Abstract
Reperfusion injury, the paradoxical tissue response that is manifested by blood flow-deprived and oxygen-starved organs following the restoration of blood flow and tissue oxygenation, has been a focus of basic and clinical research for over 4-decades. While a variety of molecular mechanisms have been proposed to explain this phenomenon, excess production of reactive oxygen species (ROS) continues to receive much attention as a critical factor in the genesis of reperfusion injury. As a consequence, considerable effort has been devoted to identifying the dominant cellular and enzymatic sources of excess ROS production following ischemia-reperfusion (I/R). Of the potential ROS sources described to date, xanthine oxidase, NADPH oxidase (Nox), mitochondria, and uncoupled nitric oxide synthase have gained a status as the most likely contributors to reperfusion-induced oxidative stress and represent priority targets for therapeutic intervention against reperfusion-induced organ dysfunction and tissue damage. Although all four enzymatic sources are present in most tissues and are likely to play some role in reperfusion injury, priority and emphasis has been given to specific ROS sources that are enriched in certain tissues, such as xanthine oxidase in the gastrointestinal tract and mitochondria in the metabolically active heart and brain. The possibility that multiple ROS sources contribute to reperfusion injury in most tissues is supported by evidence demonstrating that redox-signaling enables ROS produced by one enzymatic source (e.g., Nox) to activate and enhance ROS production by a second source (e.g., mitochondria). This review provides a synopsis of the evidence implicating ROS in reperfusion injury, the clinical implications of this phenomenon, and summarizes current understanding of the four most frequently invoked enzymatic sources of ROS production in post-ischemic tissue.
Abbreviations: A/R, anoxia-reoxygenation; AP-1, activator protein-1; BH4, tetrahydrobiopterin; BM, bone marrow; CoQ, coenzyme Q; CuZn SOD, copper–zinc superoxide dismutase; ∆ψ, membrane potential; DCF, dichlorofluorescein; DHE, dihydroethidine; DHFR, dihydrofolate reductase; DHR, dihyrdrorhodamine; DPI, diphenyliodonium; Duox, dual oxidase; EC, endothelial cell; EC-SOD, extracellular superoxide dismutase; ESR, electron spin resonance; ETC, electron transport chain; FAD, flavin adenine dinucleotide; FADH2, reduced FAD; GAG, glycosaminoglycans; α-GPD, α-glycerophosphate dehydrogenase; GPx, glutathione peroxidase; GTPCH, guanosine triphosphate cyclohydrolase I; H2O2, hydrogen peroxide; H/R, hypoxia-reoxygenation; HIF-1α, hypoxia inhibitory factor-1α; I/R, ischemia-reperfusion; IMAC, inner membrane anion channel; ICAM-1, intercellular adhesion molecule-1; IFN-γ, interferon-γ; IL-1β, interleukin-1beta; IL-6, interleukin-6; α-KDH, α-ketoglutarate dehydrogenase; LTB4, leukotriene B4; MAO, monoamine oxidase; MnSOD, manganese superoxide dismutase; MPTP, mitochondrial permeability transition pore; mtROS, mitochondrial reactive oxygen species; NAD+, Nicotinamide adenine dinucleotide (oxidized); NADH, Nicotinamide adenine dinucleotide (reduced); NADPH, Nicotinamide adenine dinucleotide phosphate; NFkB, nuclear factor kappa-B; NNT, NADP-transhydrogenase; Nox, NADPH oxidase; NO, nitric oxide; , nitrite ion; NOS, nitric oxide synthase; eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase; mtNOS, mitochondrial nitric oxide synthase; nNOS, neuronal nitric oxide synthase; O2, molecular oxygen; , superoxide anion; PDH, pyruvate dehydrogenase; PKC, protein kinase C; PR-39, synthetic peptide inhibitor of Nox; Prx, peroxiredoxin; PAF, platelet activating factor; PEG-, polyethylene glycol conjugated; RBC, red blood cell; RET, reverse electron transport; RIRR, ROS-induced ROS release; ROS, reactive oxygen species; SOD, superoxide dismutase; TCA, tricarboxyl acid; TNF-α, tumor necrosis factor-α; Trx, thioredoxin; UCP, uncoupling protein; XDH, xanthine dehydrogenase; XO, xanthine oxidase; XOR, xanthine oxidoreductase (XD+XO)
Keywords: Ischemia-reperfusion, Hypoxia-reoxygenation, Xanthine oxidase, NADPH oxidase, Uncoupled nitric oxide synthase, Mitochondria
Graphical abstract
Highlights
-
•
Reperfusion injury is implicated in a variety of human diseases and disorders.
-
•
Evidence implicating ROS in reperfusion injury continues to grow.
-
•
Several enzymes are candidate sources of ROS in post-ischemic tissue.
-
•
Inter-enzymatic ROS-dependent signaling enhances the oxidative stress caused by I/R.
.
1. Introduction
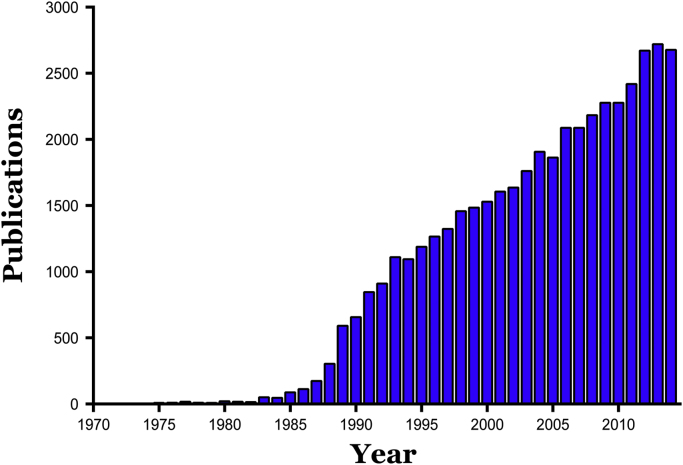
It is well known that an impairment of blood flow (ischemia) can result in tissue injury and organ dysfunction, with duration and severity of the ischemic insult determining the reversibility of the injury response and ultimate survival of the tissue [1], [2], [3], [4]. Ischemic tissue injury is generally attributed to a profound and lengthy period of tissue hypoxia and the consequent depletion of cellular ATP. It has long been appreciated that survival of ischemic tissue can be ensured by the timely restoration of blood flow (reperfusion), which should serve to minimize the magnitude of the hypoxic insult, allow for the replenishment of cellular ATP levels, re-establish ionic balance within the cell, and ultimately result in full restoration of organ function. However, the predictable beneficial influence of early reperfusion on tissue recovery following ischemia was challenged in the 1970s by reports describing a paradoxical enhancement of the injury response following reperfusion (or reoxygenation) of ischemic (or hypoxic) tissue [5], [6]. This led to the proposal by Hearse et al. [6] that the sudden reintroduction of molecular oxygen to energy- (and oxygen-) starved tissue results in a unique type of injury response that is not manifested during the period of hypoxic stress. The discovery of this reoxygenation-dependent injury response, which is now commonly called “reperfusion injury”, opened a new field of scientific investigation that has grown rapidly and consistently to this day (Fig. 1).
Fig. 1.
Publication frequency of articles dealing with ischemia-reperfusion injury from 1970 through 2014. Based on PubMed search (June, 2015) using search term “ischemia-reperfusion injury” or “reperfusion injury”.
Since its inception, the concept of reperfusion injury has steadily gained attention as an underlying component of a variety of human diseases and disorders, and it has received similar notice in the field of veterinary medicine [7], [8]. As a consequence, much attention has been devoted to defining the molecular and cellular basis of the unique injury response that results when ischemic tissues are reperfused [1], [2], [9]. In the early 1980s, oxygen-derived free radicals (now more commonly referred to as reactive oxygen species, ROS) were proposed as potential mediators of reperfusion injury. The premise that highly reactive and unstable molecules like ROS could account for reperfusion injury was quickly embraced, in large part because it was consistent with the observation that the injury response was dependent on the reintroduction of molecular oxygen. Furthermore, evidence was soon provided to support the notion that, with reperfusion of ischemic tissue, an imbalance is created between the rate of generation of ROS and the tissue's ability to detoxify these reactive species [10], [11]. In the 35 years since ROS were first implicated in reperfusion injury, the molecular basis and pathophysiological significance of this ROS-dependent response has been extensively studied, providing new insights into the enzymatic and cellular sources of the ROS, the magnitude of ROS production elicited by reperfusion (reoxygenation), and how ROS production ultimately leads to tissue injury. This review addresses how the concept of ROS-mediated reperfusion injury has evolved over the past 3-plus decades and it examines the evidence that has accumulated over this period to support or refute the existence of ROS-mediated reperfusion injury in different organ systems, as well as its relevance to different pathological states.
2. Reperfusion induced organ dysfunction/injury
The continued expansion of the scientific literature on ischemia-reperfusion (I/R) injury over the past 40 years is likely a reflection of several factors, including the implication of this mechanism of tissue injury in a growing list of organs, the development and use of in vitro models that mimic the condition of hypoxia followed by reoxygenation (H/R), and the implication of reperfusion injury in an expanding list of human diseases and clinical conditions. Reperfusion injury has been described and studied in most organs in the body, with reports describing this mechanism of injury in the heart [12], brain [13], skeletal muscle [14], skin [15], lung [16], eye [17], spinal cord [18], intestine [19], liver [20], kidney [21], uterus [22], ovary [23], testicle [24], penis [25], and joints [26]. While this assortment of tissues shares some characteristic features of the injury response to I/R, such as necrosis, apoptosis, impaired microvascular function, and edema, there is considerable diversity in the responses between tissues that reflect the unique functional properties of the affected organ. Some examples of these unique functional responses to I/R include arrhythmias and stunning (transient depression of cardiac contractility) for the heart [27], [28], behavioral deficits for brain [13], paralysis for spinal cord [18], depressed motility and bacterial translocation (with endotoxemia) for intestine [29], visual impairment and blindness for eye [30], depressed glomerular filtration rate and proteinuria for kidney [21], and infertility for testes and ovaries [23], [24].
In vitro models have proven to be useful for determining the responses of single cell populations to I/R [31], [32], [33], [34]. These models, which expose isolated, purified cell populations to hypoxia (or anoxia) and reoxygenation (H/R and A/R), have capitalized on the creation of well-defined and precisely controlled conditions to determine whether the tissue injury responses elicited by I/R in vivo can be recapitulated by single cell populations. While the conditions used to simulate I/R in vitro are arguably artificial due to the absence of other relevant cell populations and non-physiological environmental conditions (e.g., pO2, pH), the in vitro models have shown a remarkable level of consistency in reproducing the phenotypic responses of tissues to I/R [32]. Similarities in the in vitro and in vivo responses to I/R (H/R) have been demonstrated using cardiac myocytes [35], intestinal enterocytes [36], alveolar epithelium [34], neurons [37], hepatocytes [38], adipocytes [39], and arterial grafts [40]. Endothelial cell (EC) monolayers subjected to H/R have proven to be extraordinarily accurate in simulating the diverse microvascular alterations that are elicited by H/R, including (1) an enhanced production of ROS [41], (2) increased expression of adhesion molecules with a consequent increase in the adhesivity of EC to leukocytes (neutrophils and T-lymphocytes) [42], [43], (3) diminished EC barrier function [44], and (4) the development of a procoagulant/prothrombotic phenotype [45] (Fig. 2). Indeed, the adhesive interactions between post-hypoxic EC monolayers and blood cells, such as neutrophils and T-lymphocytes, have been extensively used as a surrogate for the inflammatory responses elicited by I/R [33], [42]. Growing recognition of the importance of cell–cell interactions in the pathogenesis of I/R injury has led to efforts to increase the complexity of the in vitro models through the development of multicellular co-culture systems, such as a neurovascular model comprised of co-cultured neurons, astrocytes, and cerebral microvascular EC to simulate the blood–brain barrier (BBB) [46]. The neurovascular unit model exhibits a more robust barrier function (reduced permeability) compared to EC monolayers and this more restrictive barrier is compromised to a greater degree in response to H/R [46], suggesting that having neurons and astrocytes as neighbors of EC more adequately simulates the properties of the intact BBB as well as its response to I/R. Perhaps the most tangible benefit of the in vitro models of I/R injury has been their utility in dissecting the complex array of signaling molecules that underlie the diverse cellular changes and injury responses that are ultimately manifested in postischemic tissue [47], [48]. This reductionist approach to the study of I/R injury has contributed to the continued growth and interest in this field of investigation.
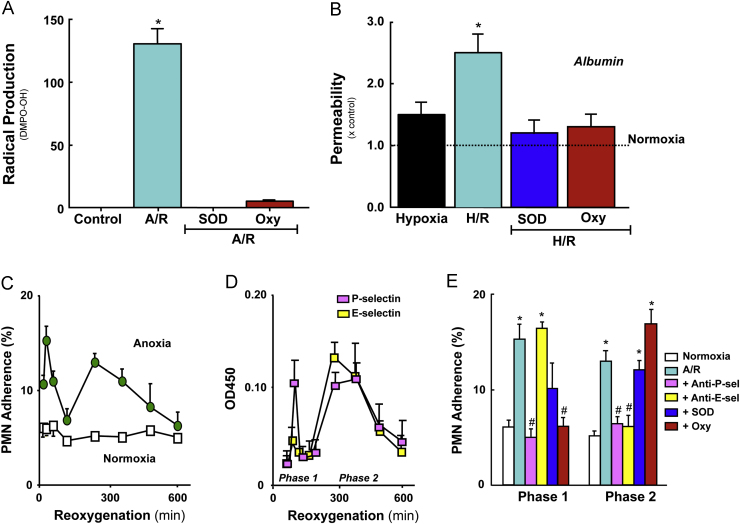
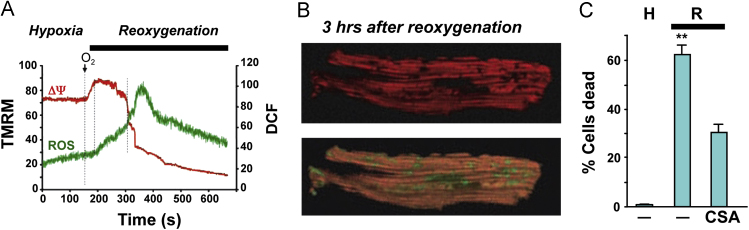
Fig. 2.
Responses of vascular endothelial cells (EC) to anoxia/reoxygenation (A/R) or hypoxia/reoxygenation (H/R). (Panel A) Radical production by human aortic EC exposed to 60 min anoxia and 10 min reoxygenation. Measurements derived from electron paramagnetic resonance using the spin trap 5,5-dimethyl-1-pyrroline N-oxide (DMPO). The large signal detected after reoxygenation was not evident when the EC were treated with either superoxide dismutase (SOD) or oxypurinol (Oxy). Data from Zweier et al. [110]. (Panel B) Albumin permeability across monolayers of bovine pulmonary artery EC exposed to 90 min hypoxia, with or without 90 reoxygenation. While hypoxia per se did not increase monolayer permeability, H/R elicited a significant increase. The H/R-induced permeability response was prevented by pretreatment with either SOD or oxypurinol (Oxy). Data from Inauen et al. [44]. (Panel C) Neutrophil (PMN) adhesion response on human umbilical vein endothelial cells (HUVEC) exposed to 60 min anoxia, followed by 30–600 min reoxygenation. A biphasic adhesion response was noted with an initial peak (phase 1) at 30 min and a later peak (phase 2) at 240 min. (Panel D) Biphasic increases in endothelial expression of P- and E-selectin on HUVEC exposed to A/R (as described for Panel C). (Panel E) PMN adhesion on HUVEC monolayers exposed to A/R (as per Panel C) following treatment with a blocking antibody to either P-selectin (anti-P-sel) or E-selectin (anti-E-sel), SOD or Oxy. Data in Panels C–E from Ichikawa et al. [42].
It is now generally accepted that reperfusion injury has the potential to jeopardize the functional recovery of patients that experience transient disruption of blood perfusion to a single tissue or multiple organs, either as a consequence of a medical/surgical procedure (e.g., organ transplantation and thrombolytic therapy) or in response to a disease process (e.g., acute kidney injury, neonatal necrotizing enterocolitis) (Table 1) [1], [49]. Conditions that are not traditionally considered to be associated with transient episodes of ischemia and/or hypoxia, such sickle cell disease [50], osteoarthritis [26], and Alzheimer's disease [51], are now receiving attention as a clinical manifestation of I/R injury. The periodic episodes of painful vasoocclusion and reperfusion that is characteristic of a sickle cell crisis have been likened to I/R injury [52]. Similarly, reperfusion injury has been implicated in the pathophysiology of obstructive sleep apnea, a condition associated with transient periods of airway obstruction (and hypoxia) followed by reoxygenation [53]. Finally, there is also growing evidence in animal models that hepatic I/R promotes the metastasis of cancerous cells to the liver from the pancreas [54] and colorectum [55]. While reperfusion injury is being assigned a role in the pathogenesis of a growing list of clinical conditions, the relative importance of reperfusion-dependent vs reperfusion-independent mechanisms in the overall morbidity and mortality of these conditions remains poorly defined.
Table 1.
Clinical conditions associated with ischemia-reperfusion injury.
|
Single organ involvement | |
|---|---|
| Organ | Condition |
| Central nervous system | Stroke |
| Perinatal hypoxic-ischemic encephalopathy | |
| Alzheimer's disease | |
| Heart | Acute coronary syndrome |
| Joints | Osteoarthritis |
| Gastrointestinal tract | Gastric ulcers |
| Neonatal necrotizing enterocolitis | |
| Volvulus | |
| Kidney | Acute kidney injury |
| Multiple organ involvement | |
| Condition | Manifestations |
| Hemorrhage & resuscitation | Multiple organ failure |
| Sickle cell disease | Acute chest syndrome, priapism |
| Sleep apnea | Oxidative damage, hypertension |
| Medical or surgical procedure-related | |
| Condition | Manifestations |
| Thrombolytic therapy | Stroke, myocardial infarction |
| Organ transplantation | Acute graft failure |
| Cardiopulmonary bypass | Lung injury, heart failure |
| Coronary angioplasty | Heart failure |
| Arterial tourniquet release | Local tissue injury |
| Thoracoabdominal aortic surgery | Spinal cord injury |
| Compartment syndrome decompression | Limb edema, muscle injury |
| Testicular or ovarian torsion/detorsion | Infertility |
3. Reactive oxygen species contribute to reperfusion injury
The concept that ROS play a role in the injury response to I/R is largely based on three lines of evidence: (1) interventions that enhance ROS scavenging and/or detoxification protect against reperfusion injury, (2) artificial generation of ROS in otherwise normal tissue recapitulates the injury response to I/R, and (3) detection of enhanced ROS production and their characteristic cellular 'footprints' in post-ischemic tissues. The early ROS scavenging studies employed native superoxide distmutase (SOD) in the presence or absence of catalase to demonstrate protection against injury in both in vivo and ex vivo models of I/R [11], [56], [57]. Experimental limitations related to the relatively small size and short circulating half-life of ROS scavenging enzymes led to the development and application of polyethylene-glycol(PEG)-conjugatedforms of both SOD [58] and catalase [59], both of which proved to be effective in some I/R models. Synthetic, low-molecular weight SOD mimetics like tempol were also developed to overcome problems (e.g., antigenicity) associated with the native enzyme that limited its clinical utility, and these have proven to be effective in several I/R models [60], [61], [62], [63]. Mutant mice that are either deficient in or overexpress ROS scavenging enzymes, such as SOD, catalase, and glutathione peroxidase, have also provided results that are consistent with a role for ROS as mediators of I/R injury in different organs [64], [65], [66], [67], [68]. Furthermore, cellular localization of the source of ROS that contributes to the I/R-induced injury response has been addressed using mutant mice that overexpress or exhibit a deficiency in one of the three different SOD isoenzymes, i.e., the cytosolic copper–zinc SOD (CuZn SOD), the mitochondria-associated manganese SOD (MnSOD), and extracellular SOD (EC-SOD)[69], [70]. Direct gene transfer of the cDNA encoding SOD has also been shown to afford protection against I/R injury [71]. Native SOD, PEG-SOD, SOD-mimetics, and genetic overexpression of SOD or other ROS scavenging enzymes have also been applied, with considerable success, in different in vitro models of hypoxia (or anoxia)-reoxygenation [42], [69], [72], [73], [74], [75], [76], [77].
Additional support for the involvement of ROS in I/R injury has come from studies describing phenotypic responses of cells or tissues exposed directly to ROS (or a ROS-generating enzyme) that recapitulate the responses elicited by I/R [19], [78]. Hydrogen peroxide, a mild and relatively stable oxidant that is generated in tissues (cells) exposed to I/R (H/R), has been extensively used as a representative ROS to assess the response of cells to oxidative stress. At pathophysiologically relevant concentrations (10–100 μM), H2O2 can elicit most of the phenotypic changes in endothelial function that are evidenced in postischemic (posthypoxic) tissues (endothelial cell monolayers) including endothelial barrier dysfunction (increased vascular permeability) [79], increased expression of endothelial cell adhesion molecules and enhanced leukocyte–endothelial cell adhesion [80], [81], increased production of inflammatory mediators (e.g., platelet activating factor) [81], and the induction of a procoagulant, prothrombotic phenotype [82]. Cardiac myocytes [83] and vascular smooth muscle [84] also respond to H2O2 in a manner that is consistent with I/R or H/R, while other cell types (e.g., mast cells) do not [85]. The molecular basis for the cellular responses elicited by hydrogen peroxide and the role of this mild oxidant as a signaling second messenger have been extensively characterized [86].
The premise that ROS are generated following I/R was initially based on the detection of chemical products generated by the reaction of ROS with cellular lipids, proteins, and other molecules [10], [87], [88]. The products of lipid peroxidation (e.g., malondialdehyde, conjugated dienes, and hydroxynonenol) have been widely used as ‘footprints’ of ROS generation in different models of I/R [2], [19], [34], [89]. Oxidation of cellular sulfhydryl groups and the generation of oxidized glutathione (GSSG) have also been offered as evidence of oxidative stress and redox imbalance following I/R in different tissues [90], [91], [92], [93], [94]. Oxidant-sensitive fluorochromes (e.g., dihyrdrorhodamine 123 (DHR), dihydroethidine (DHE), and dichlorofluorescein (DCF)) have been used to visualize and quantify ROS production in postischemic tissues and in monolayers of cultured cells [95], [96], [97], [98], [99]. While DCF and DHE exhibit differential sensitivities to hydrogen peroxide and superoxide, respectively, electron spin resonance (ESR) spectroscopy and spin trapping has become the ‘gold standard’ for detection and identification of different ROS produced by tissues and cells in response to I/R (Fig. 2). This approach, which has been applied to heart [100], [101], kidney [102], skin [103], retina [104], lung [105], intestine [106], liver [107], and monolayers of cultured cells [108], [109], has revealed that the enhanced ROS production elicited by I/R is detected immediately (within 20 s) following reperfusion and that superoxide () is the parent radical that serves as a precursor for the hydroxyl radical, carbon-centered radicals and other secondary species [2], [110]. The more recent application of proteomic and genomic mapping to tissues (or cells) exposed to I/R (or H/R) is providing novel insights into the responses of specific proteins and genes to the oxidative stress that accompanies this condition [111], [112], [113], [114].
3.1. Sources of ROS in post-ischemic tissue
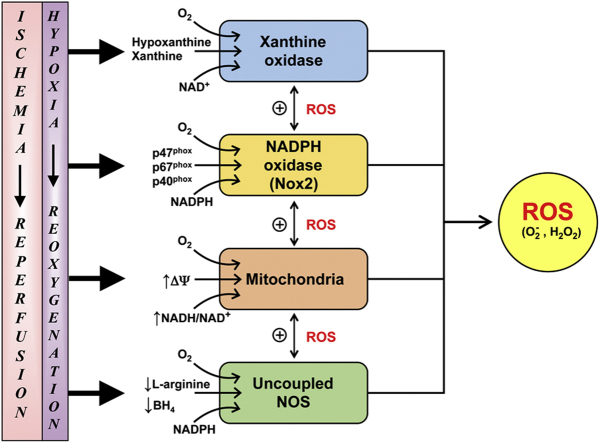
The oxidative stress elicited in tissues/cells following I/R (or H/R) has been linked to a variety of different sources of ROS. Non-enzymatic sources of ROS, such as hemoglobin and myoglobin (both of which can be released into extracellular fluid after trauma), have received some attention as potential mediators of I/R-induced oxidative stress [115], [116]. However, most studies have attributed the accelerated ROS production in post-ischemic tissues to one or more enzymes that are capable of reducing molecular oxygen to form superoxide and/or hydrogen peroxide, with the subsequent release of ROS into the intracellular and/or extracellular compartments. Table 2 summarizes the enzymatic sources of ROS that have been implicated in I/R pathogenesis in different organs. An examination of the literature reveals that the enzyme systems most commonly invoked to explain the accelerated ROS production in postischemic tissues are xanthine oxidase, NADPH oxidase, the mitochondrial electron transport chain, and uncoupled nitric oxide synthase. A brief description and assessment of the involvement of these enzyme systems in reperfusion injury follows.
Table 2.
Potential sources of reactive oxygen species in tissues exposed to ischemia and reperfusion.
| ROS source | Tissue implicated | References |
|---|---|---|
| Xanthine oxidase | Intestine, lung, heart, skin,eye, brain, skeletal muscle,liver, pancreas, stomach, testes,kidney, joints, spinal cord | [11], [475], [476], [477] |
| [478], [479] | ||
| [138], [480], [481], [482] | ||
| [483], [484], [485] | ||
| NADPH oxidase | Intestine, lung, heart, eyebrain, stomach, liver, kidneytestes | [214], [486], [487], [207] |
| [242], [204], [488], [489] | ||
| 490] | ||
| Mitochondria | Intestine, lung, heart, brain, skeletal muscle, liver, kidney, stomach, testes, spinal cord | [239], [491], [328], [492] |
| [493], [494], [495] | ||
| [496], [497], [498] | ||
| Nitric oxide synthase | Liver, heart, (aortic endothelial cells) | [423], [427], [429], [499] |
| Cytochrome P450 | Lung, heart, kidney | [500], [501], [502] |
| Lipoxygenase/cyclooxygenase | Brain,eye, pancreas, stomach | [503], [478], [480], [504] |
| Monoamine oxidase | Heart, kidney, brain | [352], [505], [506], [507] |
3.1.1. Xanthine oxidase
Xanthine oxidoreductase (XOR) is a complex molybdoflavoenzyme that controls the rate-limiting step of purine catabolism, i.e., the hydroxylation of xanthine to uric acid. The mammalian form of this enzyme exists in two interconvertible forms, xanthine dehydrogenase (XDH) and xanthine oxidase (XO), with XDH as the predominant form in normal healthy tissue. XDH preferably uses NAD+ as an electron acceptor, while XO uses O2 as the terminal electron acceptor thereby exhibiting the ability to generate ROS. XO has been extensively studied as a potential source of ROS in tissues and isolated cells exposed I/R or H/R. More organ systems have been probed for the contribution of this ROS source than any other potential source (Table 2), which likely reflects the fact that safe, inexpensive and effective drugs that inhibit the enzyme (e.g., allopurinol) have long been available. Despite the large number of reports describing a beneficial effect of xanthine oxidase inhibitors in different post-ischemic tissues, there are also studies that have failed to show protection following XO inhibition in tissues such as the heart [117], lung [118], and liver [91]. Some of the inconsistent findings have been attributed to differences in XOR abundance/activity between animal species. For example, rabbit heart, which is not protected against reperfusion injury by allopurinol treatment, is virtually devoid of XO activity [117], much like the human heart [119]. However, intestine and liver, which exhibit significant and ubiquitous expression of XOR across species (including humans) [120], [121], have yielded more consistent findings regarding a role for the enzyme in reperfusion injury [122], [123].
While whole tissue homogenates of organs (e.g., heart and brain) from human and some experimental animals have failed to exhibit significant XOR activity, immunohistochemical studies have revealed high levels of XOR antigen in vascular endothelium in these tissues [124], [125]. Furthermore, the immunohistochemical studies have rather consistently demonstrated that the enzyme is concentrated on the outer surface of the endothelial cell plasma membrane [124], [125]. This observation has lead to the proposal that the XOR localized on the surface of endothelial cells is derived from plasma [126]. Animal studies have revealed that circulating XO levels are significantly elevated following exposure of the liver and/or intestine to I/R [126], [127], and that the XO leaking into plasma can bind to surface glycosaminoglycans (GAG) on vascular endothelial cells in tissues distant from the liver and/or gut [128]. The GAG-dependent binding of XO to vascular endothelium is heparin-reversible until the enzyme is endocytosed by the cell [129], [130]. A pathophysiological role for the XO released into plasma from these enzyme-rich tissues is supported by the observation that the XO level achieved in plasma is sufficient (with adequate substrate) to produce severe endothelial cell injury in vitro [126] and that immunoblockade of the circulating XO has been shown to protect the lung vasculature from the deleterious effects of gut I/R [127]. The binding of circulating XO to vascular endothelial cells has been invoked to explain the effectiveness of xanthine oxidase inhibitors in blunting the I/R injury response in tissues that exhibit low XO activity [131]. Nonetheless, there are a number of reports that demonstrate a role for xanthine oxidase in the phenotypic changes that occur in cultured vascular endothelial cells exposed to A/R or H/R [31], [42], [110] (Fig. 2).
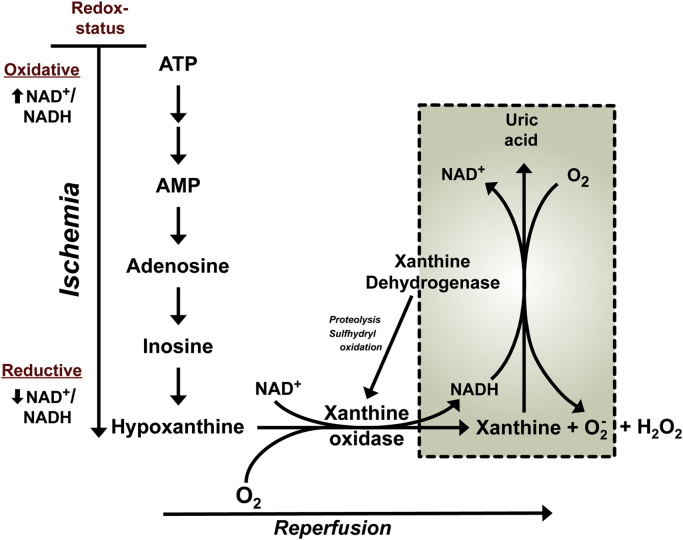
The hypothesis that xanthine oxidase is a major source of ROS following I/R was initially proposed to explain the enhanced vascular permeability response to reperfusion in cat small intestine, following a 1 h period of low-flow ischemia [11]. The original version of this hypothesis (Fig. 3) proposed that the ischemic insult results in depletion of the energy charge of the cell, an accumulation of hypoxanthine from the catabolism of ATP, and a concomitant conversion (mediated by limited proteolysis and/or sulfhydryl oxidation) of the XDH isoform to XO. With the restoration of blood flow and tissue oxygen tension at reperfusion, it was predicted that the readmitted oxygen and accumulated hypoxanthine would react with XO to produce a burst of superoxide. Since allopurinol and superoxide dismutase (SOD) treatments were equally effective in blunting the I/R-induced vascular permeability response, it was concluded that XO-derived superoxide and/or secondary radical species were largely responsible for the endothelial barrier dysfunction.
Fig. 3.
Potential mechanisms of ROS production by XOR in tissues exposed to ischemia and reperfusion. In the setting of ischemia, ATP is catabolized to hypoxanthine and the dehydrogenase form of XOR (XDH) is converted, via limited proteolysis and sulfhydryl oxidation, to the oxidase form (XO). Upon reperfusion, the restored tissue O2 reacts with hypoxanthine (or xanthine) and XO to generate both superoxide () and hydrogen peroxide (H2O2), which can consequently interact to yield more reactive secondary species [11], [133]. The conversion of XDH to XO may not be required for ROS production following reperfusion (see boxed area of figure). During ischemia, the redox status of the tissue is altered from an oxidative state (higher level of NAD+ relative to NADH) to a reductive state (higher NADH relative to NAD+). This altered redox state has been shown to enhance the generation of from XDH in the presence of xanthine [141].
While X-ray crystallography and site-directed mutagenesis studies have significantly improved our understanding of the changes in enzyme structure and function that occur when XDH is converted to XO [132], considerable uncertainty remains regarding the magnitude and kinetics of conversion of XDH to XO that is elicited by ischemia, and whether this conversion process is a requirement for XO-dependent ROS production during reperfusion. Initial reports of XDH to XO conversion in rat intestine suggested a very rapid rate of conversion i.e., requiring about 60 s for complete conversion to the ROS producing XO form [133]. However, subsequent studies have revealed that XO accounts for 19% of total enzyme (XDH+XO) activity under control (non-ischemic) conditions, and that XO activity increases by approximately 13% per hour of intestinal ischemia [134]. The issue of XDH to XO conversion during ischemia has been more extensively evaluated in liver. However, disparate findings have been reported for this tissue, with some reports describing significant conversion during ischemia, while others describe little or no conversion following prolonged ischemia [135], [136]. There appears to be a growing consensus that the conversion of XDH to XO is not a rate-limiting determinant of ROS production upon reperfusion of ischemic tissue, particularly in liver [123], [137]. This contention is supported by the observation that the hepatocellular injury response to I/R precedes the conversion of XDH to XO [136], [138]. A possible explanation for the enhanced superoxide production in the absence of XDH to XO conversion during I/R is the observation that XDH exhibits NADH oxidase activity under acidic conditions (pH ~6.5), wherein XDH oxidizes NADH rather than xanthine [123], [139]. In this regard, it is noteworthy that it has been reported that the NADH oxidase of XDH has the capacity to generate superoxide at 4-times the rate of XO [139]. However, while allopurinol can inhibit the production of superoxide by XO, the drug has no effect on the NADH oxidase activity of XDH [139], [140]. Finally, a recent analysis of XDH from chicken liver that has the unique property of being locked in the dehydrogenase form has revealed that XDH has the capacity to generate large quantities of superoxide (at approximately half the rate of XO in the presence of xanthine) and this is regulated by the relative levels of NAD+ to NADH, with more produced under reduced conditions when a higher proportion of the NAD(H) pool is in the reduced state [141]. Since XDH may remain the dominant form of the enzyme at the time of reperfusion and the tissue likely remains in a reductive state (low NAD+ to NADH ratio) in the early reperfusion period, XDH may be a quantitatively more important source of than XO during this period (inset of Fig. 3).
In addition to the post-translational modification of XDH mediated by limited proteolysis and sulfhydryl oxidation, there is also evidence supporting a role for transcriptional regulation of the enzyme in response to I/R. Hypoxia and cytokines are two relevant stimuli that have been linked to increased XDH transcription. Endothelial and epithelial cells exposed to hypoxia respond with increases in mRNA and total activity for XO [142], [143], with some studies linking these hypoxia-induced responses to interleukin-6 mediated activation of the JAK-STAT signaling pathway [143]. XO activity is also regulated by oxygen tension at the post-translational level, as evidenced by studies demonstrating an inverse relationship between O2 tension and XO activity [142], [144]. O2 tension-mediated modulation of XO activity appears to involve enzyme phosphorylation by p38 kinase [145]. A variety of cytokines, including IL-1, IFN-γ, IL-6 and TNF-α, have been shown to increase XDH/XO mRNA and to increase total activity of the enzyme in different cell populations [146], [147], [148]. Inasmuch as cytokine accumulation, resulting from mast cell degranulation and macrophage activation, occurs rapidly following I/R, these inflammatory mediators may exert a significant influence on XO-mediated ROS production following I/R.
Hypoxanthine and xanthine are two well-characterized products of purine metabolism that accumulate in ischemic tissues. A recent comparative metabolomic analysis designed to identify the metabolic signatures in different tissues (liver, kidney, heart, and brain) subjected to I/R revealed a substantial accumulation of only three metabolites, hypoxanthine, xanthine, and succinate [149], confirming the availability of the purine metabolites for XO-mediated ROS production at reperfusion. However, the need for an accumulation of hypoxanthine and xanthine in post-ischemic tissue to drive XO-mediated ROS production remains unclear. It has been estimated that hypoxanthine concentration in the mucosa of normally perfused healthy intestine is approximately 20 μM and that bowel ischemia results in an approximate 10-fold increase (to 200 μM) in the concentration of this purine [150]. However, because the Km of hypoxanthine for XO in the gut is estimated to be approximately 11 μM [150], it would appear that the concentration of hypoxanthine in both normal and ischemic gut may not be rate-limiting for ROS production by XO in this tissue. Comparable or higher hypoxanthine levels have been reported, both in the pre-ischemic and ischemic state, in other tissues (e.g., brain) [151], suggesting that the absence of a critical role for hypoxanthine accumulation for XO-mediated ROS production at the time of reperfusion may not be unique to the intestine. These observations and conclusions are inconsistent with findings reported for postischemic heart, in which there are two lines of evidence supporting the view that substrate accumulation initiates and controls XO-mediated ROS production following I/R. First, it has been shown that the time-course of ROS production elicited by I/R in isolated rat hearts is closely correlated with the kinetics of XO substrate accumulation [152]. Second, pharmacological inhibition of adenosine metabolism and transport (and the consequent modulation of tissue xanthine and hypoxanthine levels) has revealed a role for hypoxanthine and xanthine accumulation in determining the severity of the myocardial injury response to I/R [153]. Furthermore, blockade of xanthine oxidase substrate formation via pharmacologic inhibition of adenosine deaminase prevents XO-dependent ROS production (measured by electron paramagnetic resonance spin trapping) and the contractile dysfunction that accompanies reperfusion, while exogenous administration of hypoxanthine and xanthine reversed the protective effects of adenosine deaminase inhibition [154]. However, a limitation of the experimental strategy of pharmacological inhibition of XO substrate accumulation is that it also results in the buildup of adenosine, which is known to exert a protective effect against I/R-induced inflammation and tissue injury [155], [156].
Different treatment strategies have been employed to assess the contribution of xanthine oxidase to I/R injury, including purine (allopurinol and oxypurinol) [11], [157] and non-purine (febuxostat) [158] enzyme inhibitors, dietary tungsten supplementation and/or molybdenum deficiency [159], [160], and gene deletion [161]. Xanthine oxidase deficiency, an uncommon autosomal recessive disorder in humans, generally results in kidney dysfunction and failure [162]. Similar kidney responses have been reported in both homozygous and heterozygous XDH knockout mice [161], [163]. Consequently, in the absence of tissue-specific conditional knockouts for XO, genetic approaches have had little or no impact to date in defining the role of XO-dependent ROS production in I/R injury [164]. A large majority of the reports addressing the contribution of XO to reperfusion injury are based on the use of allopurinol or its more water-soluble metabolite, oxypurinol [1]. While allopurinol/oxypurinol treatment has proven to be highly effective in inhibiting XO activity and ROS production, and in reducing reperfusion injury in several model systems, some concerns have been raised regarding non-specific actions of the drugs. For example, it has been proposed that allopurinol and oxypurinol may offer protection against I/R injury by acting as radical scavengers [165], [166]. However, reports describing this action of the XO inhibitors have employed concentrations that far exceed the doses needed to inhibit the enzyme. Extracellular fluid (plasma and lymph) collected from animals receiving the more commonly used allopurinol/oxypurinol treatment regimens for XO inhibition does not exhibit an enhanced antioxidant or scavenging potential [167]. Additional concerns regarding allopurinol/oxypurinol include the inability of the drugs to block the NADH oxidase activity of XO [168] and to inhibit XO activity when the enzyme is bound to GAGs on the endothelial cell surface [169]. The non-purine XO inhibitor febuxostat appears to overcome some of the limitations of allopurinol/oxypurinol. The potency of febuxostat is 3-orders of magnitude greater than allopurinol and it appears to be highly effective in inhibiting endothelium-associated XO [170]. Febuxostat has been used in a limited number of I/R injury studies of intestine [171] and kidney [158], showing protection in both studies. A comparison of allopurinol vs febuxostat effects in a rat model of local and remote intestinal I/R injury revealed better protection with febuxostat for some of the indices of inflammation and tissue injury [171].
Much attention has been recently devoted to the ability of XO to function as a nitrate/nitrite reductase, which enables the enzyme to catalyze the one electron reduction of nitrite to nitric oxide (NO) [123], [145], [164]. Studies employing purified enzyme have revealed the generation of NO from XO in the presence of , with xanthine, NADH, or aldehyde serving as electron donors [172], [173]. Nitrite reduction by XO is enhanced under anoxic/hypoxic conditions and by acidosis [174]. Furthermore, NO generation via this reductive process is inhibited by allopurinol and oxypurinol [172], [173]. The pathophysiological relevance of the NO generated by XO in conditions such as I/R and inflammation remains unclear. However, the capacity of this enzyme to generate both superoxide and NO has lead to the proposal that XO may be an important source of peroxynitrite in postischemic and inflamed tissue [175]. Others have proposed that the NO produced during periods of ischemia may minimize the ROS-mediated damage that results at the time of reperfusion due to the ability of NO to promote vasodilation and inhibit inflammation [164]. The most convincing evidence supporting a pathophysiological role for the nitrite reductase function of XO comes from studies that show protection against I/R injury following nitrite administration. The protective effect of nitrite-derived NO in heart [176], [177], kidney[178], lung [179], and liver [180] models of I/R injury are not observed following inhibition of XO with either allopurinol or oxypurinol, suggesting that the enzyme plays a critical role in the generation of NO by .
A characteristic response of the vasculature to I/R is an enhanced recruitment of inflammatory cells, particularly neutrophils [160], [181], [182]. Similarly, endothelial cell monolayers exposed to either H/R or A/R exhibit an increased adhesivity to neutrophils (Fig. 2) [42], [43], [183]. Xanthine oxidase, more than any other potential source of ROS, has been implicated in the leukocyte recruitment that occurs following I/R in vivo and A/R (or H/R) in vitro. The substantial neutrophil recruitment (typically measured as an increased tissue myeloperoxidase activity) observed in tissues exposed to I/R [171], [184], [185], the enhanced leukocyte–endothelial cell adhesion in post-ischemic venules [43], [160], [181], [182], and increased neutrophil adhesion to post-hypoxic endothelial cell monolayers [42], [43], [183], [186] (Fig. 2) are all significantly attenuated by treatment with an XO inhibitor, SOD, or catalase. Studies employing in vivo and in vitro models have revealed a role for both inflammatory mediator production/release [42], [43], [183], [187], [188] and increased expression of endothelial cell and leukocyte adhesion molecules [42], [43], [160] as potential links between XO-dependent ROS production and the leukocyte recruitment response to I/R. Hydrogen peroxide, which is known to initiate the production of platelet activating factor (PAF) and increased surface expression of P-selectin [81], [189], appears to play an important role in this regard. Endothelial cell monolayers exposed to A/R exhibit a biphasic neutrophil adhesion response [42], with an early (30 min) enhancement of adhesion that is transcription-independent and inhibited by treatment with either oxypurinol, catalase and blocking antibodies for either P-selectin or ICAM-1 (Fig. 2). At 240 min following reoxygenation, a second phase of neutrophil hyperadhesivity is noted that is transcription-dependent and inhibited by treatment with either a PAF receptor antagonist, E-selectin blocking antibody, or antisense oligonucleotide directed against the oxidant-sensitive transcription factors, nuclear factor kappa-B (NFkB) or activator protein-1 (AP-1) [42]. In vivo studies have confirmed a role for PAF, ICAM-1 and P-selectin as mediators of XO-dependent leukocyte recruitment in postischemic venules [160], [187], [188]. Based on these observations it has been proposed that XO-derived ROS may play a more important role in mediating the reperfusion injury response by promoting the recruitment and/or activation of leukocytes, rather than XO-derived ROS directly mediating the tissue injury response [19], [190], [191], [192], [193]. This possibility is supported by reports describing a comparable level of protection against I/R injury following either inhibition of xanthine oxidase, induction of neutropenia, or blockade of leukocyte-endothelial cell adhesion [19], [160], [194], [195], [196], [197]
3.1.2. NADPH oxidase
The Nox/Duox family of NADPH oxidases has also been implicated in the production of ROS following I/R [198], [199], [200], [201], [202]. This family of multiprotein complexes is comprised of 7 members, designated as Nox-1 to Nox-5 and as dual oxidases (Duox)-1 and -2 [202]. The Duox enzymes predominately produce hydrogen peroxide along with Nox-4, while the remaining Nox isoenzymes largely produce superoxide [199]. Although the NADPH oxidases are ubiquitously expressed, cell-specific localization of some isoenzymes (e.g., Nox-3 in inner ear, Nox-5 in spleen and testes) has been described. Nox-2 (formerly known as gp91phox) accounts for the ability of phagocytic cells (neutrophils) to generate ROS, but the enzyme is also present in all cells comprising the walls of blood vessels. Generally, the non-phagocytic Nox isoenzymes are expressed at lower levels than Nox-2 in phagocytes. Nonetheless, significant mRNA and protein levels for Nox/Duox are detected in many tissues and these enzymes are proving to be a major source of ROS in a variety of pathological conditions, including I/R [200], [201], [202]. A role for NADPH oxidase as a mediator of I/R injury has been proposed for a variety of tissues (Table 2). The proposition that Nox enzymes contribute to reperfusion injury is largely based on two lines of evidence, i.e., (1) an increased expression and/or activity of Nox in postischemic tissue [203], [204], [205], [206], [207], [208], [209], and (2) an attenuation of the I/R- (or H/R-) induced injury response and/or reduced ROS production following pharmacologic inhibition [204], [205], [206], [210], [211], [212], [213], [214] or genetic inhibition/deletion of Nox activity/protein [207], [210], [215], [216], [217], [218], [219]
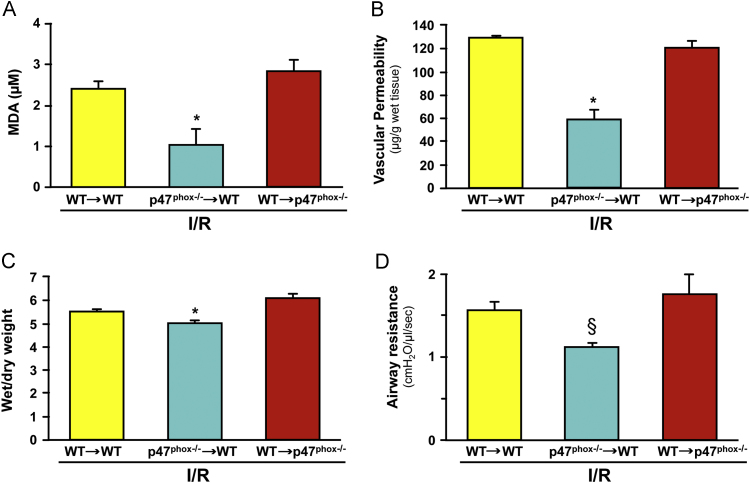
Studies implicating neutrophils in reperfusion injury provided some of the earliest evidence suggesting the involvement of Nox as a source of ROS in postischemic tissue [194], [220], [221], [222], [223], [224]. Neutrophil-dependent reperfusion injury has been implicated in a variety of tissues including heart [220], kidney [225], intestine [194], stomach [223], lung [197], skeletal muscle [196], brain [226], liver [227], skin [228], testes [229], as well as the systemic, multiple organ damage resulting from hemorrhagic shock [230], and the lung injury that accompanies reperfusion of intestine [231]. The potential involvement of neutrophils is supported by the observation that the time-course of the inflammatory cell accumulation corresponds with increased ROS generation and/or tissue injury following reperfusion [160], [232]. However, more convincing evidence has been provided by studies that show attenuated tissue injury and/or ROS production when reperfusion-induced neutrophil accumulation is blunted by either rendering the animal neutropenic [194], [220], [233], blocking neutrophil adhesion to vascular endothelial cells [194], [234], [235], or by blocking the production of (or receptors for) mediators (e.g., platelet activating factor, and leukotriene B4) that are engaged in the recruitment of leukocytes [188], [236], [237], [238]. Since inhibitors of ROS production or ROS scavengers have also been shown to be similarly effective in blunting reperfusion injury in these experimental models, it is generally assumed that the neutrophils are a major source of the ROS that mediate this response. However a reasonable alternative conclusion is that ROS generated from another cellular/enzymatic source (e.g., xanthine oxidase or mitochondria) play an essential role in the recruitment and activation of neutrophils [160], [184], [239], which ultimately mediate the tissue injury via a ROS-independent mechanism, such as proteases [240] or physical obstruction of the microvasculature [241]. The most convincing evidence supporting a role for neutrophil-associated Nox in reperfusion injury comes from studies employing bone marrow (BM) chimeras created by transplanting bone marrow from NADPH oxidase (Nox-2) deficient mice into wild type (WT) recipients. Studies of brain I/R have revealed either a partial [242] or dominant [243] role for BM-derived cells (likely leukocytes) in mediating the NADPH oxidase dependent blood brain barrier dysfunction and brain infarction following ischemic stroke. Reperfusion injury (increased vascular permeability, edema, oxidative stress, and increased airway resistance) in the lung is also attenuated in p47phox-deficient mice. Furthermore, a comparison of the responses between WT→WT, p47phox−/−→WT, and WT→p47phox−/− chimeras suggests that leukocyte-associated Nox has a dominant role in mediating lung injury following I/R [216] (Fig. 4).
Fig. 4.
Evidence implicating leukocyte-associated Nox in the lung injury response to 1 h ischemia and 2 h reperfusion. Data are presented for postischemic lungs from bone marrow chimeras produced by the transplantation of marrow from wild type (WT) mice into p47phox−/− recipients or vice versa (donor→recipient). (Panel A) Malondialdehyde (MDA) level in bronchioalveolar lavage fluid after reperfusion. (Panel B) Lung vascular permeability estimated from Evans blue accumulation in lung tissue. (Panel C) Pulmonary edema measured by wet/dry weight ratio after reperfusion. (Panel D) Lung airway resistance after reperfusion. Data derived from Yang et al. [216].
There are several lines of evidence that support a role for non-phagocytic Nox as a source of ROS following I/R (or H/R). It has been demonstrated that purified cultured cell populations, including endothelial cells [244], cardiac myocytes [245] and neurons [246] exposed to simulated I/R in vitro exhibit tissue injury-related responses that are dependent on Nox activity. For example, endothelial cells exposed to H/R show increases in NADPH oxidase expression/activity, superoxide production, NFkB activation, E-selectin expression, and adhesivity to leukocytes, with a corresponding reduction in barrier function. All of these responses are largely prevented by treatment of the endothelial cells with the Nox inhibitors, apocynin or diphenyliodonium (DPI), or with inhibitors of the signaling pathways that regulate Nox activation [244], [247], [248], [249], [250]. It has also been reported that the generation of ROS in hippocampal and cortical neurons in culture is significantly increased after oxygen/glucose deprivation and reoxygenation, but this response is absent following treatment with either apocynin or DPI and in cells derived from gp91phox knockout mice [246]. Similarly, cardiac myocytes subjected to in vitro simulated I/R display increased ROS production, enhanced lipid peroxidation, activation of redox-sensitive kinases (ERK, JNK) and cell death, all of which are prevented by DPI treatment [245]. Isolated buffer perfused organs, which minimize a potential contribution from intravascular Nox-positive cells such as PMNs, have also revealed a role for NADPH oxidase in the injury response to H/R. For example, isolated buffer perfused rat and mouse lungs exhibit enhanced ROS production following H/R and this response is blocked by treatment with either DPI or the synthetic peptide inhibitor of Nox, PR-39, with a similar attenuation of ROS production noted in isolated lungs derived from gp91phox knockout mice [251]. Other reports have similarly described a protective effect of Nox inhibitors in isolated buffer (cell free) perfused hearts exposed to H/R [252] and in buffer-perfused Langendorff preparations with hearts derived from mutant mice deficient in either Nox-1 or Nox-2 [253]. A third line of evidence that implicates non-phagocytic cell-associated Nox in I/R injury comes from studies of mice that are deficient in Nox isoforms not expressed in phagocytic cells. For example, Nox-4, which is expressed in astrocytes, neurons and microglia in the brain (but not in PMNs) [202], exhibits an increased expression following ischemic stroke [254], [255]. Mice deficient in Nox-4 are protected against stroke, with reductions in infarct size, oxidative stress, neuronal apoptosis, and BBB leakage, compared to their WT counterparts [256]. Studies of myocardial I/R injury in Nox-isoform specific knockout mice have revealed that Nox-1, Nox-2 and Nox-1/Nox-2 double knockouts have significantly reduced infarct sizes, compared to WT controls, while Nox-4 deficient mice show no protection. Since hearts from Nox-1 and Nox-2 knockout mice were similarly protected against I/R injury in the buffer perfused Langendorff model, it was concluded that the beneficial effects of these Nox isoform deletions are intrinsic to heart tissue, i.e., myocytes [253].
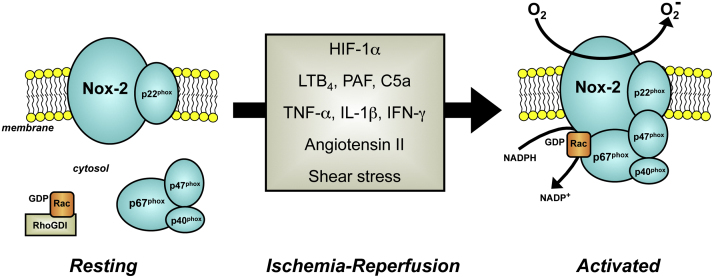
With the exception of Nox-4, all of the Nox homologs are constitutively inactive and require cell stimulation to generate ROS. In the resting state, critical protein subunits (e.g., p47phox, p40phox, and p67phox), and the small GTPase Rac1/2 of the enzyme are partitioned in the cytosol and spatially segregated from large catalytic core subunits (e.g., Nox-1 and Nox-2) that are integrated in the cell membrane (Fig. 5). Upon cell activation, the regulatory cytosolic subunits are assembled and translocated to the cell membrane where they dock with the Nox homolog. The fully assembled enzyme then generates superoxide via one-electron reduction of molecular oxygen using cytoplasmic NADPH as an electron donor [200], [202], [257]. While the signaling mechanisms that regulate NADPH oxidase assembly and activation are not completely understood, there is considerable evidence to support the potential involvement of a variety of factors in the activation of NADPH oxidase in tissues exposed to I/R. For example, the hypoxia that accompanies ischemia elicits the production and release of hypoxia inhibitory factor-1α (HIF-1α), which in turn promotes the production and activation of Nox [244], [258]. This activation mechanism is reinforced by the existence of a positive feedback loop wherein Nox-derived ROS stimulates the production of HIF-1α [201], [259]. Similarly, it has been demonstrated that the reduction in shear stress that occurs during ischemia, results in endothelial cell membrane depolarization via inactivation of ATP-sensitive potassium channels, which leads to activation of NADPH oxidase [260].
Fig. 5.
Stimuli that may act on Nox-2 positive cells following ischemia and reperfusion to elicit activation and/or induction of the enzyme. Hypoxia-inducible factor-1α (HIF-1α), leukotriene B4 (LTB4), platelet activating factor (PAF), complement component-5a (C5a), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interferon-Υ (IFN-γ).
During ischemia and at the time of reperfusion, different chemical mediators with the potential to activate NADPH oxidase are produced and released by cells. The activation of phospholipase A2 that accompanies I/R results in the production of platelet activating factor (PAF) as well as the generation of arachidonic acid and its subsequent metabolism to thromboxane and leukotrienes (e.g., LTB4), all of which engage with specific cell receptors and ultimately prime or activate NADPH oxidase [1], [261], [262], [263]. Another consequence of I/R is activation of the complement system, which has also been implicated in the increased activity of NADPH oxidase in post-ischemic tissue [203], [264]. Various cytokines (e.g., TNF-α, IL-1β) that are liberated from macrophages and mast cells following reperfusion have been shown to elicit both an increased activity and expression of NADPH oxidase [265], [266], [267], with cytokine blockade resulting in an attenuation of reperfusion-induced, NADPH oxidase-dependent ROS production [268]. Finally, angiotensin II, perhaps the most studied and best characterized stimulant for NADPH oxidase activation [269], has been implicated in reperfusion-induced NADPH oxidase activation, inflammation, and injury in several tissues [270], [271], [272], [273], [274]. These effects are mediated via engagement of the angiotensin II-type-1 receptor and likely involve the local generation of angiotensin II via angiotensin converting enzyme and chymase during I/R. Collectively, the available data in the literature suggest that I/R leads to the production of a diverse group of chemical mediators with a shared capacity to enhance both the activity and expression of Nox (Fig. 5), enabling the enzyme to generate ROS and mediate reperfusion injury.
Much of the evidence supporting a role for NADPH oxidase in reperfusion injury has come from studies employing the pharmacological inhibitors, apocynin and DPI. Apocynin exerts its inhibitory effect on Nox by interfering with membrane translocation of p47phox and p67phox, while DPI, a flavoprotein inhibitor, alters the electron transport capacity of the enzyme [200], [201], [275], [276]. Although both agents can effectively inhibit NADPH oxidase activity, they also exert other actions on cells that can make it difficult to attribute the actions specifically to Nox inhibition. For example, apocynin can act as an antioxidant, inhibit Rho kinase, and alter nitric oxide synthesis as well as arachidonic acid metabolism, while DPI inhibits a variety of flavoenzymes, including xanthine oxidase, nitric oxide synthase, and cytochrome P450 reductase, and inhibits mitochondrial enzymes [275], [276], [277], [278], [279]. The peptide inhibitors (e.g., PR-39 and gp91ds-tat) appear to offer greater specificity and some degree of selectivity to specific Nox isoforms (e.g., Nox-2 inhibition by gp91ds-tat), and there is growing interest in developing small molecule Nox inhibitors with greater specificity and isoform selectivity that can be used in human trials of ischemic disease [200], [201], [280], [281].
3.1.3. Mitochondria
Estimates of ROS production by mitochondria vary considerably, both quantitatively and qualitatively [282]. Some of this variability can be attributed to the lack of specificity and sensitivity of approaches used to measure the short lived ROS, particularly in vivo [283], [284], [285]. In addition, there are significant differences among species, tissues, cells, and even intracellular pools from which the mitochondria are derived [9], [286], [287]. Various isolation procedures may also impact mitochondrial structure and function [288], [289]. Finally, the specific experimental conditions imposed (e.g., inhibitors of electron transport and uncoupling agents) can result in abnormal mitochondrial function [290]. Given these caveats, it can be argued that the use of isolated mitochondria, sub-mitochondrial particles, or purified mitochondrial components (e.g., electron transport chain or TCA cycle enzymes) may have very little bearing on mitochondrial function in a normal cellular and/or tissue milieu [290], [291], [292]. However, reductionist approaches have proven useful for dissecting the various cellular/molecular mechanisms involved in mitochondrial function. Examples of the utility of these approaches include the TCA cycle and the chemiosmotic mechanism of oxidative phosphorylation [288]. Similarly, these approaches have greatly fueled progress in understanding the mechanisms by which mitochondria generate ROS. Specific intra-mitochondrial sites of ROS production and their targets have been identified [293], and significant advancements have been made in our understanding of the contributions of mitochondria-derived ROS to both redox signaling [294], [295], [296], [297] and I/R-induced pathology [9], [295], [296], [298], [299], [300].
Mitochondria have been implicated as a major source of I/R-induced ROS production in a variety of organs (Table 2); especially in those that are highly metabolically active, i.e., heart and brain [9], [290], [296], [298], [301]. This is not entirely surprising given the general view that mitochondria most likely generate ROS during the course of normal oxidative phosphorylation. The rapid movement of electrons through the electron transport chain (ETC) of the inner mitochondrial membrane can result in the leakage of electrons that can form via univalent reduction of O2 [282], [290], [295], [302], [303], [304], [305], [306], [307], [308]. Mitochondria contain enzymes that can generate ROS both in the matrix (e.g., TCA cycle) and in the membrane (e.g., NADPH oxidase) [290], [296], [309]. Herein, we discuss the general characteristics of the various mitochondrial sources of ROS and their potential roles in I/R.
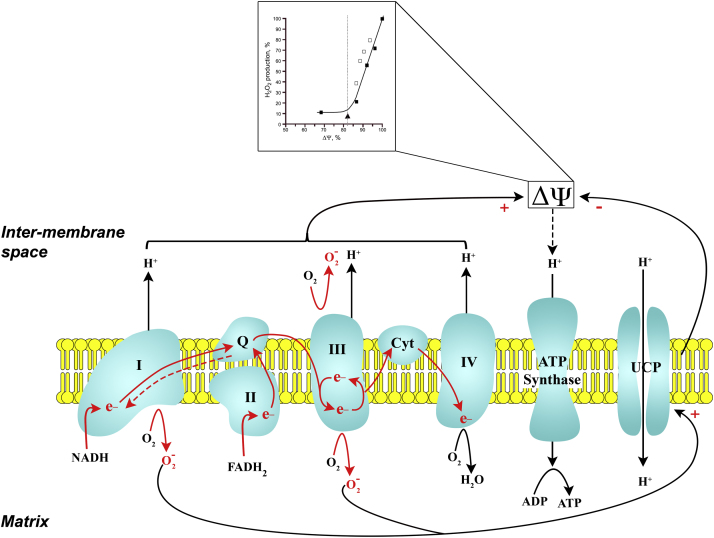
Mitochondria synthesize most of the cell's ATP by oxidative phosphorylation. Catabolism of carbohydrates (glycolysis), lipids (β-oxidation of fatty acids), and proteins (glucogenic and ketogenic pathways) yields products that enter the TCA cycle in the mitochondrial matrix. Intermediates generated during the oxidation reactions of the TCA cycle donate their reducing equivalents (H+ and electrons) to NAD+ and FADH to generate NADH and FADH2. β-oxidation of fatty acids in the mitochondrial matrix also contributes to the matrix pool of NADH and FADH2. Matrix NADH and FADH2 feed electrons and H+ into the ETC (Fig. 6). The ETC is comprised of a series of multi-subunit complexes (complexes I–IV) in the inner mitochondrial membrane that are coupled to mobile carriers (coenzyme Q and cytochrome c). The complexes and mobile carriers contain redox groups (Fe-S clusters and/or heme) that allow for the transfer of electrons along the components of the ETC [9], [290], [308]. They receive and transfer electrons in a sequence based on increasing redox potential (affinity for electrons) and decreasing free energy. NADH delivers electrons to complex I while FADH2 to complex II; electrons from these complexes are subsequently shuttled through complex III and IV. The energy released as electrons move through the ETC is used to transport H+ from the mitochondrial matrix to the inner membrane space. Since the inner mitochondrial membrane is impermeable to H+, a proton electrochemical gradient develops across the inner membrane with the inner membrane space more positive (and acidic) relative to the matrix. This proton-motive force is attributed primarily to the membrane potential (∆ψ) with a minor contribution by the pH gradient (∆pH). The proton-motive force is used to transport H+ through the membrane spanning complex, ATP synthase, thereby providing the energy for phosphorylation of ADP to form ATP.
Fig. 6.
ROS production by the electron transport chain (ETC): role of mitochondrial proton motive force (∆ψ). Electron carriers, NADH and FADH2 feed electrons (e−) into complex I and complex II of the ETC, respectively. As e− traverse through the ETC they release some of their energy to pump H+ into the intermembrane space generating a ∆ψ. The ∆ψ is used to move H+ through ATP synthase and phosphorylate ADP to ATP. At several points along the ETC (esp. complex I and III) there is a potential for e− to leak and reduce O2 to superoxide (). The rate of production is directly related to the level of ∆ψ; ∆ψ was varied using an uncoupling agent or inhibitors of ETC (inset; from Korshunov et al. [318]). A feedback mechanism exists to limit production by dissipating the ∆ψ. In this scenario, excessive production activates the uncoupling protein (UCP) allowing H+ to diffuse back into the matrix bypassing the ATP synthase. The increased efflux of H+ lowers the ∆ψ and reduces production. Red arrows, forward e− flow; red dashed arrow, reverse electron flow; Q, Coenzyme Q; Cyt, cytochrome c. (modified from Krauss et al. [326] and Chen and Zweier [298]).
Some of the mitochondrial complexes are organized into “supercomplexes” or “respirosomes”, rather than existing as independent complexes within the inner membrane. For example, complexes I, III, and IV exist both as free individual components and as supercomplexes containing various proportions of two (e.g., I–III) or three complexes (I–III–IV) [295], [310], [311], [312]. Supercomplex assembly and integrity is dependent on cardiolipin, a phospholipid present in the mitochondrial inner membrane [313], [314]. It is generally, albeit not universally [315], held that supercomplexes provide structural/functional linkages between the individual complexes thereby facilitating the tight coupling of electron transfer, with little or no leak of electrons [295], [310], [311], [312]. Thus, during normal metabolic activity most, if not all, of the electrons entering the ETC converge on cytochrome c oxidase (complex IV) where they reduce O2 to H2O. Interestingly, it has been proposed that oxidative damage to cardiolipin during I/R can result in disassembly of complex I and III from the supercomplex, thereby increasing the probability of ROS generation [295], [312].
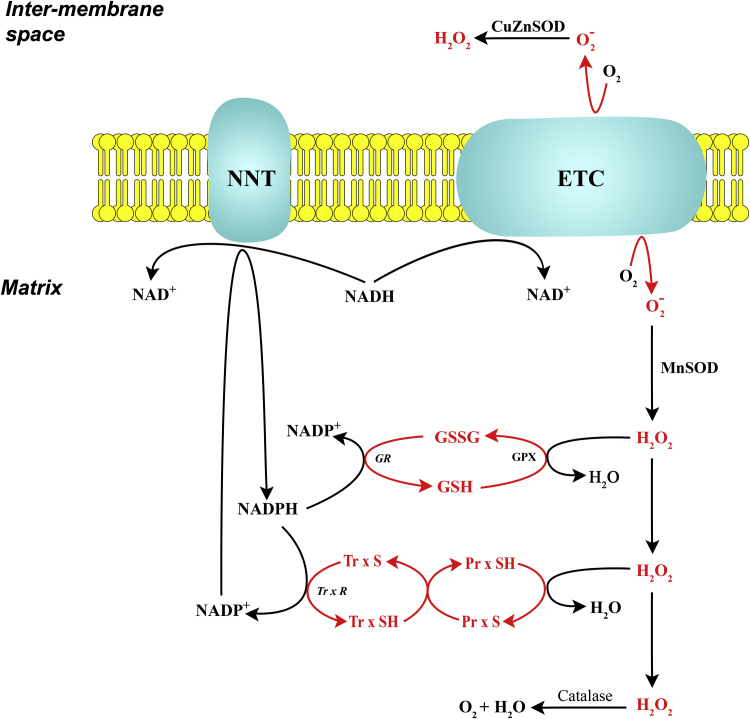
All of the components of the ETC have redox potentials that render them capable of transferring single electrons to O2 to form [295], [308]. Thus, the mitochondrial ETC is considered to be the major source of ROS during normal oxidative metabolism [9], [290], [296], [298], [308]. If mitochondria are respiring normally and generating ATP, H2O2 efflux from these organelles is minimal (0.1–0.2% of O2 consumed) [286], [295], [308], [316], [317]. Furthermore, as mitochondrial respiration rate increases, ROS production tends to decrease [286], [318]. Presumably, the low level of detectible ROS is due to the presence of an extensive intra-mitochondrial antioxidant system [282], [304] (Fig. 7). In order for mitochondrial ROS production to increase sufficiently to overcome the endogenous antioxidant system and achieve detectable levels, significant changes must occur, including exhaustion of the antioxidant systems, substrate modulation, and/or inhibition of ETC complexes [286], [318].
Fig. 7.
Mitochondrial anti-oxidant system. Superoxide () generated by the ETC (see Fig. 6 ) is converted to H2O2 by superoxide dismutase (SOD); MnSOD in the matrix and CuZnSOD in the intermembrane space, respectively. Catalase, glutathione peroxidase (GPx), and peroxiredoxin (Prx) convert H2O2 to H2O. GPx and Prx require glutathione (GSH) and thioredoxin (TrxSH) for their reduction, respectively. Glutathione disulfide (GSSG) is reduced back to GSH by glutathione reductase (GR), while TrxS is reduced back to TrxSH by thioredoxin reductase (TrxR). Both GR and TrxR depend on reducing equivalents from NADPH generated by transhydrogenase (NNT) (modified from Murphy [308] and Kowaltowski et al. [304]).
Substrate modulation or inhibition of individual mitochondrial complexes is commonly used to identify the specific complexes that are responsible for ROS production by the ETC. For example, under normal conditions, mitochondria from the heart, brain, or kidney produce larger amounts of H2O2 in the presence of succinate (targeting complex II), when compared to α-ketoglutarate (targeting complex I) [286]. Pharmacologic blockade of a distal complex results in the reduction of the proximal complexes (e.g., backup of electrons). The more reduced the proximal complexes become, the more likely these complexes will leak electrons and generate from O2. Based on these approaches, the major sites of production appear to be complexes I and III [282], [290], [304], [305], [306], [308], [319]. Complex I releases into the matrix, while complex III releases into both the matrix and the intermembrane space. Complex II which is a component of both the ETC and TCA cycle (succinate dehydrogenase) is generally dismissed as a major source of ROS, since excessive substrate (succinate) has to be supplied for complex II to generate [290]. However, it must be noted that others consider complex II as a reasonable source of electron leak, since the succinate concentrations used are within limits achieved in the heart and brain, particularly during ischemia [149], [305], [320]. In addition, complex II may be an indirect source of electron leak via complex I by reverse electron transport (RET). RET is an energy requiring process by which electrons entering complex II (via succinate oxidation), reach coenzyme Q (CoQ) and, instead of proceeding forward to complex III, are directed back to complex I [307], [308] (Fig. 6). Complex IV (cytochrome c oxidase) tends to tightly sequester electrons for the complete reduction of O2 to H2O, and thus is not believed to be a physiologic source of electron leak [321]. However, there is evidence that phosphorylation of complex IV can alter its function such that it may generate , particularly during hypoxia [322]. In summary, all the complexes have the redox components needed to generate ROS, but the general consensus holds that complexes I and III are the major potential sources under normal conditions [282], [290], [291], [306], [308].
production by complexes I and III appears to be directly related to the membrane potential (∆ψ) and/or the redox state of NADH/NAD+. Specifically, superoxide production by complex I is optimized by a high matrix NADH/NAD+ ratio (e.g., ischemia) and/or a high ∆ψ (e.g., non-phosphorylating conditions) both of which favor reduction of electron carriers [282], [305], [307], [308]. A high NADH/NAD+ ratio favors excessive movement of electrons through complex I and, if downstream electron carriers are reduced, the likelihood of superoxide production increases. Another mechanism by which complex I generates is by RET via complex II; usually requiring succinate as a substrate [305], [308]. RET is facilitated by a reduced CoQ and a high ∆ψ. The high membrane potential provides the required energy to drive electrons in reverse from CoQ to complex I (RET). This process has been noted to produce a substantial amount of in brain, heart, muscle and liver mitochondria [308]. The Q-cycle mechanism, which involves oxidation/reduction cycles of quinone, accounts for superoxide generation by complex III. This redox cycling of quinones generates unstable semiquinone intermediates at the cytoplasmic and matrix aspects of the inner mitochondrial membrane [282], [290], [291], [295], [298]. These semiquinones are believed to be the sources of generation, particularly during conditions associated with an increase in ∆ψ [295], [298]. The superoxide generated at complex III is directed to either the matrix or intermembrane space; with the latter target likely favored [282], [298].
The direct relationship between mitochondrial ∆ψ and ROS production [283], [318] provides the basis for the notion that uncoupling of oxidative phosphorylation blunts mitochondrial production (Fig. 6) [290], [308]. Mitochondrial uncoupling involves an increase in permeability of the inner membrane to H+, thereby dissipating the H+ gradient necessary for ATP synthesis. Indeed, mitochondrial uncoupling proteins (UCPs) are believed to function as regulators of mitochondrial ROS production [323]. In this scenario, excessive mitochondrial ROS production leads to an increase in UCP, which promotes protein leak back into the matrix; thereby reducing the ∆ψ (Fig. 6) [324], [325], [326]. A strategy of “mild” uncoupling is envisioned that reduces ROS production without compromising mitochondrial ATP production. Although uncoupling has been proposed as an endogenous protective mechanism in both mitochondrial physiology and pathology [323], it appears to be dysfunctional in I/R. For example, while uncoupling does reduce ROS production under basal conditions, uncoupling has no effect on ROS production by mitochondria isolated from I/R-challenged hearts [327].
It is fairly well established that I/R can alter mitochondrial structure and function. Mitochondria from I/R-challenged hearts exhibit deranged cristae, decreased number of supercomplexes and compromised ETC activity during the ischemic period. These ultrastructural and functional defects partially recover upon reperfusion, and are accompanied by increased generation [287], [292], [328], [329], [330], [331], [332]. It has been proposed that ischemic damage to complex I and III enhances their capacity to generate , setting the stage for a burst of ROS production upon reperfusion [331]. Isolated supercomplex I–III from mitochondria obtained from reperfused hearts can generate [328]. When the two individual complexes are separated from the supercomplex, both complex I and complex III still generate [328], [329]. It has been proposed that upon reperfusion, partial recovery of ETC activity without supercomplex re-assembly results in impaired oxidative phosphorylation and enhanced production [329], [330]. Moreover, the enhanced production of ROS by ETC complexes may in turn damage adjacent complexes, propagating ETC dysfunction and amplifying ROS production [292].
All of the ETC complexes have been implicated as both sources and targets of the ROS generated during I/R [287], [298], [321], [333], [334]. Consequently, considerable effort has been devoted to defining the mechanism(s) by which ETC complexes are targeted by ROS and can act as initiators of mitochondrial ROS production. Oxidant challenge of submitochondrial particles (membranes containing ETC complexes) inactivates various complexes; with hydroxyl radical being more potent than or H2O2 [335]. Other redox-sensitive mechanisms have also been implicated in I/R-induced ROS production by mitochondrial ETC, and there is evidence favoring a role for ROS-induced peroxidation of cardiolipin and/or alterations in the phosphorylation status of the complexes. Cardiolipin, a phospholipid of the inner mitochondrial membrane, is critical for normal activity of the ETC complexes [313], [314]. I/R-induced decreases in the activities of complexes I and III are associated with cardiolipin peroxidation [328], [330], [336], [337]. Liposomal delivery of exogenous intact cardiolipin, but not oxidized cardiolipin, can restore complex I and III activities. Cardiolipin is also important for stabilization of complex IV and oxidation of cardiolipin will lead to disassembly of complex IV, thereby decreasing its activity [321]. Collectively, these observations support the contention that cardiolipin is critical for complex assembly, stability, and appropriate electron transfer. I/R-induced oxidative stress results in peroxidation of cardiolipin, rendering it ineffective in stabilizing the complexes, and upon disassembly the complexes generate additional ROS causing progressive mitochondrial dysfunction [295], [321], [337], [338].
All of the ETC complexes (complexes I–V) contain potential sites for phosphorylation (e.g., serine, threonine residues), and it has been proposed that either translocated or resident kinases/phosphatases can regulate their phosphorylation status [339]. For example, the redox-sensitive δPKC translocates to the mitochondria after reperfusion of the ischemic myocardium where it initiates production [340]. Moreover, in mitochondria isolated from (1) hearts challenged with I/R or (2) cells grown under hypoxic conditions, complex IV (cytochrome c oxidase) is phosphorylated [322]. In both situations complex IV activity is compromised and there is an increase in production. The I/R-induced complex IV hyper-phosphorylation and inactivation, enhanced ROS production, and myocardial injury are mitigated by pharmacologic inhibition of protein kinase A [322], [341]. Of note, the hypoxia-induced increase in mitochondrial PKA activity has been attributed to local generation of ROS [342]. Based on the available data, it has been proposed that during ischemia the ETC complexes are de-phosphorylated (at least complex IV) by phosphatases (priming) and, with reperfusion, the primed complexes generate excessive ROS [9].
ETC complexes may also serve as the initial generators of ROS in postischemic tissue. As mentioned above, the activities of the ETC complexes are diminished during ischemia and then recover upon reperfusion [329]. When the reactivation of complex I is delayed, the reperfusion-induced generation of H2O2 is prevented and myocardial infarct size is reduced [343]. Additional support for a primary role for ETC complexes in initiating mitochondrial ROS production stems from a metabolomic analysis of various tissues subjected to ischemia [149]. A common metabolite that was increased in ischemic heart, brain, kidney, and liver was the TCA cycle intermediate, succinate. The increase in succinate accumulation was attributed to a reversal of succinate dehydrogenase activity by a switch to anaerobic metabolism during ischemia. Prevention of succinate accumulation during the ischemic period abolished mitochondrial H2O2 production upon reperfusion and ameliorated the injury in heart and brain. Moreover, pharmacologic inhibition of complex I has led to the proposal that the oxidation of succinate by complex II upon reperfusion results in RET through complex I and ROS generation [149]. Interesingly, succinate accumulation in the neonatal brain after ischemia/hypoxia (I/H) appears to be beneficial in limiting infarct size [344]. Unilateral common carotid artery occlusion followed by systemic hypoxia (no reperfusion) resulted in transiently increased succinate concentrations in the penumbral region within 90 min and significantly reduced infarct size at 4 days post-I/H. The protective effect of succinate was attributed to the ability of this metabolite to induce angiogenesis. Collectively, these observations indicate that the increase in succinate levels during ischemia results in enhanced mitochondrial ROS production and more tissue injury upon reperfusion, yet in the absence of reperfusion succinate appears to prevent expansion of ischemic injury by restoring the energy status in the affected region via angiogenesis.
There are a variety of other mitochondrial sources of ROS that may also contribute to the accelerated ROS production associated with I/R. For example, Nox4 has been implicated as a source of mitochondrial ROS generation [290], [296], presumably due to its localization in mitochondria [345], [346]. However, Nox4 has also been localized to the plasma membrane as well as ER and microsomal membranes [346], [347]. As addressed above, the role of Nox-4 in I/R-induced tissue injury is controversial, with some studies demonstrating protection [256] and others showing no protection [253] against the injury response in Nox-4 deficient mice. Despite the absence of a consensus regarding the role of Nox-4 in I/R-induced oxidant stress and tissue injury, the multiple loci of Nox-4 makes it difficult to ascribe a specific role for mitochondria-associated Nox-4 as a mediator of this response.
Monoamine oxidase (MAO), localized on the outer mitochondrial membrane, is known to generate H2O2 as an obligatory by-product of oxidative deamination of neurotransmitters and other biogenic amines [290], [295], [296], [348]. As expected, MAO is prevalent in brain neurons; but it is also expressed in peripheral tissues (e.g., heart, kidney, liver, and intestine). There are two isoforms with different substrate specificities; MAO-A (e.g., norepinephrine and dopamine) and MAO-B (e.g., serotonin and histamine). Overexpression of cardiac MAO or dopamine administration can trigger H2O2 production by cardiomyocyte mitochondria [285], [349], while knockdown of MAO-A (microRNA) reduces neuroblastoma cell ROS production by 50% [350]. Of particular relevance are the observations that pharmacologic blockade of MAO can ameliorate I/R-induced injury to the heart [351], [352] and brain [353], [354].
The p66Shc protein is an isoform of the adaptor protein ShcA that can be activated by ROS (among other apoptotic stimuli) and, in turn, generate ROS [290], [295]. There appear to be cytoplasmic, ER, and mitochondrial pools of inactive p66Shc [355], [356]. In response to oxidative stress signals, there is an increase in the mitochondrial pool of p66Shc [356] where it interacts with cytochrome c of the ETC [355]. Active p66Shc diverts electrons from the ETC by oxidizing cytochrome c and reducing O2 to /H2O2 [355], [356]. In addition to generating ROS, it can also inhibit transcriptional upregulation of antioxidant enzymes [357]. Genetic ablation of p66Shc decreases oxidant stress in various cells and tissues [358], [359], [360]. Furthermore, mice deficient in p66Shc exhibit less I/R injury in heart [359] or skeletal muscle [358].
The structurally related pyruvate dehydrogenase (PDH) and α-ketoglutarate dehydrogenase (α-KDH) complexes, as well as other NAD-linked dehydrogenases, are capable of producing /H2O2 under appropriate experimental conditions (e.g., high substrate and increased NADH/NAD+ ratio) [361], [362], [363]. The ROS generating capabilities of PDH and α-KDH are attributed to the dihydrolipoamide dehydrogenase subunit, common to both [364]. Under optimum conditions, the maximum achievable rates of ROS generation by PDH and α-KDH into the matrix can exceed that of complex I [361]. α-glycerophosphate dehydrogenase (α-GPD) is another NAD-linked dehydrogenase that is localized to the outer surface of the inner membrane and capable of generating ROS primarily into the intermembrane space [317], [362]. It has been proposed that the NAD-linked dehydrogenases may make a significant contribution to mitochondrial ROS production in postischemic tissue, since the mitochondrial NADH/NAD+ ratio is elevated in response to ischemia/hypoxia [362]. However, the only experimental evidence that supports this contention is the observation that pharmacologic inhibition of α-KDH can protect against hypoxia- or H2O2-induced microglial ROS production and death [365].
The TCA enzymes located in the mitochondrial matrix, particularly aconitase, are sensitive to oxidant-mediated inactivation [366]. Consequently, aconitase inactivation is often used as a marker for mitochondrial oxidant stress. Aconitase has also been proposed as a source of ROS [290], [298]. The enzyme contains an iron-sulfur cluster in its active site, which can be disassembled upon exposure to /H2O2, releasing free Fe++ and H2O2 to generate hydroxyl radicals via Fenten chemistry [367], [368]. An assessment of aconitase stability and activity in intact mitochondria isolated from hearts subjected to 30 min coronary occlusion followed by reperfusion has revealed a reduction in aconitase activity, with no evidence of disassembly of the iron-sulfur cluster or degradation of the enzyme [369]. These finding do not preclude a possible role for aconitase-derived oxidants in I/R injury after longer durations (>30 min) of ischemia.
Irrespective of the source, the net production of ROS by mitochondria is determined by the relative rates of ROS generation (Fig. 6) and scavenging (Fig. 7) [300], [304]. is dismutated to H2O2 by the SOD isoenzymes; Mn–SOD in the matrix and CuZn–SOD in the inner membrane space and cytosol. H2O2 is converted to H2O by the glutathione peroxidase (GPx) and peroxiredoxin (Prx)/thioredoxin (Trx) systems. Both of these thiol-based scavenging systems are dependent on a reducing environment maintained by a high NADPH/NADP+ ratio. The generation NADPH from NADP+ is, in turn, dependent on several mitochondrial enzymes, such as, NADP-transhydrogenase (NNT), which converts NADP to NADPH (Fig. 7). Interestingly, both electron transport through the ETC (increased potential for ROS production) and the activity of NNT (increased anti-oxidant status) are dependent on NADH (e.g., Krebs cycle). It has been proposed that this coupling minimizes net ROS production by energized mitochondria that are generating ATP; referred to as the “redox-optimized ROS balance” [306], [370], [371], [372]. In mitochondria isolated from I/R-challenged hearts, the redox-optimized ROS balance is altered in favor of ROS production [327]. During ischemia, the mitochondrial NADPH pool is decreased and does not recover upon reperfusion. Predictably, mitochondrial ROS production is increased after I/R; a response that is attributed to depletion of the thiol-based antioxidant pool [327]. In addition, I/R challenge of thioredoxin reductase 2 deficient hearts enhances both mitochondrial and myocardial dysfunction; effects that are corrected by thiol supplementation [373]. Finally, the protection against I/R-induced cardiac arrhythmia and infarction that is afforded by exercise has been attributed to enhanced glutathione reductase activity [374].
Of the numerous potential sources of mitochondrial ROS production during I/R, the ETC appears to play a dominant role and the most extensively studied [9], [290], [296], [298], [301]. The tendency of various ETC complexes to generate ROS in response to ROS exposure underlies the notion that mitochondrial ROS production is a self-amplifying process. This amplification of ROS production by the ETC has been termed “ROS-induced ROS release” (RIRR) [290], [375], [376], [377], [378], [379], [380], [381]. Laser-excitation of cardiac mitochondria results in a gradual increase in ROS production, and when a “threshold” level is achieved, a rapid loss of ∆ψ and a burst in ROS production ensues [375], [377]. The time required to reach threshold levels is indirectly proportional to glutathione levels [375], indicating that once the mitochondrial ROS scavenging mechanisms are overwhelmed a burst of ROS production occurs [372]. A similar phenomenon has been noted in cardiomyocytes exposed to hypoxia followed by reoxygenation (Fig. 8) [382]. The coincidence of the increased ROS production with the collapse of ∆ψ is inconsistent with the prevailing view that ROS production increases exponentially with increases in ∆ψ (see inset in Fig. 6). The only explanation that has been offered for the unexpected findings is the induction of “extreme disequilibrium” by H/R [290]. Interestingly, an analogous situation is noted when mitochondria are exposed to uncoupling agents. As discussed above, uncoupling of the ETC reduces ∆ψ and ROS production by mitochondria derived from normal hearts, but not in mitochondria from hearts subjected to I/R [327].
Fig. 8.
Hypoxia/reoxygenation (H/R): mitochondrial ROS-induced ROS release leading to cell death. (Panel A) H/R-induced increase in ROS and decrease in membrane potential (∆ψ) of a single mitochondria of a cardiomyocyte. Immediately upon reoxygenation there is an increase ∆ψ and gradual increase in ROS. Once a threshold level of ROS is achieved, ROS generation increases sharply and ∆ψ falls precipitously. (Panel B) H/R-induced loss of ∆ψ and ROS in a network of mitochondria within a cardiomyocyte. Numerous mitochondria that are completely depolarized (dark areas in upper panel) are associated with ROS production (green areas in lower panel). (Panel C) H/R-induced cell death. Several hours after reoxygenation about 2/3 of the cardiomyocytes are dead. The number of cells dying after H/R is reduced by an inhibitor of MTP (cyclosporine A; CSA). Adapted from Juhaszova et al. [382].
Since the mitochondria in cardiomyocytes are arranged in a three-dimensional lattice near the myofilaments, both the ∆ψ depolarization and ROS production spread to adjacent mitochondria within the myocyte after exposure to H/R (Fig. 8). The spread of ROS from one mitochondrion to another is attributed to the opening of pores/channels in the inner mitochondrial membrane, which allows for inter-mitochondrial signaling. Both an inner membrane anion channel (IMAC) and the mitochondrial permeability transition pore (MPTP) have been implicated in RIRR within mitochondrial networks [290], [378]. These two channels appear to operate sequentially; contingent on the thiol redox status of the mitochondrial matrix and cytoplasm [383]. Moderate reductions in the GSH/GSSG ratio open the IMAC, while more severe reductions open the MPTP [383]. Opening the IMAC is associated with transient depolarizations that develop into sustained oscillations in ∆ψ and ROS that spread within mitochondrial networks, eventually becoming a cell-wide phenomenon. In cardiomyocytes, these oscillations in RIRR and ∆ψ induce abnormal electrical events (e.g., action potential duration) [377] which can be linked to arrhythmias in intact hearts subjected to various oxidant stresses, including I/R [378], [384], [385]. Opening the MPTP is associated with a more drastic and sustained depolarization, with the eventual collapse of ∆ψ, a burst of ROS production, and ultimately cell death (Fig. 8) [375]. There is a direct relationship between the number of mitochondria exhibiting ∆ψ collapse/ROS production and myocyte dysfunction/death [382], [386], [387]. Interestingly, a variety of pharmaceutical agents shown to exert protection against I/R-induced infarcts in brain and heart appear to increase the threshold for ROS-induced MPTP opening [382].
In silico approaches incorporating in vivo and ex vivo data predict that IMAC and MPTP work in tandem to initiate and propagate mitochondrial network excitability [388]. Specifically, the model predicts that mild transients elicit opening of IMAC and oscillations in ∆ψ. Once the thiol-scavenging systems are overwhelmed, there is a larger burst of /H2O2, which opens the MPTP and leads to more dramatic and sustained depolarizations (and eventual collapse) of the ∆ψ and ultimately mitochondrial (and eventually cellular) demise. However an alternative mechanism has been proposed for RIRR within mitochondrial networks that involve only one channel/pore, the MPTP. In this scheme, the transient oscillatory waves and ∆ψ transients are attributed to reversible openings of the MPTP [290], [389]. Again, once the redox balance is tipped in favor of ROS, the MPTP opening remains stable resulting in propagation of the redox and ∆ψ waves throughout the mitochondrial networks [290].
A major obstacle to identifying the specific channels/pores by which RIRR is transmitted throughout the mitochondrial network is uncertainty about the molecular identity of the MPTP [390], [391] and IMAC [377]. Current bias favors the F-ATP synthase (complex V) as a major component of the MPTP [392], while the molecular structure of the IMAC remains more ambiguous [290]. The current approach used to determine the contribution of a particular channel/probe largely relies on drugs that are assumed to specifically target relevant structures and/or their modulators. Another problem clouding this issue is the specificity of the probes used to measure ROS waves or transients. Transient generation by mitochondria has been described in a variety of cell types and these “flashes” are enhanced in cardiomyocytes challenged with A/R [393]. However, it is unclear whether the probe is detecting or pH transients [389], [394]. Thus, a firmer understanding of the mechanisms involved in RIRR during I/R await the development of probes that can accurately and specifically assess transient generation by mitochondria as well as identification of the structural equivalent of the relevant channel/pore.
3.1.4. Nitric oxide synthase (NOS)
There are three recognized isoforms of NOS, neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS) [395], [396]. In general, eNOS and nNOS are constitutively expressed, while iNOS is inducible. As their designation implies, eNOS is primarily expressed in endothelial cells and nNOS is primarily localized to brain/neurons. However, their organ/cellular distribution is more ubiquitous, e.g., both nNOS and eNOS are present in cardiomyocytes. Moreover, the subcellular localization of eNOS and nNOS can change with cell activation [397]. Finally, while iNOS is primarily induced in immune cells, such as macrophages, its expression can be increased in diverse cell populations and tissues [396], [398]. The existence of a mitochondrial NOS isoform (mtNOS) has been proposed, but this is still debated [295], [399]. A specific gene for mtNOS has not been identified and mitochondria-associated NOS (when noted) may actually be one of the recognized isoforms, e.g., nNOS or iNOS [400], [401].
All NOS isoforms generate nitric oxide (NO) via the oxidation of L-arginine. In general, eNOS and nNOS generate small amounts of NO primarily for physiologic cell signaling, while iNOS produces larger amounts that have potentially detrimental (e.g., cytotoxicity) consequences for target cells/tissues. NO, either derived from eNOS (or nNOS) or exogenously administered as pharmaceutical NO-donors, appears to be protective against I/R-induced injury in different organs of experimental animals [402], [403], [404], [405], [406] and humans [407]. The general consensus is that NO is protective in IR by virtue of its anti-oxidant (neutralization of ) and anti-inflammatory (inhibition of neutrophil adhesion/emigration) [406,[408], [409] properties. Some of the findings that appear to disagree with the beneficial effects of NO in I/R have been attributed, in part, to mis-localization and timing of the NO supplementation [410]. In addition, the beneficial effects of NOS activity can be negated (or even reversed) when NOS is converted to a generating enzyme; the net result is a reduction in cellular NO with a concomitant increase in [395], [396], [411], [412]. This enzymatic conversion is referred to as NOS uncoupling and it has been implicated in I/R-induced ROS generation.
All NOS isoforms contain both an oxygenase and reductase domain (Fig. 9) [395], [396]. The reductase domain contains the flavins (FAD and FMN) and binds NADPH, while the oxygenase domain contains heme and tetrahydrobiopterin (BH4) and binds arginine. Arginine serves as the substrate, O2 and NADPH as co-substrates, while BH4 serves as a cofactor (Fig. 9B). Normally, electrons from NADPH are transferred through the reductase domain (via flavins) to the prosthetic heme in the oxidase domain. O2 binds to the heme and is reduced to form a ferrous–dioxygen complex; a step facilitated by BH4. This activation of O2 allows for arginine oxidation and results in the formation of NO and citrulline. Thus, O2 reduction at the heme catalytic site is normally coupled to the generation NO [395], [396], [411]. When electron flow to O2 is “uncoupled” from arginine oxidation, the reduced O2 is released from the heme as . NOS uncoupling can involve structural (dimer disassembly) and/or functional (insufficient cofactors) alterations of the enzyme. While the uncoupling of NOS can be demonstrated with each of the three isomers, the pathologic consequences of eNOS uncoupling has received the most attention.
Fig. 9.
Monomeric and dimeric forms of NOS. (Panel A) NOS monomers can transfer electrons from NADPH to the flavins in the reductase domain where limited amounts of can be formed. NOS monomers cannot bind the cofactor (BH4) or the substrate (arginine) and cannot generate NO. (Panel B) Formation of a NOS dimer requires heme, which allows for the transfer of electrons from the reductase domain of one monomer to the oxidase domain of the adjacent monomer. When sufficient arginine and BH4 are present, NOS dimers couple their heme, allowing for O2 reduction and the synthesis of NO. From Forstermann and Sessa [396] where the structure and catalytic function of NOS are discussed in greater detail.
NOS is present in cells either as a monomer or homodimer. The dimer couples O2 reduction to the synthesis of NO because the transfer of electrons occurs in trans from the reductase of one monomer to the oxygenase of the adjacent monomer. The monomer in isolation cannot couple O2 reduction to NO synthesis and generates instead [395], [396]. Since the activity of NOS as a monomer is relatively weak, only small amounts of are most likely formed [395]. However, the net result may not be inconsequential, i.e., a decrease in cellular NO with a concomitant increase in would favor ROS-induced damage to cellular constituents. It has been argued that once a dimer is formed it is relatively stable and significant reversal to the monomeric form is unlikely to occur in vivo [395]. This contention is supported by the observation that, in a cell-free system or in isolated endothelial cells, NOS uncoupling and production can occur in the absence of any dimer conversion to monomers [413]. However, in both in vivo and in vitro models of I/R the dimer/monomer ratio falls and can be rescued by maneuvers that re-couple NOS and prevent I/R injury [414], [415]. Regardless of the relative importance of dimer disassembly, significant functional uncoupling of NOS can occur when either substrates or cofactors are limiting.
BH4, a NOS cofactor required for NO synthesis and release [411], [416], is synthesized from GTP via a pathway in which GTP cyclohydrolase I (GTPCH) is the rate-limiting enzyme; cellular BH4 levels are directly correlated with GTPCH protein [417]. A strict stoichiometric relationship between BH4 and eNOS dictates whether NOS is predominatly a NO or generating enzyme [411], [416], [418]. Decreases in the local BH4/NOS ratio increase the probability of NOS producing [411], [419]. Thus, simply increasing eNOS protein levels in endothelial cells either as a result of increasing shear stress [420] or overexpression of eNOS [419] without concomitant increases in GTPCH protein/activity and BH4 leads to NOS uncoupling, i.e., decrease in dimer/monomer ratio and production [419], [420]. However, oxidative stress (e.g., I/R) decreases cellular BH4 levels, reflecting the oxidation of BH4 to BH2 [411], [416]. BH2 can occupy the BH4 binding site on NOS, but it is not catalytically active, resulting in NOS uncoupling. Thus, in response to oxidative stress, a positive feedback loop can be initiated in which production by uncoupled NOS leads to further BH4 oxidation to BH2 and the propagation of NOS uncoupling (analogous to mitochondrial RIRR). In the face of this stress, cells are capable of maintaining BH4 levels by recycling BH2 to BH4 via dihydrofolate reductase (DHFR) [421]. Based on these responses, it is proposed that the ratio of BH4 to BH2 plays a more important role in maintaining NOS coupling than the absolute level of BH4 [421].
The impact of BH4 levels on NOS uncoupling has been assessed in both in vitro and in vivo models of I/R. BH4 content and NOS activity in isolated rat hearts subjected to ischemia fall in parallel and these changes are accompanied by large increases in NOS-derived production [422]. A reduction in the BH4/BH2 ratio and NOS uncoupling has also been noted in an in vitro model of I/R (Fig. 10) [423]. The I/R-induced reduction in BH₄ has been attributed to generated by either xanthine oxidase [423] or NADPH oxidase [424]. BH4 supplementation can re-couple NOS and ameliorate the I/R-induced cardiac inflammation and tissue damage [414], [425], [426], [427]. BH4 supplementation, either directly or via over-expression of GTPCH, is also effective in preventing cardiomyocyte or endothelial cell cytotoxicity in in vitro models of A/R [415], [423], [426]. Similarly, substrates for BH4 synthesis (sepiaterin and folate) have been shown to protect against I/R-induced myocardial inflammation and contractile dysfunction [428], [429]. Similar observations have been noted in I/R models in the kidney [430], liver [431], and skeletal muscle [432]. Finally, if BH4 levels are reduced and eNOS is uncoupled prior to I/R, the resultant injury responses in both liver and heart are exacerbated [433].
Fig. 10.
Role of BH4 in NOS uncoupling in endothelial cells during H/R in vitro (Panels A–C) and I/R-induced cardiac injury in vivo (Panel D). (Panel A) Intracellular production (DHE-derived fluorescence intensity) is increased in endothelial cells subjected to H/R. *p<0.002 vs control (normoxia). (Panel B) BH4 decreases while BH2 increases in endothelial cells subjected to H/R. *p<0.004 vs control (normoxia). (Panel C) NO production by endothelial cells is decreased during H/R and is partially rescued by BH4 supplementation. *p<0.002 vs control (normoxia) #p<0.002 vs H/R. (Panel D) Liposomal BH4 (6R BH4) reduces myocardial infarction (as percent of area at risk; AAR) in rat hearts subjected to I/R. Control represents I/R and vehicle represents empty liposome. *p<0.001 vs control. Panels A–C modified from De Pascali et al. [423] while panel D modified from Xie et al. [508].
Arginine, serving as the nitrogen donor, is required for the synthesis of NO by NOS. Arginine is also a substrate for arginase present in mammalian tissues in two isoforms, arginase I and II [434], [435]. Arginase I is a cytosolic enzyme that generates urea for detoxification of ammonia and, while primarily localized in hepatocytes, is also present in other cell types (e.g., endothelium, cardiomyocyte, and vascular smooth muscle). Arginase II is a ubiquitously expressed mitochondrial enzyme that generates ornithine, the precursor to polyamines that are involved in cell proliferation, stress responses, and general maintenance. Since the two isoforms share close to 60% homology [434], [435], isoform-specific inhibitors are lacking [435], [436]. This makes it difficult to ascribe a role for either isoform and thus herein both isoforms will simply be referred to as arginase.
It is widely held that when arginase activity is increased it can consume an amount of arginine that is sufficient to limit NO production by NOS [396], [416], [434], [435]. This “arginine steal” mechanism not only decreases NO production but also increases generation by uncoupling NOS. After reperfusion of ischemic liver [437] or heart [434], [438], [439], arginase activity increases, arginine levels and NO production decrease, and generation increases, providing support for an “arginine steal” mechanism of NOS uncoupling. Pharmacologic inhibition of arginase or arginine supplementation restores the ability of NOS to generate NO and ameliorates various indices of tissue injury. Endothelium-dependent vasodilation is impaired by I/R in porcine coronary arteries [440] and in the radial arteries of patients with coronary artery disease [441]; an effect that is ameliorated by arginase inhibition. Finally, inhibition of arginase in blood-perfused, but not saline-perfused, hearts has been shown to rescue I/R-induced myocardial dysfunction [442]. When RBCs from wild type mice were used to perfuse hearts from eNOS deficient mice, arginase blockade was effective in ameliorating the I/R-induced dysfunction. However, when RBCs derived from eNOS deficient mice were used in wild type heart preparations, arginase blockade was not effective. These observations suggest that RBCs contain functional eNOS, which can significantly increase NO bioactivity in the reperfused myocardium.
There are conflicting reports concerning the importance of arginase in NOS uncoupling. For example, in liver, brain, and kidney, arginase activity is not increased when measured 24 h after reperfusion [443], [444]. This can be attributed, in part, to the biphasic changes in arginase activity following I/R; there is a transient increase in activity which returns to basal levels by 24 h of reperfusion [445], [446], [447], followed by a second increase in enzyme activity days later [439], [447], [448]. Moreover, the “arginine steal” mechanism does not appear to play an important role in NOS uncoupling in kidney parenchyma; neither arginase administration [449], [450] nor arginine supplementation [448] alters the changes in renal histopathology and inflammation induced by I/R. Whether this diminished capacity for an “arginine steal” is related to the fact that the kidney is the predominant site of arginine production in the body is unclear. Finally, in both rats and pigs, administration of either arginine or BH4 alone, either prior to or at the time of reperfusion, do not affect the extent of myocardial infarction induced by I/R; infarct size was reduced only following treatment with a combination of arginine and BH4 [451]. It was proposed that arginine alone was ineffective because arginase activity was increased by I/R. However, it is possible that the combination of arginine and BH4 is required to effectively couple NOS. Systemic administration of arginine/BH4 was shown to be effective in the rat, but not in the pig, where intracoronary administration was required for an effect [451]. This was attributed to the 10-fold greater collateral flow in the rat than pig, thus allowing the systemically administered NOS couplers more ready access to the area at risk than in the rat. From the above, it is apparent that more work is warranted to clarify the role of arginase in NOS uncoupling, keeping in mind the biphasic changes in arginase activity, the species and route of administration of inhibitors, and the organ system under study.
4. Interactions between ROS producing enzymes
Most studies addressing the role of oxidative stress in experimental models of reperfusion injury have focused on (and implicated) a single enzymatic source of ROS. However, there are several reports that describe a similar protective effect of multiple treatments that target different sources of ROS, such as mitochondrial complex 1 (rotenone) xanthine oxidase (allopurinol) or Nox (apocynin) [268], [452], [453], [454], [455], [456]. While the ability of the different agents to each afford nearly complete protection in a model of I/R injury may reflect some degree of non-specificity of the reagents, a more likely explanation is redox signaling that enables ROS produced by one enzymatic source to activate and enhance ROS production by a second source. The possibility of such interactions between the four major sources of ROS that have been implicated in I/R injury is supported by evidence that H2O2, a readily diffusible primary or secondary product of all four enzymatic sources, can act as an intracellular messenger that mediates ROS-induced ROS production, thereby propagating its own accumulation within cells [457]. The net result of such a signaling mechanism would be a significant amplification of ROS production in response to a pro-oxidative insult or condition. While it remains unclear whether interactions between ROS sources does indeed make a quantitatively important contribution to the magnitude of the oxidative stress elicited by I/R, there is ample evidence (summarized in several recent reviews) [458], [459], [460] to suggest that ROS-induced ROS production warrants attention in this field of investigation. Some noteworthy examples of the interactions that may occur between ROS sources after I/R are briefly discussed below.
Interactions between Nox and mitochondria have been extensively studied. This work has revealed that NADPH oxidase (Nox-1 or -2) activation can lead to enhanced mitochondrial ROS (mtROS) formation via a signaling mechanism that involves Nox-derived hydrogen peroxide activating ATP sensitive K+ channels in the mitochondrial membrane and opening the MPTP, with the subsequent release of mtROS into the cytosol [461]. Similarly, an enhancement of mtROS production elicited by hypoxia has been shown to activate Nox via a protein kinase C dependent mechanism that yields the phosphorylation of cytosolic Nox subunits and their subsequent translocation to the cell membrane [460], [462]. The two-way interactions, or cross-talk, between mitochondria and Noxs has given rise to the concept that activation of one enzymatic source of ROS can activate other sources and a vicious cycle of enhanced cellular ROS production in response to stimuli such as I/R. The critical role of mitochondrial ROS in such a signaling process that amplifies cellular ROS production in response to either angiotensin II [463] or hypoxia [462] is supported by studies that have employed targeted over-expression or inhibition of either MnSOD or glutathione peroxidase in mitochondria. Furthermore, it has been demonstrated that depletion of mitochondrial SOD (MnSOD) enhances Nox activity, while MnSOD overexpression blunts the activation of Nox [463].
Enhanced mitochondrial ROS production has also been linked to the conversion of XDH to XO in a rat model of volume overload (VO) induced heart failure [464]. The study revealed that XO inhibition with allopurinol attenuates the mitochondrial dysfunction that is associated with VO and that cyclic stretch of cardiomyocytes activates XO through a mechanism dependent on mitochondrial ROS. These findings are consistent with a self-perpetuating cycle by which activated XO produces ROS that damage mitochondria, that in turn causes further ROS production and XO activation [464].
While there is considerable evidence (described above) that implicates XO-derived ROS in the recruitment and activation of leukocytes, less is known about the potential for leukocyte-associated Nox to activate endothelial cell XO and vice verse. The potential for such an interaction is supported by studies demonstrating that activated adherent neutrophils elicit the conversion of XDH to XO in endothelial cells and induce endothelial cell injury via a XO dependent mechanism [465], [466], [467]. Activated leukocytes, when firmly adherent to endothelial cells, result in a significant increase in the intracellular concentration of H2O2, of which approximately 60% is derived from the leukocyte and 40% from endothelial cell XO [467]. The possibility that neutrophil (Nox)-derived ROS mediates these responses was addressed by treating the activated neutrophils with antioxidant enzymes (SOD, catalase), which failed to confer protection. Similarly, exposure of endothelial cells to relevant concentrations of exogenous H2O2 failed to recapitulate the responses elicited by activated neutrophils [465]. However, some reports have attributed the neutrophil-mediated XDH to XO conversion to neutrophil-derived elastase [468], while other studies fail to implicate elastase, but invoke a role for tyrosine kinase activation or increased intracellular calcium in the endothelial cell response [466], [467]. Consequently, while the cross-talk between adherent activated neutrophils and endothelial cell XO may not be explained by ROS-activated ROS production, it appears to involve a mechanism of XO activation that is related to some other chemical and/or physical signal emanating from the leukocyte.
Nitric oxide synthase also appears to modulate the activation of XO. The increased XO activity detected in pulmonary endothelial cells exposed to hypoxia alone or H/R is significantly enhanced following treatment of the cells with a NOS inhibitor, while the change in XO activity is blunted by L-arginine supplementation or when the cells are exposed to NO donating agents, suggesting that NO exerts an inhibitory influence on XO activity [469], [470]. The ability of NO to inactivate XO has also been demonstrated in interferon gamma-stimulated macrophages [471]. While the precise mechanism for this inhibitory action of NO on XO remains unclear, the observed responses have been attributed to the binding of NO to the iron moiety of XO or its sulfhydryl groups [469], [471]. In the presence of NOS uncoupling, an entirely different interaction between NOS and XO is observed. As described above, the xanthine oxidase-mediated ROS produced by aortic endothelial cells exposed to H/R results in both tetrahydrobiopterin depletion and S-glutathionylation, which leads to eNOS uncoupling and further ROS production [423]. A similar BH4 depletion dependent mechanism likely results when H/R initially elicits the production of superoxide from either mitochondria or Nox [472]. Regardless of the initial source of superoxide, the consequent increase in ROS production by uncoupled NOS, when combined to reduced availability of NO for inactivation of XO-derived superoxide, would result in a more profound oxidative stress in response to H/R.
5. Summary and conclusions
Progress in the field of reperfusion research has been rapid and significant over the past 3–4 decades. The concept that ROS are produced at an accelerated rate in tissues subjected to I/R and that the accumulation of ROS contributes to reperfusion injury is now well established and supported by a large and growing body of evidence. A large part of this progress can be attributed to continued improvement and refinement of the technologies and experimental approaches used to characterize the tissue/cellular responses to I/R, including mutant mice with an altered expression of ROS-related proteins, reagents that selectively inhibit specific enzymatic sources of ROS production, and more precise methods for detection and quantification of ROS production in intact living tissue, cultured cell monolayers, and subcellular compartments (e.g., mitochondria). As a result of these advancements and the more widespread use of cell culture models, the field has transitioned from an early emphasis on the direct damaging effects of ROS to a focus on molecular aspects of cell dysfunction resulting from the activation of ROS-dependent signaling events that underlie reperfusion injury.
Notable progress has been made in defining the potential contribution of different enzymatic sources of ROS production in tissues/cells exposed to I/R (H/R). Early studies in this field placed emphasis on XO as a major source of ROS after I/R. However, the recognition that some tissues exhibit low to negligible expression/activity of this enzyme provided the impetus to evaluate the contribution of other potential ROS sources, including Nox and mitochondria. Uncoupled NOS and other enzymes (see Table 2) that were not extensively addressed in the narrative above have also been ascribed a role in reperfusion injury. While there is ample evidence in the literature to tentatively assign a dominant role for specific sources of ROS production in certain tissues (e.g., XO in the XOR-rich intestine, mitochondria in the metabolically active heart and brain), the available body of knowledge in this field does not yet justify definitive conclusions in this regard. Drawing such conclusions are made all the more difficult because of the potential for extensive cross-talk between these enzymes and their susceptibility to ROS-activated ROS production. The relative contributions of the different enzymes to I/R-induced ROS production in a given tissue is also likely to be influenced by the physiologic status of the tissue, e.g., with inflamed tissues producing a different pattern of enzyme involvement due to transcriptional up-regulation of certain ROS-producing enzymes (e.g., XO, Nox, and NOS) by cytokines. Similarly, chronic conditions that promote mitochondrial biogenesis (e.g., skeletal muscle following aerobic exercise training) may also influence the relative roles of the different enzymes to I/R-induced ROS production. The recognition that there are redundant and interactive sources of ROS that are activated in response to I/R is an important consideration in future efforts to design novel therapies that are directed towards targeting ROS to attenuate or prevent reperfusion injury.
Despite the long and growing list of clinical conditions that have been linked to reperfusion injury (Table 1) and the expansive literature that implicates ROS in this injury response, the clinical benefit of ROS-targeted therapies have largely proven to be absent or very limited. [473] While clear explanations for the failure to effectively extend the positive preclinical findings to the clinical setting are not readily apparent, preclinical models that inadequately mimic the complexities of the human disease counterpart is one likely explanation. In this regard, a notable deficiency in the field of reperfusion research is the prevalence of studies that focus on the injury response and underlying mechanisms in otherwise healthy animals. Epidemiological evidence clearly demonstrates that individuals who are most likely to experience an ischemic episode have one or more risk factors (e.g., hypertension, hypercholesterolemia, obesity, diabetes, and cigarette smoking) for cardiovascular disease. Each of these risk factors is known to induce a low-grade pro-inflammatory and pro-oxidative environment that renders tissues more vulnerable to the deleterious effects of a secondary oxidative and inflammatory stress such as I/R [474]. The few published studies that address the responses to I/R in the presence of risk factor(s) have revealed a more robust production of ROS and greater tissue damage following reperfusion, compared to the responses elicited by I/R in the absence of the risk factor(s). However, it remains unclear whether the relative contributions of the different ROS producing enzymes to the I/R-induced oxidative stress differ due to the presence or absence of a risk factor for cardiovascular disease. While this limitation is not unique to the field of reperfusion research, it does offer a challenge for future investigators in this area to refine experimental models so that they more accurately mimic the conditions that exist in most patients who experience reperfusion injury. Furthermore, this issue warrants more attention in order to ensure that meaningful progress can be made towards the translation of preclinical findings to the clinical setting, and for the eventual discovery of novel ROS-directed drugs that can be used to effectively treat reperfusion injury.
Conflict of interest
None.
Acknowledgments
The authors are grateful for the assistance of Janice Russell and Hafsa Mohammed Zareen in preparing the illustrations that accompany this manuscript. DNG is supported by a Grant from the National Heart Lung and Blood Institute (HL26441-32) and PRK is supported by a KACST Grant of Saudi Arabia (11-MED1672-20).
Footnotes
Supported by a Grant from the National Heart Lung and Blood Institute (HL26441) and the KACST of Saudi Arabia (11-MED1672-20).
References
- 1.Carden D.L., Granger D.N. Pathophysiology of ischaemia-reperfusion injury. J. Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Raedschelders K., Ansley D.M., Chen D.D. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol. Ther. 2012;133:230–255. doi: 10.1016/j.pharmthera.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Costantino C., Corday E., Lang T.W., Meerbaum S., Brasch J., Kaplan L., Rubins S., Gold H., Osher J. Revascularization after 3 h of coronary arterial occlusion: effects on regional cardiac metabolic function and infarct size. Am. J. Cardiol. 1975;36:368–384. doi: 10.1016/0002-9149(75)90492-0. [DOI] [PubMed] [Google Scholar]
- 4.Kloner R.A., Ganote C.E., Whalen D.A., Jr., Jennings R.B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am. J. Pathol. 1974;74:399–422. [PMC free article] [PubMed] [Google Scholar]
- 5.Reimer K.A., Lowe J.E., Rasmussen M.M., Jennings R.B. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–794. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 6.Hearse D.J., Humphrey S.M., Chain E.B. Abrupt reoxygenation of the anoxic potassium-arrested perfused rat heart: a study of myocardial enzyme release. J. Mol. Cell. Cardiol. 1973;5:395–407. doi: 10.1016/0022-2828(73)90030-8. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez L.M., Moeser A.J., Blikslager A.T. Animal models of ischemia-reperfusion-induced intestinal injury: progress and promise for translational research. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;308:G63–G75. doi: 10.1152/ajpgi.00112.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore R.M. Clinical relevance of intestinal reperfusion injury in horses. J. Am. Vet. Med. Assoc. 1997;211:1362–1366. [PubMed] [Google Scholar]
- 9.Sanderson T.H., Reynolds C.A., Kumar R., Przyklenk K., Huttemann M. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 2013;47:9–23. doi: 10.1007/s12035-012-8344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guarnieri C., Flamigni F., Caldarera C.M. Role of oxygen in the cellular damage induced by re-oxygenation of hypoxic heart. J. Mol. Cell. Cardiol. 1980;12:797–808. doi: 10.1016/0022-2828(80)90081-4. [DOI] [PubMed] [Google Scholar]
- 11.Granger D.N., Rutili G., McCord J.M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81:22–29. [PubMed] [Google Scholar]
- 12.Downey J.M. Free radicals and their involvement during long-term myocardial ischemia and reperfusion. Annu. Rev. Physiol. 1990;52:487–504. doi: 10.1146/annurev.ph.52.030190.002415. [DOI] [PubMed] [Google Scholar]
- 13.Jung J.E., Kim G.S., Chen H., Maier C.M., Narasimhan P., Song Y.S., Niizuma K., Katsu M., Okami N., Yoshioka H., Sakata H., Goeders C.E., Chan P.H. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol. Neurobiol. 2010;41:172–179. doi: 10.1007/s12035-010-8102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalogeris T., Baines C.P., Krenz M., Korthuis R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell. Mol. Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W.Z., Baynosa R.C., Zamboni W.A. Update on ischemia-reperfusion injury for the plastic surgeon. Plast. Reconstr. Surg. 2011;128:685e–692e. doi: 10.1097/PRS.0b013e318230c57b. [DOI] [PubMed] [Google Scholar]
- 16.Weyker P.D., Webb C.A., Kiamanesh D., Flynn B.C. Lung ischemia reperfusion injury: a bench-to-bedside review. Semin. Cardiothorac. Vasc. Anesth. 2013;17:28–43. doi: 10.1177/1089253212458329. [DOI] [PubMed] [Google Scholar]
- 17.Nishijima K., Kiryu J., Tsujikawa A., Miyamoto K., Honjo M., Tanihara H., Nonaka A., Yamashiro K., Katsuta H., Miyahara S., Honda Y., Ogura Y. Platelets adhering to the vascular wall mediate postischemic leukocyte-endothelial cell interactions in retinal microcirculation. Investig. Ophthalmol. Vis. Sci. 2004;45:977–984. doi: 10.1167/iovs.03-0526. [DOI] [PubMed] [Google Scholar]
- 18.Zhu P., Li J.X., Fujino M., Zhuang J., Li X.K. Development and treatments of inflammatory cells and cytokines in spinal cord ischemia-reperfusion injury. Mediat. Inflamm. 2013;2013:701970. doi: 10.1155/2013/701970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granger D.N. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am. J. Physiol. 1988;255:H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- 20.Ito Y., Abril E.R., Bethea N.W., McCuskey M.K., Cover C., Jaeschke H., McCuskey R.S. Mechanisms and pathophysiological implications of sinusoidal endothelial cell gap formation following treatment with galactosamine/endotoxin in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G211–G218. doi: 10.1152/ajpgi.00312.2005. [DOI] [PubMed] [Google Scholar]
- 21.Snoeijs M.G., van Heurn L.W., Buurman W.A. Biological modulation of renal ischemia-reperfusion injury. Curr. Opin. Organ Transpl. 2010;15:190–199. doi: 10.1097/MOT.0b013e32833593eb. [DOI] [PubMed] [Google Scholar]
- 22.Du H., Naqvi H., Taylor H.S. Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev. 2012;21:3324–3331. doi: 10.1089/scd.2011.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celik M., Aksoy A.N., Aksoy H., Aksoy Y., Halici Z. Sildenafil reduces ischemia-reperfusion injury in rat ovary: biochemical and histopathological evaluation. Gynecol. Obstet. Investig. 2014;78:162–167. doi: 10.1159/000363747. [DOI] [PubMed] [Google Scholar]
- 24.Ozturk H., Ozturk H., Terzi E.H., Bugdayci G., Duran A. Interleukin 10 reduces testicular damage in experimental testicular ischemia/reperfusion injury. Urology. 2014;83(508):e501–e506. doi: 10.1016/j.urology.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Parsons B.A., Kalejaiye O., Mohammed M., Persad R.A. The penile tourniquet. Asian J. Androl. 2013;15:364–367. doi: 10.1038/aja.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vos L.M., Slater J.J., Leijsma M.K., Stegenga B. Does hypoxia-reperfusion injury occur in osteoarthritis of the temporomandibular joint? J. Orofac. Pain. 2012;26:233–239. [PubMed] [Google Scholar]
- 27.Braunwald E., Kloner R.A. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation. 1982;66:1146–1149. doi: 10.1161/01.cir.66.6.1146. [DOI] [PubMed] [Google Scholar]
- 28.Ferdinandy P., Schulz R., Baxter G.F. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol. Rev. 2007;59:418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 29.Vollmar B., Menger M.D. Intestinal ischemia/reperfusion: microcirculatory pathology and functional consequences. Langenbecks Arch. Surg. 2011;396:13–29. doi: 10.1007/s00423-010-0727-x. [DOI] [PubMed] [Google Scholar]
- 30.Osborne N.N., Casson R.J., Wood J.P., Chidlow G., Graham M., Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Inauen W., Granger D.N., Meininger C.J., Schelling M.E., Granger H.J., Kvietys P.R. An in vitro model of ischemia/reperfusion-induced microvascular injury. Am. J. Physiol. 1990;259:G134–G139. doi: 10.1152/ajpgi.1990.259.1.G134. [DOI] [PubMed] [Google Scholar]
- 32.Kvietys P.R., Granger D.N. Endothelial cell monolayers as a tool for studying microvascular pathophysiology. Am. J. Physiol. 1997;273:G1189–G1199. doi: 10.1152/ajpgi.1997.273.6.G1189. [DOI] [PubMed] [Google Scholar]
- 33.Kokura S., Yoshida N., Yoshikawa T. Anoxia/reoxygenation-induced leukocyte-endothelial cell interactions. Free Radic. Biol. Med. 2002;33:427–432. doi: 10.1016/s0891-5849(02)00852-3. [DOI] [PubMed] [Google Scholar]
- 34.Li C., Jackson R.M. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am. J. Physiol. Cell. Physiol. 2002;282:C227–C241. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- 35.Okada H., Lai N.C., Kawaraguchi Y., Liao P., Copps J., Sugano Y., Okada-Maeda S., Banerjee I., Schilling J.M., Gingras A.R., Asfaw E.K., Suarez J., Kang S.M., Perkins G.A., Au C.G., Israeli-Rosenberg S., Manso A.M., Liu Z., Milner D.J., Kaufman S.J., Patel H.H., Roth D.M., Hammond H.K., Taylor S.S., Dillmann W.H., Goldhaber J.I., Ross R.S. Integrins protect cardiomyocytes from ischemia/reperfusion injury. J. Clin. Investig. 2013;123:4294–4308. doi: 10.1172/JCI64216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Mandat Schultz A., Bonnard A., Barreau F., Aigrain Y., Pierre-Louis C., Berrebi D., Peuchmaur M. Expression of tlr-2, tlr-4, nod2 and pnf-kappab in a neonatal rat model of necrotizing enterocolitis. PloS One. 2007;2:e1102. doi: 10.1371/journal.pone.0001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu D., Guo H., Griffin J.H., Fernandez J.A., Zlokovic B.V. Protein s confers neuronal protection during ischemic/hypoxic injury in mice. Circulation. 2003;107:1791–1796. doi: 10.1161/01.CIR.0000058460.34453.5A. [DOI] [PubMed] [Google Scholar]
- 38.Ozaki M., Haga S., Zhang H.Q., Irani K., Suzuki S. Inhibition of hypoxia/reoxygenation-induced oxidative stress in hgf-stimulated antiapoptotic signaling: role of pi3-k and akt kinase upon rac1. Cell. Death Differ. 2003;10:508–515. doi: 10.1038/sj.cdd.4401172. [DOI] [PubMed] [Google Scholar]
- 39.Hong S.J., Park E., Xu W., Jia S., Galiano R.D., Mustoe T.A. Response of human mature adipocytes to hypoxia-reoxygenation. Cytotherapy. 2014;16:1656–1665. doi: 10.1016/j.jcyt.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Veres G., Hegedus P., Barnucz E., Zoller R., Klein S., Schmidt H., Radovits T., Korkmaz S., Karck M., Szabo G. Endothelial dysfunction of bypass graft: Direct comparison of in vitro and in vivo models of ischemia-reperfusion injury. PloS One. 2015;10:e0124025. doi: 10.1371/journal.pone.0124025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terada L.S., Willingham I.R., Rosandich M.E., Leff J.A., Kindt G.W., Repine J.E. Generation of superoxide anion by brain endothelial cell xanthine oxidase. J. Cell. Physiol. 1991;148:191–196. doi: 10.1002/jcp.1041480202. [DOI] [PubMed] [Google Scholar]
- 42.Ichikawa H., Flores S., Kvietys P.R., Wolf R.E., Yoshikawa T., Granger D.N., Aw T.Y. Molecular mechanisms of anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Circ. Res. 1997;81:922–931. doi: 10.1161/01.res.81.6.922. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida N., Granger D.N., Anderson D.C., Rothlein R., Lane C., Kvietys P.R. Anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Am. J. Physiol. 1992;262:H1891–H1898. doi: 10.1152/ajpheart.1992.262.6.H1891. [DOI] [PubMed] [Google Scholar]
- 44.Inauen W., Payne D.K., Kvietys P.R., Granger D.N. Hypoxia/reoxygenation increases the permeability of endothelial cell monolayers: role of oxygen radicals. Free Radic. Biol. Med. 1990;9:219–223. doi: 10.1016/0891-5849(90)90031-d. [DOI] [PubMed] [Google Scholar]
- 45.Yin J., Luo X.G., Yu W.J., Liao J.Y., Shen Y.J., Zhang Z.W. Antisense oligodeoxynucleotide against tissue factor inhibits human umbilical vein endothelial cells injury induced by anoxia-reoxygenation. Cell. Physiol. Biochem. 2010;25:477–490. doi: 10.1159/000303053. [DOI] [PubMed] [Google Scholar]
- 46.Xue Q., Liu Y., Qi H., Ma Q., Xu L., Chen W., Chen G., Xu X. A novel brain neurovascular unit model with neurons, astrocytes and microvascular endothelial cells of rat. Int. J. Biol. Sci. 2013;9:174–189. doi: 10.7150/ijbs.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aslam M., Schluter K.D., Rohrbach S., Rafiq A., Nazli S., Piper H.M., Noll T., Schulz R., Gunduz D. Hypoxia-reoxygenation-induced endothelial barrier failure: role of rhoa, rac1 and myosin light chain kinase. J. Physiol. 2013;591:461–473. doi: 10.1113/jphysiol.2012.237834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J., Hong Z., Zeng C., Yu Q., Wang H. NADPH oxidase 4 promotes cardiac microvascular angiogenesis after hypoxia/reoxygenation in vitro. Free Radic. Biol. Med. 2014;69:278–288. doi: 10.1016/j.freeradbiomed.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 49.Eltzschig H.K., Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat. Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hebbel R.P. Ischemia-reperfusion injury in sickle cell anemia: relationship to acute chest syndrome, endothelial dysfunction, arterial vasculopathy, and inflammatory pain. Hematol. Oncol. Clin. N. Am. 2014;28:181–198. doi: 10.1016/j.hoc.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Zheng G.Q., Wang X.M., Wang Y., Wang X.T. Tau as a potential novel therapeutic target in ischemic stroke. J. Cell. Biochem. 2010;109:26–29. doi: 10.1002/jcb.22408. [DOI] [PubMed] [Google Scholar]
- 52.Osarogiagbon U.R., Choong S., Belcher J.D., Vercellotti G.M., Paller M.S., Hebbel R.P. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 2000;96:314–320. [PubMed] [Google Scholar]
- 53.Ryan S., Taylor C.T., McNicholas W.T. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 54.Yoshimoto K., Tajima H., Ohta T., Okamoto K., Sakai S., Kinoshita J., Furukawa H., Makino I., Hayashi H., Nakamura K., Oyama K., Inokuchi M., Nakagawara H., Itoh H., Fujita H., Takamura H., Ninomiya I., Kitagawa H., Fushida S., Fujimura T., Wakayama T., Iseki S., Shimizu K. Increased e-selectin in hepatic ischemia-reperfusion injury mediates liver metastasis of pancreatic cancer. Oncol. Rep. 2012;28:791–796. doi: 10.3892/or.2012.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lenglet S., Mach F., Montecucco F. Matrix metalloproteinase-9: a deleterious link between hepatic ischemia-reperfusion and colorectal cancer. World J. Gastroenterol. 2012;18:7131–7133. doi: 10.3748/wjg.v18.i48.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shlafer M., Kane P.F., Kirsh M.M. Superoxide dismutase plus catalase enhances the efficacy of hypothermic cardioplegia to protect the globally ischemic, reperfused heart. J. Thorac. Cardiovasc. Surg. 1982;83:830–839. [PubMed] [Google Scholar]
- 57.Korthuis R.J., Granger D.N., Townsley M.I., Taylor A.E. The role of oxygen-derived free radicals in ischemia-induced increases in canine skeletal muscle vascular permeability. Circ. Res. 1985;57:599–609. doi: 10.1161/01.res.57.4.599. [DOI] [PubMed] [Google Scholar]
- 58.Tamura Y., Chi L.G., Driscoll E.M., Jr., Hoff P.T., Freeman B.A., Gallagher K.P., Lucchesi B.R. Superoxide dismutase conjugated to polyethylene glycol provides sustained protection against myocardial ischemia/reperfusion injury in canine heart. Circ. Res. 1988;63:944–959. doi: 10.1161/01.res.63.5.944. [DOI] [PubMed] [Google Scholar]
- 59.Armstead W.M., Mirro R., Thelin O.P., Shibata M., Zuckerman S.L., Shanklin D.R., Busija D.W., Leffler C.W. Polyethylene glycol superoxide dismutase and catalase attenuate increased blood–brain barrier permeability after ischemia in piglets. Stroke J. Cereb. Circ. 1992;23:755–762. doi: 10.1161/01.str.23.5.755. [DOI] [PubMed] [Google Scholar]
- 60.McDonald M.C., Zacharowski K., Bowes J., Cuzzocrea S., Thiemermann C. Tempol reduces infarct size in rodent models of regional myocardial ischemia and reperfusion. Free Radic. Biol. Med. 1999;27:493–503. doi: 10.1016/s0891-5849(99)00100-8. [DOI] [PubMed] [Google Scholar]
- 61.Rak R., Chao D.L., Pluta R.M., Mitchell J.B., Oldfield E.H., Watson J.C. Neuroprotection by the stable nitroxide tempol during reperfusion in a rat model of transient focal ischemia. J. Neurosurg. 2000;92:646–651. doi: 10.3171/jns.2000.92.4.0646. [DOI] [PubMed] [Google Scholar]
- 62.Salvemini D., Wang Z.Q., Zweier J.L., Samouilov A., Macarthur H., Misko T.P., Currie M.G., Cuzzocrea S., Sikorski J.A., Riley D.P. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 63.Duann P., Datta P.K., Pan C., Blumberg J.B., Sharma M., Lianos E.A. Superoxide dismutase mimetic preserves the glomerular capillary permeability barrier to protein. J. Pharmacol. Exp. Ther. 2006;316:1249–1254. doi: 10.1124/jpet.105.092957. [DOI] [PubMed] [Google Scholar]
- 64.Kinouchi H., Epstein C.J., Mizui T., Carlson E., Chen S.F., Chan P.H. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing cuzn superoxide dismutase. Proc. Natl. Acad. Sci. USA. 1991;88:11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen E.P., Bittner H.B., Davis R.D., Folz R.J., Van Trigt P. Extracellular superoxide dismutase transgene overexpression preserves postischemic myocardial function in isolated murine hearts. Circulation. 1996;94:II412–II417. [PubMed] [Google Scholar]
- 66.Horie Y., Wolf R., Flores S.C., McCord J.M., Epstein C.J., Granger D.N. Transgenic mice with increased copper/zinc-superoxide dismutase activity are resistant to hepatic leukostasis and capillary no-reflow after gut ischemia/reperfusion. Circ. Res. 1998;83:691–696. doi: 10.1161/01.res.83.7.691. [DOI] [PubMed] [Google Scholar]
- 67.Li G., Chen Y., Saari J.T., Kang Y.J. Catalase-overexpressing transgenic mouse heart is resistant to ischemia-reperfusion injury. Am. J. Physiol. 1997;273:H1090–H1095. doi: 10.1152/ajpheart.1997.273.3.H1090. [DOI] [PubMed] [Google Scholar]
- 68.Ishibashi N., Weisbrot-Lefkowitz M., Reuhl K., Inouye M., Mirochnitchenko O. Modulation of chemokine expression during ischemia/reperfusion in transgenic mice overproducing human glutathione peroxidases. J. Immunol. 1999;163:5666–5677. [PubMed] [Google Scholar]
- 69.Sasaki T., Shimizu T., Koyama T., Sakai M., Uchiyama S., Kawakami S., Noda Y., Shirasawa T., Kojima S. Superoxide dismutase deficiency enhances superoxide levels in brain tissues during oxygenation and hypoxia-reoxygenation. J. Neurosci. Res. 2011;89:601–610. doi: 10.1002/jnr.22581. [DOI] [PubMed] [Google Scholar]
- 70.Park J.W., Qi W.N., Cai Y., Zelko I., Liu J.Q., Chen L.E., Urbaniak J.R., Folz R.J. Skeletal muscle reperfusion injury is enhanced in extracellular superoxide dismutase knockout mouse. Am. J. Physiol. Hear. Circ. Physiol. 2005;289:H181–H187. doi: 10.1152/ajpheart.00458.2004. [DOI] [PubMed] [Google Scholar]
- 71.Li Q., Bolli R., Qiu Y., Tang X.L., Guo Y., French B.A. Gene therapy with extracellular superoxide dismutase protects conscious rabbits against myocardial infarction. Circulation. 2001;103:1893–1898. doi: 10.1161/01.cir.103.14.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu J., Hou J., Xia Z.Y., Zeng W., Wang X., Li R., Ke C., Xu J., Lei S., Xia Z. Recombinant ptd-cu/zn sod attenuates hypoxia-reoxygenation injury in cardiomyocytes. Free Radic. Res. 2013;47:386–393. doi: 10.3109/10715762.2013.780286. [DOI] [PubMed] [Google Scholar]
- 73.Gebhardt B.R., Ries J., Caspary W.F., Boehles H., Stein J. Superoxide: a major factor for stress protein induction in reoxygenation injury in the intestinal cell line caco-2. Digestion. 1999;60:238–245. doi: 10.1159/000007664. [DOI] [PubMed] [Google Scholar]
- 74.Gao H., Korthuis R.J., Benoit J.N. Hypoxia/reoxygenation selectively impairs alpha 1b-adrenoceptor function in small mesenteric arteries. Am. J. Physiol. 1996;271:G820–G823. doi: 10.1152/ajpgi.1996.271.5.G820. [DOI] [PubMed] [Google Scholar]
- 75.Hu Q., Ziegelstein R.C. Hypoxia/reoxygenation stimulates intracellular calcium oscillations in human aortic endothelial cells. Circulation. 2000;102:2541–2547. doi: 10.1161/01.cir.102.20.2541. [DOI] [PubMed] [Google Scholar]
- 76.Yamada J., Yoshimura S., Yamakawa H., Sawada M., Nakagawa M., Hara S., Kaku Y., Iwama T., Naganawa T., Banno Y., Nakashima S., Sakai N. Cell permeable ros scavengers, tiron and tempol, rescue pc12 cell death caused by pyrogallol or hypoxia/reoxygenation. Neurosci. Res. 2003;45:1–8. doi: 10.1016/s0168-0102(02)00196-7. [DOI] [PubMed] [Google Scholar]
- 77.Lepore D.A., Shinkel T.A., Fisicaro N., Mysore T.B., Johnson L.E., d'Apice A.J., Cowan P.J. Enhanced expression of glutathione peroxidase protects islet beta cells from hypoxia-reoxygenation. Xenotransplantation. 2004;11:53–59. doi: 10.1111/j.1399-3089.2004.00082.x. [DOI] [PubMed] [Google Scholar]
- 78.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc. Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 79.Lum H., Barr D.A., Shaffer J.R., Gordon R.J., Ezrin A.M., Malik A.B. Reoxygenation of endothelial cells increases permeability by oxidant-dependent mechanisms. Circ. Res. 1992;70:991–998. doi: 10.1161/01.res.70.5.991. [DOI] [PubMed] [Google Scholar]
- 80.Bradley J.R., Johnson D.R., Pober J.S. Endothelial activation by hydrogen peroxide. Selective increases of intercellular adhesion molecule-1 and major histocompatibility complex class i. Am. J. Pathol. 1993;142:1598–1609. [PMC free article] [PubMed] [Google Scholar]
- 81.Lewis M.S., Whatley R.E., Cain P., McIntyre T.M., Prescott S.M., Zimmerman G.A. Hydrogen peroxide stimulates the synthesis of platelet-activating factor by endothelium and induces endothelial cell-dependent neutrophil adhesion. J. Clin. Investig. 1988;82:2045–2055. doi: 10.1172/JCI113825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kvietys P.R., Granger D.N. Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic. Biol. Med. 2012;52:556–592. doi: 10.1016/j.freeradbiomed.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sabri A., Byron K.L., Samarel A.M., Bell J., Lucchesi P.A. Hydrogen peroxide activates mitogen-activated protein kinases and Na+–H+ exchange in neonatal rat cardiac myocytes. Circ. Res. 1998;82:1053–1062. doi: 10.1161/01.res.82.10.1053. [DOI] [PubMed] [Google Scholar]
- 84.Faraci F.M. Reactive oxygen species: influence on cerebral vascular tone. J. Appl. Physiol. 2006;100:739–743. doi: 10.1152/japplphysiol.01044.2005. [DOI] [PubMed] [Google Scholar]
- 85.Peden D.B., Dailey L., DeGraff W., Mitchell J.B., Lee J.G., Kaliner M.A., Hohman R.J. Hydrogen peroxide effects on rat mast cell function. Am. J. Physiol. 1994;267:L85–L93. doi: 10.1152/ajplung.1994.267.1.L85. [DOI] [PubMed] [Google Scholar]
- 86.Breton-Romero R., Lamas S. Hydrogen peroxide signaling in vascular endothelial cells. Redox Biol. 2014;2:529–534. doi: 10.1016/j.redox.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schoenberg M.H., Fredholm B.B., Haglund U., Jung H., Sellin D., Younes M., Schildberg F.W. Studies on the oxygen radical mechanism involved in the small intestinal reperfusion damage. Acta Physiol. Scand. 1985;124:581–589. doi: 10.1111/j.1748-1716.1985.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 88.Kogure K., Watson B.D., Busto R., Abe K. Potentiation of lipid peroxides by ischemia in rat brain. Neurochem. Res. 1982;7:437–454. doi: 10.1007/BF00965496. [DOI] [PubMed] [Google Scholar]
- 89.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J. Gastroenterol. Hepatol. 2011;26(Suppl. 1):S173–S179. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- 90.Tatarkova Z., Aplan P., Matejovicova M., Lehotsky J., Dobrota D., Flameng W. Effect of ischemia and reperfusion on protein oxidation in isolated rabbit hearts. Physiol. Res. 2005;54:185–191. [PubMed] [Google Scholar]
- 91.Metzger J., Lauterburg B.H. Effect of allopurinol on oxidant stress and hepatic function following ischemia and reperfusion in the rat. Liver. 1988;8:344–349. doi: 10.1111/j.1600-0676.1988.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 92.Siems W., Kowalewski J., Werner A., Schimke I., Gerber G. Radical formation in the rat small intestine during and following ischemia. Free Radic. Res. Commun. 1989;7:347–353. doi: 10.3109/10715768909087961. [DOI] [PubMed] [Google Scholar]
- 93.Jackson R.M., Veal C.F. Effects of hypoxia and reoxygenation on lung glutathione system. Am. J. Physiol. 1990;259:H518–H524. doi: 10.1152/ajpheart.1990.259.2.H518. [DOI] [PubMed] [Google Scholar]
- 94.Shivakumar B.R., Kolluri S.V., Ravindranath V. Glutathione homeostasis in brain during reperfusion following bilateral carotid artery occlusion in the rat. Mol. Cell. Biochem. 1992;111:125–129. doi: 10.1007/BF00229583. [DOI] [PubMed] [Google Scholar]
- 95.al-Mehdi A., Shuman H., Fisher A.B. Fluorescence microtopography of oxidative stress in lung ischemia-reperfusion. Lab. Investig. J. Tech. Methods Pathol. 1994;70:579–587. [PubMed] [Google Scholar]
- 96.Kurose I., Wolf R.E., Grisham M.B., Granger D.N. Hypercholesterolemia enhances oxidant production in mesenteric venules exposed to ischemia/reperfusion. Arterioscler. Thromb. Vasc. Biol. 1998;18:1583–1588. doi: 10.1161/01.atv.18.10.1583. [DOI] [PubMed] [Google Scholar]
- 97.Pasdois P., Beauvoit B., Tariosse L., Vinassa B., Bonoron-Adele S., Dos Santos P. Effect of diazoxide on flavoprotein oxidation and reactive oxygen species generation during ischemia-reperfusion: a study on langendorff-perfused rat hearts using optic fibers. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H2088–H2097. doi: 10.1152/ajpheart.01345.2007. [DOI] [PubMed] [Google Scholar]
- 98.Rui T., Cepinskas G., Feng Q., Kvietys P.R. Delayed preconditioning in cardiac myocytes with respect to development of a proinflammatory phenotype: role of sod and nos. Cardiovasc. Res. 2003;59:901–911. doi: 10.1016/s0008-6363(03)00502-9. [DOI] [PubMed] [Google Scholar]
- 99.Beetsch J.W., Park T.S., Dugan L.L., Shah A.R., Gidday J.M. Xanthine oxidase-derived superoxide causes reoxygenation injury of ischemic cerebral endothelial cells. Brain Res. 1998;786:89–95. doi: 10.1016/s0006-8993(97)01407-8. [DOI] [PubMed] [Google Scholar]
- 100.Zweier J.L., Flaherty J.T., Weisfeldt M.L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci. USA. 1987;84:1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garlick P.B., Davies M.J., Hearse D.J., Slater T.F. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ. Res. 1987;61:757–760. doi: 10.1161/01.res.61.5.757. [DOI] [PubMed] [Google Scholar]
- 102.Kadkhodaee M., Hanson G.R., Towner R.A., Endre Z.H. Detection of hydroxyl and carbon-centred radicals by epr spectroscopy after ischaemia and reperfusion of the rat kidney. Free Radic. Res. 1996;25:31–42. doi: 10.3109/10715769609145654. [DOI] [PubMed] [Google Scholar]
- 103.Miller C.W., Chen G., Janzen E.G. Detection of free radicals in reperfused dog skin flaps using electron paramagnetic resonance spectroscopy: a pilot study. Microsurgery. 1999;19:171–175. doi: 10.1002/(sici)1098-2752(1999)19:4<171::aid-micr2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 104.Ophir A., Berenshtein E., Kitrossky N., Berman E.R., Photiou S., Rothman Z., Chevion M. Hydroxyl radical generation in the cat retina during reperfusion following ischemia. Exp. Eye Res. 1993;57:351–357. doi: 10.1006/exer.1993.1134. [DOI] [PubMed] [Google Scholar]
- 105.Egemnazarov B., Sydykov A., Schermuly R.T., Weissmann N., Stasch J.P., Sarybaev A.S., Seeger W., Grimminger F., Ghofrani H.A. Novel soluble guanylyl cyclase stimulator bay 41-2272 attenuates ischemia-reperfusion-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:L462–L469. doi: 10.1152/ajplung.90377.2008. [DOI] [PubMed] [Google Scholar]
- 106.Nilsson U.A., Aberg J., Aneman A., Lundgren O. Feline intestinal ischemia and reperfusion: relation between radical formation and tissue damage. Eur. Surg. Res. 1993;25:20–29. doi: 10.1159/000129253. [DOI] [PubMed] [Google Scholar]
- 107.Kono H., Woods C.G., Maki A., Connor H.D., Mason R.P., Rusyn I., Fujii H. Electron spin resonance and spin trapping technique provide direct evidence that edaravone prevents acute ischemia-reperfusion injury of the liver by limiting free radical-mediated tissue damage. Free Radic. Res. 2006;40:579–588. doi: 10.1080/10715760600606374. [DOI] [PubMed] [Google Scholar]
- 108.Nilsson U.A., Olsson L.I., Thor H., Moldeus P., Bylund-Fellenius A.C. Detection of oxygen radicals during reperfusion of intestinal cells in vitro. Free Radic. Biol. Med. 1989;6:251–259. doi: 10.1016/0891-5849(89)90052-x. [DOI] [PubMed] [Google Scholar]
- 109.Arroyo C.M., Carmichael A.J., Bouscarel B., Liang J.H., Weglicki W.B. Endothelial cells as a source of oxygen-free radicals. An esr study. Free Radic. Res. Commun. 1990;9:287–296. doi: 10.3109/10715769009145687. [DOI] [PubMed] [Google Scholar]
- 110.Zweier J.L., Broderick R., Kuppusamy P., Thompson-Gorman S., Lutty G.A. Determination of the mechanism of free radical generation in human aortic endothelial cells exposed to anoxia and reoxygenation. J. Biol. Chem. 1994;269:24156–24162. [PubMed] [Google Scholar]
- 111.Kumar V., Kleffmann T., Hampton M.B., Cannell M.B., Winterbourn C.C. Redox proteomics of thiol proteins in mouse heart during ischemia/reperfusion using icat reagents and mass spectrometry. Free Radic. Biol. Med. 2013;58:109–117. doi: 10.1016/j.freeradbiomed.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 112.Black K.M., Barnett R.J., Bhasin M.K., Daly C., Dillon S.T., Libermann T.A., Levitsky S., McCully J.D. Microarray and proteomic analysis of the cardioprotective effects of cold blood cardioplegia in the mature and aged male and female. Physiol. Genom. 2012;44:1027–1041. doi: 10.1152/physiolgenomics.00011.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cadete V.J., Lin H.B., Sawicka J., Wozniak M., Sawicki G. Proteomic analysis of right and left cardiac ventricles under aerobic conditions and after ischemia/reperfusion. Proteomics. 2012;12:2366–2377. doi: 10.1002/pmic.201100604. [DOI] [PubMed] [Google Scholar]
- 114.Ikejiri A.T., Somaio Neto F., Chaves J.C., Bertoletto P.R., Teruya R., Bertoletto E.R., Taha M.O., Fagundes D.J. Gene expression profile of oxidative stress in the lung of inbred mice after intestinal ischemia/reperfusion injury. Acta Cir. Bras. 2014;29:186–192. doi: 10.1590/S0102-86502014000300007. [DOI] [PubMed] [Google Scholar]
- 115.McLeod L.L., Alayash A.I. Detection of a ferrylhemoglobin intermediate in an endothelial cell model after hypoxia-reoxygenation. Am. J. Physiol. 1999;277:H92–H99. doi: 10.1152/ajpheart.1999.277.1.H92. [DOI] [PubMed] [Google Scholar]
- 116.Xu F., Mack C.P., Quandt K.S., Shlafer M., Massey V., Hultquist D.E. Pyrroloquinoline quinone acts with flavin reductase to reduce ferryl myoglobin in vitro and protects isolated heart from re-oxygenation injury. Biochem. Biophys. Res. Commun. 1993;193:434–439. doi: 10.1006/bbrc.1993.1642. [DOI] [PubMed] [Google Scholar]
- 117.Downey J.M., Miura T., Eddy L.J., Chambers D.E., Mellert T., Hearse D.J., Yellon D.M. Xanthine oxidase is not a source of free radicals in the ischemic rabbit heart. J. Mol. Cell. Cardiol. 1987;19:1053–1060. doi: 10.1016/s0022-2828(87)80350-4. [DOI] [PubMed] [Google Scholar]
- 118.Kennedy T.P., Rao N.V., Hopkins C., Pennington L., Tolley E., Hoidal J.R. Role of reactive oxygen species in reperfusion injury of the rabbit lung. J. Clin. Investig. 1989;83:1326–1335. doi: 10.1172/JCI114019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eddy L.J., Stewart J.R., Jones H.P., Engerson T.D., McCord J.M., Downey J.M. Free radical-producing enzyme, xanthine oxidase, is undetectable in human hearts. Am. J. Physiol. 1987;253:H709–H711. doi: 10.1152/ajpheart.1987.253.3.H709. [DOI] [PubMed] [Google Scholar]
- 120.Auscher C., Amory N., van der Kemp P., Delbarre F. Xanthine oxidase activity in human intestines. Histochemical and radiochemical study. Adv. Exp. Med. Biol. 1979;122B:197–201. doi: 10.1007/978-1-4684-8559-2_33. [DOI] [PubMed] [Google Scholar]
- 121.Della Corte E., Gozzetti G., Novello F., Stirpe F. Properties of the xanthine oxidase from human liver. Biochim. Biophys. Acta. 1969;191:164–166. doi: 10.1016/0005-2744(69)90327-1. [DOI] [PubMed] [Google Scholar]
- 122.Parks D.A., Granger D.N. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol. Scand. Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- 123.Harrison R. Structure and function of xanthine oxidoreductase: where are we now? Free Radic. Biol. Med. 2002;33:774–797. doi: 10.1016/s0891-5849(02)00956-5. [DOI] [PubMed] [Google Scholar]
- 124.Jarasch E.D., Bruder G., Heid H.W. Significance of xanthine oxidase in capillary endothelial cells. Acta Physiol. Scand. Suppl. 1986;548:39–46. [PubMed] [Google Scholar]
- 125.Vickers S., Schiller H.J., Hildreth J.E., Bulkley G.B. Immunoaffinity localization of the enzyme xanthine oxidase on the outside surface of the endothelial cell plasma membrane. Surgery. 1998;124:551–560. [PubMed] [Google Scholar]
- 126.Yokoyama Y., Beckman J.S., Beckman T.K., Wheat J.K., Cash T.G., Freeman B.A., Parks D.A. Circulating xanthine oxidase: potential mediator of ischemic injury. Am. J. Physiol. 1990;258:G564–G570. doi: 10.1152/ajpgi.1990.258.4.G564. [DOI] [PubMed] [Google Scholar]
- 127.Terada L.S., Dormish J.J., Shanley P.F., Leff J.A., Anderson B.O., Repine J.E. Circulating xanthine oxidase mediates lung neutrophil sequestration after intestinal ischemia-reperfusion. Am. J. Physiol. 1992;263:L394–L401. doi: 10.1152/ajplung.1992.263.3.L394. [DOI] [PubMed] [Google Scholar]
- 128.Adachi T., Fukushima T., Usami Y., Hirano K. Binding of human xanthine oxidase to sulphated glycosaminoglycans on the endothelial-cell surface. Biochem. J. 1993;289:523–527. doi: 10.1042/bj2890523. (Pt 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tan S., Yokoyama Y., Dickens E., Cash T.G., Freeman B.A., Parks D.A. Xanthine oxidase activity in the circulation of rats following hemorrhagic shock. Free Radic. Biol. Med. 1993;15:407–414. doi: 10.1016/0891-5849(93)90040-2. [DOI] [PubMed] [Google Scholar]
- 130.Houston M., Estevez A., Chumley P., Aslan M., Marklund S., Parks D.A., Freeman B.A. Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J. Biol. Chem. 1999;274:4985–4994. doi: 10.1074/jbc.274.8.4985. [DOI] [PubMed] [Google Scholar]
- 131.Battelli M.G., Bolognesi A., Polito L. Pathophysiology of circulating xanthine oxidoreductase: new emerging roles for a multi-tasking enzyme. Biochim. Biophys. Acta. 2014;1842:1502–1517. doi: 10.1016/j.bbadis.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 132.Nishino T., Okamoto K., Eger B.T., Pai E.F., Nishino T. Mammalian xanthine oxidoreductase – mechanism of transition from xanthine dehydrogenase to xanthine oxidase. FEBS J. 2008;275:3278–3289. doi: 10.1111/j.1742-4658.2008.06489.x. [DOI] [PubMed] [Google Scholar]
- 133.McCord J.M. Oxygen-derived free radicals in postischemic tissue injury. N Engl. J. Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 134.Parks D.A., Williams T.K., Beckman J.S. Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: a reevaluation. Am. J. Physiol. 1988;254:G768–G774. doi: 10.1152/ajpgi.1988.254.5.G768. [DOI] [PubMed] [Google Scholar]
- 135.Frederiks W.M., Bosch K.S. The proportion of xanthine oxidase activity of total xanthine oxidoreductase activity in situ remains constant in rat liver under various (patho)physiological conditions. Hepatology. 1996;24:1179–1184. doi: 10.1002/hep.510240533. [DOI] [PubMed] [Google Scholar]
- 136.Engerson T.D., McKelvey T.G., Rhyne D.B., Boggio E.B., Snyder S.J., Jones H.P. Conversion of xanthine dehydrogenase to oxidase in ischemic rat tissues. J. Clin. Investig. 1987;79:1564–1570. doi: 10.1172/JCI112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nishino T. The conversion of xanthine dehydrogenase to xanthine oxidase and the role of the enzyme in reperfusion injury. J. Biochem. 1994;116:1–6. doi: 10.1093/oxfordjournals.jbchem.a124480. [DOI] [PubMed] [Google Scholar]
- 138.Adkison D., Hollwarth M.E., Benoit J.N., Parks D.A., McCord J.M., Granger D.N. Role of free radicals in ischemia-reperfusion injury to the liver. Acta Physiol. Scand. Suppl. 1986;548:101–107. [PubMed] [Google Scholar]
- 139.Sanders S.A., Eisenthal R., Harrison R. Nadh oxidase activity of human xanthine oxidoreductase – generation of superoxide anion. Eur. J. Biochem./FEBS. 1997;245:541–548. doi: 10.1111/j.1432-1033.1997.00541.x. [DOI] [PubMed] [Google Scholar]
- 140.Zhang Z., Blake D.R., Stevens C.R., Kanczler J.M., Winyard P.G., Symons M.C., Benboubetra M., Harrison R. A reappraisal of xanthine dehydrogenase and oxidase in hypoxic reperfusion injury: the role of nadh as an electron donor. Free Radic. Res. 1998;28:151–164. doi: 10.3109/10715769809065801. [DOI] [PubMed] [Google Scholar]
- 141.Lee M.C., Velayutham M., Komatsu T., Hille R., Zweier J.L. Measurement and characterization of superoxide generation from xanthine dehydrogenase: a redox-regulated pathway of radical generation in ischemic tissues. Biochemistry. 2014;53:6615–6623. doi: 10.1021/bi500582r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Terada L.S., Piermattei D., Shibao G.N., McManaman J.L., Wright R.M. Hypoxia regulates xanthine dehydrogenase activity at pre- and posttranslational levels. Arch. Biochem. Biophys. 1997;348:163–168. doi: 10.1006/abbi.1997.0367. [DOI] [PubMed] [Google Scholar]
- 143.Wang G., Qian P., Jackson F.R., Qian G., Wu G. Sequential activation of jaks, stats and xanthine dehydrogenase/oxidase by hypoxia in lung microvascular endothelial cells. Int. J. Biochem. Cell. Biol. 2008;40:461–470. doi: 10.1016/j.biocel.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Poss W.B., Huecksteadt T.P., Panus P.C., Freeman B.A., Hoidal J.R. Regulation of xanthine dehydrogenase and xanthine oxidase activity by hypoxia. Am. J. Physiol. 1996;270:L941–L946. doi: 10.1152/ajplung.1996.270.6.L941. [DOI] [PubMed] [Google Scholar]
- 145.Berry C.E., Hare J.M. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J. Physio. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dupont G.P., Huecksteadt T.P., Marshall B.C., Ryan U.S., Michael J.R., Hoidal J.R. Regulation of xanthine dehydrogenase and xanthine oxidase activity and gene expression in cultured rat pulmonary endothelial cells. J. Clin. Investig. 1992;89:197–202. doi: 10.1172/JCI115563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Falciani F., Ghezzi P., Terao M., Cazzaniga G., Garattini E. Interferons induce xanthine dehydrogenase gene expression in l929 cells. Biochem. J. 1992;285:1001–1008. doi: 10.1042/bj2851001. (Pt 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pfeffer K.D., Huecksteadt T.P., Hoidal J.R. Xanthine dehydrogenase and xanthine oxidase activity and gene expression in renal epithelial cells. Cytokine and steroid regulation. J. Immunol. 1994;153:1789–1797. [PubMed] [Google Scholar]
- 149.Chouchani E.T., Pell V.R., Gaude E., Aksentijevic D., Sundier S.Y., Robb E.L., Logan A., Nadtochiy S.M., Ord E.N., Smith A.C., Eyassu F., Shirley R., Hu C.H., Dare A.J., James A.M., Rogatti S., Hartley R.C., Eaton S., Costa A.S., Brookes P.S., Davidson S.M., Duchen M.R., Saeb-Parsy K., Shattock M.J., Robinson A.J., Work L.M., Frezza C., Krieg T., Murphy M.P. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ros. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mousson B., Desjacques P., Baltassat P. Measurement of xanthine oxidase activity in some human tissues. An optimized method. Enzyme. 1983;29:32–43. doi: 10.1159/000469601. [DOI] [PubMed] [Google Scholar]
- 151.Hagberg H., Andersson P., Lacarewicz J., Jacobson I., Butcher S., Sandberg M. Extracellular adenosine, inosine, hypoxanthine, and xanthine in relation to tissue nucleotides and purines in rat striatum during transient ischemia. J. Neurochem. 1987;49:227–231. doi: 10.1111/j.1471-4159.1987.tb03419.x. [DOI] [PubMed] [Google Scholar]
- 152.Xia Y., Zweier J.L. Substrate control of free radical generation from xanthine oxidase in the postischemic heart. J. Biol. Chem. 1995;270:18797–18803. doi: 10.1074/jbc.270.32.18797. [DOI] [PubMed] [Google Scholar]
- 153.Abd-Elfattah A.S., Jessen M.E., Lekven J., Doherty N.E., 3rd, Brunsting L.A., Wechsler A.S. Myocardial reperfusion injury. Role of myocardial hypoxanthine and xanthine in free radical-mediated reperfusion injury. Circulation. 1988;78:III224–III235. [PubMed] [Google Scholar]
- 154.Xia Y., Khatchikian G., Zweier J.L. Adenosine deaminase inhibition prevents free radical-mediated injury in the postischemic heart. J. Biol. Chem. 1996;271:10096–10102. doi: 10.1074/jbc.271.17.10096. [DOI] [PubMed] [Google Scholar]
- 155.Laubach V.E., French B.A., Okusa M.D. Targeting of adenosine receptors in ischemia-reperfusion injury. Expert Opin. Ther. Targets. 2011;15:103–118. doi: 10.1517/14728222.2011.541441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Grisham M.B., Hernandez L.A., Granger D.N. Adenosine inhibits ischemia-reperfusion-induced leukocyte adherence and extravasation. Am. J. Physiol. 1989;257:H1334–H1339. doi: 10.1152/ajpheart.1989.257.5.H1334. [DOI] [PubMed] [Google Scholar]
- 157.Puett D.W., Forman M.B., Cates C.U., Wilson B.H., Hande K.R., Friesinger G.C., Virmani R. Oxypurinol limits myocardial stunning but does not reduce infarct size after reperfusion. Circulation. 1987;76:678–686. doi: 10.1161/01.cir.76.3.678. [DOI] [PubMed] [Google Scholar]
- 158.Tsuda H., Kawada N., Kaimori J.Y., Kitamura H., Moriyama T., Rakugi H., Takahara S., Isaka Y. Febuxostat suppressed renal ischemia-reperfusion injury via reduced oxidative stress. Biochem. Biophys. Res. Commun. 2012;427:266–272. doi: 10.1016/j.bbrc.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 159.Smith S.M., Grisham M.B., Manci E.A., Granger D.N., Kvietys P.R. Gastric mucosal injury in the rat. Role of iron and xanthine oxidase. Gastroenterology. 1987;92:950–956. doi: 10.1016/0016-5085(87)90969-3. [DOI] [PubMed] [Google Scholar]
- 160.Victorino G.P., Ramirez R.M., Chong T.J., Curran B., Sadjadi J. Ischemia-reperfusion injury in rats affects hydraulic conductivity in two phases that are temporally and mechanistically separate. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2164–H2171. doi: 10.1152/ajpheart.00419.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ohtsubo T., Rovira I.I., Starost M.F., Liu C., Finkel T. Xanthine oxidoreductase is an endogenous regulator of cyclooxygenase-2. Circ. Res. 2004;95:1118–1124. doi: 10.1161/01.RES.0000149571.96304.36. [DOI] [PubMed] [Google Scholar]
- 162.Levartovsky D., Lagziel A., Sperling O., Liberman U., Yaron M., Hosoya T., Ichida K., Peretz H. Xdh gene mutation is the underlying cause of classical xanthinuria: a second report. Kidney Int. 2000;57:2215–2220. doi: 10.1046/j.1523-1755.2000.00082.x. [DOI] [PubMed] [Google Scholar]
- 163.Cheung K.J., Tzameli I., Pissios P., Rovira I., Gavrilova O., Ohtsubo T., Chen Z., Finkel T., Flier J.S., Friedman J.M. Xanthine oxidoreductase is a regulator of adipogenesis and ppargamma activity. Cell. Metab. 2007;5:115–128. doi: 10.1016/j.cmet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 164.Cantu-Medellin N., Kelley E.E. Xanthine oxidoreductase-catalyzed reactive species generation: a process in critical need of reevaluation. Redox Biol. 2013;1:353–358. doi: 10.1016/j.redox.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Das D.K., Engelman R.M., Clement R., Otani H., Prasad M.R., Rao P.S. Role of xanthine oxidase inhibitor as free radical scavenger: a novel mechanism of action of allopurinol and oxypurinol in myocardial salvage. Biochem. Biophys. Res. Commun. 1987;148:314–319. doi: 10.1016/0006-291x(87)91112-0. [DOI] [PubMed] [Google Scholar]
- 166.Moorhouse P.C., Grootveld M., Halliwell B., Quinlan J.G., Gutteridge J.M. Allopurinol and oxypurinol are hydroxyl radical scavengers. FEBS Lett. 1987;213:23–28. doi: 10.1016/0014-5793(87)81458-8. [DOI] [PubMed] [Google Scholar]
- 167.Zimmerman B.J., Parks D.A., Grisham M.B., Granger D.N. Allopurinol does not enhance antioxidant properties of extracellular fluid. Am. J. Physiol. 1988;255:H202–H206. doi: 10.1152/ajpheart.1988.255.1.H202. [DOI] [PubMed] [Google Scholar]
- 168.Sanders S.A., Harrison R., Eisenthal R. The reaction of human xanthine dehydrogenase with nadh. Biochem. Soc. Trans. 1997;25:517S. doi: 10.1042/bst025517s. [DOI] [PubMed] [Google Scholar]
- 169.Kelley E.E., Trostchansky A., Rubbo H., Freeman B.A., Radi R., Tarpey M.M. Binding of xanthine oxidase to glycosaminoglycans limits inhibition by oxypurinol. J. Biol. Chem. 2004;279:37231–37234. doi: 10.1074/jbc.M402077200. [DOI] [PubMed] [Google Scholar]
- 170.Malik U.Z., Hundley N.J., Romero G., Radi R., Freeman B.A., Tarpey M.M., Kelley E.E. Febuxostat inhibition of endothelial-bound xo: Implications for targeting vascular ros production. Free Radic. Biol. Med. 2011;51:179–184. doi: 10.1016/j.freeradbiomed.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shafik A.N. Febuxostat improves the local and remote organ changes induced by intestinal ischemia/reperfusion in rats. Dig. Dis. Sci. 2013;58:650–659. doi: 10.1007/s10620-012-2391-1. [DOI] [PubMed] [Google Scholar]
- 172.Millar T.M., Stevens C.R., Benjamin N., Eisenthal R., Harrison R., Blake D.R. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 173.Li H., Samouilov A., Liu X., Zweier J.L. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrate reduction: evaluation of its role in nitrite and nitric oxide generation in anoxic tissues. Biochemistry. 2003;42:1150–1159. doi: 10.1021/bi026385a. [DOI] [PubMed] [Google Scholar]
- 174.Maia L.B., Pereira V., Mira L., Moura J.J. Nitrite reductase activity of rat and human xanthine oxidase, xanthine dehydrogenase, and aldehyde oxidase: evaluation of their contribution to no formation in vivo. Biochemistry. 2015;54:685–710. doi: 10.1021/bi500987w. [DOI] [PubMed] [Google Scholar]
- 175.Godber B.L., Doel J.J., Sapkota G.P., Blake D.R., Stevens C.R., Eisenthal R., Harrison R. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J. Biol. Chem. 2000;275:7757–7763. doi: 10.1074/jbc.275.11.7757. [DOI] [PubMed] [Google Scholar]
- 176.Baker J.E., Su J., Fu X., Hsu A., Gross G.J., Tweddell J.S., Hogg N. Nitrite confers protection against myocardial infarction: role of xanthine oxidoreductase, NADPH oxidase and k(atp) channels. J. Mol. Cell. Cardiol. 2007;43:437–444. doi: 10.1016/j.yjmcc.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Webb A., Bond R., McLean P., Uppal R., Benjamin N., Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc. Natl. Acad. Sci. USA. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tripatara P., Patel N.S., Webb A., Rathod K., Lecomte F.M., Mazzon E., Cuzzocrea S., Yaqoob M.M., Ahluwalia A., Thiemermann C. Nitrite-derived nitric oxide protects the rat kidney against ischemia/reperfusion injury in vivo: role for xanthine oxidoreductase. J. Am. Soc. Nephrol.: JASN. 2007;18:570–580. doi: 10.1681/ASN.2006050450. [DOI] [PubMed] [Google Scholar]
- 179.Sugimoto R., Okamoto T., Nakao A., Zhan J., Wang Y., Kohmoto J., Tokita D., Farver C.F., Tarpey M.M., Billiar T.R., Gladwin M.T., McCurry K.R. Nitrite reduces acute lung injury and improves survival in a rat lung transplantation model. Am. J. Transpl.: Off. J. Am. Soc. Transpl. Am. Soc. Transpl. Surg. 2012;12:2938–2948. doi: 10.1111/j.1600-6143.2012.04169.x. [DOI] [PubMed] [Google Scholar]
- 180.Lu P., Liu F., Yao Z., Wang C.Y., Chen D.D., Tian Y., Zhang J.H., Wu Y.H. Nitrite-derived nitric oxide by xanthine oxidoreductase protects the liver against ischemia-reperfusion injury. Hepatobiliary Pancreat. Dis. Int.: HBPD Int. 2005;4:350–355. [PubMed] [Google Scholar]
- 181.Granger D.N., Benoit J.N., Suzuki M., Grisham M.B. Leukocyte adherence to venular endothelium during ischemia-reperfusion. Am. J. Physiol. 1989;257:G683–G688. doi: 10.1152/ajpgi.1989.257.5.G683. [DOI] [PubMed] [Google Scholar]
- 182.Suematsu M., DeLano F.A., Poole D., Engler R.L., Miyasaka M., Zweifach B.W., Schmid-Schonbein G.W. Spatial and temporal correlation between leukocyte behavior and cell injury in postischemic rat skeletal muscle microcirculation. Lab. Investig. J. Tech. Methods Pathol. 1994;70:684–695. [PubMed] [Google Scholar]
- 183.Wiles M.E., Hechtman H.B., Morel N.M., Shepro D. Hypoxia reoxygenation-induced injury of cultured pulmonary microvessel endothelial cells. J. Leukoc. Biol. 1993;53:490–497. doi: 10.1002/jlb.53.5.490. [DOI] [PubMed] [Google Scholar]
- 184.Grisham M.B., Hernandez L.A., Granger D.N. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am. J. Physiol. 1986;251:G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- 185.Zimmerman B.J., Grisham M.B., Granger D.N. Role of oxidants in ischemia/reperfusion-induced granulocyte infiltration. Am. J. Physiol. 1990;258:G185–G190. doi: 10.1152/ajpgi.1990.258.2.G185. [DOI] [PubMed] [Google Scholar]
- 186.Michiels C., Arnould T., Houbion A., Remacle J. Human umbilical vein endothelial cells submitted to hypoxia-reoxygenation in vitro: implication of free radicals, xanthine oxidase, and energy deficiency. J. Cell. Physiol. 1992;153:53–61. doi: 10.1002/jcp.1041530109. [DOI] [PubMed] [Google Scholar]
- 187.Kubes P., Ibbotson G., Russell J., Wallace J.L., Granger D.N. Role of platelet-activating factor in ischemia/reperfusion-induced leukocyte adherence. Am. J. Physiol. 1990;259:G300–G305. doi: 10.1152/ajpgi.1990.259.2.G300. [DOI] [PubMed] [Google Scholar]
- 188.Kurose I., Argenbright L.W., Wolf R., Lianxi L., Granger D.N. Ischemia/reperfusion-induced microvascular dysfunction: role of oxidants and lipid mediators. Am. J. Physiol. 1997;272:H2976–H2982. doi: 10.1152/ajpheart.1997.272.6.H2976. [DOI] [PubMed] [Google Scholar]
- 189.Patel K.D., Zimmerman G.A., Prescott S.M., McEver R.P., McIntyre T.M. Oxygen radicals induce human endothelial cells to express gmp-140 and bind neutrophils. J. Cell. Biol. 1991;112:749–759. doi: 10.1083/jcb.112.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Meneshian A., Bulkley G.B. The physiology of endothelial xanthine oxidase: from urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirculation. 2002;9:161–175. doi: 10.1038/sj.mn.7800136. [DOI] [PubMed] [Google Scholar]
- 191.Vega V.L., Mardones L., Maldonado M., Nicovani S., Manriquez V., Roa J., Ward P.H. Xanthine oxidase released from reperfused hind limbs mediate kupffer cell activation, neutrophil sequestration, and hepatic oxidative stress in rats subjected to tourniquet shock. Shock. 2000;14:565–571. doi: 10.1097/00024382-200014050-00012. [DOI] [PubMed] [Google Scholar]
- 192.Matsumura F., Yamaguchi Y., Goto M., Ichiguchi O., Akizuki E., Matsuda T., Okabe K., Liang J., Ohshiro H., Iwamoto T., Yamada S., Mori K., Ogawa M. Xanthine oxidase inhibition attenuates kupffer cell production of neutrophil chemoattractant following ischemia-reperfusion in rat liver. Hepatology. 1998;28:1578–1587. doi: 10.1002/hep.510280618. [DOI] [PubMed] [Google Scholar]
- 193.Otamiri T. Oxygen radicals, lipid peroxidation, and neutrophil infiltration after small-intestinal ischemia and reperfusion. Surgery. 1989;105:593–597. [PubMed] [Google Scholar]
- 194.Hernandez L.A., Grisham M.B., Twohig B., Arfors K.E., Harlan J.M., Granger D.N. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am. J. Physiol. 1987;253:H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- 195.Smith J.K., Carden D.L., Korthuis R.J. Role of xanthine oxidase in postischemic microvascular injury in skeletal muscle. Am. J. Physiol. 1989;257:H1782–H1789. doi: 10.1152/ajpheart.1989.257.6.H1782. [DOI] [PubMed] [Google Scholar]
- 196.Carden D.L., Smith J.K., Korthuis R.J. Neutrophil-mediated microvascular dysfunction in postischemic canine skeletal muscle. Role of granulocyte adherence. Circ. Res. 1990;66:1436–1444. doi: 10.1161/01.res.66.5.1436. [DOI] [PubMed] [Google Scholar]
- 197.Adkins W.K., Taylor A.E. Role of xanthine oxidase and neutrophils in ischemia-reperfusion injury in rabbit lung. J. Appl. Physiol. 1990;69:2012–2018. doi: 10.1152/jappl.1990.69.6.2012. [DOI] [PubMed] [Google Scholar]
- 198.Brandes R.P. Vascular functions of NADPH oxidases. Hypertension. 2010;56:17–21. doi: 10.1161/HYPERTENSIONAHA.108.120295. [DOI] [PubMed] [Google Scholar]
- 199.Brandes R.P., Weissmann N., SchrodeR K. NADPH oxidases in cardiovascular disease. Free Radic. Biol. Med. 2010;49:687–706. doi: 10.1016/j.freeradbiomed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 200.Lambeth J.D., Krause K.H., Clark R.A. Nox enzymes as novel targets for drug development. Sem. Immunopathol. 2008;30:339–363. doi: 10.1007/s00281-008-0123-6. [DOI] [PubMed] [Google Scholar]
- 201.Kleikers P.W., Wingler K., Hermans J.J., Diebold I., Altenhofer S., Radermacher K.A., Janssen B., Gorlach A., Schmidt H.H. NADPH oxidases as a source of oxidative stress and molecular target in ischemia/reperfusion injury. J. Mol. Med. 2012;90:1391–1406. doi: 10.1007/s00109-012-0963-3. [DOI] [PubMed] [Google Scholar]
- 202.Kahles T., Brandes R.P. Which NADPH oxidase isoform is relevant for ischemic stroke? The case for nox 2. Antioxid. Redox Signal. 2013;18:1400–1417. doi: 10.1089/ars.2012.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Simone S., Rascio F., Castellano G., Divella C., Chieti A., Ditonno P., Battaglia M., Crovace A., Staffieri F., Oortwijn B., Stallone G., Gesualdo L., Pertosa G., Grandaliano G. Complement-dependent NADPH oxidase enzyme activation in renal ischemia/reperfusion injury. Free Radic. Biol. Med. 2014;74:263–273. doi: 10.1016/j.freeradbiomed.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 204.Nakagiri A., Sunamoto M., Murakami M. NADPH oxidase is involved in ischaemia/reperfusion-induced damage in rat gastric mucosa via ros production – role of NADPH oxidase in rat stomachs. Inflammopharmacology. 2007;15:278–281. doi: 10.1007/s10787-007-1587-z. [DOI] [PubMed] [Google Scholar]
- 205.Choi D.H., Kim J.H., Lee K.H., Kim H.Y., Kim Y.S., Choi W.S., Lee J. Role of neuronal NADPH oxidase 1 in the peri-infarct regions after stroke. PloS One. 2015;10:e0116814. doi: 10.1371/journal.pone.0116814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Miller A.A., Dusting G.J., Roulston C.L., Sobey C.G. NADPH-oxidase activity is elevated in penumbral and non-ischemic cerebral arteries following stroke. Brain Res. 2006;1111:111–116. doi: 10.1016/j.brainres.2006.06.082. [DOI] [PubMed] [Google Scholar]
- 207.Yokota H., Narayanan S.P., Zhang W., Liu H., Rojas M., Xu Z., Lemtalsi T., Nagaoka T., Yoshida A., Brooks S.E., Caldwell R.W., Caldwell R.B. Neuroprotection from retinal ischemia/reperfusion injury by nox2 NADPH oxidase deletion. Investig. Ophthalmol. Vis. Sci. 2011;52:8123–8131. doi: 10.1167/iovs.11-8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Doerries C., Grote K., Hilfiker-Kleiner D., Luchtefeld M., Schaefer A., Holland S.M., Sorrentino S., Manes C., Schieffer B., Drexler H., Landmesser U. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ. Res. 2007;100:894–903. doi: 10.1161/01.RES.0000261657.76299.ff. [DOI] [PubMed] [Google Scholar]
- 209.Gan X., Su G., Zhao W., Huang P., Luo G., Hei Z. The mechanism of sevoflurane preconditioning-induced protections against small intestinal ischemia reperfusion injury is independent of mast cell in rats. Mediat. Inflamm. 2013;2013:378703. doi: 10.1155/2013/378703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chen H., Song Y.S., Chan P.H. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J. Cereb. Blood Flow. Metab.: Off. J. Int. Soc. Cereb. Blood Flow. Metab. 2009;29:1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Uysal A., Sahna E., Ozguler I., Burma O., Ilhan N. Effects of apocynin, an NADPH oxidase inhibitor, on levels of adma, mpo, inos and tlr4 induced by myocardial ischemia reperfusion. Perfusion. 2014 doi: 10.1177/0267659114559260. [DOI] [PubMed] [Google Scholar]
- 212.Lee I., Dodia C., Chatterjee S., Zagorski J., Mesaros C., Blair I.A., Feinstein S.I., Jain M., Fisher A.B. A novel nontoxic inhibitor of the activation of NADPH oxidase reduces reactive oxygen species production in mouse lung. J. Pharmacol. Exp. Ther. 2013;345:284–296. doi: 10.1124/jpet.112.201079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Paterniti I., Galuppo M., Mazzon E., Impellizzeri D., Esposito E., Bramanti P., Cuzzocrea S. Protective effects of apocynin, an inhibitor of NADPH oxidase activity, in splanchnic artery occlusion and reperfusion. J. Leukoc. Biol. 2010;88:993–1003. doi: 10.1189/jlb.0610322. [DOI] [PubMed] [Google Scholar]
- 214.Korthuis R.J., Gute D.C., Blecha F., Ross C.R. Pr-39, a proline/arginine-rich antimicrobial peptide, prevents postischemic microvascular dysfunction. Am. J. Physiol. 1999;277:H1007–H1013. doi: 10.1152/ajpheart.1999.277.3.H1007. [DOI] [PubMed] [Google Scholar]
- 215.Loukogeorgakis S.P., van den Berg M.J., Sofat R., Nitsch D., Charakida M., Haiyee B., de Groot E., MacAllister R.J., Kuijpers T.W., Deanfield J.E. Role of NADPH oxidase in endothelial ischemia/reperfusion injury in humans. Circulation. 2010;121:2310–2316. doi: 10.1161/CIRCULATIONAHA.108.814731. [DOI] [PubMed] [Google Scholar]
- 216.Yang Z., Sharma A.K., Marshall M., Kron I.L., Laubach V.E. NADPH oxidase in bone marrow-derived cells mediates pulmonary ischemia-reperfusion injury. Am. J. Respir. Cell. Mol. Biol. 2009;40:375–381. doi: 10.1165/rcmb.2008-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ishikawa M., Stokes K.Y., Zhang J.H., Nanda A., Granger D.N. Cerebral microvascular responses to hypercholesterolemia: roles of NADPH oxidase and p-selectin. Circ. Res. 2004;94:239–244. doi: 10.1161/01.RES.0000111524.05779.60. [DOI] [PubMed] [Google Scholar]
- 218.Harada H., Hines I.N., Flores S., Gao B., McCord J., Scheerens H., Grisham M.B. Role of NADPH oxidase-derived superoxide in reduced size liver ischemia and reperfusion injury. Arch. Biochem. Biophys. 2004;423:103–108. doi: 10.1016/j.abb.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 219.Kahles T., Luedike P., Endres M., Galla H.J., Steinmetz H., Busse R., Neumann-Haefelin T., Brandes R.P. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke J. Cereb. Circ. 2007;38:3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- 220.Romson J.L., Hook B.G., Kunkel S.L., Abrams G.D., Schork M.A., Lucchesi B.R. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- 221.Mullane K.M., Read N., Salmon J.A., Moncada S. Role of leukocytes in acute myocardial infarction in anesthetized dogs: relationship to myocardial salvage by anti-inflammatory drugs. J. Pharmacol. Exp. Ther. 1984;228:510–522. [PubMed] [Google Scholar]
- 222.Rowe G.T., Eaton L.R., HesS M.L. Neutrophil-derived, oxygen free radical-mediated cardiovascular dysfunction. J. Mol. Cell. Cardiol. 1984;16:1075–1079. doi: 10.1016/s0022-2828(84)80020-6. [DOI] [PubMed] [Google Scholar]
- 223.Smith S.M., Holm-Rutili L., Perry M.A., Grisham M.B., Arfors K.E., Granger D.N., Kvietys P.R. Role of neutrophils in hemorrhagic shock-induced gastric mucosal injury in the rat. Gastroenterology. 1987;93:466–471. doi: 10.1016/0016-5085(87)90907-3. [DOI] [PubMed] [Google Scholar]
- 224.Kuzuya T., Hoshida S., Nishida M., Kim Y., Fuji H., Kitabatake A., Kamada T., Tada M. Role of free radicals and neutrophils in canine myocardial reperfusion injury: myocardial salvage by a novel free radical scavenger, 2-octadecylascorbic acid. Cardiovasc. Res. 1989;23:323–330. doi: 10.1093/cvr/23.4.323. [DOI] [PubMed] [Google Scholar]
- 225.Linas S.L., Shanley P.F., Whittenburg D., Berger E., Repine J.E. Neutrophils accentuate ischemia-reperfusion injury in isolated perfused rat kidneys. Am. J. Physiol. 1988;255:F728–F735. doi: 10.1152/ajprenal.1988.255.4.F728. [DOI] [PubMed] [Google Scholar]
- 226.Matsuo Y., Onodera H., Shiga Y., Nakamura M., Ninomiya M., Kihara T., Kogure K. Correlation between myeloperoxidase-quantified neutrophil accumulation and ischemic brain injury in the rat. Effects of neutrophil depletion. Stroke J. Cereb. Circ. 1994;25:1469–1475. doi: 10.1161/01.str.25.7.1469. [DOI] [PubMed] [Google Scholar]
- 227.Jaeschke H., Farhood A., Smith C.W. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J.: Off. Publ. Fed. Am. Soc. Exp. Biol. 1990;4:3355–3359. [PubMed] [Google Scholar]
- 228.Tosa Y., Lee W.P., Kollias N., Randolph M.A., May J.W., Jr. Monoclonal antibody to intercellular adhesion molecule 1 protects skin flaps against ischemia-reperfusion injury: an experimental study in rats. Plast. Reconstr. Surg. 1998;101:1586–1594. doi: 10.1097/00006534-199805000-00023. discussion 1595–1586. [DOI] [PubMed] [Google Scholar]
- 229.Lysiak J.J., Turner S.D., Nguyen Q.A., Singbartl K., Ley K., Turner T.T. Essential role of neutrophils in germ cell-specific apoptosis following ischemia/reperfusion injury of the mouse testis. Biol. Reprod. 2001;65:718–725. doi: 10.1095/biolreprod65.3.718. [DOI] [PubMed] [Google Scholar]
- 230.Vedder N.B., Fouty B.W., Winn R.K., Harlan J.M., Rice C.L. Role of neutrophils in generalized reperfusion injury associated with resuscitation from shock. Surgery. 1989;106:509–516. [PubMed] [Google Scholar]
- 231.Schmeling D.J., Caty M.G., Oldham K.T., Guice K.S., Hinshaw D.B. Evidence for neutrophil-related acute lung injury after intestinal ischemia-reperfusion. Surgery. 1989;106:195–201. discussion 201–192. [PubMed] [Google Scholar]
- 232.Smith E.F., 3rd, Egan J.W., Bugelski P.J., Hillegass L.M., Hill D.E., Griswold D.E. Temporal relation between neutrophil accumulation and myocardial reperfusion injury. Am. J. Physiol. 1988;255:H1060–H1068. doi: 10.1152/ajpheart.1988.255.5.H1060. [DOI] [PubMed] [Google Scholar]
- 233.Hellberg P.O., Kallskog T.O. Neutrophil-mediated post-ischemic tubular leakage in the rat kidney. Kidney Int. 1989;36:555–561. doi: 10.1038/ki.1989.230. [DOI] [PubMed] [Google Scholar]
- 234.Bowes M.P., Zivin J.A., Rothlein R. Monoclonal antibody to the icam-1 adhesion site reduces neurological damage in a rabbit cerebral embolism stroke model. Exp. Neurol. 1993;119:215–219. doi: 10.1006/exnr.1993.1023. [DOI] [PubMed] [Google Scholar]
- 235.Simpson P.J., Todd R.F., 3rd, Fantone J.C., Mickelson J.K., Griffin J.D., Lucchesi B.R. Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti-mo1, anti-cd11b) that inhibits leukocyte adhesion. J. Clin. Investig. 1988;81:624–629. doi: 10.1172/JCI113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kubes P., Suzuki M., Granger D.N. Platelet-activating factor-induced microvascular dysfunction: role of adherent leukocytes. Am. J. Physiol. 1990;258:G158–G163. doi: 10.1152/ajpgi.1990.258.1.G158. [DOI] [PubMed] [Google Scholar]
- 237.Smith E.F., 3rd, Griswold D.E., Egan J.W., Hillegass L.M., DiMartino M.J. Reduction of myocardial damage and polymorphonuclear leukocyte accumulation following coronary artery occlusion and reperfusion by the thromboxane receptor antagonist bm 13.505. J. Cardiovasc. Pharmacol. 1989;13:715–722. [PubMed] [Google Scholar]
- 238.Klausner J.M., Paterson I.S., Goldman G., Kobzik L., Rodzen C., Lawrence R., Valeri C.R., Shepro D., Hechtman H.B. Postischemic renal injury is mediated by neutrophils and leukotrienes. Am. J. Physiol. 1989;256:F794–F802. doi: 10.1152/ajprenal.1989.256.5.F794. [DOI] [PubMed] [Google Scholar]
- 239.Ichikawa H., Takagi T., Uchiyama K., Higashihara H., Katada K., Isozaki Y., Naito Y., Yoshida N., Yoshikawa T. Rotenone, a mitochondrial electron transport inhibitor, ameliorates ischemia-reperfusion-induced intestinal mucosal damage in rats. Redox Rep. 2004;9:313–316. doi: 10.1179/135100004225006795. [DOI] [PubMed] [Google Scholar]
- 240.Inauen W., Granger D.N., Meininger C.J., Schelling M.E., Granger H.J., Kvietys P.R. Anoxia-reoxygenation-induced, neutrophil-mediated endothelial cell injury: Role of elastase. Am. J. Physiol. 1990;259:H925–H931. doi: 10.1152/ajpheart.1990.259.3.H925. [DOI] [PubMed] [Google Scholar]
- 241.del Zoppo G.J., Schmid-Schonbein G.W., Mori E., Copeland B.R., Chang C.M. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke J. Cereb. Circ. 1991;22:1276–1283. doi: 10.1161/01.str.22.10.1276. [DOI] [PubMed] [Google Scholar]
- 242.Walder C.E., Green S.P., Darbonne W.C., Mathias J., Rae J., Dinauer M.C., Curnutte J.T., Thomas G.R. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke J. Cereb. Circ. 1997;28:2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- 243.Tang X.N., Zheng Z., Giffard R.G., Yenari M.A. Significance of marrow-derived nicotinamide adenine dinucleotide phosphate oxidase in experimental ischemic stroke. Ann. Neurol. 2011;70:606–615. doi: 10.1002/ana.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Rupin A., Paysant J., Sansilvestri-Morel P., Lembrez N., Lacoste J.M., Cordi A., Verbeuren T.J. Role of NADPH oxidase-mediated superoxide production in the regulation of e-selectin expression by endothelial cells subjected to anoxia/reoxygenation. Cardiovasc. Res. 2004;63:323–330. doi: 10.1016/j.cardiores.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 245.Borchi E., Parri M., Papucci L., Becatti M., Nassi N., Nassi P., Nediani C. Role of NADPH oxidase in h9c2 cardiac muscle cells exposed to simulated ischaemia-reperfusion. J. Cell. Mol. Med. 2009;13:2724–2735. doi: 10.1111/j.1582-4934.2008.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Abramov A.Y., Scorziello A., Duchen M.R. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J. Neurosci.: Off. J. Soc. Neurosci. 2007;27:1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Paysant J.R., Rupin A., Verbeuren T.J. Effect of NADPH oxidase inhibition on e-selectin expression induced by concomitant anoxia/reoxygenation and tnf-alpha. Endothel.: J. Endothel. Cell Res. 2002;9:263–271. doi: 10.1080/10623320214737. [DOI] [PubMed] [Google Scholar]
- 248.Gibson C.L., Srivastava K., Sprigg N., Bath P.M., Bayraktutan U. Inhibition of rho-kinase protects cerebral barrier from ischaemia-evoked injury through modulations of endothelial cell oxidative stress and tight junctions. J. Neurochem. 2014;129:816–826. doi: 10.1111/jnc.12681. [DOI] [PubMed] [Google Scholar]
- 249.Dodd-o J.M., Welsh L.E., Salazar J.D., Walinsky P.L., Peck E.A., Shake J.G., Caparrelli D.J., Ziegelstein R.C., Zweier J.L., Baumgartner W.A., Pearse D.B. Effect of NADPH oxidase inhibition on cardiopulmonary bypass-induced lung injury. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H927–H936. doi: 10.1152/ajpheart.01138.2003. [DOI] [PubMed] [Google Scholar]
- 250.Zehendner C.M., Librizzi L., Hedrich J., Bauer N.M., Angamo E.A., de Curtis M., Luhmann H.J. Moderate hypoxia followed by reoxygenation results in blood-brain barrier breakdown via oxidative stress-dependent tight-junction protein disruption. PloS One. 2013;8:e82823. doi: 10.1371/journal.pone.0082823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Al-Mehdi A.B., Zhao G., Dodia C., Tozawa K., Costa K., Muzykantov V., Ross C., Blecha F., Dinauer M., Fisher A.B. Endothelial NADPH oxidase as the source of oxidants in lungs exposed to ischemia or high K+ Circ. Res. 1998;83:730–737. doi: 10.1161/01.res.83.7.730. [DOI] [PubMed] [Google Scholar]
- 252.Donoso P., Finkelstein J.P., Montecinos L., Said M., Sanchez G., Vittone L., Bull R. Stimulation of nox2 in isolated hearts reversibly sensitizes ryr2 channels to activation by cytoplasmic calcium. J. Mol. Cell. Cardiol. 2014;68:38–46. doi: 10.1016/j.yjmcc.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 253.Braunersreuther V., Montecucco F., Asrih M., Pelli G., Galan K., Frias M., Burger F., Quindere A.L., Montessuit C., Krause K.H., Mach F., Jaquet V. Role of NADPH oxidase isoforms nox1, nox2 and nox4 in myocardial ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2013;64:99–107. doi: 10.1016/j.yjmcc.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 254.McCann S.K., Dusting G.J., Roulston C.L. Early increase of nox4 NADPH oxidase and superoxide generation following endothelin-1-induced stroke in conscious rats. J. Neurosci. Res. 2008;86:2524–2534. doi: 10.1002/jnr.21700. [DOI] [PubMed] [Google Scholar]
- 255.Vallet P., Charnay Y., Steger K., Ogier-Denis E., Kovari E., Herrmann F., Michel J.P., Szanto I. Neuronal expression of the NADPH oxidase nox4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 256.Kleinschnitz C., Grund H., Wingler K., Armitage M.E., Jones E., Mittal M., Barit D., Schwarz T., Geis C., Kraft P., Barthel K., Schuhmann M.K., Herrmann A.M., Meuth S.G., Stoll G., Meurer S., Schrewe A., Becker L., Gailus-Durner V., Fuchs H., Klopstock T., de Angelis M.H., Jandeleit-Dahm K., Shah A.M., Weissmann N., Schmidt H.H. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8(9):e1000479. doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Pendyala S., Usatyuk P.V., Gorshkova I.A., Garcia J.G., Natarajan V. Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid. Redox Signal. 2009;11:841–860. doi: 10.1089/ars.2008.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mittal M., Roth M., Konig P., Hofmann S., Dony E., Goyal P., Selbitz A.C., Schermuly R.T., Ghofrani H.A., Kwapiszewska G., Kummer W., Klepetko W., Hoda M.A., Fink L., Hanze J., Seeger W., Grimminger F., Schmidt H.H., Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit nox4 in the pulmonary vasculature. Circ. Res. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 259.Kietzmann T., Gorlach A. Reactive oxygen species in the control of hypoxia-inducible factor-mediated gene expression. Sem. Cell. Dev. Biol. 2005;16:474–486. doi: 10.1016/j.semcdb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 260.Zhang Q., Matsuzaki I., Chatterjee S., Fisher A.B. Activation of endothelial NADPH oxidase during normoxic lung ischemia is katp channel dependent. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L954–L961. doi: 10.1152/ajplung.00210.2005. [DOI] [PubMed] [Google Scholar]
- 261.Granger D.N., Korthuis R.J. Physiologic mechanisms of postischemic tissue injury. Annu. Rev. Physiol. 1995;57:311–332. doi: 10.1146/annurev.ph.57.030195.001523. [DOI] [PubMed] [Google Scholar]
- 262.Fraser P.A. The role of free radical generation in increasing cerebrovascular permeability. Free Radic. Biol. Med. 2011;51:967–977. doi: 10.1016/j.freeradbiomed.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 263.Dewald B., Baggiolini M. Activation of NADPH oxidase in human neutrophils. Synergism between fmlp and the neutrophil products paf and ltb4. Biochem. Biophys. Res. Commun. 1985;128:297–304. doi: 10.1016/0006-291x(85)91678-x. [DOI] [PubMed] [Google Scholar]
- 264.Arumugam T.V., Shiels I.A., Woodruff T.M., Granger D.N., Taylor S.M. The role of the complement system in ischemia-reperfusion injury. Shock. 2004;21:401–409. doi: 10.1097/00024382-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 265.Park H.S., Chun J.N., Jung H.Y., Choi C., Bae Y.S. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc. Res. 2006;72:447–455. doi: 10.1016/j.cardiores.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 266.Miller F.J., Jr., Filali M., Huss G.J., Stanic B., Chamseddine A., Barna T.J., Lamb F.S. Cytokine activation of nuclear factor kappa b in vascular smooth muscle cells requires signaling endosomes containing nox1 and clc-3. Circ. Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 267.Barth B.M., Stewart-Smeets S., Kuhn T.B. Proinflammatory cytokines provoke oxidative damage to actin in neuronal cells mediated by rac1 and NADPH oxidase. Mol. Cell. Neurosci. 2009;41:274–285. doi: 10.1016/j.mcn.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Gao X., Zhang H., Belmadani S., Wu J., Xu X., Elford H., Potter B.J., Zhang C. Role of tnf-alpha-induced reactive oxygen species in endothelial dysfunction during reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2242–H2249. doi: 10.1152/ajpheart.00587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nguyen Dinh Cat A., Montezano A.C., Burger D., Touyz R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid. Redox Signal. 2013;19:1110–1120. doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kusaka I., Kusaka G., Zhou C., Ishikawa M., Nanda A., Granger D.N., Zhang J.H., Tang J. Role of at1 receptors and nad(p)h oxidase in diabetes-aggravated ischemic brain injury. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H2442–H2451. doi: 10.1152/ajpheart.01169.2003. [DOI] [PubMed] [Google Scholar]
- 271.Riaz A.A., Wang Y., Schramm R., Sato T., Menger M.D., Jeppsson B., Thorlacius H. Role of angiotensin II in ischemia/reperfusion-induced leukocyte-endothelium interactions in the colon. FASEB J.: Off. Publ. Fed. Am. Soc. Exp. Biol. 2004;18:881–883. doi: 10.1096/fj.03-0502fje. [DOI] [PubMed] [Google Scholar]
- 272.Wu B., Lin R., Dai R., Chen C., Wu H., Hong M. Valsartan attenuates oxidative stress and nf-kappab activation and reduces myocardial apoptosis after ischemia and reperfusion. Eur. J. Pharmacol. 2013;705:140–147. doi: 10.1016/j.ejphar.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 273.Nakagiri A., Sunamoto M., Takeuchi K., Murakami M. Evidence for the involvement of NADPH oxidase in ischemia/reperfusion-induced gastric damage via angiotensin ii. J. Physiol. Pharmacol.: Off. J. Pol. Physiol. Soc. 2010;61:171–179. [PubMed] [Google Scholar]
- 274.Oudot A., Vergely C., Ecarnot-Laubriet A., Rochette L. Angiotensin II activates NADPH oxidase in isolated rat hearts subjected to ischaemia-reperfusion. Eur. J. Pharmacol. 2003;462:145–154. doi: 10.1016/s0014-2999(03)01315-3. [DOI] [PubMed] [Google Scholar]
- 275.McCann S.K., Roulston C.L. NADPH oxidase as a therapeutic target for neuroprotection against ischaemic stroke: future perspectives. Brain Sci. 2013;3:561–598. doi: 10.3390/brainsci3020561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Brandes R.P., Weissmann N., Schroder K. Nox family NADPH oxidases in mechano-transduction: mechanisms and consequences. Antioxid. Redox Signal. 2014;20:887–898. doi: 10.1089/ars.2013.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Heumuller S., Wind S., Barbosa-Sicard E., Schmidt H.H., Busse R., Schroder K., Brandes R.P. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 278.Aldieri E., Riganti C., Polimeni M., Gazzano E., Lussiana C., Campia I., Ghigo D. Classical inhibitors of nox NAD(P)H oxidases are not specific. Curr. Drug Metab. 2008;9:686–696. doi: 10.2174/138920008786049285. [DOI] [PubMed] [Google Scholar]
- 279.Li Y., Trush M.A. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem. Biophys. Res. Commun. 1998;253:295–299. doi: 10.1006/bbrc.1998.9729. [DOI] [PubMed] [Google Scholar]
- 280.Rey F.E., Cifuentes M.E., Kiarash A., Quinn M.T., Pagano P.J. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(−) and systolic blood pressure in mice. Circ. Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 281.Altenhofer S., Radermacher K.A., Kleikers P.W., Wingler K., Schmidt H.H. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid. Redox Signal. 2015;23:406–427. doi: 10.1089/ars.2013.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Drose S., Brandt U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv. Exp. Med. Biol. 2012;748:145–169. doi: 10.1007/978-1-4614-3573-0_6. [DOI] [PubMed] [Google Scholar]
- 283.Starkov A.A., Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J. Neurochem. 2003;86:1101–1107. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- 284.Logan A., Cocheme H.M., Li Pun P.B., Apostolova N., Smith R.A., Larsen L., Larsen D.S., James A.M., Fearnley I.M., Rogatti S., Prime T.A., Finichiu P.G., Dare A., Chouchani E.T., Pell V.R., Methner C., Quin C., McQuaker S.J., Krieg T., Hartley R.C., Murphy M.P. Using exomarkers to assess mitochondrial reactive species in vivo. Biochim. Biophys. Acta. 2014;1840:923–930. doi: 10.1016/j.bbagen.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 285.Kaludercic N., Deshwal S., Di Lisa F. Reactive oxygen species and redox compartmentalization. Front. Physiol. 2014;5:285. doi: 10.3389/fphys.2014.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Tahara E.B., Navarete F.D., Kowaltowski A.J. Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic. Biol. Med. 2009;46:1283–1297. doi: 10.1016/j.freeradbiomed.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 287.Lesnefsky E.J., Moghaddas S., Tandler B., Kerner J., Hoppel C.L. Mitochondrial dysfunction in cardiac disease: ischemia – reperfusion, aging, and heart failure. J. Mol. Cell. Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 288.Picard M., Taivassalo T., Gouspillou G., Hepple R.T. Mitochondria: isolation, structure and function. J. Physiol. 2011;589:4413–4421. doi: 10.1113/jphysiol.2011.212712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Chomova M., Tatarkova Z., Dobrota D., Racay P. Ischemia-induced inhibition of mitochondrial complex I in rat brain: effect of permeabilization method and electron acceptor. Neurochem. Res. 2012;37:965–976. doi: 10.1007/s11064-011-0689-6. [DOI] [PubMed] [Google Scholar]
- 290.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ros) and ros-induced ros release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Jastroch M., Divakaruni A.S., Mookerjee S., Treberg J.R., Brand M.D. Mitochondrial proton and electron leaks. Essays Biochem. 2010;47:53–67. doi: 10.1042/bse0470053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Solaini G., Harris D.A. Biochemical dysfunction in heart mitochondria exposed to ischaemia and reperfusion. Biochem. J. 2005;390:377–394. doi: 10.1042/BJ20042006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Bleier L., Wittig I., Heide H., Steger M., Brandt U., Drose S. Generator-specific targets of mitochondrial reactive oxygen species. Free Radic. Biol. Med. 2015;78:1–10. doi: 10.1016/j.freeradbiomed.2014.10.511. [DOI] [PubMed] [Google Scholar]
- 294.Hamanaka R.B., Chandel N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lenaz G. Mitochondria and reactive oxygen species. Which role in physiology and pathology? Adv. Exp. Med. Biol. 2012;942:93–136. doi: 10.1007/978-94-007-2869-1_5. [DOI] [PubMed] [Google Scholar]
- 296.Kalogeris T., Bao Y., Korthuis R.J. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Holzerova E., Prokisch H. Mitochondria: much ado about nothing? How dangerous is reactive oxygen species production? Int. J. Biochem. Cell. Biol. 2015;63:16–20. doi: 10.1016/j.biocel.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Chen Y.R., Zweier J.L. Cardiac mitochondria and reactive oxygen species generation. Circ. Res. 2014;114:524–537. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Loor G., Kondapalli J., Iwase H., Chandel N.S., Waypa G.B., Guzy R.D., Vanden Hoek T.L., Schumacker P.T. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochim. Biophys. Acta. 2011;1813:1382–1394. doi: 10.1016/j.bbamcr.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Trachootham D., Lu W., Ogasawara M.A., Nilsa R.D., Huang P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Cardoso S., Correia S., Carvalho C., Candeias E., Placido A.I., Duarte A.I., Seica R.M., Moreira P.I. Perspectives on mitochondrial uncoupling proteins-mediated neuroprotection. J. Bioenerg. Biomembr. 2015;47:119–131. doi: 10.1007/s10863-014-9580-x. [DOI] [PubMed] [Google Scholar]
- 302.Loschen G., Flohe L., Chance B. Respiratory chain linked H(2)O(2) production in pigeon heart mitochondria. FEBS Lett. 1971;18:261–264. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- 303.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kowaltowski A.J., de Souza-Pinto N.C., Castilho R.F., Vercesi A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 305.Starkov A.A. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N. Y. Acad. Sci. 2008;1147:37–52. doi: 10.1196/annals.1427.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Nickel A., Kohlhaas M., Maack C. Mitochondrial reactive oxygen species production and elimination. J. Mol. Cell. Cardiol. 2014;73:26–33. doi: 10.1016/j.yjmcc.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 307.Andreyev A.Y., Kushnareva Y.E., Starkov A.A. Mitochondrial metabolism of reactive oxygen species. Biochemistry. 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 308.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid. Redox Signal. 2005;7:1140–1149. doi: 10.1089/ars.2005.7.1140. [DOI] [PubMed] [Google Scholar]
- 310.Lanciano P., Khalfaoui-Hassani B., Selamoglu N., Ghelli A., Rugolo M., Daldal F. Molecular mechanisms of superoxide production by complex III: a bacterial versus human mitochondrial comparative case study. Biochim. Biophys. Acta. 2013;1827:1332–1339. doi: 10.1016/j.bbabio.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Lenaz G., Baracca A., Barbero G., Bergamini C., Dalmonte M.E., Del Sole M., Faccioli M., Falasca A., Fato R., Genova M.L., Sgarbi G., Solaini G. Mitochondrial respiratory chain super-complex I-III in physiology and pathology. Biochim. Biophys. Acta. 2010;1797:633–640. doi: 10.1016/j.bbabio.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 312.Genova M.L., Lenaz G. Functional role of mitochondrial respiratory supercomplexes. Biochim. Biophys. Acta. 2014;1837:427–443. doi: 10.1016/j.bbabio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 313.Pfeiffer K., Gohil V., Stuart R.A., Hunte C., Brandt U., Greenberg M.L., Schagger H. Cardiolipin stabilizes respiratory chain supercomplexes. J. Biol. Chem. 2003;278:52873–52880. doi: 10.1074/jbc.M308366200. [DOI] [PubMed] [Google Scholar]
- 314.Mileykovskaya E., Dowhan W. Cardiolipin-dependent formation of mitochondrial respiratory supercomplexes. Chem. Phys. Lipids. 2014;179:42–48. doi: 10.1016/j.chemphyslip.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Trouillard M., Meunier B., Rappaport F. Questioning the functional relevance of mitochondrial supercomplexes by time-resolved analysis of the respiratory chain. Proc. Natl. Acad. Sci. USA. 2011;108:E1027–E1034. doi: 10.1073/pnas.1109510108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Brown G.C., Borutaite V. There is no evidence that mitochondria are the main source of reactive oxygen species in mammalian cells. Mitochondrion. 2012;12:1–4. doi: 10.1016/j.mito.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 317.Brand M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Korshunov S.S., Skulachev V.P., Starkov A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 319.Quinlan C.L., Perevoshchikova, Hey-Mogensen M., Orr A.L., Brand M.D. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013;1:304–312. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Quinlan C.L., Orr A.L., Perevoshchikova, Treberg J.R., Ackrell B.A., Brand M.D. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J. Biol. Chem. 2012;287:27255–27264. doi: 10.1074/jbc.M112.374629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Musatov A., Robinson N.C. Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Radic. Res. 2012;46:1313–1326. doi: 10.3109/10715762.2012.717273. [DOI] [PubMed] [Google Scholar]
- 322.Prabu S.K., Anandatheerthavarada H.K., Raza H., Srinivasan S., Spear J.F., Avadhani N.G. Protein kinase a-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury. J. Biol. Chem. 2006;281:2061–2070. doi: 10.1074/jbc.M507741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Cardoso S., Correia S., Carvalho C., Candeias E., Placido A.I., Duarte A.I., Seica R.M., Moreira P.I. Perspectives on mitochondrial uncoupling proteins-mediated neuroprotection. J. Bioenerg. Biomembr. 2015;47:119–131. doi: 10.1007/s10863-014-9580-x. [DOI] [PubMed] [Google Scholar]
- 324.Speijer D. How the mitochondrion was shaped by radical differences in substrates: what carnitine shuttles and uncoupling tell us about mitochondrial evolution in response to ros. Bioessays. 2014;36:634–643. doi: 10.1002/bies.201400033. [DOI] [PubMed] [Google Scholar]
- 325.Echtay K.S., Roussel D., St-Pierre J., Jekabsons M.B., Cadenas S., Stuart J.A., Harper J.A., Roebuck S.J., Morrison A., Pickering S., Clapham J.C., Brand M.D. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 326.Krauss S., Zhang C.Y., Lowell B.B. The mitochondrial uncoupling-protein homologues. Nat. Rev. Mol. Cell. Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- 327.Quarrie R., Lee D.S., Reyes L., Erdahl W., Pfeiffer D.R., Zweier J.L., Crestanello J.A. Mitochondrial uncoupling does not decrease reactive oxygen species production after ischemia-reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H996–H1004. doi: 10.1152/ajpheart.00189.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Paradies G., Petrosillo G., Pistolese M., Di Venosa N., Federici A., Ruggiero F.M. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ. Res. 2004;94:53–59. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- 329.Lee H.L., Chen C.L., Yeh S.T., Zweier J.L., Chen Y.R. Biphasic modulation of the mitochondrial electron transport chain in myocardial ischemia and reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H1410–H1422. doi: 10.1152/ajpheart.00731.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Gadicherla A.K., Stowe D.F., Antholine W.E., Yang M., Camara A.K. Damage to mitochondrial complex I during cardiac ischemia reperfusion injury is reduced indirectly by anti-anginal drug ranolazine. Biochim. Biophys. Acta. 2012;1817:419–429. doi: 10.1016/j.bbabio.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Chen Q., Moghaddas S., Hoppel C.L., Lesnefsky E.J. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am. J. Physiol. Cell. Physiol. 2008;294:C460–C466. doi: 10.1152/ajpcell.00211.2007. [DOI] [PubMed] [Google Scholar]
- 332.Korge P., Ping P., Weiss J.N. Reactive oxygen species production in energized cardiac mitochondria during hypoxia/reoxygenation: modulation by nitric oxide. Circ. Res. 2008;103:873–880. doi: 10.1161/CIRCRESAHA.108.180869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Starkov A.A., Andreyev A.Y., Zhang S.F., Starkova N.N., Korneeva M., Syromyatnikov M., Popov V.N. Scavenging of H2O2 by mouse brain mitochondria. J. Bioenerg. Biomembr. 2014;46:471–477. doi: 10.1007/s10863-014-9581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Drose S., Brandt U., Wittig I. Mitochondrial respiratory chain complexes as sources and targets of thiol-based redox-regulation. Biochim. Biophys. Acta. 2014;1844:1344–1354. doi: 10.1016/j.bbapap.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 335.Zhang Y., Marcillat O., Giulivi C., Ernster L., Davies K.J. The oxidative inactivation of mitochondrial electron transport chain components and atpase. J. Biol. Chem. 1990;265:16330–16336. [PubMed] [Google Scholar]
- 336.Petrosillo G., Ruggiero F.M., Di Venosa N., Paradies G. Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: role of reactive oxygen species and cardiolipin. FASEB J. 2003;17:714–716. doi: 10.1096/fj.02-0729fje. [DOI] [PubMed] [Google Scholar]
- 337.Petrosillo G., Di Venosa N., Ruggiero F.M., Pistolese M., D'Agostino D., Tiravanti E., Fiore T., Paradies G. Mitochondrial dysfunction associated with cardiac ischemia/reperfusion can be attenuated by oxygen tension control. Role of oxygen-free radicals and cardiolipin. Biochim. Biophys. Acta. 2005;1710:78–86. doi: 10.1016/j.bbabio.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 338.Brown D.A., Sabbah H.N., Shaikh S.R. Mitochondrial inner membrane lipids and proteins as targets for decreasing cardiac ischemia/reperfusion injury. Pharmacol. Ther. 2013;140:258–266. doi: 10.1016/j.pharmthera.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 339.Huttemann M., Lee I., Samavati L., Yu H., Doan J.W. Regulation of mitochondrial oxidative phosphorylation through cell signaling. Biochim. Biophys. Acta. 1773;2007:1701–1720. doi: 10.1016/j.bbamcr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 340.Churchill E.N., Szweda L.I. Translocation of deltapkc to mitochondria during cardiac reperfusion enhances superoxide anion production and induces loss in mitochondrial function. Arch. Biochem. Biophys. 2005;439:194–199. doi: 10.1016/j.abb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 341.Fang J.K., Prabu S.K., Sepuri N.B., Raza H., Anandatheerthavarada H.K., Galati D., Spear J., Avadhani N.G. Site specific phosphorylation of cytochrome c oxidase subunits I, IVI1 and VB in rabbit hearts subjected to ischemia/reperfusion. FEBS Lett. 2007;581:1302–1310. doi: 10.1016/j.febslet.2007.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Srinivasan S., Spear J., Chandran K., Joseph J., Kalyanaraman B., Avadhani N.G. Oxidative stress induced mitochondrial protein kinase a mediates cytochrome c oxidase dysfunction. PLoS One. 2013;8:e77129. doi: 10.1371/journal.pone.0077129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Chouchani E.T., Methner C., Nadtochiy S.M., Logan A., Pell V.R., Ding S., James A.M., Cocheme H.M., Reinhold J., Lilley K.S., Partridge L., Fearnley I.M., Robinson A.J., Hartley R.C., Smith R.A., Krieg T., Brookes P.S., Murphy M.P. Cardioprotection by s-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 2013;19:753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Hamel D., Sanchez M., Duhamel F., Roy O., Honore J.C., Noueihed B., Zhou T., Nadeau-Vallee M., Hou X., Lavoie J.C., Mitchell G., Mamer O.A., Chemtob S. G-protein-coupled receptor 91 and succinate are key contributors in neonatal postcerebral hypoxia-ischemia recovery. Arterioscler. Thromb. Vasc. Biol. 2014;34:285–293. doi: 10.1161/ATVBAHA.113.302131. [DOI] [PubMed] [Google Scholar]
- 345.Block K., Gorin Y., Abboud H.E. Subcellular localization of nox4 and regulation in diabetes. Proc. Natl. Acad. Sci. USA. 2009;106:14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Kuroda J., Ago T., Matsushima S., Zhai P., Schneider M.D., Sadoshima J. NADPH oxidase 4 (nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. USA. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Zhang L., Nguyen M.V., Lardy B., Jesaitis A.J., Grichine A., Rousset F., Talbot M., Paclet M.H., Qian G., Morel F. New insight into the nox4 subcellular localization in hek293 cells: first monoclonal antibodies against nox4. Biochimie. 2011;93:457–468. doi: 10.1016/j.biochi.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 348.Kaludercic N., Mialet-Perez J., Paolocci N., Parini A., Di Lisa F. Monoamine oxidases as sources of oxidants in the heart. J. Mol. Cell. Cardiol. 2014;73:34–42. doi: 10.1016/j.yjmcc.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Villeneuve C., Guilbeau-Frugier C., Sicard P., Lairez O., Ordener C., Duparc T., De Paulis D., Couderc B., Spreux-Varoquaux O., Tortosa F., Garnier A., Knauf C., Valet P., Borchi E., Nediani C., Gharib A., Ovize M., Delisle M.B., Parini A., Mialet-Perez J. P53-pgc-1alpha pathway mediates oxidative mitochondrial damage and cardiomyocyte necrosis induced by monoamine oxidase-a upregulation: role in chronic left ventricular dysfunction in mice. Antioxid. Redox Signal. 2013;18:5–18. doi: 10.1089/ars.2011.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Fitzgerald J.C., Ugun-Klusek A., Allen G., De Girolamo L.A., Hargreaves I., Ufer C., Abramov A.Y., Billett E.E. Monoamine oxidase-a knockdown in human neuroblastoma cells reveals protection against mitochondrial toxins. FASEB J. 2014;28:218–229. doi: 10.1096/fj.13-235481. [DOI] [PubMed] [Google Scholar]
- 351.Pchejetski D., Kunduzova O., Dayon A., Calise D., Seguelas M.H., Leducq N., Seif I., Parini A., Cuvillier O. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase a-associated cardiac cell apoptosis. Circ. Res. 2007;100:41–49. doi: 10.1161/01.RES.0000253900.66640.34. [DOI] [PubMed] [Google Scholar]
- 352.Bianchi P., Kunduzova O., Masini E., Cambon C., Bani D., Raimondi L., Seguelas M.H., Nistri S., Colucci W., Leducq N., Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation. 2005;112:3297–3305. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- 353.Li C., Feng J.J., Wu Y.P., Zhang G.Y. Cerebral ischemia-reperfusion induces gapdh s-nitrosylation and nuclear translocation. Biochemistry. 2012;77:671–678. doi: 10.1134/S0006297912060156. [DOI] [PubMed] [Google Scholar]
- 354.Matsui Y., Kumagae Y. Monoamine oxidase inhibitors prevent striatal neuronal necrosis induced by transient forebrain ischemia. Neurosci. Lett. 1991;126:175–178. doi: 10.1016/0304-3940(91)90547-7. [DOI] [PubMed] [Google Scholar]
- 355.Giorgio M., Migliaccio E., Orsini F., Paolucci D., Moroni M., Contursi C., Pelliccia G., Luzi L., Minucci S., Marcaccio M., Pinton P., Rizzuto R., Bernardi P., Paolucci F., Pelicci P.G. Electron transfer between cytochrome c and p66shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 356.Paneni F., Mocharla P., Akhmedov A., Costantino S., Osto E., Volpe M., Luscher T.F., Cosentino F. Gene silencing of the mitochondrial adaptor p66(shc) suppresses vascular hyperglycemic memory in diabetes. Circ. Res. 2012;111:278–289. doi: 10.1161/CIRCRESAHA.112.266593. [DOI] [PubMed] [Google Scholar]
- 357.Nemoto S., Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 358.Zaccagnini G., Martelli F., Fasanaro P., Magenta A., Gaetano C., Di Carlo A., Biglioli P., Giorgio M., Martin-Padura I., Pelicci P.G., Capogrossi M.C. P66shca modulates tissue response to hindlimb ischemia. Circulation. 2004;109:2917–2923. doi: 10.1161/01.CIR.0000129309.58874.0F. [DOI] [PubMed] [Google Scholar]
- 359.Carpi A., Menabo R., Kaludercic N., Pelicci P., Di Lisa F., Giorgio M. The cardioprotective effects elicited by p66(shc) ablation demonstrate the crucial role of mitochondrial ros formation in ischemia/reperfusion injury. Biochim. Biophys. Acta. 2009;1787:774–780. doi: 10.1016/j.bbabio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 360.Di Lisa F., Kaludercic N., Carpi A., Menabo R., Giorgio M. Mitochondrial pathways for ros formation and myocardial injury: the relevance of p66(shc) and monoamine oxidase. Basic Res. Cardiol. 2009;104:131–139. doi: 10.1007/s00395-009-0008-4. [DOI] [PubMed] [Google Scholar]
- 361.Quinlan C.L., Goncalves R.L., Hey-Mogensen M., Yadava N., Bunik V.I., Brand M.D. The 2-oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I. J. Biol. Chem. 2014;289:8312–8325. doi: 10.1074/jbc.M113.545301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Adam-Vizi V., Tretter L. The role of mitochondrial dehydrogenases in the generation of oxidative stress. Neurochem. Int. 2013;62:757–763. doi: 10.1016/j.neuint.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 363.Starkov A.A. An update on the role of mitochondrial alpha-ketoglutarate dehydrogenase in oxidative stress. Mol. Cell. Neurosci. 2013;55:13–16. doi: 10.1016/j.mcn.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Kareyeva A.V., Grivennikova V.G., Vinogradov A.D. Mitochondrial hydrogen peroxide production as determined by the pyridine nucleotide pool and its redox state. Biochim. Biophys. Acta. 1817;2012:1879–1885. doi: 10.1016/j.bbabio.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 365.Huang H.M., Ou H.C., Chen H.L., Hou R.C., Jeng K.C. Protective effect of alpha-keto-beta-methyl-n-valeric acid on bv-2 microglia under hypoxia or oxidative stress. Ann. N. Y. Acad. Sci. 2005;1042:272–278. doi: 10.1196/annals.1338.049. [DOI] [PubMed] [Google Scholar]
- 366.Tretter L., Adam-Vizi V. Inhibition of krebs cycle enzymes by hydrogen peroxide: a key role of [alpha]-ketoglutarate dehydrogenase in limiting nadh production under oxidative stress. J. Neurosci. 2000;20:8972–8979. doi: 10.1523/JNEUROSCI.20-24-08972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Vasquez-Vivar J., Kalyanaraman B., Kennedy M.C. Mitochondrial aconitase is a source of hydroxyl radical. An electron spin resonance investigation. J. Biol. Chem. 2000;275:14064–14069. doi: 10.1074/jbc.275.19.14064. [DOI] [PubMed] [Google Scholar]
- 368.Bulteau A.L., Ikeda-Saito M., Szweda L.I. Redox-dependent modulation of aconitase activity in intact mitochondria. Biochemistry. 2003;42:14846–14855. doi: 10.1021/bi0353979. [DOI] [PubMed] [Google Scholar]
- 369.Bulteau A.L., Lundberg K.C., Ikeda-Saito M., Isaya G., Szweda L.I. Reversible redox-dependent modulation of mitochondrial aconitase and proteolytic activity during in vivo cardiac ischemia/reperfusion. Proc. Natl. Acad. Sci. USA. 2005;102:5987–5991. doi: 10.1073/pnas.0501519102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Cortassa S., O'Rourke B., Aon M.A. Redox-optimized ros balance and the relationship between mitochondrial respiration and ros. Biochim. Biophys. Acta. 2014;1837:287–295. doi: 10.1016/j.bbabio.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Aon M.A., Cortassa S., O'Rourke B. Redox-optimized ros balance: a unifying hypothesis. Biochim. Biophys. Acta. 2010;1797:865–877. doi: 10.1016/j.bbabio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Aon M.A., Stanley B.A., Sivakumaran V., Kembro J.M., O'Rourke B., Paolocci N., Cortassa S. Glutathione/thioredoxin systems modulate mitochondrial h2o2 emission: an experimental-computational study. J. Gen. Physiol. 2012;139:479–491. doi: 10.1085/jgp.201210772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Horstkotte J., Perisic T., Schneider M., Lange P., Schroeder M., Kiermayer C., Hinkel R., Ziegler T., Mandal P.K., David R., Schulz S., Schmitt S., Widder J., Sinowatz F., Becker B.F., Bauersachs J., Naebauer M., Franz W.M., Jeremias I., Brielmeier M., Zischka H., Conrad M., Kupatt C. Mitochondrial thioredoxin reductase is essential for early postischemic myocardial protection. Circulation. 2011;124:2892–2902. doi: 10.1161/CIRCULATIONAHA.111.059253. [DOI] [PubMed] [Google Scholar]
- 374.Frasier C.R., Moukdar F., Patel H.D., Sloan R.C., Stewart L.M., Alleman R.J., La Favor J.D., Brown D.A. Redox-dependent increases in glutathione reductase and exercise preconditioning: role of NADPH oxidase and mitochondria. Cardiovasc. Res. 2013;98:47–55. doi: 10.1093/cvr/cvt009. [DOI] [PubMed] [Google Scholar]
- 375.Zorov D.B., Filburn C.R., Klotz L.O., Zweier J.L., Sollott S.J. Reactive oxygen species (ros)-induced ros release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial ros-induced ros release: an update and review. Biochim. Biophys. Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 377.Aon M.A., Cortassa S., Marban E., O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 378.Aon M.A., Cortassa S., Akar F.G., Brown D.A., Zhou L., O'Rourke B. From mitochondrial dynamics to arrhythmias. Int. J. Biochem. Cell. Biol. 2009;41:1940–1948. doi: 10.1016/j.biocel.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Zhou L., O'Rourke B. Cardiac mitochondrial network excitability: insights from computational analysis. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H2178–H2189. doi: 10.1152/ajpheart.01073.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Brady N.R., Elmore S.P., van Beek J.J., Krab K., Courtoy P.J., Hue L., Westerhoff H.V. Coordinated behavior of mitochondria in both space and time: a reactive oxygen species-activated wave of mitochondrial depolarization. Biophys. J. 2004;87:2022–2034. doi: 10.1529/biophysj.103.035097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Brady N.R., Hamacher-Brady A., Westerhoff H.V., Gottlieb R.A. A wave of reactive oxygen species (ros)-induced ros release in a sea of excitable mitochondria. Antioxid. Redox Signal. 2006;8:1651–1665. doi: 10.1089/ars.2006.8.1651. [DOI] [PubMed] [Google Scholar]
- 382.Juhaszova M., Zorov D.B., Kim S.H., Pepe S., Fu Q., Fishbein K.W., Ziman B.D., Wang S., Ytrehus K., Antos C.L., Olson E.N., Sollott S.J. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J. Clin. Investig. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Aon M.A., Cortassa S., Maack C., O'Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J. Biol. Chem. 2007;282:21889–21900. doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Slodzinski M.K., Aon M.A., O'Rourke B. Glutathione oxidation as a trigger of mitochondrial depolarization and oscillation in intact hearts. J. Mol. Cell. Cardiol. 2008;45:650–660. doi: 10.1016/j.yjmcc.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Biary N., Xie C., Kauffman J., Akar F.G. Biophysical properties and functional consequences of reactive oxygen species (ros)-induced ros release in intact myocardium. J. Physiol. 2011;589:5167–5179. doi: 10.1113/jphysiol.2011.214239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Berkich D.A., Salama G., LaNoue K.F. Mitochondrial membrane potentials in ischemic hearts. Arch. Biochem. Biophys. 2003;420:279–286. doi: 10.1016/j.abb.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 387.Honda H.M., Korge P., Weiss J.N. Mitochondria and ischemia/reperfusion injury. Ann. N. Y. Acad. Sci. 2005;1047:248–258. doi: 10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- 388.Yang L., Korge P., Weiss J.N., Qu Z. Mitochondrial oscillations and waves in cardiac myocytes: insights from computational models. Biophys. J. 2010;98:1428–1438. doi: 10.1016/j.bpj.2009.12.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Hou T., Wang X., Ma Q., Cheng H. Mitochondrial flashes: new insights into mitochondrial ros signalling and beyond. J. Physiol. 2014;592:3703–3713. doi: 10.1113/jphysiol.2014.275735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Bernardi P., Di Lisa F. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J. Mol. Cell. Cardiol. 2015;78:100–106. doi: 10.1016/j.yjmcc.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Ong S.B., Samangouei P., Kalkhoran S.B., Hausenloy D.J. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J. Mol. Cell. Cardiol. 2015;78:23–34. doi: 10.1016/j.yjmcc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 392.Bernardi P., Di Lisa F., Fogolari F., Lippe G. From atp to ptp and back: a dual function for the mitochondrial atp synthase. Circ. Res. 2015;116:1850–1862. doi: 10.1161/CIRCRESAHA.115.306557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Wang W., Fang H., Groom L., Cheng A., Zhang W., Liu J., Wang X., Li K., Han P., Zheng M., Yin J., Mattson M.P., Kao J.P., Lakatta E.G., Sheu S.S., Ouyang K., Chen J., Dirksen R.T., Cheng H. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Schwarzlander M., Murphy M.P., Duchen M.R., Logan D.C., Fricker M.D., Halestrap A.P., Muller F.L., Rizzuto R., Dick T.P., Meyer A.J., Sweetlove L.J. Mitochondrial 'flashes': a radical concept rephined. Trends Cell. Biol. 2012;22:503–508. doi: 10.1016/j.tcb.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 395.Forstermann U., Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 396.Forstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. 837a-837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Oess S., Icking A., Fulton D., Govers R., Muller-Esterl W. Subcellular targeting and trafficking of nitric oxide synthases. Biochem. J. 2006;396:401–409. doi: 10.1042/BJ20060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Zhang Y.H., Casadei B. Sub-cellular targeting of constitutive nos in health and disease. J. Mol. Cell. Cardiol. 2012;52:341–350. doi: 10.1016/j.yjmcc.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 399.Zaobornyj T., Ghafourifar P. Strategic localization of heart mitochondrial nos: a review of the evidence. Am. J. Physiol. Heart Circ. Physiol. 2012;303:H1283–H1293. doi: 10.1152/ajpheart.00674.2011. [DOI] [PubMed] [Google Scholar]
- 400.Lopez L.C., Escames G., Tapias V., Utrilla P., Leon J., Acuna-Castroviejo D. Identification of an inducible nitric oxide synthase in diaphragm mitochondria from septic mice: its relation with mitochondrial dysfunction and prevention by melatonin. Int. J. Biochem. Cell. Biol. 2006;38:267–278. doi: 10.1016/j.biocel.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 401.Kanai A.J., Pearce L.L., Clemens P.R., Birder L.A., VanBibber M.M., Choi S.Y., de Groat W.C., Peterson J. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc. Natl. Acad. Sci. USA. 2001;98:14126–14131. doi: 10.1073/pnas.241380298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Godinez-Rubi M., Rojas-Mayorquin A.E., Ortuno-Sahagun D. Nitric oxide donors as neuroprotective agents after an ischemic stroke-related inflammatory reaction. Oxid. Med. Cell. Longev. 2013;2013:297357. doi: 10.1155/2013/297357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Schulz R., Kelm M., Heusch G. Nitric oxide in myocardial ischemia/reperfusion injury. Cardiovasc. Res. 2004;61:402–413. doi: 10.1016/j.cardiores.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 404.Malek M., Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Ren. Inj. Prev. 2015;4:20–27. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Duranski M.R., Greer J.J., Dejam A., Jaganmohan S., Hogg N., Langston W., Patel R.P., Yet S.F., Wang X., Kevil C.G., Gladwin M.T., Lefer D.J. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J. Clin. Investig. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Kurose I., Wolf R., Grisham M.B., Granger D.N. Modulation of ischemia/reperfusion-induced microvascular dysfunction by nitric oxide. Circ. Res. 1994;74:376–382. doi: 10.1161/01.res.74.3.376. [DOI] [PubMed] [Google Scholar]
- 407.Roberts B.W., Mitchell J., Kilgannon J.H., Chansky M.E., Trzeciak S. Nitric oxide donor agents for the treatment of ischemia/reperfusion injury in human subjects: a systematic review. Shock. 2013;39:229–239. doi: 10.1097/SHK.0b013e31827f565b. [DOI] [PubMed] [Google Scholar]
- 408.Phillips L., Toledo A.H., Lopez-Neblina F., Anaya-Prado R., Toledo-Pereyra L.H. Nitric oxide mechanism of protection in ischemia and reperfusion injury. J. Investig. Surg. 2009;22:46–55. doi: 10.1080/08941930802709470. [DOI] [PubMed] [Google Scholar]
- 409.Kubes P., Suzuki M., Granger D.N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Farah C., Reboul C. No better way to protect the heart during ischemia-reperfusion: to be in the right place at the right time. Front. Pediatr. 2015;3:6. doi: 10.3389/fped.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Alkaitis M.S., Crabtree M.J. Recoupling the cardiac nitric oxide synthases: tetrahydrobiopterin synthesis and recycling. Curr. Heart Fail. Rep. 2012;9:200–210. doi: 10.1007/s11897-012-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Zhang Y., Tocchetti C.G., Krieg T., Moens A.L. Oxidative and nitrosative stress in the maintenance of myocardial function. Free Radic. Biol. Med. 2012;53:1531–1540. doi: 10.1016/j.freeradbiomed.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 413.Whitsett J., Martasek P., Zhao H., Schauer D.W., Hatakeyama K., Kalyanaraman B., Vasquez-Vivar J. Endothelial cell superoxide anion radical generation is not dependent on endothelial nitric oxide synthase-serine 1179 phosphorylation and endothelial nitric oxide synthase dimer/monomer distribution. Free Radic. Biol. Med. 2006;40:2056–2068. doi: 10.1016/j.freeradbiomed.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 414.Masano T., Kawashima S., Toh R., Satomi-Kobayashi S., Shinohara M., Takaya T., Sasaki N., Takeda M., Tawa H., Yamashita T., Yokoyama M., Hirata K. Beneficial effects of exogenous tetrahydrobiopterin on left ventricular remodeling after myocardial infarction in rats: the possible role of oxidative stress caused by uncoupled endothelial nitric oxide synthase. Circ. J. 2008;72:1512–1519. doi: 10.1253/circj.cj-08-0072. [DOI] [PubMed] [Google Scholar]
- 415.Leucker T.M., Ge Z.D., Procknow J., Liu Y., Shi Y., Bienengraeber M., Warltier D.C., Kersten J.R. Impairment of endothelial-myocardial interaction increases the susceptibility of cardiomyocytes to ischemia/reperfusion injury. PloS One. 2013;8:e70088. doi: 10.1371/journal.pone.0070088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Kietadisorn R., Juni R.P., Moens A.L. Tackling endothelial dysfunction by modulating nos uncoupling: new insights into its pathogenesis and therapeutic possibilities. Am. J. Physiol. Endocrinol. Metab. 2012;302:E481–E495. doi: 10.1152/ajpendo.00540.2011. [DOI] [PubMed] [Google Scholar]
- 417.Tatham A.L., Crabtree M.J., Warrick N., Cai S., Alp N.J., Channon K.M. GTP cyclohydrolase I expression, protein, and activity determine intracellular tetrahydrobiopterin levels, independent of gtp cyclohydrolase feedback regulatory protein expression. J. Biol. Chem. 2009;284:13660–13668. doi: 10.1074/jbc.M807959200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Crabtree M.J., Tatham A.L., Al-Wakeel Y., Warrick N., Hale A.B., Cai S., Channon K.M., Alp N.J. Quantitative regulation of intracellular endothelial nitric-oxide synthase (enos) coupling by both tetrahydrobiopterin-enos stoichiometry and biopterin redox status: insights from cells with tet-regulated gtp cyclohydrolase i expression. J. Biol. Chem. 2009;284:1136–1144. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- 419.Bendall J.K., Alp N.J., Warrick N., Cai S., Adlam D., Rockett K., Yokoyama M., Kawashima S., Channon K.M. Stoichiometric relationships between endothelial tetrahydrobiopterin, endothelial no synthase (enos) activity, and enos coupling in vivo: Insights from transgenic mice with endothelial-targeted gtp cyclohydrolase 1 and enos overexpression. Circ. Res. 2005;97:864–871. doi: 10.1161/01.RES.0000187447.03525.72. [DOI] [PubMed] [Google Scholar]
- 420.Widder J.D., Chen W., Li L., Dikalov S., Thony B., Hatakeyama K., Harrison D.G. Regulation of tetrahydrobiopterin biosynthesis by shear stress. Circ. Res. 2007;101:830–838. doi: 10.1161/CIRCRESAHA.107.153809. [DOI] [PubMed] [Google Scholar]
- 421.Crabtree M.J., Tatham A.L., Hale A.B., Alp N.J., Channon K.M. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J. Biol. Chem. 2009;284:28128–28136. doi: 10.1074/jbc.M109.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Dumitrescu C., Biondi R., Xia Y., Cardounel A.J., Druhan L.J., Ambrosio G., Zweier J.L. Myocardial ischemia results in tetrahydrobiopterin (bh4) oxidation with impaired endothelial function ameliorated by bh4. Proc. Natl. Acad. Sci. USA. 2007;104:15081–15086. doi: 10.1073/pnas.0702986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.De Pascali F., Hemann C., Samons K., Chen C.A., Zweier J.L. Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and s-glutathionylation. Biochemistry. 2014;53:3679–3688. doi: 10.1021/bi500076r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Siu K.L., Lotz C., Ping P., Cai H. Netrin-1 abrogates ischemia/reperfusion-induced cardiac mitochondrial dysfunction via nitric oxide-dependent attenuation of nox4 activation and recoupling of nos. J. Mol. Cell. Cardiol. 2015;78:174–185. doi: 10.1016/j.yjmcc.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Yamashiro S., Noguchi K., Matsuzaki T., Miyagi K., Nakasone J., Sakanashi M., Koja K. Beneficial effect of tetrahydrobiopterin on ischemia-reperfusion injury in isolated perfused rat hearts. J. Thorac. Cardiovasc. Surg. 2002;124:775–784. doi: 10.1067/mtc.2002.124393. [DOI] [PubMed] [Google Scholar]
- 426.Verma S., Maitland A., Weisel R.D., Fedak P.W., Pomroy N.C., Li S.H., Mickle D.A., Li R.K., Rao V. Novel cardioprotective effects of tetrahydrobiopterin after anoxia and reoxygenation: identifying cellular targets for pharmacologic manipulation. J. Thorac. Cardiovasc. Surg. 2002;123:1074–1083. doi: 10.1067/mtc.2002.121687. [DOI] [PubMed] [Google Scholar]
- 427.Perkins K.A., Pershad S., Chen Q., McGraw S., Adams J.S., Zambrano C., Krass S., Emrich J., Bell B., Iyamu M., Prince C., Kay H., Teng J.C., Young L.H. The effects of modulating enos activity and coupling in ischemia/reperfusion (i/r) Naunyn Schmiedebergs Arch. Pharmacol. 2012;385:27–38. doi: 10.1007/s00210-011-0693-z. [DOI] [PubMed] [Google Scholar]
- 428.Tiefenbacher C.P., Lee C.H., Kapitza J., Dietz V., Niroomand F. Sepiapterin reduces postischemic injury in the rat heart. Pflug. Arch. 2003;447:1–7. doi: 10.1007/s00424-003-1131-y. [DOI] [PubMed] [Google Scholar]
- 429.Moens A.L., Champion H.C., Claeys M.J., Tavazzi B., Kaminski P.M., Wolin M.S., Borgonjon D.J., Van Nassauw L., Haile A., Zviman M., Bedja D., Wuyts F.L., Elsaesser R.S., Cos P., Gabrielson K.L., Lazzarino G., Paolocci N., Timmermans J.P., Vrints C.J., Kass D.A. High-dose folic acid pretreatment blunts cardiac dysfunction during ischemia coupled to maintenance of high-energy phosphates and reduces postreperfusion injury. Circulation. 2008;117:1810–1819. doi: 10.1161/CIRCULATIONAHA.107.725481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Legrand M., Kandil A., Payen D., Ince C. Effects of sepiapterin infusion on renal oxygenation and early acute renal injury after suprarenal aortic clamping in rats. J. Cardiovasc. Pharmacol. 2011;58:192–198. doi: 10.1097/FJC.0b013e31821f8ec3. [DOI] [PubMed] [Google Scholar]
- 431.Hara Y., Teramoto K., Ishidate K., Arii S. Cytoprotective function of tetrahydrobiopterin in rat liver ischemia/reperfusion injury. Surgery. 2006;139:377–384. doi: 10.1016/j.surg.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 432.Wang W.Z., Fang X.H., Stephenson L.L., Khiabani K.T., Zamboni W.A. Effects of supplementation of bh4 after prolonged ischemia in skeletal muscle. Microsurgery. 2007;27:200–205. doi: 10.1002/micr.20331. [DOI] [PubMed] [Google Scholar]
- 433.King A.L., Polhemus D.J., Bhushan S., Otsuka H., Kondo K., Nicholson C.K., Bradley J.M., Islam K.N., Calvert J.W., Tao Y.X., Dugas T.R., Kelley E.E., Elrod J.W., Huang P.L., Wang R., Lefer D.J. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc. Natl. Acad. Sci. USA. 2014;111:3182–3187. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Pernow J., Jung C. Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal? Cardiovasc. Res. 2013;98:334–343. doi: 10.1093/cvr/cvt036. [DOI] [PubMed] [Google Scholar]
- 435.Rabelo L.A., Ferreira F.O., Nunes-Souza V., da Fonseca L.J., Goulart M.O. Arginase as a critical prooxidant mediator in the binomial endothelial dysfunction-atherosclerosis. Oxid. Med. cell. Longev. 2015;2015:924860. doi: 10.1155/2015/924860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Girard-Thernier C., Pham T.N., Demougeot C. The promise of plant-derived substances as inhibitors of arginase. Mini Rev. Med. Chem. 2015;15:798–808. doi: 10.2174/1389557515666150511153852. [DOI] [PubMed] [Google Scholar]
- 437.Abu-Amara M., Yang S.Y., Seifalian A., Davidson B., Fuller B. The nitric oxide pathway – evidence and mechanisms for protection against liver ischaemia reperfusion injury. Liver Int.: Off. J. Int. Assoc. Study Liver. 2012;32:531–543. doi: 10.1111/j.1478-3231.2012.02755.x. [DOI] [PubMed] [Google Scholar]
- 438.Tratsiakovich Y., Yang J., Gonon A.T., Sjoquist P.O., Pernow J. Arginase as a target for treatment of myocardial ischemia-reperfusion injury. Eur. J. Pharmacol. 2013;720:121–123. doi: 10.1016/j.ejphar.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 439.Schreckenberg R., Weber P., Cabrera-Fuentes H.A., Steinert I., Preissner K.T., Bencsik P., Sarkozy M., Csonka C., Ferdinandy P., Schulz R., Schluter K.D. Mechanism and consequences of the shift in cardiac arginine metabolism following ischaemia and reperfusion in rats. Thromb. Haemost. 2015;113:482–493. doi: 10.1160/TH14-05-0477. [DOI] [PubMed] [Google Scholar]
- 440.Hein T.W., Zhang C., Wang W., Chang C.I., Thengchaisri N., Kuo L. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: counteracting role of arginase. FASEB J.: Off. Publ. Fed. Am. Soc. Exp. Biol. 2003;17:2328–2330. doi: 10.1096/fj.03-0115fje. [DOI] [PubMed] [Google Scholar]
- 441.Kovamees O., Shemyakin A., Pernow J. Effect of arginase inhibition on ischemia-reperfusion injury in patients with coronary artery disease with and without diabetes mellitus. PloS One. 2014;9:e103260. doi: 10.1371/journal.pone.0103260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Yang J., Gonon A.T., Sjoquist P.O., Lundberg J.O., Pernow J. Arginase regulates red blood cell nitric oxide synthase and export of cardioprotective nitric oxide bioactivity. Proc. Natl. Acad. Sci. USA. 2013;110:15049–15054. doi: 10.1073/pnas.1307058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Erbas H., Aydogdu N., Kaymak K. Effects of n-acetylcysteine on arginase, ornithine and nitric oxide in renal ischemia-reperfusion injury. Pharmacol. Res.: Off. J. Ital. Pharmacol. Society. 2004;50:523–527. doi: 10.1016/j.phrs.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 444.Quirie A., Demougeot C., Bertrand N., Mossiat C., Garnier P., Marie C., Prigent-Tessier A. Effect of stroke on arginase expression and localization in the rat brain. Eur. J. Neurosci. 2013;37:1193–1202. doi: 10.1111/ejn.12111. [DOI] [PubMed] [Google Scholar]
- 445.Jeyabalan G., Klune J.R., Nakao A., Martik N., Wu G., Tsung A., Geller D.A. Arginase blockade protects against hepatic damage in warm ischemia-reperfusion. Nitric oxide: Biol. Chem./Off. J. Nitric Oxide Soc. 2008;19:29–35. doi: 10.1016/j.niox.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Reid K.M., Tsung A., Kaizu T., Jeyabalan G., Ikeda A., Shao L., Wu G., Murase N., Geller D.A. Liver i/r injury is improved by the arginase inhibitor, n(omega)-hydroxy-nor-l-arginine (nor-noha) Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G512–G517. doi: 10.1152/ajpgi.00227.2006. [DOI] [PubMed] [Google Scholar]
- 447.Gronros J., Kiss A., Palmer M., Jung C., Berkowitz D., Pernow J. Arginase inhibition improves coronary microvascular function and reduces infarct size following ischaemia-reperfusion in a rat model. Acta Physiol. 2013;208:172–179. doi: 10.1111/apha.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Mohamed Abd E., Lasheen N.N. Comparative study on the protective role of vitamin c and l-arginine in experimental renal ischemia reperfusion in adult rats. Int. J. Physiol. Pathophysiol. Pharmacol. 2014;6:153–165. [PMC free article] [PubMed] [Google Scholar]
- 449.Nijveldt R.J., Prins H.A., van Kemenade F.J., Teerlink T., van Lambalgen A.A., Boelens P.G., Rauwerda J.A., van Leeuwen P.A. Low arginine plasma levels do not aggravate renal blood flow after experimental renal ischaemia/reperfusion. Eur. J. Vasc. Endovasc. Surg.: Off. J. Eur. Soc. Vasc. Surg. 2001;22:232–239. doi: 10.1053/ejvs.2001.1444. [DOI] [PubMed] [Google Scholar]
- 450.Prins H.A., Nijveldt R.J., Gasselt D.V., van Kemenade F., Teerlink T., van Lambalgen A.A., Rauwerda J.A., van Leeuwen P.A. The flux of arginine after ischemia-reperfusion in the rat kidney. Kidney Int. 2002;62:86–93. doi: 10.1046/j.1523-1755.2002.00409.x. [DOI] [PubMed] [Google Scholar]
- 451.Tratsiakovich Y., Gonon A.T., Kiss A., Yang J., Bohm F., Tornvall P., Settergren M., Channon K.M., Sjoquist P.O., Pernow J. Myocardial protection by co-administration of l-arginine and tetrahydrobiopterin during ischemia and reperfusion. Int. J. Cardiol. 2013;169:83–88. doi: 10.1016/j.ijcard.2013.08.075. [DOI] [PubMed] [Google Scholar]
- 452.Mozaffari M.S., Baban B., Liu J.Y., Abebe W., Sullivan J.C., El-Marakby A. Mitochondrial complex i and nad(p)h oxidase are major sources of exacerbated oxidative stress in pressure-overloaded ischemic-reperfused hearts. Basic Res. Cardiol. 2011;106:287–297. doi: 10.1007/s00395-011-0150-7. [DOI] [PubMed] [Google Scholar]
- 453.Liu P.G., He S.Q., Zhang Y.H., Wu J. Protective effects of apocynin and allopurinol on ischemia/reperfusion-induced liver injury in mice. World J. Gastroenterol. 2008;14:2832–2837. doi: 10.3748/wjg.14.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Baudry N., Laemmel E., Vicaut E. In vivo reactive oxygen species production induced by ischemia in muscle arterioles of mice: involvement of xanthine oxidase and mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H821–H828. doi: 10.1152/ajpheart.00378.2007. [DOI] [PubMed] [Google Scholar]
- 455.Angelos M.G., Kutala V.K., Torres C.A., He G., Stoner J.D., Mohammad M., Kuppusamy P. Hypoxic reperfusion of the ischemic heart and oxygen radical generation. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H341–H347. doi: 10.1152/ajpheart.00223.2005. [DOI] [PubMed] [Google Scholar]
- 456.Choi E.K., Jung H., Kwak K.H., Yeo J., Yi S.J., Park C.Y., Ryu T.H., Jeon Y.H., Park K.M., Lim D.G. Effects of allopurinol and apocynin on renal ischemia-reperfusion injury in rats. Transpl. Proc. 2015;47:1633–1638. doi: 10.1016/j.transproceed.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 457.Cai H. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ. Res. 2005;96:818–822. doi: 10.1161/01.RES.0000163631.07205.fb. [DOI] [PubMed] [Google Scholar]
- 458.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta. 2010;1797:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 460.Schulz E., Wenzel P., Munzel T., Daiber A. Mitochondrial redox signaling: interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid. Redox Signal. 2014;20:308–324. doi: 10.1089/ars.2012.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Doughan A.K., Harrison D.G., Dikalov S.I. Molecular mechanisms of angiotensin ii-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 462.Rathore R.Z.Y., Niu C.F., Liu Q.H., Korde A., Ho Y.S., Wang Y.X. Hypoxia activates NADPH oxidase to increase [ros]i and [Ca2+]I through the mitochondrial ros-pkcepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic. Biol. Med. 2008;45:1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Dikalova A.E., Bikineyeva A.T., Budzyn K., Nazarewicz R.R., McCann L., Lewis W., Harrison D.G., Dikalov S.I. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Gladden J.D., Zelickson B.R., Wei C.C., Ulasova E., Zheng J., Ahmed M.I., Chen Y., Bamman M., Ballinger S., Darley-Usmar V., Dell'Italia L.J. Novel insights into interactions between mitochondria and xanthine oxidase in acute cardiac volume overload. Free Radic. Biol. Med. 2011;51:1975–1984. doi: 10.1016/j.freeradbiomed.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Phan S.H., Gannon D.E., Varani J., Ryan U.S., Ward P.A. Xanthine oxidase activity in rat pulmonary artery endothelial cells and its alteration by activated neutrophils. Am. J. Pathol. 1989;134:1201–1211. [PMC free article] [PubMed] [Google Scholar]
- 466.Wakabayashi Y., Fujita H., Morita I., Kawaguchi H., Murota S. Conversion of xanthine dehydrogenase to xanthine oxidase in bovine carotid artery endothelial cells induced by activated neutrophils: involvement of adhesion molecules. Biochim. Biophys. Acta. 1995;1265:103–109. doi: 10.1016/0167-4889(94)00202-p. [DOI] [PubMed] [Google Scholar]
- 467.Murota S.I., Fujita H., Morita I., Wakabayashi Y. Adhesion molecule mediated endothelial cell injury elicited by activated leukocytes. Ann. N. Y. Acad. Sci. 1995;748:133–147. doi: 10.1111/j.1749-6632.1994.tb17313.x. [DOI] [PubMed] [Google Scholar]
- 468.Phan S.H., Gannon D.E., Ward P.A., Karmiol S. Mechanism of neutrophil-induced xanthine dehydrogenase to xanthine oxidase conversion in endothelial cells: evidence of a role for elastase. Am. J. Respir. Cell. Mol. Biol. 1992;6:270–278. doi: 10.1165/ajrcmb/6.3.270. [DOI] [PubMed] [Google Scholar]
- 469.Hassoun P.M., Yu F.S., Zulueta J.J., White A.C., Lanzillo J.J. Effect of nitric oxide and cell redox status on the regulation of endothelial cell xanthine dehydrogenase. Am. J. Physiol. 1995;268:L809–L817. doi: 10.1152/ajplung.1995.268.5.L809. [DOI] [PubMed] [Google Scholar]
- 470.Cote C.G., Yu F.S., Zulueta J.J., Vosatka R.J., Hassoun P.M. Regulation of intracellular xanthine oxidase by endothelial-derived nitric oxide. Am. J. Physiol. 1996;271:L869–L874. doi: 10.1152/ajplung.1996.271.5.L869. [DOI] [PubMed] [Google Scholar]
- 471.Rinaldo J.E., Clark M., Parinello J., Shepherd V.L. Nitric oxide inactivates xanthine dehydrogenase and xanthine oxidase in interferon-gamma-stimulated macrophages. Am. J. Respir. Cell. Mol. Biol. 1994;11:625–630. doi: 10.1165/ajrcmb.11.5.7524568. [DOI] [PubMed] [Google Scholar]
- 472.Gremmels H., Bevers L.M., Fledderus J.O., Braam B., van Zonneveld A.J., Verhaar M.C., Joles J.A. Oleic acid increases mitochondrial reactive oxygen species production and decreases endothelial nitric oxide synthase activity in cultured endothelial cells. Eur. J. Pharmacol. 2015;751:67–72. doi: 10.1016/j.ejphar.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 473.Camara A.K., Lesnefsky E.J., Stowe D.F. Potential therapeutic benefits of strategies directed to mitochondria. Antioxid. Redox Signal. 2010;13:279–347. doi: 10.1089/ars.2009.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Granger D.N., Rodrigues S.F., Yildirim A., Senchenkova E.Y. Microvascular responses to cardiovascular risk factors. Microcirculation. 2010;17:192–205. doi: 10.1111/j.1549-8719.2009.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Allison R.C., Kyle J., Adkins W.K., Prasad V.R., McCord J.M., Taylor A.E. Effect of ischemia reperfusion or hypoxia reoxygenation on lung vascular permeability and resistance. J Appl. Physiol. 1990;69:597–603. doi: 10.1152/jappl.1990.69.2.597. [DOI] [PubMed] [Google Scholar]
- 476.Chambers D.E., Parks D.A., Patterson G., Roy R., McCord J.M., Yoshida S., Parmley L.F., Downey J.M. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J. Mol. Cell. Cardiol. 1985;17:145–152. doi: 10.1016/s0022-2828(85)80017-1. [DOI] [PubMed] [Google Scholar]
- 477.Im M.J., Shen W.H., Pak C.J., Manson P.N., Bulkley G.B., Hoopes J.E. Effect of allopurinol on the survival of hyperemic island skin flaps. Plast. Reconstr. Surg. 1984;73:276–278. doi: 10.1097/00006534-198402000-00023. [DOI] [PubMed] [Google Scholar]
- 478.Rieger J.M., Shah A.R., Gidday J.M. Ischemia-reperfusion injury of retinal endothelium by cyclooxygenase- and xanthine oxidase-derived superoxide. Exp. Eye Res. 2002;74:493–501. doi: 10.1006/exer.2001.1156. [DOI] [PubMed] [Google Scholar]
- 479.Patt A., Harken A.H., Burton L.K., Rodell T.C., Piermattei D., Schorr W.J., Parker N.B., Berger E.M., Horesh I.R., Terada L.S. Xanthine oxidase-derived hydrogen peroxide contributes to ischemia reperfusion-induced edema in gerbil brains. J. Clin. Investig. 1988;81:1556–1562. doi: 10.1172/JCI113488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Hotter G., Closa D., Gelpi E., Prats N., Rosello-Catafau J. Role of xanthine oxidase and eicosanoids in development of pancreatic ischemia-reperfusion injury. Inflammation. 1995;19:469–478. doi: 10.1007/BF01534580. [DOI] [PubMed] [Google Scholar]
- 481.Perry M.A., Wadhwa S., Parks D.A., Pickard W., Granger D.N. Role of oxygen radicals in ischemia-induced lesions in the cat stomach. Gastroenterology. 1986;90:362–367. doi: 10.1016/0016-5085(86)90933-9. [DOI] [PubMed] [Google Scholar]
- 482.Akgur F.M., Kilinc K., Aktug T., Olguner M. The effect of allopurinol pretreatment before detorting testicular torsion. J. Urol. 1994;151:1715–1717. doi: 10.1016/s0022-5347(17)35351-x. [DOI] [PubMed] [Google Scholar]
- 483.Paller M.S., Hoidal J.R., Ferris T.F. Oxygen free radicals in ischemic acute renal failure in the rat. J. Clin. Investig. 1984;74:1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Woodruff T., Blake D.R., Freeman J., Andrews F.J., Salt P., Lunec J. Is chronic synovitis an example of reperfusion injury? Ann. Rheum. Dis. 1986;45:608–611. doi: 10.1136/ard.45.7.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Qayumi A.K., Janusz M.T., Dorovini-Zis K., Lyster D.M., Jamieson W.R., Poostizadeh A., Feeley E.J., Nikbakht-Sangari M. Additive effect of allopurinol and deferoxamine in the prevention of spinal cord injury caused by aortic crossclamping. J. Thorac. Cardiovasc. Surg. 1994;107:1203–1209. [PubMed] [Google Scholar]
- 486.Fisher A.B., Al-Mehdi A.B., Muzykantov V. Activation of endothelial NADPH oxidase as the source of a reactive oxygen species in lung ischemia. Chest. 1999;116:25S–26S. doi: 10.1378/chest.116.suppl_1.25s. [DOI] [PubMed] [Google Scholar]
- 487.Ikeda Y., Young L.H., Scalia R., Ross C.R., Lefer A.M. Pr-39, a proline/arginine-rich antimicrobial peptide, exerts cardioprotective effects in myocardial ischemia-reperfusion. Cardiovasc. Res. 2001;49:69–77. doi: 10.1016/s0008-6363(00)00226-1. [DOI] [PubMed] [Google Scholar]
- 488.Lehnert M., Arteel G.E., Smutney O.M., Conzelmann L.O., Zhong Z., Thurman R.G., Lemasters J.J. Dependence of liver injury after hemorrhage/resuscitation in mice on NADPH oxidase-derived superoxide. Shock. 2003;19:345–351. doi: 10.1097/00024382-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 489.Jang H.S., Kim J.I., Kim J., Na Y.K., Park J.W., Park K.M. Bone marrow derived cells and reactive oxygen species in hypertrophy of contralateral kidney of transient unilateral renal ischemia-induced mouse. Free Radic. Res. 2012;46:903–911. doi: 10.3109/10715762.2012.686664. [DOI] [PubMed] [Google Scholar]
- 490.Sener T.E., Yuksel M., Ozyilmaz-Yay N., Ercan F., Akbal C., Simsek F., Sener G. Apocynin attenuates testicular ischemia-reperfusion injury in rats. J. Pediatr. Surg. 2014;50:1382–1387. doi: 10.1016/j.jpedsurg.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 491.Powell C.S., Jackson R.M. Mitochondrial complex I, aconitase, and succinate dehydrogenase during hypoxia-reoxygenation: modulation of enzyme activities by mnsod. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L189–L198. doi: 10.1152/ajplung.00253.2002. [DOI] [PubMed] [Google Scholar]
- 492.Piantadosi C.A., Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke J. Cereb. Circ. 1996;27:327–331. doi: 10.1161/01.str.27.2.327. [DOI] [PubMed] [Google Scholar]
- 493.Tran T.P., Tu H., Liu J., Muelleman R.L., Li Y.L. Mitochondria-derived superoxide links to tourniquet-induced apoptosis in mouse skeletal muscle. PloS One. 2012;7:e43410. doi: 10.1371/journal.pone.0043410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Gonzalez-Flecha B., Cutrin J.C., Boveris A. Time course and mechanism of oxidative stress and tissue damage in rat liver subjected to in vivo ischemia-reperfusion. J. Clin. Investig. 1993;91:456–464. doi: 10.1172/JCI116223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Gonzalez-Flecha B., Boveris A. Mitochondrial sites of hydrogen peroxide production in reperfused rat kidney cortex. Biochim. Biophys. Acta. 1995;1243:361–366. doi: 10.1016/0304-4165(94)00160-y. [DOI] [PubMed] [Google Scholar]
- 496.Muthuraman A., Ramesh M., Chauhan A. Mitochondrial dependent apoptosis: ameliorative effect of flunarizine on ischemia-reperfusion of celiac artery-induced gastric lesions in the rat. Dig. Dis. Sci. 2011;56:2244–2251. doi: 10.1007/s10620-011-1607-0. [DOI] [PubMed] [Google Scholar]
- 497.Lysiak J.J., Zheng S., Woodson R., Turner T.T. Caspase-9-dependent pathway to murine germ cell apoptosis: mediation by oxidative stress, bax, and caspase 2. Cell. Tissue Res. 2007;328:411–419. doi: 10.1007/s00441-006-0341-y. [DOI] [PubMed] [Google Scholar]
- 498.Roseborough G., Gao D., Chen L., Trush M.A., Zhou S., Williams G.M., Wei C. The mitochondrial k-atp channel opener, diazoxide, prevents ischemia-reperfusion injury in the rabbit spinal cord. Am. J. Pathol. 2006;168:1443–1451. doi: 10.2353/ajpath.2006.050569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Elrod J.W., Duranski M.R., Langston W., Greer J.J., Tao L., Dugas T.R., Kevil C.G., Champion H.C., Lefer D.J. Enos gene therapy exacerbates hepatic ischemia-reperfusion injury in diabetes: a role for enos uncoupling. Circ. Res. 2006;99:78–85. doi: 10.1161/01.RES.0000231306.03510.77. [DOI] [PubMed] [Google Scholar]
- 500.Bysani G.K., Kennedy T.P., Ky N., Rao N.V., Blaze C.A., Hoidal J.R. Role of cytochrome p-450 in reperfusion injury of the rabbit lung. J. Clin. Investig. 1990;86:1434–1441. doi: 10.1172/JCI114859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Granville D.J., Tashakkor B., Takeuchi C., Gustafsson A.B., Huang C., Sayen M.R., Wentworth P., Jr., Yeager M., Gottlieb R.A. Reduction of ischemia and reperfusion-induced myocardial damage by cytochrome p450 inhibitors. Proc. Natl. Acad. Sci. USA. 2004;101:1321–1326. doi: 10.1073/pnas.0308185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Paller M.S., Jacob H.S. Cytochrome p-450 mediates tissue-damaging hydroxyl radical formation during reoxygenation of the kidney. Proc. Natl. Acad. Sci. USA. 1994;91:7002–7006. doi: 10.1073/pnas.91.15.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Wakatsuki A., Izumiya C., Okatani Y., Sagara Y. Oxidative damage in fetal rat brain induced by ischemia and subsequent reperfusion. Relation to arachidonic acid peroxidation. Biol. Neonate. 1999;76:84–91. doi: 10.1159/000014145. [DOI] [PubMed] [Google Scholar]
- 504.Yoshikawa M., Mukai Y., Okada Y., Yoshioka Y., Tsunoda S., Tsutsumi Y., Okada N., Aird W.C., Doi T., Nakagawa S. Ligand-independent assembly of purified soluble magic roundabout (robo4), a tumor-specific endothelial marker. Protein Expr. Purif. 2008;61:78–82. doi: 10.1016/j.pep.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 505.Kunduzova O.R., Bianchi P., Parini A., Cambon C. Hydrogen peroxide production by monoamine oxidase during ischemia/reperfusion. Eur. J. Pharmacol. 2002;448:225–230. doi: 10.1016/s0014-2999(02)01913-1. [DOI] [PubMed] [Google Scholar]
- 506.Suzuki T., Akaike N., Ueno K., Tanaka Y., Himori N. Mao inhibitors, clorgyline and lazabemide, prevent hydroxyl radical generation caused by brain ischemia/reperfusion in mice. Pharmacology. 1995;50:357–362. doi: 10.1159/000139304. [DOI] [PubMed] [Google Scholar]
- 507.Simonson S.G., Zhang J., Canada A.T., Jr, Su Y.F., Benveniste H., Piantadosi C.A. Hydrogen peroxide production by monoamine oxidase during ischemia-reperfusion in the rat brain. J. Cereb. Blood Flow. Metab.: Off. J. Int. Soc. Cereb. Blood Flow. Metab. 1993;13:125–134. doi: 10.1038/jcbfm.1993.15. [DOI] [PubMed] [Google Scholar]
- 508.Xie L., Talukder M.A., Sun J., Varadharaj S., Zweier J.L. Liposomal tetrahydrobiopterin preserves enos coupling in the post-ischemic heart conferring in vivo cardioprotection. J. Mol. Cell. Cardiol. 2015;86:14–22. doi: 10.1016/j.yjmcc.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]