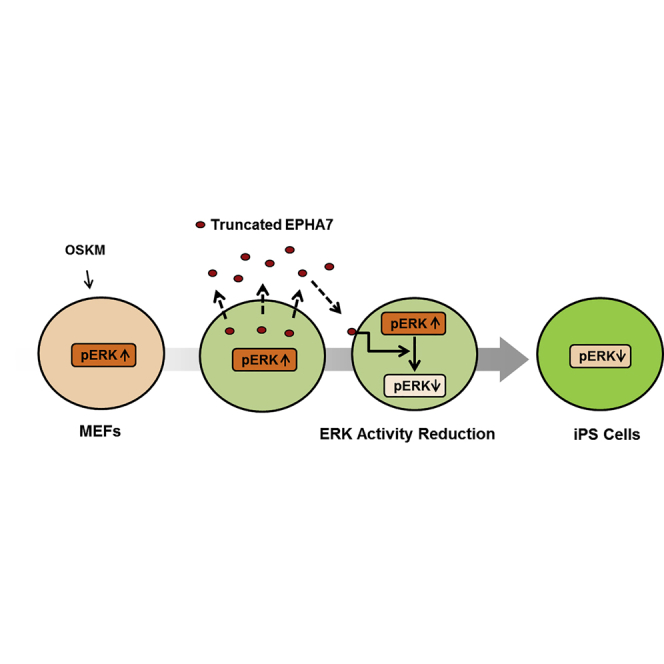
Summary
The role of secreted molecules in cellular reprogramming has been poorly understood. Here we identify a truncated form of ephrin receptor A7 (EPHA7) as a key regulator of reprogramming. Truncated EPHA7 is prominently upregulated and secreted during reprogramming. EPHA7 expression is directly regulated by OCT3/4. EphA7 knockdown results in marked reduction of reprogramming efficiency, and the addition of truncated EPHA7 is able to restore it. ERK activity is markedly reduced during reprogramming, and the secreted, truncated EPHA7 is responsible for ERK activity reduction. Remarkably, treatment of EphA7-knockdown MEFs with the ERK pathway inhibitor restores reprogramming efficiency. Analyses show that truncated EPHA7-induced ERK activity reduction plays an important role in the middle phase of reprogramming. Thus, our findings uncover the importance of secreted EPHA7-induced ERK activity reduction in reprogramming.
Graphical Abstract
Highlights
-
•
EPHA7 is prominently upregulated during reprogramming
-
•
Secreted, truncated EPHA7 contributes to promoting reprogramming
-
•
Truncated EPHA7 drives reprogramming by inducing ERK1/2 activity reduction
-
•
Truncated EPHA7 plays an important role in the middle phase of reprogramming
Nishida and colleagues show that truncated EPHA7 promotes cellular reprogramming by inducing ERK1/2 activity reduction. Truncated EPHA7 is transiently upregulated and secreted during reprogramming. The downregulation of ERK1/2 activity by secreted, truncated EPHA7 is required for efficient reprogramming. These findings uncover the importance of secreted EPHA7-induced ERK1/2 activity reduction in reprogramming.
Introduction
Somatic cells can be reprogrammed to a pluripotent state (induced pluripotent stem [iPS] cells) by defined transcription factors (Oct3/4, Sox2, Klf4, and c-Myc, hereafter referred to as OSKM) (Takahashi and Yamanaka, 2006). iPS cells are almost equivalent to embryonic stem cells (ESCs) in their properties (Okita et al., 2007, Mikkelsen et al., 2008). Many studies have been done to elucidate the mechanism of reprogramming (Stadtfeld et al., 2008, Samavarchi-Tehrani et al., 2010, Buganim et al., 2012), and several factors that affect reprogramming efficiency have also been revealed (Huangfu et al., 2008, Onder et al., 2012, Costa et al., 2013). But the importance of cell-cell interactions and secreted, extracellular molecules in reprogramming has not been extensively studied.
To approach this problem, we focused on EPHRIN receptor (EPH)/EPHRIN (EFN) signaling pathways. EPH receptors and EPHRINs are both anchored to the plasma membrane and form an important cell communication system with roles in normal physiology and disease pathogenesis (Pasquale, 2010). The EPH/EPHRIN signals propagate bidirectionally into both the EPH-expressing cells (forward signaling) and the EPHRIN-expressing cells (reverse signaling) to elicit different effects on each cell (Pasquale, 2008). Several EPH receptors and EPHRINs have truncated forms, which are secreted and function to propagate or repress signals (Aasheim et al., 2000, Dawson et al., 2007, Wykosky et al., 2008). Recently, some of EPH receptors and EPHRINs have been identified as regulators of stem and progenitor cell proliferation (Holmberg et al., 2005, Holmberg et al., 2006, Jiao et al., 2008, Nomura et al., 2010). However, whether EPH/EPHRIN signaling pathways regulate cell reprogramming has been unknown.
In this study, we found that EPHA7, among EPH and EFN members, is most prominently upregulated during reprogramming and that secreted, truncated EPHA7 plays a crucial role in reprogramming through inducing ERK activity reduction.
Results
EPHA7 Is Prominently Upregulated during Mouse Embryonic Fibroblast Reprogramming
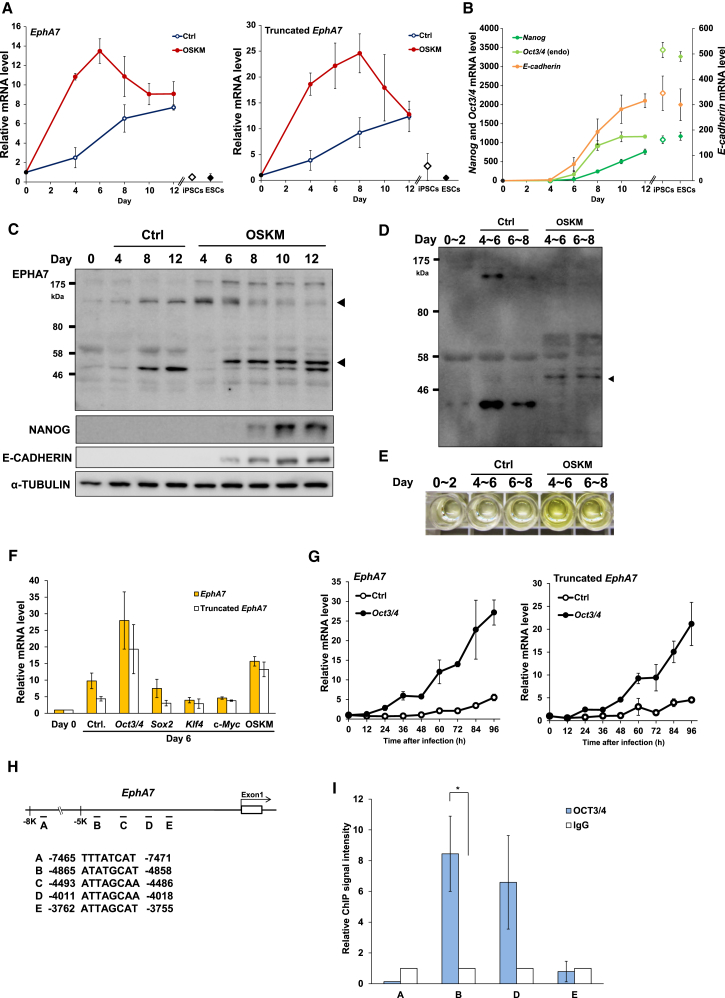
We measured the changes in the expression levels of Eph- and Efn-family genes during OSKM-induced reprogramming of mouse embryonic fibroblasts (MEFs) and found that EphA7 shows the most prominent upregulation. The upregulation of EphA7 preceded the induction of E-cadherin and the pluripotency markers Nanog and Oct3/4 (Figures 1A, 1B, S1A, and S1B). Because there exists a secreted, truncated form of EPHA7 (truncated EPHA7), which is a splice variant and lacks kinase activity (Dawson et al., 2007, Oricchio et al., 2011), we examined truncated EphA7. Truncated EphA7 was markedly upregulated from early periods of reprogramming (Figure 1A). But the expression levels of EphA7 and truncated EphA7 were low in established iPS cells and ESCs (Figure 1A).
Figure 1.
EPHA7 Is Upregulated during MEF Reprogramming
(A) qRT-PCR analysis of EphA7 (full-length plus truncated EphA7) and truncated EphA7. Ctrl (control), mCherry instead of OSKM.
(B) qRT-PCR analysis of Nanog, endogenous Oct3/4, and E-cadherin.
(C) Immunoblotting analysis for EPHA7, NANOG, E-CADHERIN, and α-TUBULIN (as a loading control). Triangles indicate full-length EPHA7 (top) and truncated EPHA7 (bottom).
(D) Immunoblotting analysis of conditioned media. A triangle indicates truncated EPHA7.
(E) ELISA of the conditioned medium for EPHA7.
(F) qRT-PCR analysis of EphA7 and truncated EphA7. mCherry (Ctrl), Oct3/4, Sox2, Klf4, c-Myc, or OSKM was introduced to MEFs at day 0.
(G) qRT-PCR analysis of both full-length EphA7 and truncated EphA7. Oct3/4 was introduced to MEFs at time 0. Ctrl, mCherry.
(H) Potential OCT3/4 biding sites in the upstream region of EphA7.
(I) Chromatin fragments of the OCT3/4 introduced MEFs were immunoprecipitated with control immunoglobulin G (IgG) or anti-OCT3/4 antibody. Each site was quantified by qRT-PCR. The values with control IgG were set to 1. Data are shown as mean ± SEM (sites B and D, n = 4 [∗p < 0.05]; site E, n = 3; site A, n = 2 independent experiments; site C, not detected). The p values were calculated using Student’s unpaired two-tailed t tests.
In (A), (B), (F), and (G), data are shown as mean ± SEM (n = 3 independent experiments).
The protein level of full-length EPHA7 was increased markedly at day 4 and then gradually decreased, and truncated EPHA7 protein was markedly increased at day 6 (Figure 1C). Analyses of the conditioned medium indicated that truncated EPHA7 protein was secreted during reprogramming (Figures 1D and 1E).
We next examined which factor is responsible for the upregulation of EphA7. Ectopic expression of Oct3/4 alone, but not Sox2, Klf4, or c-Myc, in MEFs resulted in marked increases in the expression levels of both full-length EphA7 and truncated EphA7 (Figure 1F), indicating that OCT3/4 plays a major role in the induction of EphA7. The analysis showed that OCT3/4 strongly upregulated the expression of both full-length and truncated EphA7 within 24 hr (Figure 1G). Chromatin immunoprecipitation assays showed that OCT3/4 directly bound to at least one site among five potential OCT3/4-binding sites (Figure 1H) (Nishimoto et al., 2003) in the upstream region of EphA7 (Figure 1I), suggesting that OCT3/4 directly regulates the expression of EphA7. OCT3/4 weakly bound to the EphA7 promoter region in ESCs (Figure S1C), consistent with the low expression of EphA7 in ESCs.
Truncated EPHA7 Plays a Crucial Role in Reprogramming
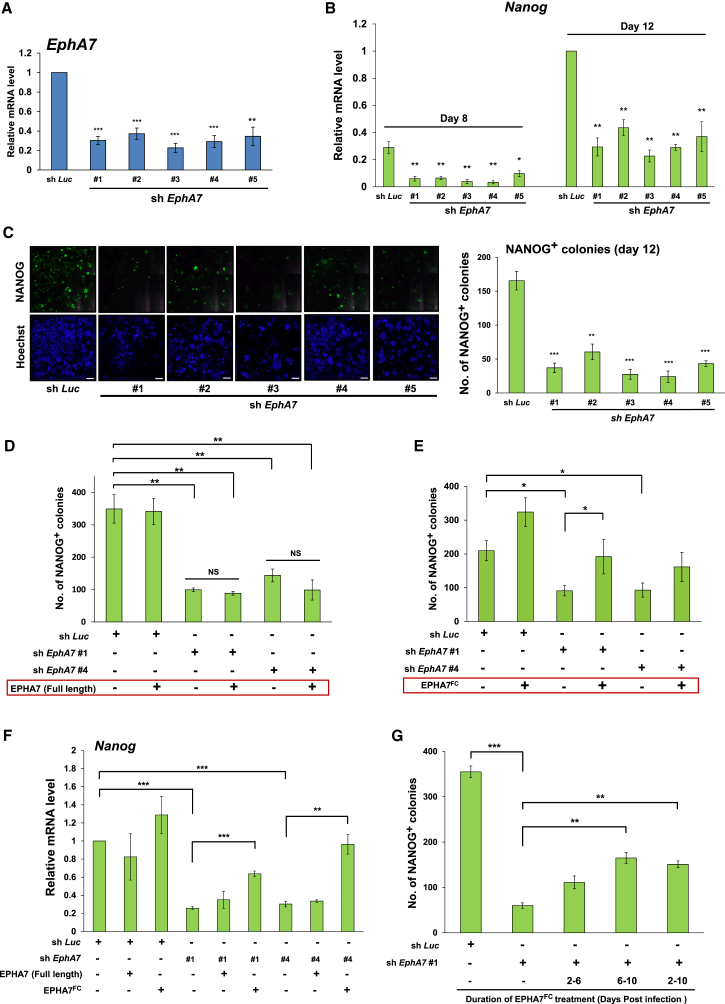
We then examined the effect of EphA7 knockdown on the reprogramming efficiency. EphA7 mRNA levels were markedly reduced by each EphA7 small hairpin RNA (shRNA) (Figure 2A). The protein levels of both full-length and truncated EPHA7 and the amounts of secreted, truncated EPHA7 protein were markedly reduced by each EphA7 shRNA (Figure S2A). EphA7 knockdown resulted in marked reduction in the mRNA and protein levels of NANOG (Figures 2B and S2A) and the numbers of alkaline phosphatase–positive colonies and NANOG-positive colonies (Figures 2C and S2B). The efficiency of OSK-mediated reprogramming was also markedly reduced by EphA7 knockdown (Figure S2C). These results show that EPHA7 promotes reprogramming.
Figure 2.
Truncated EPHA7 (EPHA7FC), but Not Full-Length EPHA7, Enhances Reprogramming Efficiency
(A) Knockdown efficiencies of EphA7 shRNAs. EphA7 mRNA levels at day 6 were determined. Luciferase shRNA (sh Luc), control.
(B) Effect of EphA7 shRNAs on Nanog mRNA levels during reprogramming.
(C) Effect of EphA7 shRNAs on the number of NANOG-positive colonies. Left: representative images at day 12 (left). Top: NANOG-positive colonies; bottom: Hoechst. The scale bars represent 2 mm. Right: quantification.
(D) Effect of exogenous expression of full-length EPHA7 on reprogramming efficiency. Full-length EphA7 or mCherry (Ctrl) was expressed in control shRNA-treated, OSKM-introduced MEFs (sh Luc) or in EphA7 shRNA-treated, OSKM-introduced MEFs (sh EphA7 #1 and #4). In (D), (E), and (G), quantification of the NANOG-positive colonies is shown.
(E) Effect of the addition of truncated EPHA7 protein to the culture medium on reprogramming efficiency. EPHA7FC (a truncated form of EPHA7; 5 μg/ml) was added to the culture medium at day 6 and incubated until day 10.
(F) Effect of the exogenous expression of full-length EphA7 or the addition of truncated EPHA7 protein to the culture medium on Nanog mRNA levels during reprogramming.
(G) Effect of the addition of truncated EPHA7 protein (EPHA7FC) to the culture medium at the different time points.
In (A)–(G), data are shown as mean ± SEM (n = 3 independent experiments; n = 4 independent experiments in [C]; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). The p values were calculated using Student’s unpaired two-tailed t tests.
We examined whether introduction of EPHA7 could reverse the decreased reprogramming efficiency of EphA7-knockdown cells. When full-length EPHA7 was introduced to EphA7-knockdown cells, the reprogramming efficiency was not improved (Figures 2D, 2F, and S2D). In contrast, the addition of EPHA7FC, which is a truncated form of EPHA7 protein, to the culture medium markedly increased reprogramming efficiency (Figures 2E, 2F, S2E, and S2F). These results indicate that secreted, truncated EPHA7, but not full-length EPHA7, contributes to promoting cell reprogramming. The addition of EPHA7FC enhanced the reprogramming efficiency of control MEFs (Figures 2E and 2F). We then examined whether truncated EPHA7 acts on specific phases of reprogramming or all the way along reprogramming. Data showed that the number of NANOG-positive colonies was significantly increased when EPHA7FC was added after day 6 (Figure 2G), suggesting that truncated EPHA7 has a stronger effect on the middle and/or late phases than on the early phase.
Knockdown of EfnA3, which shows the most prominent upregulation during reprogramming among Ephrin genes, did not significantly affect reprogramming efficiency (Figure S2G). This result, together with the above finding that truncated EPHA7, which is shown to function to inhibit EPH signaling (Dawson et al., 2007, Oricchio et al., 2011), but not full-length EPHA7, plays a positive role in reprogramming, suggests that inhibition of EPH signaling is important for reprogramming. Because there are many other ligands for EPHA7, knockdown of EfnA3 may not produce a large effect.
Truncated EPHA7 Promotes Cell Reprogramming by Inducing ERK Activity Reduction
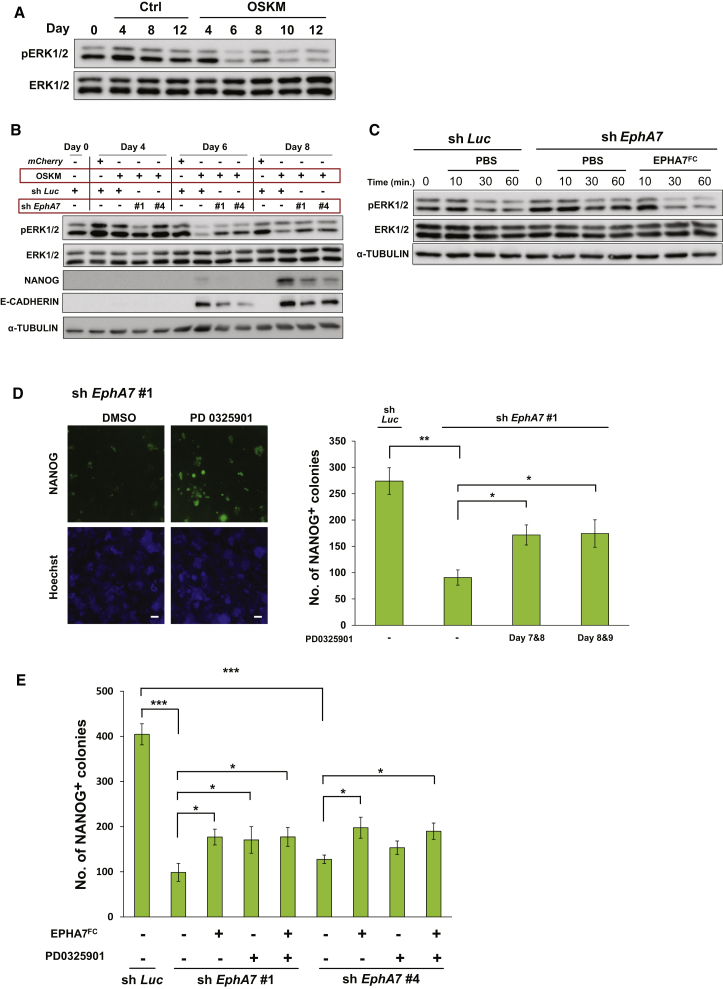
It has previously been shown that secreted, truncated EPHA7 inhibits EPH signaling, which induces the phosphorylation and activation of ERK1/2 in lymphomas (Oricchio et al., 2011), and that the levels of phosphorylated ERK1/2 (pERK1/2) are increased when mouse ESCs lose pluripotency and start to differentiate (Kim et al., 2012). We thus reasoned that truncated EPHA7 would regulate cellular reprogramming by controlling ERK1/2 activity. We then examined pERK1/2 levels during reprogramming and found that pERK1/2 levels were markedly reduced after day 6 of reprogramming in parallel with EPHA7 upregulation (Figure 3A, OSKM; Figures 1A–1E).
Figure 3.
Truncated EPHA7 Promotes MEF Reprogramming through Inducing ERK Activity Reduction
(A) Immunoblotting analysis for pERK1/2 in mCherry (Ctrl) or OSKM-introduced MEFs. The samples here were the same as those used in Figure 1C.
(B) Immunoblotting analysis for pERK1/2 levels in EphA7 shRNA-treated cells.
(C) Effect of the addition of truncated EPHA7 on pERK1/2 levels. EPHA7FC (5 μg/ml) was added to the culture medium in OSKM-introduced MEFs treated with EphA7 shRNA #4 or sh Luc at day 8.
(D) EphA7 shRNA (#1)-treated, OSKM-introduced MEFs were treated with the MEK inhibitor PD0325901 (0.5 μM) for 2 days as indicated and examined for the number of Nanog-positive colonies at day 12. Left: representative images; right: quantification. The scale bars represent 1 mm.
(E) Effect of the addition of truncated EPHA7 and/or the MEK inhibitor (PD0325901) treatment on reprogramming efficiency. EPHA7FC was added to the culture medium from day 6 to day 10. PD0325901 treatment was from day 7 to day 8. The number of NANOG-positive colonies at day 12 was examined.
In (D) and (E), data are shown as mean ± SEM (n = 3 independent experiments; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). The p values were calculated using Student’s unpaired two-tailed t tests.
The analysis revealed that the reduction of pERK1/2 levels during reprogramming was suppressed in EphA7-knockdown cells (Figure 3B). pERK1/2 levels were reduced to the lowest level at day 6, when the induction of E-CADHERIN began to occur (Figure 3B). Remarkably, the addition of EPHA7FC to the culture medium reversed the EphA7 knockdown-induced suppression of pERK1/2 reduction and thus caused pERK1/2 reduction again (Figure 3C).
Because ERK1/2 activity reduction is likely to play a role in promoting cellular reprogramming, we examined whether treatment with the specific inhibitor of MEK, an activator of ERK1/2, gives the same effect as the addition of truncated EPHA7 on reprogramming. The results showed that incubation of EphA7-knockdown cells with the MEK inhibitor PD0325901 during the reprogramming process significantly restored reprogramming efficiency (Figures 3D and S3A). Moreover, PD0325901 treatment enhanced the reprogramming efficiency of control MEFs (Figure S3B). These effects were the same as those of the addition of truncated EPHA7 (see Figure 2E). Furthermore, EPHA7FC and MEK inhibition did not have additive effects on reprogramming efficiency (Figure 3E). These results support our idea that truncated EPHA7 promotes cellular reprogramming by inducing ERK1/2 activity reduction.
Inhibition of MEK is shown to be helpful to ESC establishment and maintenance (Ying et al., 2008, Shi et al., 2008, Silva et al., 2009). To examine whether truncated EPHA7 has an effect on pluripotency maintenance, ESCs were treated with EPHA7FC, and mRNA levels of pluripotency markers, such as Nanog and Klf2, were examined. Data showed that EPHA7FC did not increase the expression of pluripotency marker genes, while treatment with PD0325901 or 2i (PD0325901 and CHIR99021; GSK-3β inhibitor) significantly increased their expression (Figure S3C). Furthermore, EPHA7FC treatment did not reduce pERK1/2 levels in ESCs (Figure S3D). Thus, the regulation of ERK1/2 activity by truncated EPHA7 may not be involved in pluripotency maintenance in ESCs.
Pre-reprogrammed Cells Secrete Truncated EPHA7 and Support SSEA-1 Negative Cells to Progress toward a Pluripotent State
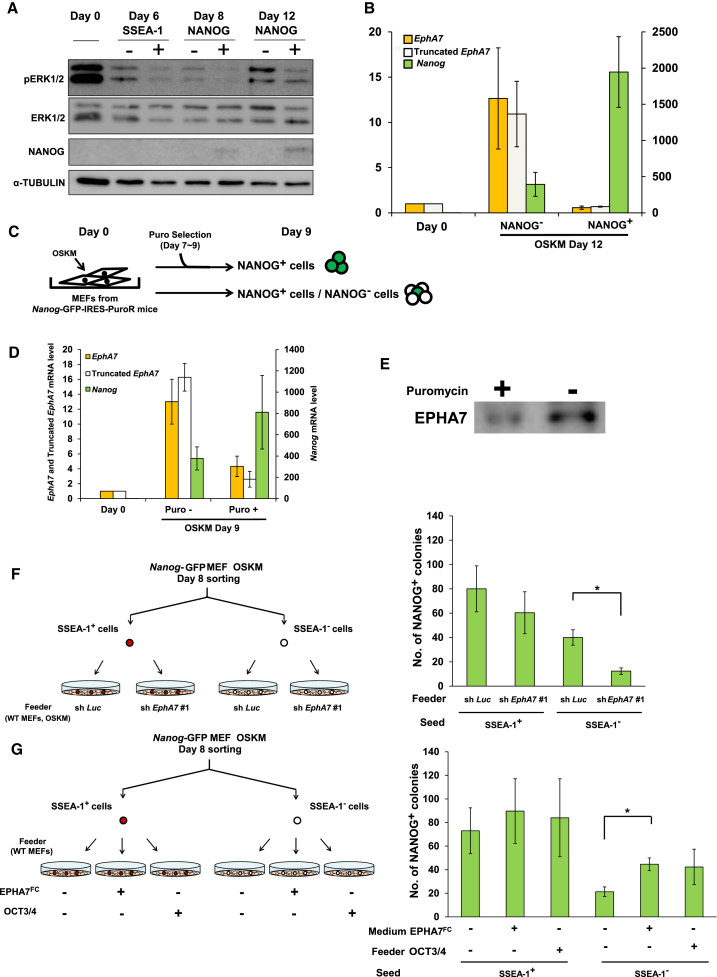
We examined pERK1/2 levels of cells, which were sorted by the expression intensity of SSEA-1, an early pluripotency marker, or that of NANOG (Figure S4A). The analysis revealed that pERK1/2 levels were reduced to a greater extent in SSEA-1- and NANOG-positive cells than in SSEA-1- and Nanog-negative cells, respectively (Figure 4A), suggesting that the progress of reprogramming is accompanied by the reduction of pERK1/2 levels.
Figure 4.
Truncated EPHA7 Is Expressed and Secreted from Pre-reprogramming Cells and Supports SSEA-1-Negative Cells to Progress toward a Pluripotent State
(A) Immunoblotting analysis for pERK1/2 levels of the sorted samples (shown in Figure S4A).
(B) qRT-PCR analysis of truncated EphA7 in the sorted samples.
(C) A scheme for the puromycin selection experiment. OSKM were introduced into MEFs, derived from Nanog-GFP-IRES-PuroR mice, and the cells were treated with puromycin (1 μg/ml) from day 7 to day 9 for selection.
(D) qRT-PCR analysis of Nanog, EphA7 and truncated EphA7 from cells treated with puromycin (NANOG+ cells) or without puromycin (NANOG− cells/NANOG+ cells). In (B) and (D), data are shown as mean ± SEM (n = 3 independent experiments).
(E) Immunoblotting analysis of conditioned media from cells treated with puromycin (NANOG+ cells) or without puromycin (NANOG− cells/NANOG+ cells).
(F) Effect of truncated EPHA7-expressing pre-reprogrammed cells on reprogramming of SSEA-1+ and SSEA-1− cells. Left: a scheme for the experiment. OSKM-introduced Nanog-GFP MEFs were sorted into SSEA-1+ and SSEA-1− cells at day 8 and plated on control shRNA-treated, OSKM-introduced WT MEFs (sh Luc) or on EphA7 shRNA-treated, OSKM-introduced WT MEFs (sh EphA7 #1). Right: the number of NANOG-positive colonies after 4 days of incubation.
(G) Effect of truncated EPHA7 on reprogramming of SSEA-1+ and SSEA-1− cells. Left: a scheme for the experiments. OSKM-introduced Nanog-GFP MEFs were sorted into SSEA-1+ and SSEA-1− cells at day 8 and plated on control WT MEFs or OCT3/4-overexpressing WT MEFs. EPHA7FC (5 μg/ml) or PBS was added to the culture medium. Right: the number of NANOG-positive colonies after 4 days of incubation. In (F) and (G), data are shown as mean ± SEM (n = 3 independent experiments; ∗p < 0.05). The p values were calculated using Student’s unpaired two-tailed t tests.
We then found that both full-length EphA7 and truncated EphA7 were expressed to a much higher extent in NANOG-negative cells than in NANOG-positive cells at the late time point (day 12) of reprogramming (Figure 4B). Moreover, we performed puromycin selection experiments by using MEFs derived from Nanog-GFP-IRES-PuroR mice (Okita et al., 2007), which express GFP and the puromycin-resistant gene under the control of the Nanog promoter (Figure 4C). We treated the cells with puromycin from day 7 to day 9 after OSKM introduction. Data also showed that both full-length EphA7 and truncated EphA7 were expressed to a much higher extent in NANOG-negative (puro−) cells than in NANOG-positive (puro+) cells (Figure 4D) and that the amount of secreted, truncated EPHA7 in the culture medium was greater in non-puromycin-added cell cultures than in puromycin-added ones (Figure 4E). These results suggest that EPHA7 expression was markedly decreased in fully reprogrammed cells.
We asked whether pre-reprogrammed cells expressing truncate EPHA7 are able to support other cells to progress toward a pluripotent state, and which kind of cells were affected by truncated EPHA7. We sorted OSKM-introduced Nanog-GFP MEFs into SSEA-1-positive and SSEA-1-negative cells at days 8 and 12. Sorted cells were then plated on the control shRNA-treated, OSKM-introduced wild-type (WT) MEFs or EphA7 shRNA-treated, OSKM-introduced WT MEFs, and the number of Nanog-GFP colonies was examined. The results showed that the number of Nanog-GFP positive colonies was reduced when sorted cells were cultured on the EphA7-knockdown feeder cells at both days 8 and 12 (Figures 4F and S4B). The extent of the reduction of Nanog-GFP positive colonies by EphA7 knockdown in feeder cells was larger in SSEA-1-negative cells than in SSEA-1-positive cells at day 8. Next, SSEA-1-positive and SSEA-1-negative cells from OSKM-introduced Nanog-GFP MEFs were sorted at day 8 and plated on MEF feeder cells, and PBS or EPHA7FC was added to the culture medium. We also prepared OCT3/4-overexpressing MEFs as feeder cells as OCT3/4 is responsible for the upregulation of truncated EphA7. The results showed that treatment with truncated EPHA7 or the use of OCT3/4-overexpressing feeder cells enhanced the generation of Nanog-positive colonies, especially from SSEA-1 negative cells (Figure 4G). Taken together, our results suggest that truncated EPHA7 is expressed and secreted from pre-reprogrammed cells and supports SSEA-1-negative cells to progress toward a pluripotent state.
Discussion
Here, we have demonstrated that EPHA7 is upregulated during the early and middle periods of MEF reprogramming and that secreted, truncated EPHA7 promotes MEF reprogramming through inducing ERK1/2 activity reduction. EPH receptors and EPHRINs are critical regulators of cell-cell interactions and known to transmit bidirectional signaling. Moreover, several of EPH receptors and EPHRINs have their truncated forms, which are secreted to regulate EPH/EPHRIN signaling. Our results demonstrate that a truncated form of EPHA7, but not full-length EPHA7, plays a crucial role in reprogramming and that truncated EPHA7 is secreted to the culture medium to influence surrounding cells and promote their reprogramming.
Previous studies have demonstrated that the inhibition of ERK1/2 activity plays a role in acquiring and maintaining the naive pluripotent state of ESCs and iPS cells (Silva et al., 2008, Ying et al., 2008). However, neither ERK1/2 activity changes during reprogramming nor their role in reprogramming has been addressed. Our data show that the reduction of ERK1/2 activity by truncated EPHA7 occurs in the middle phase (around day 6) of reprogramming and is essential for driving reprogramming.
Our results show that pre-reprogrammed cells mainly express and secrete truncated EPHA7 and that truncated EPHA7 has a stronger effect on SSEA-1-negative cells than on SSEA-1-positive cells. Notably, the reduction of the pERK/1/2 levels began at day 6 of reprogramming, SSEA-1-positive cells also started to emerge at day 6, and pERK1/2 levels remained higher in SSEA-1-negative cells than in SSEA-1-positive cells at day 6. Collectively, our results suggest that EPHA7-induced ERK activity reduction has a stronger effect on the middle phase of reprogramming and supports SSEA-1-negative cells to progress toward a pluripotent state.
The expression of truncated EPHA7 during reprogramming is transient, unlike most of the reprogramming-related genes that have high expression levels in mature ESCs or iPS cells. Microarray data in earlier reports (Mikkelsen et al., 2008, Sridharan et al., 2009, Samavarchi-Tehrani et al., 2010) also indicated that EPHA7 was mainly expressed in reprograming cells but not in ESCs or iPS cells. Moreover, our results show that the regulation of ERK1/2 activity by truncated EPHA7 is specific to cell reprogramming and may not be involved in pluripotency maintenance in ESCs and that the expression of eph receptors and ephrins is quite low in ESCs and iPS cells, indicating that EPH-EPHRIN signaling might not be involved in the regulation of the ERK1/2 pathway in ESCs.
Our analysis has demonstrated that the ERK activity reduction is not a result of the completion of reprogramming but is a crucial event during reprogramming, which drives reprogramming. This study identifies a secreted factor responsible for the ERK1/2 activity reduction during reprogramming. Thus, our findings reveal the importance of cell-cell communications during reprogramming.
Experimental Procedures
Cell Culture Protocols
The MEFs for microarray, qRT-PCR analyses (experiments for Figures 1A, S1A, and S1B) and establishment of iPS cells (Figure S2F) were from E13.5 ICR mice. In other experiments, MEFs were derived by mating ICR mice and Nanog-GFP-IRES-PuroR mice (provided by RIKEN BRC through the national Bio-Resource Project of MEXT) (Okita et al., 2007). The MEFs were maintained in DMEM)containing 10% fetal bovine serum (FBS). The Plat-E cells were maintained in DMEM containing 10% FBS, puromycin (1 μg/ml), and blasticidin S (10 μg/ml). HEK293T cells were maintained in DMEM containing 10% FBS and kanamycin (250 μg/ml). Cells undergoing reprogramming and ESCs (HT7) were cultured in a standard mouse ES medium containing 7.5% knockout serum replacement (Invitrogen), 7.5% HyClone standard FBS (Thermo) and 1,000 U/ml leukemia inhibitory factor (LIF) (Millipore). ESCs and mature iPS cells (iPS-MEF-Ng-20D-17; provided by the RIKEN BRC through the Project for Realization of Regenerative Medicine and the National Bio-Resource Project of MEXT) were cultured and passed on the mitomycin-C-treated MEF feeder cells in mouse ES medium with 1,500 U/ml LIF. All mouse experiments were conducted in accordance with the Regulation on Animal Experimentation at Kyoto University and approved by the Animal Experimentation Committee of Kyoto University.
Generation of Mouse iPS Cells
In each gelatin-coated 35 mm dish, 1 × 105 MEFs were seeded. The next day, cells were infected with the retroviruses in DMEM containing 10% FBS (day 0). Sixteen hours after infection, the medium was replaced with mouse ES medium. On day 2, the cells were plated onto new gelatin-coated 35 mm dishes, and ES medium was changed every 2 days. For the knockdown experiments, MEFs were infected with lentiviruses 2 days before infection with the retroviruses. For the rescue experiments, we added mouse recombinant EPHA7-Fc (EPHA7FC, R&D Systems, 608-A7-200) or PD0325901 (WAKO, 163-24001). EPHA7-Fc protein has the extracellular domain of mouse EPHA7 (Ala30-Pro549) and thus acts as truncated, secreted EPHA7.
Acknowledgments
This work was supported by JST, CREST (to E.N.); the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to E.N.); and the Japanese Government (MEXT) Scholarship Program (to J.L.).
Published: October 1, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.09.001.
Supplemental Information
References
- Aasheim H.C., Munthe E., Funderud S., Smeland E.B., Beiske K., Logtenberg T. A splice variant of human ephrin-A4 encodes a soluble molecule that is secreted by activated human B lymphocytes. Blood. 2000;95:221–230. [PubMed] [Google Scholar]
- Buganim Y., Faddah D.A., Cheng A.W., Itskovich E., Markoulaki S., Ganz K., Klemm S.L., van Oudenaarden A., Jaenisch R. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell. 2012;150:1209–1222. doi: 10.1016/j.cell.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Y., Ding J., Theunissen T.W., Faiola F., Hore T.A., Shliaha P.V., Fidalgo M., Saunders A., Lawrence M., Dietmann S. NANOG-dependent function of TET1 and TET2 in establishment of pluripotency. Nature. 2013;495:370–374. doi: 10.1038/nature11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D.W., Hong J.S., Shen R.R., French S.W., Troke J.J., Wu Y.Z., Chen S.S., Gui D., Regelson M., Marahrens Y. Global DNA methylation profiling reveals silencing of a secreted form of Epha7 in mouse and human germinal center B-cell lymphomas. Oncogene. 2007;26:4243–4252. doi: 10.1038/sj.onc.1210211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg J., Armulik A., Senti K.-A., Edoff K., Spalding K., Momma S., Cassidy R., Flanagan J.G., Frisén J. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes Dev. 2005;19:462–471. doi: 10.1101/gad.326905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg J., Genander M., Halford M.M., Annerén C., Sondell M., Chumley M.J., Silvany R.E., Henkemeyer M., Frisén J. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125:1151–1163. doi: 10.1016/j.cell.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Huangfu D., Maehr R., Guo W., Eijkelenboom A., Snitow M., Chen A.E., Melton D.A. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat. Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J.W., Feldheim D.A., Chen D.F. Ephrins as negative regulators of adult neurogenesis in diverse regions of the central nervous system. Proc. Natl. Acad. Sci. USA. 2008;105:8778–8783. doi: 10.1073/pnas.0708861105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.O., Kim S.H., Cho Y.Y., Nadas J., Jeong C.H., Yao K., Kim D.J., Yu D.H., Keum Y.S., Lee K.Y. ERK1 and ERK2 regulate embryonic stem cell self-renewal through phosphorylation of Klf4. Nat. Struct. Mol. Biol. 2012;19:283–290. doi: 10.1038/nsmb.2217. [DOI] [PubMed] [Google Scholar]
- Mikkelsen T.S., Hanna J., Zhang X., Ku M., Wernig M., Schorderet P., Bernstein B.E., Jaenisch R., Lander E.S., Meissner A. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto M., Miyagi S., Katayanagi T., Tomioka M., Muramatsu M., Okuda A. The embryonic Octamer factor 3/4 displays distinct DNA binding specificity from those of other Octamer factors. Biochem. Biophys. Res. Commun. 2003;302:581–586. doi: 10.1016/s0006-291x(03)00218-3. [DOI] [PubMed] [Google Scholar]
- Nomura T., Göritz C., Catchpole T., Henkemeyer M., Frisén J. EphB signaling controls lineage plasticity of adult neural stem cell niche cells. Cell Stem Cell. 2010;7:730–743. doi: 10.1016/j.stem.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K., Ichisaka T., Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Onder T.T., Kara N., Cherry A., Sinha A.U., Zhu N., Bernt K.M., Cahan P., Marcarci B.O., Unternaehrer J., Gupta P.B. Chromatin-modifying enzymes as modulators of reprogramming. Nature. 2012;483:598–602. doi: 10.1038/nature10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oricchio E., Nanjangud G., Wolfe A.L., Schatz J.H., Mavrakis K.J., Jiang M., Liu X., Bruno J., Heguy A., Olshen A.B. The Eph-receptor A7 is a soluble tumor suppressor for follicular lymphoma. Cell. 2011;147:554–564. doi: 10.1016/j.cell.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale E.B. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Pasquale E.B. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat. Rev. Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavarchi-Tehrani P., Golipour A., David L., Sung H.K., Beyer T.A., Datti A., Woltjen K., Nagy A., Wrana J.L. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell. 2010;7:64–77. doi: 10.1016/j.stem.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Shi Y., Do J.T., Desponts C., Hahm H.S., Schöler H.R., Ding S. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2:525–528. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Silva J., Barrandon O., Nichols J., Kawaguchi J., Theunissen T.W., Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J., Nichols J., Theunissen T.W., Guo G., van Oosten A.L., Barrandon O., Wray J., Yamanaka S., Chambers I., Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan R., Tchieu J., Mason M.J., Yachechko R., Kuoy E., Horvath S., Zhou Q., Plath K. Role of the murine reprogramming factors in the induction of pluripotency. Cell. 2009;136:364–377. doi: 10.1016/j.cell.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M., Maherali N., Breault D.T., Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Wykosky J., Palma E., Gibo D.M., Ringler S., Turner C.P., Debinski W. Soluble monomeric EphrinA1 is released from tumor cells and is a functional ligand for the EphA2 receptor. Oncogene. 2008;27:7260–7273. doi: 10.1038/onc.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Q.L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.