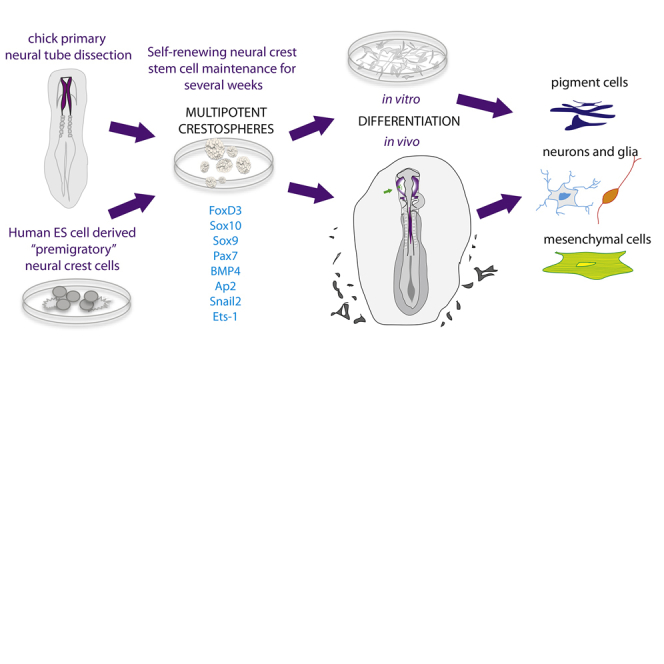
Summary
Premigratory neural crest cells comprise a transient, embryonic population that arises within the CNS, but subsequently migrates away and differentiates into many derivatives. Previously, premigratory neural crest could not be maintained in a multipotent, adhesive state without spontaneous differentiation. Here, we report conditions that enable maintenance of neuroepithelial “crestospheres” that self-renew and retain multipotency for weeks. Moreover, under differentiation conditions, these cells can form multiple derivatives in vitro and in vivo after transplantation into chick embryos. Similarly, human embryonic stem cells directed to a neural crest fate can be maintained as crestospheres and subsequently differentiated into several derivatives. By devising conditions that maintain the premigratory state in vitro, these results demonstrate that neuroepithelial neural crest precursors are capable of long-term self-renewal. This approach will help uncover mechanisms underlying their developmental potential, differentiation and, together with the induced pluripotent stem cell techniques, the pathology of human neurocristopathies.
Graphical Abstract
Highlights
-
•
Long-term maintenance of premigratory chick neural crest cells as crestospheres
-
•
A self-renewing population of multipotent neuroepithelial neural crest stem cells
-
•
Crestospheres differentiate into neural crest derivatives in vitro and in vivo
-
•
Long-term maintenance of human ESC-derived crestospheres for several weeks
Introduction
The neural crest is a uniquely vertebrate cell population characterized by its remarkable ability to form numerous differentiated derivatives, as diverse as facial skeleton and peripheral nervous system. From a stem cell biology perspective, the neural crest is an ideal embryonic source of multipotent stem cells for the purposes of regenerative medicine. Numerous studies have successfully isolated cell lines that differentiate into neural crest derivatives either in vitro or after transplantation into the embryo (Curchoe et al., 2010, Lee et al., 2007, Trentin et al., 2004). However, it remains controversial whether neural crest cells are indeed true stem cells or simply a transient multipotent progenitor population.
A cardinal feature of stem cells is their ability to give rise to multiple lineages and to self-renew. In contrast to true stem cells, primary cultures of embryonic neural crest cells examined to date have only limited capacity to self-renew in vitro (Stemple and Anderson, 1992, Trentin et al., 2004). Likewise, neural crest cells derived from peripheral nerve or facial skin have self-renewal capacity but tend to form a limited set of cell types reflecting their tissue of origin (Adameyko et al., 2009, Fernandes et al., 2004, Johnston et al., 2013, Li et al., 2007, Morrison et al., 1999). In general, adult neural-crest-derived “stem cells” have limited ability for long-term self-renewal or differentiation into a large repertoire of derivatives (Dupin and Coelho-Aguiar, 2013, Kruger et al., 2002). This raises the important question of whether a true neural crest stem cell that is multipotent and capable of self-renewal can be identified in either the embryo or adult.
Embryonic neural crest cells arise from the dorsal portion of the developing CNS around the time of neural tube closure. At this premigratory phase, neural crest precursors are characterized by combined expression of transcription factors such as FOXD3, SOXE, SNAIL, and AP2α (Khudyakov and Bronner-Fraser, 2009). They subsequently undergo an epithelial to mesenchymal transition (EMT), enabling them to leave the neural tube, and migrate extensively throughout the embryo. Single cell lineage analyses in vivo (Baggiolini et al., 2015, Bronner-Fraser and Fraser, 1988) and clonal analyses in vitro (Calloni et al., 2009) have shown that the majority of embryonic neural crest cells are multipotent. Indeed, premigratory precursors contribute not only to neural crest, but also to dorsal neural tube lineages (Baggiolini et al., 2015, Bronner-Fraser and Fraser, 1989). After emigration, however, their developmental potential is thought to become restricted by cues present in the environment and final site of localization. Finally, neural crest cells differentiate into many cell types that far exceed the repertoire traditionally considered as “ectodermal,” including sensory and autonomic neurons and glia, bone and cartilage of the face, smooth muscle cells, adipocytes, melanocytes, and various endocrine cells (Dupin and Coelho-Aguiar, 2013).
Development of new methods for generating neural crest cells with self-renewal capacity is complicated by the transience of the embryonic premigratory crest stage. Our goal was to maintain, for extended time periods in vitro, primary neural crest stem cells derived from the embryo or human embryonic stem cells (hESCs) in a self-renewing state that reflects their premigratory character; under appropriate conditions, these can then be differentiated into multiple derivatives. Such long-term maintenance of neural crest stem cells is useful not only for regenerative medicine, but also for understanding neural-crest-related birth defects.
Results
Crestosphere Culture Conditions
Reasoning that neural crest precursors residing within the dorsal neural tube are most likely to have stem cell properties and given elegant classical studies on avian neural crest development, we used cranial to vagal chick embryonic neural tubes from five- to eight-somite stage embryos as starting material. We sought to find appropriate culture conditions that would maintain a molecular profile similar to premigratory crest cells and support maintenance of the self-renewing and multipotent neural crest state, thus mimicking the time point when they are premigratory and reside within an adhesive neuroepithelium.
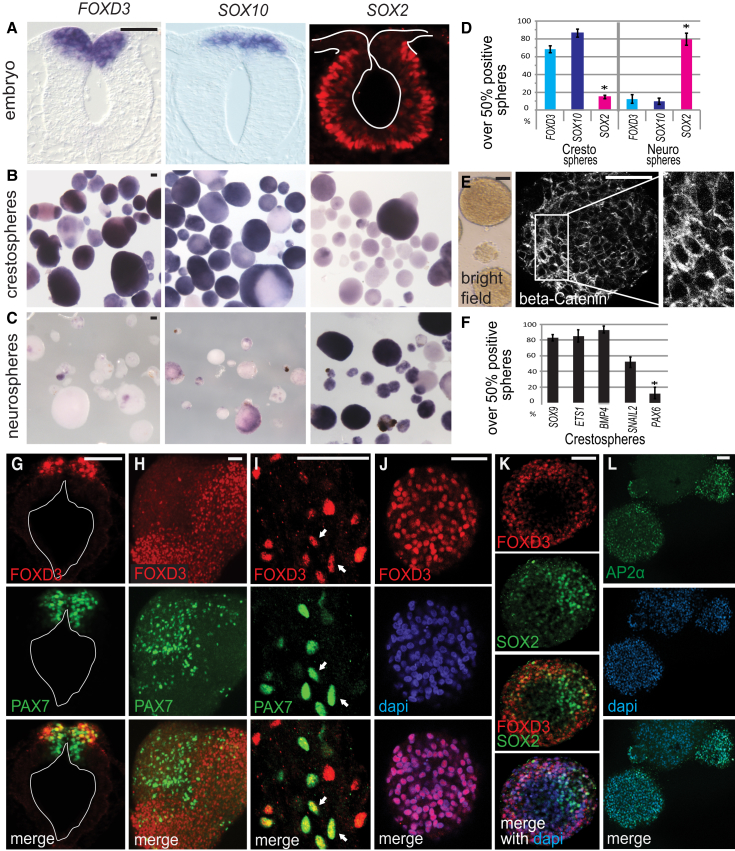
To determine optimal conditions for enabling culture of these embryonic cells as epithelial “crestospheres,” we tested the effects of different concentrations of growth factors and signaling molecules on expression of SOX10, FOXD3, and SOX2. SOX10 and FOXD3 are strongly expressed by premigratory neural crest (Figure 1A) and associated with maintenance of multipotency (Kim et al., 2003, Kim et al., 2014, Nitzan et al., 2013, Teng et al., 2008). In contrast, neural stem cell marker SOX2 is downregulated in dorsal relative to ventral neural tube regions (Figure 1A), albeit required at low levels for neural crest EMT (Cimadamore et al., 2011). Transcript levels were compared with those in whole embryo lysates using qPCR (see the Supplemental Results; Figure S1A). Because traditional neural stem cell medium, containing epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) (Molofsky et al., 2003) failed to sustain expression of FOXD3 and SOX10 (Figures 1C, 1D, and S1A–S1C), we turned to modified growth medium containing bFGF, retinoic acid (RA), and insulin-like growth factor1 (IGF1) previously used to support self-renewal of stem cells derived from embryonic sciatic nerve (Morrison et al., 1999), gut (Molofsky et al., 2003), or migratory neural crest (Mundell and Labosky, 2011) and implicated in aspects of neural crest development (Kerosuo and Bronner-Fraser, 2012, Martínez-Morales et al., 2011).
Figure 1.
Chick Crestospheres Mimic Premigratory Neural Crest Cells
(A) Expression of the neural crest markers FOXD3 and SOX10 mRNA and SOX2 protein, highest in the ventral neural tube, in HH9 chick embryos.
(B and C) Highly enriched RNA expression of premigratory neural crest markers in 2-week-old crestospheres (B) compared with neurospheres (C) derived from equivalently staged neural tubes that express high mRNA levels of the neural marker SOX2.
(D) Quantification of spheres that consist of over 50% positive cells for each marker shows a significant difference between crestospheres (n = 5) and neurospheres (n = 6).
(E) The epithelial characteristics of floating crestospheres revealed by a brightfield image and β-Catenin staining showing adherens junctions typical of epithelial cells (magnified in inset).
(F) High expression of additional premigratory neural crest markers quantified from in situ hybridization (n = 3). Low PAX6 levels reflect neural cells.
(G) FOXD3 and PAX7 show only partially overlapping protein expression in the chick cranial neural tube.
(H) A large crestosphere with both FOXD3 and PAX7 protein-expressing domains.
(I) High-magnification image showing partially overlapping expression of FOXD3 and PAX7 protein in a sphere (white arrows mark characteristic examples).
(J) Confocal image of a small crestosphere exclusively expressing FOXD3.
(K) Confocal image showing largely nonoverlapping protein expression of high SOX2 and FOXD3 in a sphere.
(L) Heterogenous protein expression of AP2α in crestospheres. Scale bar represents 50 μm.
Neural crest marker expression was significantly increased when cells were grown in the presence of bFGF, IGF1, and RA. Of the concentrations tested, 20 ng/ml of bFGF and IGF1 plus 60 nM (19 mg/ml) RA were optimal to maintain high levels of FOXD3 and SOX10 as assessed by qPCR and in situ hybridization. Concomitantly, these conditions yielded low SOX2 levels (Figures 1B, 1D, S1A–S1C, and S1F; Supplemental Results). In contrast, similar cells grown under neurosphere-promoting conditions contained high SOX2 but low neural crest marker expression (Figures 1C and 1D). Crestospheres are epithelial in character, as evidenced by their morphology and β-Catenin expression, which outlines adherens junctions on their membranes (Figure 1E) similar to that seen in neuroepithelial cells prior to neural crest emigration (Figure S1E). Cultures prepared from either dorsal or entire neural tubes had equivalent ability to form crestospheres, suggesting that the culture conditions support neural crest at the expense of neural fate (Figure S1D).
Neural Crest Marker Expression in Crestospheres Reflects Endogenous Expression In Vivo
We next examined expression of additional premigratory neural crest (SOX9, BMP4, ETS-1, SNAIL2) and neural (PAX6) markers in crestopheres by in situ hybridization. The results reveal heterogeneous but high transcript levels for neural crest markers but only low levels of neural markers (Figures 1F and S1G). For cellular resolution, we performed immunostaining followed by confocal microscopy of crestospheres and compared expression patterns to those in the embryo. Neural crest marker expression in vivo initiates in the dorsal neural tube in a characteristic sequence. Expression of neural plate border gene PAX7 initiates at gastrula stages and is maintained in premigratory (Figure 1G) and early migrating neural crest cells (Basch et al., 2006). FOXD3 is expressed by the dorsal-most subset of PAX7+ cells (Figure 1G). Similarly within crestospheres, PAX7+ and FOXD3+ domains are intermixed, with some double-labeled but other single-labeled cells (Figures 1H and 1I). Some crestospheres consist entirely of FOXD3-expressing cells (Figure 1J). SOX2 expression is very low in dorsal compared with more ventral regions of the neural tube (Figure 1A) similar to crestospheres where FOXD3+ cells clearly segregate from high SOX2-expressing regions (Figure 1K), similar to segregation of PAX7+ and PAX6+ cells (Figure S1H). Other neural crest genes such as AP2α and SNAIL also exhibit high and heterogeneous expression (Figures 1L and S1I). Thus, whereas crestospheres contain a heterogeneous cell population, they are highly enriched in neural crest cells.
Cells Can Be Maintained as Self-Renewing Crestospheres for Several Weeks
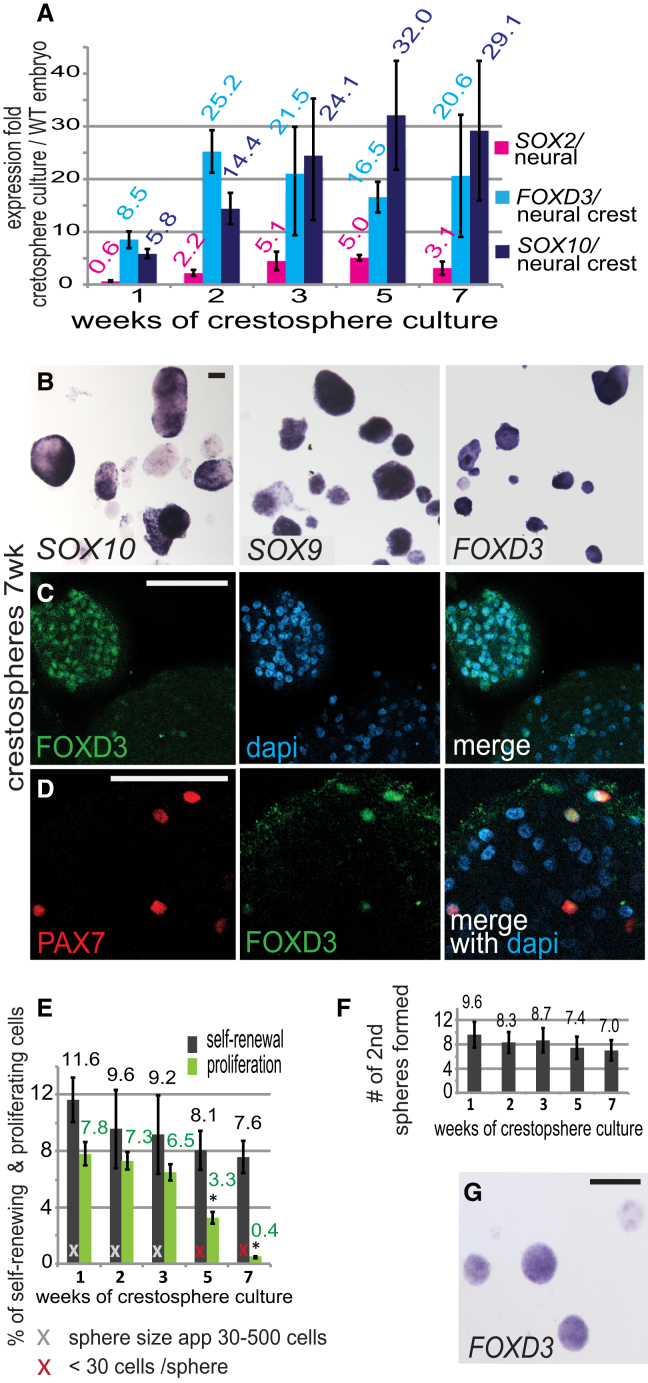
In the embryo, neural crest cells are transiently premigratory for ∼5 hr prior to initiating emigration. To test whether crestospheres maintained self-renewal capacity in the long term, we evaluated expression of neural crest markers and sphere-forming ability as a function of time. FOXD3 and SOX10 RNA expression persisted for up to 7 weeks in culture, the last time point examined (Figure 2A). Figure S2A shows examples of high, medium, and low expression by qPCR. Similarly, FOXD3, SOX10, SOX9, and PAX7 were expressed after 7 weeks of culture, although the frequency of high expressing spheres decreased by week 7 (Figures 2B–2D and S2B). Moreover, serial replating of pooled cultures revealed that ∼10% of the cells in crestosphere cultures have the ability to self-renew and form new spheres from single cells (Figure 2E). Within 1 week, each primary sphere on average formed eight new secondary spheres (Figure 2F). Importantly, crestospheres formed from single cells retained expression of FOXD3 (Figure 2G). Self-renewal capacity, as measured by ability of single cells to form new crestospheres, remained constant over the 7-week culture period. However, the cell proliferation rate, as assayed by phosphohistone3 staining, declined dramatically after 3 weeks, dropping from ∼7% to 4% by week 5 and <1% by week 7. Consequently, crestospheres became progressively smaller during the course of the self-renewal assay (Figure 2E), possibly reflecting a slowdown of the proliferation rate in the transit amplifying cell population.
Figure 2.
Long-Term Maintenance of Crestospheres
(A) FOXD3 and SOX10 mRNA expression was maintained throughout the 7-week crestosphere culture, initiated from pooled neural folds (n = 6; shown as average values).
(B–D) In situ hybridization (B) and immunostaining (C, D) reveal high and heterogeneous expression of premigratory neural crest markers in 7-week-old crestospheres.
(E) Sphere forming assay from single cells shows maintenance of self-renewal capacity through 7 weeks of crestosphere culture (week 1, n = 10; weeks 2, 3, and 7, n = 6; week 5, n = 12), with a drop in the proliferation rate after 5 weeks (n = 3).
(F) Secondary sphere formation is maintained throughout 7 weeks of crestosphere culture (n = 6).
(G) FOXD3 mRNA-expressing crestospheres formed from single cells during the primary sphere-forming assay.
Crestospheres Form Migratory Cells and Neural Crest Derivatives In Vitro and In Vivo
Migration of crestospheres was triggered by plating onto an adhesion-promoting surface after withdrawal of stem cell medium. We compared the distances migrated by cells emanating from crestospheres with those from explanted neural tubes. The results show that both cell populations are able to undergo EMT and migrate in a similar fashion as a function of time (Figures S3A–S3C; Supplemental Results).
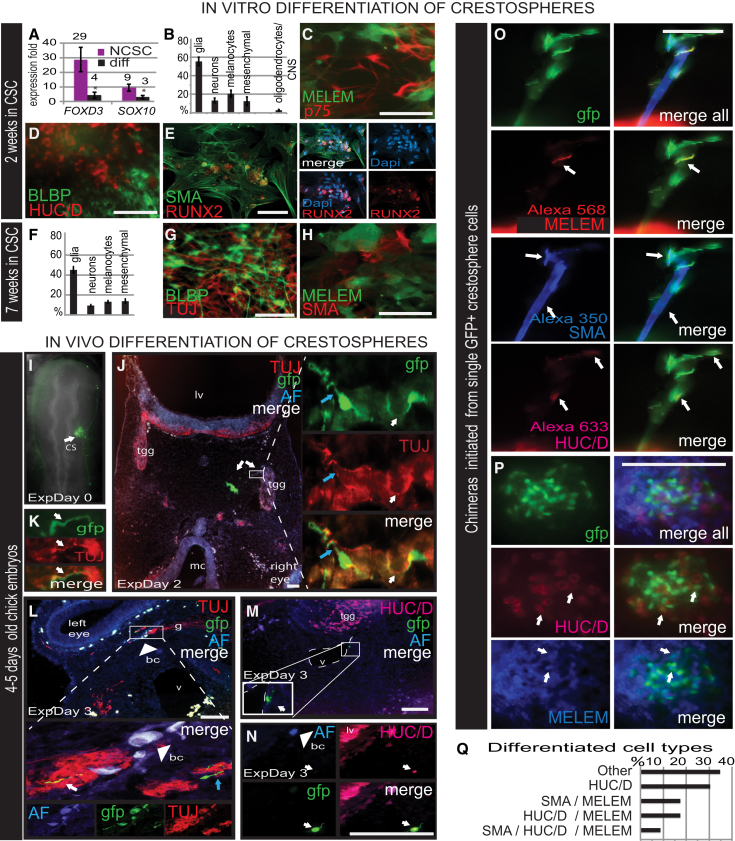
After transfer to differentiation conditions, expression of FOXD3 and SOX10 decreased in crestospheres derived from pooled cultures of 2-week-old spheres (Figure 3A); numerous neural crest derivatives were observed by immunostaining. Glial cells (BLBP) were predominant; roughly 10%–20% of cells positive for markers for neurons (HUC/D, TUJ1, ISLET), melanocytes (MELEM), mesenchymal smooth muscle (SMA), and cartilage (RUNX2), and ∼30% of the cells were HNK1+. By increasing the FBS from 1% to 10%, the percentage of mesenchymal cells increased significantly from 12% to 42% (SEM = 7.7, p = 0.004; data not shown). In contrast, very few oligodendrocytes, depicted with the O4 marker, were observed in crestospheres compared with neurospheres, suggesting a low presence of CNS cells in the former (see Figures 3B–3E and S3D–S3I and Supplemental Results), likely reflecting the shared lineage of premigratory neural crest and dorsal neural tube cells in vivo (Bronner-Fraser and Fraser, 1989, Baggiolini et al., 2015). The differentiation ability of crestospheres was maintained throughout the 7-week culture period (Figures 3F–3H and S3G).
Figure 3.
Multipotent Chick Crestospheres Differentiate into Multiple Neural Crest Derivatives
(A) qPCR shows a dramatic decrease in FOXD3 and SOX10 expression levels after 7 days of in vitro differentiation (n = 3).
(B–H) Seven day in vitro differentiation of either 2-week (B–E) or 7-week (F–H) cultured crestospheres results in production of melanoblasts (MELEM), neurons (TUJ-1, HUC/D), cells expressing P75, a common marker of many neural crest derived cells, glial cells (BCLP) as well as smooth muscle cells (SMA) and cartilage (RUNX2). Quantification of different derivatives shows similar results for 2- (B) and 7- (F) week spheres (n ranges: week 2, from 7 to 13; week 7, from 3 to 7). In contrast, few CNS-derived cells were observed as shown by oligodendrocyte marker 04.
(I) In vivo transplantation of 2-week cultured GFP crestospheres (CS) into the head mesenchyme of HH10 chick embryo (Day 0).
(J–L) GFP-positive cells were observed in the trigeminal ganglion (TGG) (J and K) and in a nerve bundle next to the left eye (L) 2 and 3 days after the transplantation, respectively. GFP+ cells express neuronal marker TUJ-1 in cell body and overlapping axons (white arrow); TUJ-negative presumptive Schwann cells are intertwined with the neuronal axons (blue arrow).
(M) GFP+ cells lining a blood vessel.
(N) Perinuclear HUC/D staining shows a GFP+ neuron in the mesenchyme below the lateral ventricle (lv). Arrowhead marks autofluorescent (AF) blood cells (bc) visible in all three channels. g, ganglia; mc, mouth cavity.
(O and P) Clonal GFP+ cells derived from individual premigratory neural crest cells grown as crestopheres give rise to neurons, melanocytes, and smooth muscle.
(Q) Summary of different derivatives formed. CSC, crestosphere culture. Scale bar represents 100 μm.
To examine differentiation in vivo, we transplanted 2- to 3-week-old crestospheres derived from GFP transgenic chick embryos onto cranial crest migration pathways of HH9-10 chick embryos. After 2 or 3 days in ovo, we noted GFP-labeled cells within normal neural crest derivatives, forming trigeminal neurons, putative Schwann cells, as well as smooth muscle surrounding blood vessels (Figures 3I–3N).
Clonal Chimeric Cultures Verify Multipotency of the Cultured Crestosphere Cells
To examine crestosphere developmental potential at the individual cell level, we examined the differentiative potential of single GFP+ cells, cocultured with unlabeled crestosphere cells. These clonal GFP cells contributed to multiple cell types, differentiating into neurons, melanocytes, and smooth muscle cells, demonstrating that crestosphere culture conditions are conducive to maintenance of multipotentiality of individual neural crest stem cells (Figures 3O–3Q; Supplemental Results).
Multipotency of hESC-Derived Neural Crest Cells
hESCs can be directed toward a neural crest cell fate from neural rosettes (Bajpai et al., 2010, Lee et al., 2007). However, these cells do not remain in a stem-like state but rather spontaneously detach from the rosettes, migrate and differentiate into neural crest derivatives, similar to neural crest behavior in the embryo.
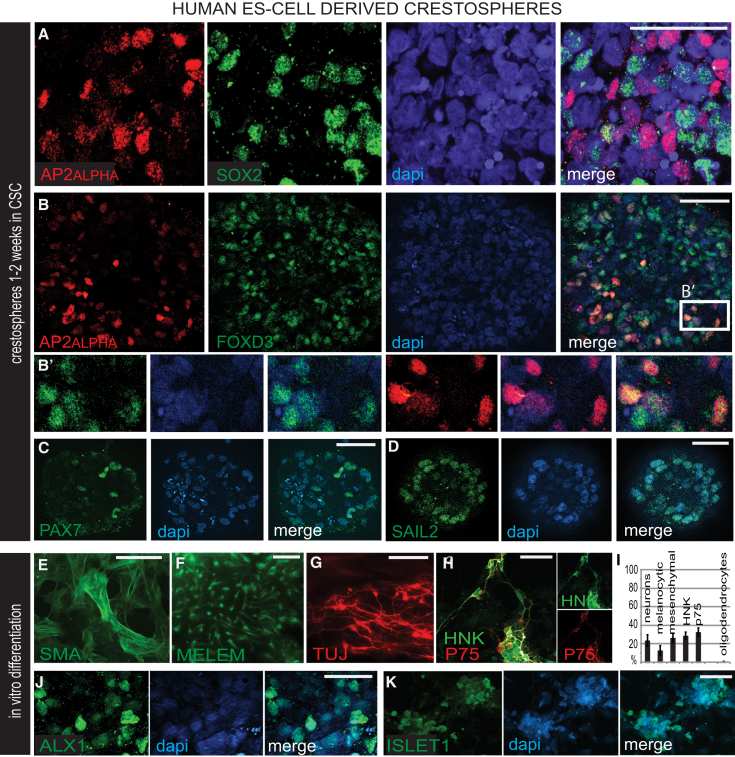
To test whether hESCs can form crestospheres, we exposed the hESC lines H7 (Figure 4) and H9, directed toward a neural crest fate, to crestosphere conditions. The results show that these spheres express premigratory human neural crest markers AP2α, FOXD3, PAX7, and SNAIL when cultured for 1–2 weeks. Whereas expression of AP2α and FOXD3 partially overlap, they segregate from SOX2 (Figures 4A–4D). Like chick cells, ∼30% of human crestospheres were HNK+/P75+ after 1 week in differentiation conditions and formed neural, glial, melanocytic, and mesenchymal neural crest derivatives after 2 weeks (Figures 4E–4K and S3J).
Figure 4.
hESC-Derived Premigratory Neural Crest Cells Maintained in Crestosphere Cultures
(A) Confocal images showing AP2α+ neural crest and SOX2+ neural cells are largely nonoverlapping in a human crestosphere after 2 weeks of crestosphere stem cell culture (CSC).
(B) Double immunostaining with AP2α and FOXD3 reveals only partial overlap of the markers in a human crestosphere shown clearly in magnification box (B′).
(C and D) Expression of PAX7 and SNAIL in human crestospheres.
(E–H, J, and K) Human crestospheres differentiated for 1 week into mesenchymal smooth muscle cells (SMA), melanoblasts (MELEM), neurons (TUJ-1), P75+ and HNK+ neural crest derivatives, cartilage (ALX1), and sensory neurons (ISLET1) after 10 days of crestosphere culture.
(I) The various derivatives formed are similar to those observed in chick crestospheres (n ranges from 7 to 14). Scale bar represents 50 μm.
Discussion
Here, we present culture conditions for maintaining premigratory neural crest cells in a self-renewing, multipotent state. For decades, investigators have been culturing migratory neural crest cells derived from embryonic dorsal neural tubes or folds. However, it was not previously possible to maintain these cells in an adhesive, premigratory state without spontaneous differentiation.
Our simple culture conditions allow maintenance of neural tube or human ES-derived cells as adhesive, neuroepithelial cells, as evidenced by their morphology and presence of β-Catenin in adherens junctions. These culture conditions appear to stop time and maintain the self-renewing state of the crestospheres for several weeks, although their proliferation rate begins to exhaust by 7 weeks. Switching into differentiation-promoting conditions shows that crestospheres are multipotent, forming neurons, glia, melanocytes, cartilage, and smooth muscle. Crestosphere cells can also contribute to migratory neural crest streams in vivo and subsequently differentiate into derivatives like peripheral neurons, smooth muscle, and other cell types. Expression of early neural crest markers FOXD3, SOX10, SOX9, BMP4, ETS-1, PAX7, SNAIL2, and AP2α was used to assay the neural crest cell state. Multiple loss-of-function studies and lineage analyses have suggested that these transcription factors are critical in early steps of neural crest development and specification (Barembaum and Bronner, 2013, Honoré et al., 2003, Kim et al., 2003, Luo et al., 2003, Mundell and Labosky, 2011, Murdoch et al., 2012, Nitzan et al., 2013, Teng et al., 2008, Tien et al., 2015).
There has been controversy regarding whether subsets of premigratory neural crest cells are predetermined to form distinct cell types or multipotent and capable of forming numerous derivatives. Recently, this has been elegantly put to rest by single cell lineage analysis using Confetti transgenic mice. By labeling individual premigratory or migrating neural crest cells, the authors conclusively demonstrate that the majority of both are multipotent (Baggiolini et al., 2015), consistent with previous intracellular dye injections experiments in chick embryos (Bronner-Fraser and Fraser, 1988, Bronner-Fraser and Fraser, 1989). Similarly, in vitro clonal analysis has demonstrated that many individual clones formed from early migrating neural crest cells can form numerous derivatives (Baroffio et al., 1988, Calloni et al., 2009, Sieber-Blum et al., 1993). This suggests that at least some cell fate decisions take place during neural crest migration. Our results support this, as crestospheres initiated from single cells retained expression of FOXD3, reflecting their self-renewal ability as multipotent stem-like cells. Moreover, our clonal analysis shows that individual crestosphere clones can differentiate into neural, melanocytic, and mesenchymal cell types, reflecting their multipotency. Interestingly, our results also show that the expression of neural crest markers is heterogeneous and dynamic in crestospheres, suggesting that perhaps only a subpopulation of crestosphere cells (∼10%) are true stem cells with the ability to self-renew.
Taken together, these results demonstrate that premigratory neuroepithelial cells maintained as crestospheres: (1) have high self-renewal capacity, (2) can be maintained for long periods in a stem-like state, (3) exhibit a profile characteristic of premigratory neural crest cells prior to EMT, and (4) can form multiple derivatives under differentiation conditions. These culture conditions promise to be useful for exploring the mechanism underlying maintenance of multipotency and the drivers of specific lineage specification. Furthermore, combined with the induced pluripotent stem cell techniques aimed at obtaining neural crest cells from individual patients, these conditions promise to provide an important tool for understanding pathology of human neurocristopathies that affect early stages of neural crest development.
Experimental Procedures
Crestosphere Cultures
Dorsal or whole neural tubes from four- to eight-somite stage chick embryos were dissected and pooled from four to six embryos for each experiment. Human neural crest cells were obtained from neural rosettes as previously described (Bajpai et al., 2010) from two ESC lines (H7; H9) at a stage mimicking premigratory dorsal neural tube on day 8 of the neural crest induction protocol. The cells were then transferred to the crestosphere culture (CSC) medium (see the Supplemental Experimental Procedures for details). The hESC culture and differentiation experiments were done in accordance with USC-SCRO approved protocols.
qPCR
qPCR was performed by comparing relative expression of FOXD3, SOX10, and SOX2 in the crestopsheres to whole chick embryo lysates from 5-8 somite stage (see the Supplemental Experimental Procedures for details).
In Vitro Differentiation
Pooled 2- or 7-week-old crestosphere cultures or GFP chimeric cultures were placed in differentiation-promoting medium (1% FBS) on poly-L-lysine coated glass coverslips and incubated at 37°C for 1 week and immunostained (see the Supplemental Experimental Procedures for details).
In Vivo Transplantation
Crestospheres derived from GFP chicken embryos were cultured in CSC medium for 2–4 weeks, and transplanted in ovo into the head mesenchyme of 10–13 somite stage chick embryos. Eggs were sealed and fixed 3 days later in 4% PFA on or near 4°C and cryosectioned before immunostaining (see the Supplemental Experimental Procedures for details).
Statistics
Averages were calculated from independent biological replicates (n), except for in vitro differentiation quantifications where n equals individual counted spots on the slides (see the Supplemental Results). Error bars represent SEM values and p values were calculated by using Student’s t test.
Acknowledgments
We thank Drs. Chathurani Jayasena and Crystal Rogers and Erin Moran for technical assistance. This work was funded by DE024157 (to M.E.B.) and the Sigrid Juselius Foundation, Finnish Cultural Foundation, Jane and Aatos Erkko Foundation, Väre Foundation, and Ella and Georg Ehrnrooth Foundation (to L.K.).
Published: October 1, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Results, Supplemental Experimental Procedures, and three figures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.08.017.
Supplemental Information
References
- Adameyko I., Lallemend F., Aquino J.B., Pereira J.A., Topilko P., Müller T., Fritz N., Beljajeva A., Mochii M., Liste I. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell. 2009;139:366–379. doi: 10.1016/j.cell.2009.07.049. [DOI] [PubMed] [Google Scholar]
- Baggiolini A., Varum S., Mateos J.M., Bettosini D., John N., Bonalli M., Ziegler U., Dimou L., Clevers H., Furrer R., Sommer L. Premigratory and migratory neural crest cells are multipotent in vivo. Cell Stem Cell. 2015;16:314–322. doi: 10.1016/j.stem.2015.02.017. [DOI] [PubMed] [Google Scholar]
- Bajpai R., Chen D.A., Rada-Iglesias A., Zhang J., Xiong Y., Helms J., Chang C.-P., Zhao Y., Swigut T., Wysocka J. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature. 2010;463:958–962. doi: 10.1038/nature08733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barembaum M., Bronner M.E. Identification and dissection of a key enhancer mediating cranial neural crest specific expression of transcription factor, Ets-1. Dev. Biol. 2013;382:567–575. doi: 10.1016/j.ydbio.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroffio A., Dupin E., Le Douarin N.M. Clone-forming ability and differentiation potential of migratory neural crest cells. Proc. Natl. Acad. Sci. USA. 1988;85:5325–5329. doi: 10.1073/pnas.85.14.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch M.L., Bronner-Fraser M., García-Castro M.I. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature. 2006;441:218–222. doi: 10.1038/nature04684. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M., Fraser S.E. Cell lineage analysis reveals multipotency of some avian neural crest cells. Nature. 1988;335:161–164. doi: 10.1038/335161a0. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M., Fraser S. Developmental potential of avian trunk neural crest cells in situ. Neuron. 1989;3:755–766. doi: 10.1016/0896-6273(89)90244-4. [DOI] [PubMed] [Google Scholar]
- Calloni G.W., Le Douarin N.M., Dupin E. High frequency of cephalic neural crest cells shows coexistence of neurogenic, melanogenic, and osteogenic differentiation capacities. Proc. Natl. Acad. Sci. USA. 2009;106:8947–8952. doi: 10.1073/pnas.0903780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadamore F., Fishwick K., Giusto E., Gnedeva K., Cattarossi G., Miller A., Pluchino S., Brill L.M., Bronner-Fraser M., Terskikh A.V. Human ESC-derived neural crest model reveals a key role for SOX2 in sensory neurogenesis. Cell Stem Cell. 2011;8:538–551. doi: 10.1016/j.stem.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curchoe C.L., Maurer J., McKeown S.J., Cattarossi G., Cimadamore F., Nilbratt M., Snyder E.Y., Bronner-Fraser M., Terskikh A.V. Early acquisition of neural crest competence during hESCs neuralization. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0013890. e13890–e13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin E., Coelho-Aguiar J.M. Isolation and differentiation properties of neural crest stem cells. Cytometry A. 2013;83:38–47. doi: 10.1002/cyto.a.22098. [DOI] [PubMed] [Google Scholar]
- Fernandes K.J.L., McKenzie I.A., Mill P., Smith K.M., Akhavan M., Barnabé-Heider F., Biernaskie J., Junek A., Kobayashi N.R., Toma J.G. A dermal niche for multipotent adult skin-derived precursor cells. Nat. Cell Biol. 2004;6:1082–1093. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- Honoré S.M., Aybar M.J., Mayor R. Sox10 is required for the early development of the prospective neural crest in Xenopus embryos. Dev. Biol. 2003;260:79–96. doi: 10.1016/s0012-1606(03)00247-1. [DOI] [PubMed] [Google Scholar]
- Johnston A.P.W., Naska S., Jones K., Jinno H., Kaplan D.R., Miller F.D. Sox2-mediated regulation of adult neural crest precursors and skin repair. Stem Cell Reports. 2013;1:38–45. doi: 10.1016/j.stemcr.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerosuo L., Bronner-Fraser M. What is bad in cancer is good in the embryo: importance of EMT in neural crest development. Semin. Cell Dev. Biol. 2012;23:320–332. doi: 10.1016/j.semcdb.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khudyakov J., Bronner-Fraser M. Comprehensive spatiotemporal analysis of early chick neural crest network genes. Dev. Dyn. 2009;238:716–723. doi: 10.1002/dvdy.21881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Lo L., Dormand E., Anderson D.J. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 2003;38:17–31. doi: 10.1016/s0896-6273(03)00163-6. [DOI] [PubMed] [Google Scholar]
- Kim Y.J., Lim H., Li Z., Oh Y., Kovlyagina I., Choi I.Y., Dong X., Lee G. Generation of multipotent induced neural crest by direct reprogramming of human postnatal fibroblasts with a single transcription factor. Cell Stem Cell. 2014;15:497–506. doi: 10.1016/j.stem.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Kruger G.M., Mosher J.T., Bixby S., Joseph N., Iwashita T., Morrison S.J. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35:657–669. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Kim H., Elkabetz Y., Al Shamy G., Panagiotakos G., Barberi T., Tabar V., Studer L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat. Biotechnol. 2007;25:1468–1475. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- Li H.-Y., Say E.H.M., Zhou X.-F. Isolation and characterization of neural crest progenitors from adult dorsal root ganglia. Stem Cells. 2007;25:2053–2065. doi: 10.1634/stemcells.2007-0080. [DOI] [PubMed] [Google Scholar]
- Luo T., Lee Y.-H., Saint-Jeannet J.-P., Sargent T.D. Induction of neural crest in Xenopus by transcription factor AP2alpha. Proc. Natl. Acad. Sci. USA. 2003;100:532–537. doi: 10.1073/pnas.0237226100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Morales P.L., Diez del Corral R., Olivera-Martínez I., Quiroga A.C., Das R.M., Barbas J.A., Storey K.G., Morales A.V. FGF and retinoic acid activity gradients control the timing of neural crest cell emigration in the trunk. J. Cell Biol. 2011;194:489–503. doi: 10.1083/jcb.201011077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky A.V., Pardal R., Iwashita T., Park I.-K., Clarke M.F., Morrison S.J. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S.J., White P.M., Zock C., Anderson D.J. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- Mundell N.A., Labosky P.A. Neural crest stem cell multipotency requires Foxd3 to maintain neural potential and repress mesenchymal fates. Development. 2011;138:641–652. doi: 10.1242/dev.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch B., DelConte C., García-Castro M.I. Pax7 lineage contributions to the mammalian neural crest. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0041089. e41089–e41089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitzan E., Krispin S., Pfaltzgraff E.R., Klar A., Labosky P.A., Kalcheim C. A dynamic code of dorsal neural tube genes regulates the segregation between neurogenic and melanogenic neural crest cells. Development. 2013;140:2269–2279. doi: 10.1242/dev.093294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber-Blum M., Ito K., Richardson M.K., Langtimm C.J., Duff R.S. Distribution of pluripotent neural crest cells in the embryo and the role of brain-derived neurotrophic factor in the commitment to the primary sensory neuron lineage. J. Neurobiol. 1993;24:173–184. doi: 10.1002/neu.480240205. [DOI] [PubMed] [Google Scholar]
- Stemple D.L., Anderson D.J. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- Teng L., Mundell N.A., Frist A.Y., Wang Q., Labosky P.A. Requirement for Foxd3 in the maintenance of neural crest progenitors. Development. 2008;135:1615–1624. doi: 10.1242/dev.012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien C.L., Jones A., Wang H., Gerigk M., Nozell S., Chang C. Snail2/Slug cooperates with Polycomb repressive complex 2 (PRC2) to regulate neural crest development. Development. 2015;142:722–731. doi: 10.1242/dev.111997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentin A., Glavieux-Pardanaud C., Le Douarin N.M., Dupin E. Self-renewal capacity is a widespread property of various types of neural crest precursor cells. Proc. Natl. Acad. Sci. USA. 2004;101:4495–4500. doi: 10.1073/pnas.0400629101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.