correlates with increased occupancy of EZH2 (Figure 2B, iii and iv), SUZ12 (Figure 2B, v and vi) and H3K27me3 (Figure 2B, vii and viii) on these sites in the absence of CHIR without a global change of EZH2 or SUZ12 levels (Figure S1F, right). This increased occupancy is also seen on another primitive streak gene, GSC (Figure S1F).
While it is well established that transient Wnt induction is important for mesendoderm differentiation from hES cells, we examined the impact on OCT4 expression in the initial 24 hr of differentiation. In the absence of CHIR, we still find upregulation of OCT4 at the protein (Figure 2C) and RNA (Figure 2D) levels. In contrast, SOX2 is not downregulated, and primitive streak genes are poorly upregulated in the absence of CHIR (Figures 2C and 2D). While not detectable at the protein level at D1 of differentiation (Figure 1B), FOXA1 and FOXA2 mRNAs were also not upregulated in the absence of Wnt activation by CHIR (Figure 2D). Similar results were obtained with an additional human iPS cell line (Figures S1G and S1H). These data suggest that Wnt signaling is critical for mesendoderm gene induction but does not impact OCT4 expression at this early stage of differentiation.
We hypothesized that Wnt signaling components may be interacting with OCT4 to promote mesendoderm induction. Using nuclear extracts, we found an enhanced physical interaction between OCT4 and β-catenin by co-immunoprecipitation analysis at D1 of differentiation using antibodies for either total or activated β-catenin (Figure 2E). These data suggest that β-catenin and OCT4 may act in concert to regulate the removal of the PRC2 complex on primitive streak and endoderm genes, priming them for expression.
OCT4 Is Indispensable for Primitive Streak Commitment and DE Differentiation
To examine the role of OCT4 during endoderm differentiation, we transiently knocked down OCT4 with siRNA in hES cells or human iPS cells prior to the start of differentiation. An OCT4 knockdown time course showed that though OCT4 mRNA level decreased 4 hr after transfection (Figure S2A, i), 18 hr was required for maximal effect at the protein level (Figure S2, ii). Therefore, OCT4 knockdown was performed 18 hr prior to differentiation in subsequent experiments. While the siRNA treatment led to a dramatic decrease in OCT4 protein (Figures 3A and S3A, i) and RNA levels (Figure S2A, i), other pluripotent markers such as NANOG and SOX2 displayed only mildly decreased expression at the protein level in the H9 hES cell line (Figure S2A, iii and iv) and remained unchanged in the human iPS cells line, CHOPWT2.1 (Figure S3A, ii and iii). OCT4 knockdown cells also retained other ES cell markers such as Tra-1-81 and SSEA3 (Figure S2A, v). Consistent with a previous study (Teo et al., 2011), the expression of primitive streak and DE markers were also detected 72 hr after OCT4 siRNA transfection in ES cell culture media, but the levels of expression for these genes were extremely low compared with the expression levels of these genes during the differentiation to mesendoderm in D1 cells (Figure S2B, ii) and DE in D3 cells (Figure S2B, iii). These data suggest that the loss of OCT4 without differentiation induction may cause transcriptional de-repression of lineage-specific genes as opposed to rapid activation to physiologically relevant levels. When OCT4 siRNA-transfected hESCs were subjected to DE differentiation, primitive streak genes, such as T, EOMES, and GSC, were not fully activated (Figures 3A, 3B, and S2C). DE genes, such as CXCR4, CD117, FOXA1, FOXA2, and SOX17, were also poorly induced (Figures 3C, S2A, vi, S2C, and S3B, i, ii, and iii). These findings were confirmed using a second OCT4 siRNA (Figure S2D). These data indicate that OCT4 expression is necessary for primitive streak and endoderm induction during hES cell and iPS cell differentiation.
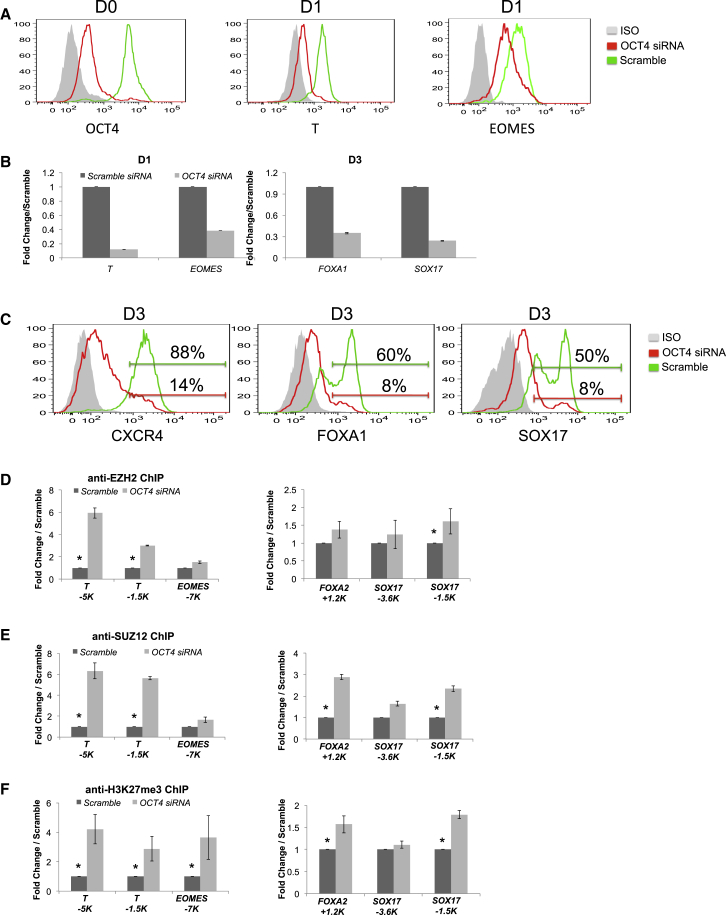
Figure 3.
OCT4 Knockdown Eliminates Endoderm Differentiation
HES cells were transfected with siRNA against OCT4 or a control scramble siRNA construct 18 hr prior to initiation of endoderm differentiation as described in Figure 1.
(A) Flow cytometry analysis of OCT4 protein levels at D0, and T and EOMES levels at D1 of endoderm differentiation.
(B) QRT-PCR gene expression analysis of T and EOMES at D1 and FOXA1 and SOX17 at D3 of differentiation. Data shown are an average three independent experiments. Statistical significance represented as ∗p < 0.05.
(C) Flow cytometry analysis of CXCR4, FOXA1, and SOX17 at D3 of endoderm differentiation.
(D–F) ChIP-qPCR analysis comparing D1 differentiation cultures treated with OCT4 or scramble siRNA on primitive streak genes (T and EOMES) and endodermal genes (FOXA2 and SOX17). (D) Anti-EZH2. (E) Anti-SUZ12. (F) Anti-H3K27me3. Sites used for ChIP-qPCR are indicated in kilobases (K) from the transcription start site. Data shown are an average from three independent experiments. Statistical significance represented as ∗p < 0.05.
OCT4 Regulates PRC2 Complex Recruitment during Mesendoderm Commitment
To determine whether OCT4 was necessary for the change in PRC2 complex occupancy of endodermal genes following differentiation, the H9 hES cell line or human iPS cell line CHOPWT2.1 was treated with OCT4 siRNA and examined at D1 of endoderm differentiation by ChIP. In the absence of OCT4, the occupancy of EZH2 and SUZ12 on primitive streak genes was increased (Figures 3D, 3E, S2E, and S3C, i) without global changes in EZH2 or SUZ12 protein levels (Figure S2F). Importantly, EZH2 and SUZ12 were significantly increased on at least one site on the FOXA2 and SOX17 loci (Figures 3D and 3E, and S3Ci), which correlated with higher levels of the H3K27me3 mark (Figures 3F and S3C, ii) and loss of FOXA1 and SOX17 gene expression at D3 of differentiation (Figures 3B, 3C, and S3B, ii and iii). These findings suggest that OCT4 is critical for PRC2 complex-mediated epigenetic pre-patterning of the master endoderm regulator SOX17 prior to gene expression.
Discussion
The pluripotency status of ES cells or iPS cells is maintained by a tightly regulated transcription factor network (Boyer et al., 2005). OCT4 is at the center of this network (Pardo et al., 2010, van den Berg et al., 2010) and is associated with chromatin modifiers to maintain epigenetic identity of pluripotent cells (Liang et al., 2008, Zhao et al., 2007). Its expression level is strictly controlled in pluripotent cells in which changes in protein level, either higher or lower, can lead to lineage-specific differentiation (Niwa et al., 2000, Wang et al., 2012).
Wnt signaling is well known as an inducer of primitive streak formation (Gadue et al., 2006) and an indispensable component for efficient endoderm differentiation (Han et al., 2011). Endogenous WNT3 expression in ES cells can predict endoderm differentiation efficiency, highlighting the importance of this pathway in endoderm induction (Jiang et al., 2013). While Wnt can directly activate primitive streak genes such as T (Gadue et al., 2006), we demonstrate a secondary effect that this pathway has on regulating OCT4 function. Activation of β-catenin was critical for increased OCT4 binding to target genes and presumably regulates the transcription status of these genes during activin A-induced differentiation. Without Wnt signaling, mesendoderm genes (T, EOMES, and GSC) and DE genes (FOXA2 and SOX17) maintain the repressive PRC2 complex and the H3K27me3 mark. These findings are supported by recent work in the mouse ES cell system where it was demonstrated that a composite Oct4-Tcf/Lef site was critical for Mesp1 induction and cardiomyocyte differentiation (Li et al., 2013). Based on our observations, we propose a preliminary working model of how OCT4 cooperates with β-catenin to evict the PRC2 complex and remove the repressive histone mark at differentiation initiation (Figure S3D).
Two recent studies have reported opposite conclusions on the effect of OCT4 knockdown in hES cell differentiation. Teo et al. (2011) demonstrated that OCT4 knockdown led to precocious upregulation of endodermal genes, while Wang et al. (2012) reported that OCT4 knockdown inhibits mesendoderm differentiation. Our data support the findings from both sides and shed light on the mechanism of these seemingly contradictory results. Like Teo et al., we also find endodermal gene expression upon OCT4 knockdown. However, the level of endodermal and primitive streak gene induction, while increased over that seen in ES cells, was orders of magnitude less than expression of these genes during normal differentiation (Figure S2B, ii, iii). In both hES cells and iPS cells, when OCT4 knockdown cells were subjected to DE differentiation, expression of primitive streak and DE genes was drastically downregulated compared with scramble siRNA transfected cells, which supported the findings of Wang et al. (2012). These seemingly contradictory phenotypes of OCT4 knockdown may underlie multiple roles for OCT4 in ES cell maintenance and differentiation. OCT4 is well known to repress differentiation genes (Boyer et al., 2005) such that lack of its expression leads to de-repression of these genes but at low levels without other differentiation induction signals. The second role of OCT4 that we uncovered is its involvement in the removal of PRC2 complex from mesendodermal genes and its requirement for the physiologic expression of these genes during directed differentiation of hES cells.
Recent studies examining the role of Oct4 showed that Oct4 is critical in lineage specification in the mouse, especially for the endodermal program and supports our results. Oct4+/− ES cells and mouse iPS cells expressing low levels of Oct4 can be maintained in the undifferentiated state and express increased levels of many pluripotency markers (Karwacki-Neisius et al., 2013, Radzisheuskaya et al., 2013). Both pluripotent stem cell lines also displayed an inhibition of differentiation into embryonic lineages. In addition, mouse blastocysts that lack Oct4 fail to upregulate primitive endoderm genes in a cell autonomous manner. Our results show that OCT4 is not only critical for mesendoderm gene activation, but also crucial for DE gene expression by pre-patterning epigenetic marks prior to gene expression.
In summary, we demonstrated that OCT4 is critical for mesendoderm differentiation by showing its involvement in eviction of the PRC2 complex and loss of the H3K27me3 mark from mesendodermal genes. The effect on SOX17 is most revealing as it demonstrates pre-patterning of this gene prior to gene expression that may be critical for eventual endoderm germ layer formation. This effect requires Wnt signaling, giving a mechanism for how an external stimulus can allow a pluripotency factor, OCT4, to behave as a differentiation factor. This study highlights the multiple roles that pluripotency genes play in maintaining the ES cell state but also in directing subsequent differentiation.
Experimental Procedures
Cell Culture and Differentiation
The H9 hES cell line was obtained from the Wicell Research Institute. The CHOPWT2.1 iPS cell line was described previously (Mills et al., 2013). hES and iPS cell culture and differentiation followed previous paper (Cheng et al., 2012, Nostro et al., 2008). hES cells and human iPS cells were cultured in DMEM/F12 supplemented with 15% knockout serum replacement (KSR) and 10 ng/ml basic fibroblast growth factor (bFGF) on mouse embryonic feeders (MEFs). hES cells and iPS cells were replated onto matrigel-coated surface to perform monolayer differentiation. Briefly, the GSK3 inhibitor CHIR99021 and activin A were added at day 0 (D0) to induce differentiation initiation. Then medium containing 0.25 ng/ml BMP4, 5 ng/ml bFGF, 10 ng/ml vascular endothelial growth factor (VEGF), and 100 ng/ml activin A was added to induce DE.
siRNA Knockdown
OCT4 and control scramble siRNAs were obtained from Integrated DNA Technologies. Sequences of siRNAs are listed in Table S1. H9 hES cells were replated onto matrigel-coated plate 24 hr before transfection. When H9 hES cell culture reached 70% confluent, siRNA transfection was carried out with Lipofectamine RNAiMAX (Invitrogen). Transfected cells were either cultured in hES media or DE differentiation culture conditions 18 hr after transfection.
ChIP-qPCR, Flow Cytometry, Immunofluorescence, Statistics, Western Blot, and Immunoprecipitation
Detailed procedures are listed in Supplement Experimental Procedures.
Author Contributions
L.Y. collected and/or assembled data, performed data analysis and interpretation, and wrote the manuscript. J.A.M. collected and/or assembled data and performed data analysis and interpretation. D.L.F. performed data analysis and interpretation and wrote the manuscript. P.G. performed data analysis and interpretation, wrote the manuscript, and gave final approval of the manuscript.
Acknowledgments
This work was supported by NIH grant R01 DK092113.
Published: September 24, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, three figures, and three tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.08.014.
Supplemental Information
References
- Bernstein B.E., Mikkelsen T.S., Xie X., Kamal M., Huebert D.J., Cuff J., Fry B., Meissner A., Wernig M., Plath K. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Boyer L.A., Lee T.I., Cole M.F., Johnstone S.E., Levine S.S., Zucker J.P., Guenther M.G., Kumar R.M., Murray H.L., Jenner R.G. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer L.A., Plath K., Zeitlinger J., Brambrink T., Medeiros L.A., Lee T.I., Levine S.S., Wernig M., Tajonar A., Ray M.K. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- Cheng X., Huber T.L., Chen V.C., Gadue P., Keller G.M. Numb mediates the interaction between Wnt and Notch to modulate primitive erythropoietic specification from the hemangioblast. Development. 2008;135:3447–3458. doi: 10.1242/dev.025916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Ying L., Lu L., Galvão A.M., Mills J.A., Lin H.C., Kotton D.N., Shen S.S., Nostro M.C., Choi J.K. Self-renewing endodermal progenitor lines generated from human pluripotent stem cells. Cell Stem Cell. 2012;10:371–384. doi: 10.1016/j.stem.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadue P., Huber T.L., Nostro M.C., Kattman S., Keller G.M. Germ layer induction from embryonic stem cells. Exp. Hematol. 2005;33:955–964. doi: 10.1016/j.exphem.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Gadue P., Huber T.L., Paddison P.J., Keller G.M. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther M.G., Frampton G.M., Soldner F., Hockemeyer D., Mitalipova M., Jaenisch R., Young R.A. Chromatin structure and gene expression programs of human embryonic and induced pluripotent stem cells. Cell Stem Cell. 2010;7:249–257. doi: 10.1016/j.stem.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Dziedzic N., Gadue P., Keller G.M., Gouon-Evans V. An endothelial cell niche induces hepatic specification through dual repression of Wnt and Notch signaling. Stem Cells. 2011;29:217–228. doi: 10.1002/stem.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Zhang D., Bursac N., Zhang Y. WNT3 is a biomarker capable of predicting the definitive endoderm differentiation potential of hESCs. Stem Cell Reports. 2013;1:46–52. doi: 10.1016/j.stemcr.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwacki-Neisius V., Göke J., Osorno R., Halbritter F., Ng J.H., Weiße A.Y., Wong F.C., Gagliardi A., Mullin N.P., Festuccia N. Reduced Oct4 expression directs a robust pluripotent state with distinct signaling activity and increased enhancer occupancy by Oct4 and Nanog. Cell Stem Cell. 2013;12:531–545. doi: 10.1016/j.stem.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K.F., Ng D.Y., Jayakumaran G., Wood G.A., Koide H., Doble B.W. β-catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell. 2011;8:214–227. doi: 10.1016/j.stem.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisada Y., Tsubooka-Yamazoe N., Shoji M., Hosoya M. Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res. (Amst.) 2012;8:274–284. doi: 10.1016/j.scr.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Li Y., Yu W., Cooney A.J., Schwartz R.J., Liu Y. Brief report: Oct4 and canonical Wnt signaling regulate the cardiac lineage factor Mesp1 through a Tcf/Lef-Oct4 composite element. Stem Cells. 2013;31:1213–1217. doi: 10.1002/stem.1362. [DOI] [PubMed] [Google Scholar]
- Liang J., Wan M., Zhang Y., Gu P., Xin H., Jung S.Y., Qin J., Wong J., Cooney A.J., Liu D., Songyang Z. Nanog and Oct4 associate with unique transcriptional repression complexes in embryonic stem cells. Nat. Cell Biol. 2008;10:731–739. doi: 10.1038/ncb1736. [DOI] [PubMed] [Google Scholar]
- Lu R., Markowetz F., Unwin R.D., Leek J.T., Airoldi E.M., MacArthur B.D., Lachmann A., Rozov R., Ma’ayan A., Boyer L.A. Systems-level dynamic analyses of fate change in murine embryonic stem cells. Nature. 2009;462:358–362. doi: 10.1038/nature08575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde K., Belting H.G., Driever W. Zebrafish pou5f1/pou2, homolog of mammalian Oct4, functions in the endoderm specification cascade. Curr. Biol. 2004;14:48–55. doi: 10.1016/j.cub.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Lyashenko N., Winter M., Migliorini D., Biechele T., Moon R.T., Hartmann C. Differential requirement for the dual functions of β-catenin in embryonic stem cell self-renewal and germ layer formation. Nat. Cell Biol. 2011;13:753–761. doi: 10.1038/ncb2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J.A., Wang K., Paluru P., Ying L., Lu L., Galvão A.M., Xu D., Yao Y., Sullivan S.K., Sullivan L.M. Clonal genetic and hematopoietic heterogeneity among human-induced pluripotent stem cell lines. Blood. 2013;122:2047–2051. doi: 10.1182/blood-2013-02-484444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry C.E., Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Niwa H., Miyazaki J., Smith A.G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- Nostro M.C., Cheng X., Keller G.M., Gadue P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2008;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo M., Lang B., Yu L., Prosser H., Bradley A., Babu M.M., Choudhary J. An expanded Oct4 interaction network: implications for stem cell biology, development, and disease. Cell Stem Cell. 2010;6:382–395. doi: 10.1016/j.stem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzisheuskaya A., Chia Gle.B., dos Santos R.L., Theunissen T.W., Castro L.F., Nichols J., Silva J.C. A defined Oct4 level governs cell state transitions of pluripotency entry and differentiation into all embryonic lineages. Nat. Cell Biol. 2013;15:579–590. doi: 10.1038/ncb2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubinoff B.E., Pera M.F., Fong C.Y., Trounson A., Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Sokol S.Y. Maintaining embryonic stem cell pluripotency with Wnt signaling. Development. 2011;138:4341–4350. doi: 10.1242/dev.066209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo A.K., Arnold S.J., Trotter M.W., Brown S., Ang L.T., Chng Z., Robertson E.J., Dunn N.R., Vallier L. Pluripotency factors regulate definitive endoderm specification through eomesodermin. Genes Dev. 2011;25:238–250. doi: 10.1101/gad.607311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson M., Liu S.J., Zou L.N., Smith Z., Meissner A., Ramanathan S. Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell. 2011;145:875–889. doi: 10.1016/j.cell.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg D.L., Snoek T., Mullin N.P., Yates A., Bezstarosti K., Demmers J., Chambers I., Poot R.A. An Oct4-centered protein interaction network in embryonic stem cells. Cell Stem Cell. 2010;6:369–381. doi: 10.1016/j.stem.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wynshaw-Boris A. The canonical Wnt pathway in early mammalian embryogenesis and stem cell maintenance/differentiation. Curr. Opin. Genet. Dev. 2004;14:533–539. doi: 10.1016/j.gde.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Wang Z., Oron E., Nelson B., Razis S., Ivanova N. Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. Cell Stem Cell. 2012;10:440–454. doi: 10.1016/j.stem.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Zhao X.D., Han X., Chew J.L., Liu J., Chiu K.P., Choo A., Orlov Y.L., Sung W.K., Shahab A., Kuznetsov V.A. Whole-genome mapping of histone H3 Lys4 and 27 trimethylations reveals distinct genomic compartments in human embryonic stem cells. Cell Stem Cell. 2007;1:286–298. doi: 10.1016/j.stem.2007.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.