Abstract
Enzyme-directed mutasynthesis is an emerging strategy for the targeted derivatization of natural products. Here, data on the synthesis of malonic acid derivatives for feeding studies in Saccharopolyspora erythraea , the mutagenesis of DEBS and bioanalytical data on the experimental investigation of studies on the biosynthetic pathway towards erythromycin are presented.
Specifications table
| Subject area | Chemistry, Biology |
| More specific subject area | Natural Products Biosynthesis |
| Type of data | Image (NMR-spectra of malonic acid derivatives), text file, figure |
| How data was acquired | NMR (Varian Mercury 400), mass spectrometry (LTQ Orbitrap) |
| Data format | Analyzed |
| Experimental factors | Synthesis products were analyzed after chromatographic purification, biosynthesis products were solid-phase extracted from fermentation broth |
| Experimental features | Analogues of biosynthetic building blocks were chemically synthesized and supplied to mutated strains of S. erythraea. LC–MS analysis of fermentation products revealed the substrate specificity of a key enzyme in polyketide biosynthesis |
| Data source location | Bochum, Germany and Mülheim an der Ruhr, Germany |
| Data accessibility | The data are supplied with this article |
Value of the data
-
•
The preparative synthesis and handling of biosynthetic building block analogs is described.
-
•
Analytical data on synthesized compounds are shown.
-
•
Data on the site-directed mutagenesis of 6-deoxyerythronolide B synthase (DEBS) in S. erythraea are presented.
1. Data, experimental design, materials and methods
The data shown here substantiate the exploration of the mutated polyketide synthase which directs the biosynthesis of erythryomcin in Saccharopolyspora erythraea. In the sixth module of this polyketide synthase, the acyltransferase domain was recently mutated to accept propargylmalonyl-SNAC (2, Fig. 1) as substrate next to the native substrate methylmalonyl-CoA [1]. The critical mutation in the acyltransferase domain was V295A, located in the heart of the active center [2]. The mutation reduced the sterical hindrance on the substrate, allowing for the accommodation of 2. Now, we explored the substrate flexibility of the DEBS AT6 V295A variant using a number of thioester-activated and differently substituted malonic acid derivatives. Biomolecular modeling was able to further the design and implementation of additional mutations in the active site of DEBS AT6, which decrease the steric constraints and improve the incorporation of the synthetic substrate 2 into the resulting polyketide. In this article, the synthesis of artificial extender unit analogs for polyketide biosynthesis and the mutagenesis of an acyltransferase domain for acceptance of these building blocks are described. Furthermore, the data on feeding experiments in S. erythraea are shown.
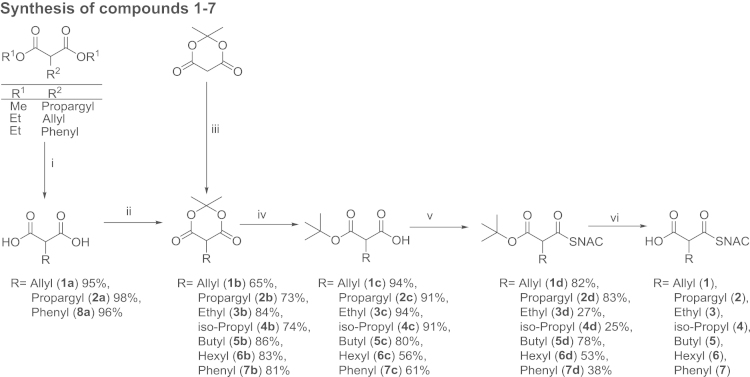
Fig. 1.
Synthesis of compounds 1–7; (i) 3.0 eq. LiOH*H2O, H2O, 18 h, RT; (ii) 1.01 eq. isoprenylacetate, 0.06 eq. H2SO4, neat, 18 h, RT; (iii) 1.0 eq. boranedimethylamine complex, 3.0 eq. aldehyde or acetone, 1 h, RT; (iv) tBuOH, 6 h, 90–100 °C; (v) 1.1 eq. CDI, 0.3 eq. DMAP, 1.2 eq. SNAC, THF, 18 h, RT; (vi) 2.5 eq. TiCl4, DCM, 6 h, RT: room temperature, CDI: N,N′-Carbonyldiimidazole, DMAP: 4-Dimethylaminopyridine, SNAC: N-acetylcysteamine.
2. General information
Unless otherwise stated, materials for chemical synthesis were obtained from commercial suppliers (Sigma Aldrich, Alfa Aesar, Fluka, Acros) in the highest purity available and used without further purification. Solvents were dried following standard procedures [3]. Solvents used for extraction and chromatography were purchased from Thermo Fisher Scientific. Flash chromatography was carried out using Acros silica gel 60 (35–70 μm mesh). Thin-layer chromatography (TLC) was performed on aluminum-backed, precoated silica gel (60 F245) from Merck with cyclohexane/EtOAc or DCM/MeOH mixtures as mobile phases. Spots were detected by staining with KMnO4 solution (5.0 g KMnO4, 33 g K2CO3, 10 mL 5% aqueous NaOH in 500 mL H2O) and subsequent heat treatment.
NMR spectra were recorded by using a Varian Mercury 400 (400 MHz, 1H; 100 MHz, 13C) spectrometer and calibrated using residual undeuterated solvent as an internal reference. Data are shown in Supplementary File 1.
High-resolution mass spectra were recorded on a LTQ Orbitrap with Accela HPLC-System (column Hypersil Gold, length 50 mm, inside diameter 1 mm, particle size 1.9 μm, ionization method: Electrospray Ionization). Products were characterized by NMR (1H, 13C) and HRMS.
For mass spectrometric detection the electrospray ionization was carried out in positive ionization mode by using a source voltage of 4 kV. The capillary voltage was set to 18 V, the capillary temperature to 275 °C, and the tube lens voltage to 115 V. Spectra were acquired in full scan centroid mode with a mass-to-charge range from 200 to 2000.
3. Synthesis of compounds 1–7
Synthesis of N-acetylcysteamine (SNAC) [4]: 20.00 g (176 mmol) cysteamine hydrochloride, 11.62 g (259 mmol) KOH (85%) and 36.97 g (440 mmol) NaHCO3 were added to 500 mL of deionized H2O. After everything was dissolved, 19.77 g (18.31 ml, 259 mmol) acetic anhydride was added dropwise at 0 °C. After stirring at room temperature for 18 h, the light rose solution was brought to pH 1 with conc. HCl and the colorless solution was extracted three times with 150 ml EtOAc. The combined organic layers were dried over Na2SO4 to obtain 20.47 g (98%) of the desired product as colorless oil.
1H NMR (400 MHz, CDCl3-d1): 1.34–1.38 (t, J=8.4 Hz, 1H), 1.97 (s, 3H), 2.60–2.66 (m, 2H), 3.36–3.40 (m, 2H), 6.33 (bs, 1H); 13C NMR (101 MHz, CDCl3-d1): 23.1, 24.5, 42.6, 170.5; HRMS: calc. for 120.04776 C4H10ONS [M+H]+; found: 120.04730 C4H10ONS [M+H]+; Rf: 0.42 (DCM/MeOH 9:1, KMnO4).
General procedure for the saponification of malonic acid diesters 1a, 2a+7a: The commercially available malonic diester was added to H2O (10 ml/g) and 3.0 eq LiOH*H2O were added at once. The solution was stirred for 18 h, then washed with 100 ml Et2O. The aqueous phase was acidified to pH 1 using conc. HCl and extracted three times with 150 ml EtOAc. The combined organic layers were dried over Na2SO4 to obtain the desired product as white solid.
2-Allyl-malonic acid (1a): 1H NMR: (400 MHz, D2O-d2) =2.49–2.51 (m, 2H), 3.17–3.21 (t, J=7.8 Hz, 1H), 5.04–5.16 (m, 2H), 5.83–5.93 (m, 1H); 13C NMR: (101 MHz, MeOD-d4) =34.1, 52.9, 117.5, 135.8; 172.5; mp: 103.3–103.6 °C; yield: 18.59 g; 95% (27.0 g scale, 134.8 mmol).
2-(Prop-2-yn-1-yl)malonic acid (2a): 1H NMR: (400 MHz, MeOD-d4) =2.31–2.32 (t, J=2.7 Hz, 1H), 2.68–2.71 (dd, J=7.6, 2.7 Hz, 2H), 3.49–3.53 (t, J=7.6 Hz, 1H, 2-H, CH); 13C NMR: (101 MHz, MeOD-d4) =19.2, 52.6, 71.2, 81.4, 171.4; mp: 141–141.6 °C; yield: 10.42 g; 98% (12.6 g scale, 74.13 mmol).
2-Phenylmalonic acid (7a): 1H NMR : (400 MHz, MeOD-d4) =4.65 (s, 1H), 4.88 (bs, 2H), 7.30–7.37 (m, 3H), 7.39–7.42 (m, 2H); 13C NMR: (101 MHz, MeOD-d4) =59.0, 128.9, 129.4, 130.3, 130.4, 135.2, 171.9; HRMS: calc.: 179.03498 C9H7O4 [M−H]; found: 179.03568 C9H7O4 [M−H]; mp: 160.4-160.5 °C; yield: 7.29 g; 96% (10.0 g scale, 42.33 mmol).
General procedure for the synthesis of Meldrum׳s acid derivatives 1b, 2b+7b [5]:
For the formation of Meldrum׳s acid derivatives 1b, 2b+8b the general procedure of Singh and Danishefsky was used [5]. 1.01 eq. isoprenylacetate was added under argon protection to the corresponding malonic acid derivative. To the resulting white slurry 0.06 eq. sulfuric acid were added dropwise at 0 °C. The resulting yellow to brown solution was stirred for 18 h to reach room temperature. 100 g ice and 10 ml 1 M HCl were added to the brown reaction mixture (at 10 g synthesis scale). The resulting precipitate was filtered and washed twice with 20 ml cold water.
In cases where the reaction mixture became solid after 18 h, water was added to form a slurry. To this slurry 100 g ice and 10 ml 1 M HCl were added (for 10 g synthesis scale). The resulting precipitate was filtered and washed twice with 20 ml of ice-cold water. The resulting white to brown product usually was directly submitted to the next synthesis step.
If material of higher purity was needed the white to brown solids obtained from the first precipitation were dissolved in a small volume MeOH at RT. After adding ice and a few drops of conc. HCl the white precipitate was filtered and washed twice with 20 ml of ice cold water.
5-Allyl-2,2-dimethyl-1,3-dioxane-4,6-dione (1b): 1H NMR: (400 MHz, CDCl3-d1) =1.76 (s, 3H), 1.79 (s, 3H), 2.86–2.90 (m, 2H), 3.57–3.60 (t, J=5.3Hz, 1H), 5.14–5.26 (m, 2H), 5.81–5.92 (m, 1H); 13C NMR: (101 MHz, CDCl3-d1) =27.2, 28.6, 30.5, 46.4, 105.1, 132.8, 165.1; HRMS: calc.: 185.08084 C9H13O4 [M+H]+;found: 185.08071 C9H13O4 [M+H]+; mp: 71 °C; Rf: 0.56 (EtOAc/cyclohexane 1:1, KMnO4); yield: 23.05 g; 65% (27.95 g scale, 193.96 mmol).
2,2-Dimethyl-5-(prop-2-yn-1-yl)-1,3-dioxane-4,6-dione (2b): 1H NMR: (400 MHz, CDCl3-d1) =1.80 (s, 3H), 1.81 (s, 3H), 2.05–2.06 (t, J=2.6 Hz, 1H), 3.02–3.04 (dd, J=4.9, 2.6 Hz, 2H), 3.67–3.96 (t, J=4.9 Hz, 1H); 13C NMR: (101 MHz, CDCl3-d1) =16.7, 27.2, 28.7, 46.1, 70.9, 79.4, 105.5, 164.1; HRMS: calc.: 183.06519 C9H11O4 [M+H]+; found: 183.06512 C9H11O4 [M+H]+; mp: 140.0–140.4 °C; Rf: 0.66 (EtOAc/cyclohexane 1:1, KMnO4); yield: 29.67 g; 73% (31.9 g scale, 224.47 mmol).
2,2-Dimethyl-5-phenyl-1,3-dioxane-4,6-dione (7b): 1H NMR: (400 MHz, CDCl3-d1) =1.75 (s, 3H), 1.87 (s, 3H), 4.77 (s, 1H), 7.28–7.31 (m, 2H), 7.37–7.45 (m, 3H); 13C NMR: (101 MHz, CDCl3-d1) =27.7, 28.7, 52.9, 105.8, 128.9, 129.2, 129.3, 130.7, 164.8; HRMS: calc.: 221.08084 C12H13O4 [M+H]+; found: 221.08113 C12H13O4 [M+H]+; mp: 140.1–142.3 decomposition; Rf: 0.18 (EtOAc/cyclohexane 1:1, KMnO4); yield: 5.96 g; 81% (6.0 g scale, 33.30 mmol).
General procedure for the reductive alkylation of Meldrum׳s acid 3b–6b [6]:
The alkylation was carried out as described by Hurubowchak and Smith [6]. Meldrum׳s acid was dissolved in abs. MeOH. Subsequently, 1.01 eq. boranedimethylamine complex were added. After the borane was dissolved completely 3.0 eq. of the corresponding aldehyde were added in 3 min at RT under a stream of N2. After 1 h the yellow reaction mixture was quenched by 100 g ice and 10 ml of 1 M HCl. The resulting suspension was filtered and washed twice with 25 ml cold water. The resulting white solid was dried in vacuo and can directly be submitted to the next reaction step.
Synthesis of Isopropyl-meldrum׳s acid (4b):
To 8 ml Acetone (freshly dried over 4 A°-molecular sieve), 4.0 g (27.75 mmol) meldrum׳s acid were added under argon atmosphere. At 0 °C 1.68 g (28.03 mmol) borane dimethylamine complex was added. After 15 min the ice bath was removed and the reaction mixture was stierred for 18 h at room temperature. The yellow solution was poured on 80 cm3 ice and acidified with 3 ml 1 N HCl. The resulting precipitate was filtered and washed twice with 20 ml ice cold H2O.
If material of higher purity was needed, the white to brown solids obtained from the first precipitation were dissolved in a minimum of MeOH at RT. After adding ice and a view drops of conc. HCl the white precipitate was filtered and washed twice with 20 ml of ice cold water.
5-Ethyl-2,2-dimethyl-1,3-dioxane-4,6-dione (3b): 1H NMR: (400 MHz, CDCl3-d1) =1.03–1.07 (t, J=7.3 Hz, 3H), 1.75 (s, 3H), 1.78 (s, 3H), 2.14–2.20 (qd, J=7.3, 4.9 Hz, 2H), 3.48–3.50 (t, J=4.9 Hz, 1H); 13C NMR: (101 MHz, CDCl3-d1) =10.9, 20.3, 27.1, 28.6, 47.3, 104.9, 165.57; HRMS: calc.: 173.08084 C8H13O4 [M+H]+; found: 173.08065 C8H13O4 [M+H]+; mp: 110–110.2 °C; Rf: 0.66 (EtOAc/cyclohexane 1:1, KMnO4); yield: 3.50 g; 84% (4.0 g scale, 27.75 mmol).
5-Isopropyl-2,2-dimethyl-1,3-dioxane-4,6-dione (4b): 1H NMR: (400 MHz, CDCl3-d1) =1.16 (s, 3H) 1.18 (s, 3H),1.73–1.74 (d, J=6.0 Hz, 6H), 2.73–2.78 (m, 1H), 3.37 (d, J=3.1 Hz, 1H); 13C NMR: (101 MHz, CDCl3-d1) =19.3, 27.6, 28.4, 29.2, 51.8, 104.8, 165.1; HRMS: calc.: 187.09649 C9H15O4 [M+H]+; found: 187.09638 C9H15O4 [M+H]+; mp: 104 °C; Rf: 0.71 (1:1 EtOAc/cyclohexane, KMnO4); yield: 3.80 g; 74% (4.0 g scale, 27.75 mmol).
5-Butyl-2,2-dimethyl-1,3-dioxane-4,6-dione (5b): 1H NMR: (400 MHz, CDCl3-d1)=0.90–0.93 (t, J=7.1 Hz), 1.32–1.47 (m, 4H), 1.73 (s, 3H), 1.78 (s, 3H), 2.07-2.13 (m, 2H), 3.47–3.50 (t, J=5.1 Hz, 1H); 13C NMR: (101 MHz, CDCl3-d1) =13.9, 22.8, 26.6, 27.1, 28.6, 28.8, 46.3, 104.9, 165.80; HRMS: calc.: 201.11214 C10H17O4 [M+H]+; found: 201.11206 C10H17O4 [M+H]+; mp: 55.6-56.1 °C; Rf: 0.73 (EtOAc/cyclohexane 1:1, KMnO4); yield: 19.31 g; 86% (18.0 g scale, 123.17 mmol).
5-Hexyl-2,2-dimethyl-1,3-dioxane-4,6-dione (6b): 1H NMR: (400 MHz, CDCl3-d1) =0.85–0.89 (t, J=6.5 Hz), 1.28–1.35 (6H), 1.40–1.47 (2H), 1.75 (3H), 1.77 (3H), 2.06–2.12 (2H), 3.47–3.50 (t, J=5.0 Hz, 1H); 13C NMR: (101 MHz, CDCl3-d1) =14.1, 22.6, 26.6, 26.9, 27.1, 28.6, 29.3, 31.56, 46.3, 104.9, 165.8; HRMS: calc.: 229.14344 C12H21O4 [M+H]+; found: 229.14332 C12H21O4 [M+H]+; Rf: 0.50 (DCM/MeOH, KMnO4); yield: 13.12 g; 83% (10.0 g scale; 69.38 mmol).
General procedure for the synthesistButylmalonic acids 1c–7c:
tBuOH (125 ml/10 g) was added to Meldrum׳s acid and heated up to 95–100 °C for 6 h (DC-control). Then tBuOH was evaporated in vacuo and the resulting oil was purified by column chromatography (PE/EtOAc 1:0→ 85:15, gradient in 5%-steps) to obtain the desired products as clear oils.
2-(tert-Butoxycarbonyl)pent-4-enoic acid (1c): 1H NMR: (400 MHz, CDCl3-d1) =1.47 (s, 9H), 2.58–2.68 (m, 2H), 3.36–3.40 (t, J=7.3 Hz, 1H), 5.07–5.16 (m, 2H), 5.73–5.83 (m, 1H); 13C NMR: (101 MHz, CDCl3-d1) =28.0, 33.2, 52.1, 82.8, 117.9, 133.8, 168.5, 174.4; HRMS: calc.: 201.11214 C10H17O4 [M+H]+, 223.09408 C10H16O4Na [M+Na]+,218.13868 C10H20O4N [M+NH4]+; found: 201.11217 C10H17O4 [M+H]+, 223.09421 C10H16O4Na [M+Na]+,218.13878 C10H20O4N [M+NH4]+; Rf: 0.55 (EtOAc/cyclohexane, KMnO4); yield: 3.01 g; 91% (3.0 g scale, 35.34 mmol).
2-(tert-Butoxycarbonyl)-pent-4-yl acid (2c): 1H NMR: (400 MHz, CDCl3-d1) =1.46 (s, 9H), 2.04 (t, J=2.6 Hz, 1H), 2.17–2.18 (s, 1H), 2.94–2.95 (d, J=2.6 Hz, 2H); 13C NMR: (101 MHz, CDCl3-d1) =23.1, 28.1, 57.2, 72.2, 78.8, 83.8, 167.7, 174.4; HRMS: calc.: 199.09649 C10H15O4 [M+H]+, 221.07843 C10H15O4Na [M+Na]+; found: 221.07845 C10H15O4, [M+Na]+; mp: 95.6–96.7 °C; Rf: 0.54 (MeOH/CHCl3 1:9, KMnO4); yield: 30.35 g; 94% (29.67 g scale, 162.8 mmol).
2-(tert-Butoxycarbonyl)butanoicacid (3c): 1H NMR: (400 MHz, CDCl3-d1) =0.97–1.01 (t, J=7.4 Hz, 3H), 1.48 (s, 9H), 1.89–1.96 (m, 2H), 3.20–3.24 (t, J=7.2 Hz, 1H); 13C NMR: (101 MHz, CDCl3-d1) =11.8, 22.8, 28.0, 53.9, 82.7, 169.4, 174.7; HRMS: calc.: 187.09758 C9H15O4 [M−H]; found: 187.09826 C9H15O4 [M−H]; mp: 53.8-54.1 °C; Rf: 0.0–0.65 (EtOAc/cyclohexane 1:1, KMnO4); yield: 7.38 g; 94% (7.17 g scale, 41.64 mmol).
2-(tert-Butoxycarbonyl)-3-methylbutanoic acid (4c): 1H NMR: 1.03–1.04 (d, J=2.2 Hz), 1.05 (d, J=2.2 Hz), 1.48 (s, 9H), 2.29–2.40 (m, 1H), 3.08–3.10 (d, J=7.7 Hz, 1H); 13C NMR: (101 MHz, CDCl3-d1) =20.3, 20.5, 28.1, 29.8, 59.2, 82.9, 169.4, 173.5; HRMS: calc: 201.11323 C10H17O4 [M−H]; found: 201.11372 C10H17O4 [M−H]; mp: 64.3–65.2 °C; Rf: 0.08-0.55 (EtOAc/cyclohexane 1:1, KMnO4); yield: 5.07 g; 91% (5.1 g scale, 27.3 mmol).
2-(tert-Butoxycarbonyl)hexanoicacid (5c): 1H NMR: (400 MHz, CDCl3-d1) =0.88–0.92 (t, J=7.0 Hz, 3H), 1.32–1.35 (m, 4H), 1.47 (s, 9H), 1.85–1.91 (m, 2H), 3.25–3.29 (t, J=7.4 Hz, 1H); 13C NMR: (101 MHz, CDCl3-d1) =13.9, 22.5, 28.0, 28.9, 29.5, 52.5, 82.6, 164.3, 174.9; HRMS: calc.: 215.12888 C11H19O4 [M−H]; found: 215.12939 C11H19O4 [M−H]; Rf: 0–0.51 (EtOAc/cyclohexane 1:1, KMnO4); yield: 2.73 g; 80% (2.8 g scale, 14.0 mmol).
2-(tert-Butoxycarbonyl)octanoicacid (6c): 1H NMR: (400 MHz, CDCl3-d1) =0.86–0.89 (t, J=6.9 Hz, 3H), 1.28–1.32 (m, 8H), 1.47 (s, 9H), 1.84–1.91 (m, 2H), 3.26–3.29 (t, J=7.3 Hz, 3H); 13C NMR: (101 MHz, CDCl3-d1) =14.2, 22.6, 27.3, 28.0, 28.9, 29.4, 31.6, 52.5, 82.7, 169.4, 175.1; HRMS: calc.: 243.16018 C13H23O4 [M−H]; found: 243.16086 C13H23O4 [M−H]; Rf: 0–0.47 (EtOAc/cyclohexane 1:1, KMnO4); yield: 8.02 g; 56% (13.2 g scale, 57.8 mmol).
3-(tert-Butoxy)-3-oxo-2-phenylpropanoic acid (7c): 1H NMR: (400 MHz, CDCl3-d1) =1.44 (s, 9H), 4.55 (s, 1H), 7.28–7.40 (m, 5H); 13C NMR: (101 MHz, CDCl3-d1) =27.9, 58.3, 83.5, 128.5, 128.9, 129.1, 132.8, 168.0, 173.1; HRMS: calc.: 237.11214 C13H16O4 [M+H]+; found: 191.10828 C12H16O2 [M-CO2]; mp: 100.9–101.3 °C; Rf: 0.0–0.50 (EtOAc/cyclohexane 1:1, KMnO4); yield: 3.26 g; 61% (5.0 g scale, 22.7 mmol).
General procedure for the thioesterfication of compounds 1d–7d:
tert-Butylcarboxylic acid was dissolved in abs. THF (10 ml/g) under argon. Subsequently, 1.2 eq. CDI was added at 0 °C, and the mixture was stirred for 30 min at 0 °C followed by 3 h at RT before 0.3 eq. DMAP and 1.3 eq. SNAC were added. After 18 h at RT the solvent was removed in vacuo and the residue was suspended 300 ml EtOAc and washed three times with 100 ml 1 M K2CO3 and twice with 100 ml 1 M HCl. The organic layer was dried over Na2SO4, and purified by column chromatography (DCM/MeOH 99:1) to obtain the desired thioesters as slightly yellow oils.
tert-Butyl 2-(((2-acetamidoethyl)thio)carbonyl)pent-4-enoate (1d): 1H NMR: (400 MHz, CDCl3-d1) =1.43 (s, 9H), 1.94 (s, 3H), 2.58–2.62 (m, 2H), 2.99–3.11 (m, 2H), 3.34–3.48 (m, 2H), 3.55–3.59 (t, J=7.5 Hz, 1H), 5.03–5.12 (m, 2H), 5.66–5.77 (m, 1H), 6.00 (bs, 1H); 13C NMR: (101 MHz, CDCl3-d1) =23.3, 28.1, 28.9, 33.5, 39.6, 60.6, 82.7, 117.9, 133.9, 167.4, 170.6, 195.5; HRMS: calc: 302.14206 C14H24O4NS[M+H]+, 324.12400 C14H23O4NNaS [M+Na]+, 319.16860 C14H27O4N2S [M+NH4]+; found: 302.14231 C14H24O4NS[M+H]+, 324.12418 C14H23O4NNaS [M+Na]+, 319.16919 C14H27O4N2S [M+NH4]+; Rf: 0.68 (DCM/MeOH 9:1, KMnO4); yield: 3.73 g; 82% (3.02 g scale, 15.06 mmol).
tert-Butyl 2-(((2-acetamidoethyl)thio)-carbonyl)pent-4-ynoate (2d): 1H NMR: (400 MHz, CDCl3-d1) =1.47 (s, 9H),1.96 (s, 3H),2.01–3.03 (t, J=2.7 Hz, 1H),2.74–2.76(dd, J=7.6, 2.7,0.6 Hz, 2H),3.09-3.12 (m, 2H),3.43-3.47 (m, 2H), 3.69-3.73 (t, J=7.6 Hz, 1H),5.87 (bs, 1H); 13C NMR: (101 MHz, CDCl3-d1)=18.8, 23.3, 27.9, 29.1, 39.5, 59.6, 70.7, 79.9, 83.3, 166.3, 170.5, 194.4; HRMS: calc.: 300.12641 C14H22O4NS [M+H]+, 322.10835 C14H22O4NSNa [M+Na]+, 317.15295 C14H25O4N2S [M+NH4]+; found: 300.12664 C14H22O4NS [M+H]+, 322.10861 C14H22O4NSNa [M+Na]+, 317.15325 C14H25O4N2S,[M+NH4]+; Rf: 0.69 (DCM/MeOH 9:1, KMnO4); yield: 83% (10.8 g scale, 54.49 mmol).
tert-Butyl 2-(((2-acetamidoethyl)thio)carbonyl)butanoate (3d): 1H NMR: (400 MHz, CDCl3-d1) =0.90–0.94 (t, J=7.4 Hz, 3H), 1.43 (s, 9H), 1.84–1.91 (m, 2H), 1.93 (s, 3H), 2.98–3.10 (m, 2H), 3.34–3.46 (m, 3H), 6.09 (bs, 1H); 13C NMR: (101 MHz, CDCl3-d1) =11.8, 22.9, 23.2, 27.9, 28.7, 39.6, 62.7, 82.3, 167.8, 170.5, 196.0; HRMS: calc.: 290.14206 C13H24O4NS[M+H]+, 312.12400 C13H23O4NNaS [M+Na]+, 307.16860 C13H27O4N2S [M+NH4]+; found: 290.14246 C13H24O4NS[M+H]+, 312.12437 C13H23O4NNaS [M+Na]+, 307.16939 C13H27O4N2S [M+NH4]+; Rf: 0.67 (DCM/MeOH 9:1, KMnO4); yield: 786 mg; 27% (1.91 g scale, 10.13 mmol).
tert-Butyl 2-(((2-acetamidoethyl)thio)carbonyl)-3-methylbutanoate (4d): 1H NMR: (400 MHz, CDCl3-d1) =0.94–0.96 (d, J=6.7 Hz, 3H), 0.98–0.99 (d, J=6.7 Hz, 3H), 1.43 (s, 9H), 1.95 (s, 3H), 2.45–2.39 (m, 1H), 2.99–3.13 (m, 2H), 3.23–3.25 (d, J=9.5 Hz, 1H), 3.36–3.50 (m, 2H), 5.87 (bs, 1H); 13C NMR: (101 MHz, CDCl3-d1) =20.5, 20.6, 23.5, 28.2, 28.9, 30.0, 39.9, 68.9, 82.5, 167.4, 170.6, 195.6; HRMS: calc.: 304.15771 C14H26O4NS[M+H]+, 326.13965 C14H25O4NNaS [M+Na]+, 321.18425 C14H29O4N2S [M+NH4]+; found: 304.15825 C14H26O4NS[M+H]+, 326.14008 C14H25O4NNaS [M+Na]+, 321.18516 C14H29O4N2S [M+NH4]+; Rf: 0.64 (DCM/MeOH 9:1, KMnO4); yield: 598 mg; 25% (1.58 g scale, 7.81 mmol).
tert-Butyl 2-(((2-acetamidoethyl)thio)carbonyl)hexanoate (5d): 1H NMR: (400 MHz, CDCl3-d1) =0.84–0.88 (t, J=7.0 Hz, 3H), 1.26–1.28 (m, 4H), 1.42 (s, 9H), 1.80–1.87 (m, 2H), 1.96 (s, 3H), 2.99–3.08 (m, 2H), 3.33–3.47 (m, 3H), 6.09 (bs, 1H); 13C NMR: (101 MHz, CDCl3-d1) =13.8, 22.4, 23.2, 27.9, 28.7, 29.2, 29.4, 39.6, 61.2, 82.3, 167.9, 170.5, 196.1; HRMS: calc.: 318.17336 C15H28O4NS [M+H]+, 340.15530 C15H27O4NNaS [M+Na]+, 335.19990 C15H31O4N2S [M+NH4]+; found: 318.17390 C15H28O4NS [M+H]+, 340.15569 C15H27O4NNaS [M+Na]+, 335.20083 C15H31O4N2S [M+NH4]+; Rf: 0.69 (DCM/MeOH 9:1, KMnO4); yield: 23.00 g; 78% (20.0 g scale, 92.48 mmol).
tert-Butyl 2-(((2-acetamidoethyl)thio)carbonyl)octanoate (6d): 1HNMR: (400 MHz, CDCl3-d1) =0.86–0.89 (t, J=6.9 Hz, 3H), 1.27–1.29 (m, 8H), 1.46 (s, 9H), 1.85–1.88 (m, 2H), 1.96 (s, 3H), 3.00–3.13 (m, 2H), 3.37–3.51 (m, 3H), 5.85 (bs, 1H); 13C NMR: (101 MHz, CDCl3-d1) =14.2, 22.7, 23.3, 27.3, 28.03, 28.06, 28.8, 29.03, 29.6, 31.6, 39.7, 61.3, 82.4, 168.0, 170.6, 196.3; HRMS: calc: 346.20466 C17H32O4NS [M+H]+, 368.18660 C17H31O4NNaS [M+Na]+, 363.23120 C17H35O4N2S [M+NH4]+; found: 346.20494 C17H32O4NS [M+H]+, 368.18686 C17H31O4NNaS [M+Na]+, 363.23182 C17H35O4N2S [M+NH4]+; Rf: 0.79 (DCM/MeOH 9:1, KMnO4); yield: 9.61 g; 53% (12.77 g scale; 52.27 mmol).
tert-Butyl 3-((2-acetamidoethyl)thio)-3-oxo-2-phenylpropanoate (7d): 1H NMR: (400 MHz, CDCl3-d1) =1.45 (s, 9H), 1.90 (s, 3H), 3.00–3.06 (m, 2H), 3.39–3.44 (m, 2H), 4.72 (s, 1H),5.83 (bs, 1H, NH), 7.35–7.41 (m, 5H); 13C NMR: (101 MHz, CDCl3-d1) =23.1, 27.9, 29.3, 39.5, 66.4, 83.0, 128.6, 128.8, 128.9, 129.5, 132.6, 166.8, 170.6, 195.0; HRMS: calc.: 338.14206 C17H24O4NS[M+H]+, 360.12400 C17H23O4NNaS [M+Na]+, 355.16860 C17H27O4N2S [M+NH4]+; found: 338.14223 C17H24O4NS[M+H]+, 360.12417 C17H23O4NNaS [M+Na]+, 355.16909 C17H27O4N2S [M+NH4]+; Rf: 0.56 (MeOH/DCM 9:1, KMnO4); yield: 1.60 g; 38% (2.9 g scale, 12.3 mmol).
General procedure for the deprotection of compounds 1–7:
The thioester was dissolved in abs. DCM (10 ml/100 mg) under argon. At 0 °C 2.5 eq. TiCl4 was dropwise added. The dark brown reaction mixture was stirred for 5 min at 0 °C, then for another 6 h at room temperature. After 6 h (DC-control) the reaction mixture was quenched with aq. Na2CO3-solution (10.0 eq. Na2CO3) in an ice bath to reach a final concentration of 0.1 M of product. The white suspension was filtered and washed twice with 10 ml MeOH. The combined solvents were evaporated at 30 °C under reduced pressure. The resulting brown solution or white slurry was transferred to polypropylene tubes and cooled for 2 h at −20 °C; after warming to 4 °C, Na2CO3 precipitated. The precipitate was removed by centrifugation at 4 °C/4000 rpm for 10 min. Subsequently, the supernatant was freeze dried. The resulting white/yellow solidwas transferred into polypropylene tubes and dissolved in SM16 medium to yield a 100 mM solution. The resulting slightly brown solution was centrifuged at 4 °C/4000 rpm for 10 min and the supernatant was sterile filtered and used directly for feeding experiments.
For analysis of the reaction product by NMR, the product was dissolved in D2O instead of SM3 medium.
2-(((2-acetamidoethyl)thio)carbonyl)pent-4-enoic acid (1): 1H NMR: (400 MHz, D2O-d2) =2.26 (s, 3H), 2.83–2.87 (m, 2H), 3.30–3.43 (m, 2H), 3.59–3.69 (m, 2H), 3.96–3.99 (t, J=7.6 Hz, 1H), 5.35–5.39 (m, 2H), 6.05–6.13 (m, 1H,); 13C NMR: (101 MHz, D2O-d2) =22.8, 28.7, 34.2, 39.1,117.6, 135.6, 174.8, 175.7, 201.2; HRMS: calc.: 246.07946 C10H16O4NS[M+H]+, 268.06140 C10H15O4NNaS [M+Na]+; found: 246.07949 C10H16O4NS [M+H]+, 268.06048 C10H15O4NNaS [M+Na]+; Rf: 0.12 (DCM/MeOH 9:1, KMnO4).
2-(((2-acetamidoethyl)thio)carbonyl)pent-4-yn acid (2): 1H NMR: (400 MHz, D2O-d2) =1.88 (s, 3H); 2.30–2.32 (t, J=2.6 Hz, 1H), 2.61–2.63 (m, 2H); 2.99–3.04 (m, 2H); 3.26–3.32 (m, 2H), 3.68-3.72 (t, J=7.6 Hz, 1H); 13C NMR: (101 MHz, D2O-d2) =19.1, 22.5, 28.6, 38.9, 174.2, 174.7, 199.6; HRMS: cal.: 244.06381 C10H14O4NS [M+H]+; found: 244.06402 C10H14O4NS [M+H]+; Rf: 0.18 (DCM/MeOH 1:9, KMnO4).
2-(((2-acetamidoethyl)thio)carbonyl)butanoicacid (3): 1H NMR: (400 MHz, D2O-d2) =1.13-1.17 (t, J=7.4 Hz, 3H), 2.04–2.11 (q, J=7.4 Hz, 2H), 2.23 (s, 3H), 3.27–3.40 (m, 2H), 3.59–3.69 (m, 2H), 3.74–3.77 (t, J=7.6 Hz, 1H); 13C NMR: (101 MHz, D2O-d2) =12.0, 22.7, 23.9, 28.7, 39.1, 66.2, 174.7, 176.5, 201.92; HRMS: cal.: 234.07946 C9H16O4NS[M+H]+, 256.06140 C9H15O4NNaS [M+Na]+; found: 234.07957 C9H16O4NS[M+H]+, 256.06095 C9H15O4NNaS [M+Na]+; Rf: 0.23 (DCM/MeOH: 9:1, KMnO4).
2-(((2-acetamidoethyl)thio)carbonyl)-3-methylbutanoic acid (4): 1H NMR: (400 MHz, MeOD-d4/D2O-d2) =0.86–0.87 (d, J=6.6 Hz, 3H), 0.92–0.94 (d, J=6.7 Hz, 3H), 1.95 (s, 3H), 2.25–2.34 (m, 1H), 3.00–3.04 (m, 2H), 3.18–3.21 (d, J=10.4 Hz, 1H), 3.18–3.32 (m, 2H); 13C NMR: (101 MHz, MeOD-d4) =20.8, 21.3, 22.6, 29.2, 31.2, 39.9, 74.5, 173.7, 174.7, 199.7; HRMS: calc.: 248.09511 C10H18O4NS [M+H]+, 270.07705 C10H17O4NNaS [M+Na]+; found: 248.09535 C10H18O4NS [M+H]+, 270.07709 C10H17O4NNaS [M+Na]+; Rf: 0.18 (DCM/MeOH 9:1, KMnO4).
2-(((2-acetamidoethyl)thio)carbonyl)hexanoicacid (5): 1H NMR: (400 MHz, CDCl3-d1) =1.12–1.16 (t, J=7.1 Hz, 3H), 1.53–1.57 (m, 4H), 2.05–2.11 (m, 2H), 2.25 (s, 3H) 3.28–3.43 (m, 2H), 3.63–3.67 (m, 2H), 3.82–3.85 (t, J=7.6 Hz, 1H); 13C NMR: (101 MHz, CDCl3-d1) =16.5, 24.9, 25.4, 31.3, 32.1, 32.7, 41.7, 67.1, 177.2, 179.1, 204.6; HRMS: cal.: 262.11076 C11H20O4NS [M+H]+, 284.09270 C11H19O4NNaS [M+Na]+; found: 262.11083 C11H20O4NS [M+H]+, 284.09226 C11H19O4NNaS [M+Na]+; Rf: 0.13 (DCM/MeOH 9:1, KMnO4).
2-(((2-acetamidoethyl)thio)carbonyl)octanoicacid (6): 1H NMR: (400 MHz, D2O-d2/MeOD-d4) =0.83-0.86 (m, 3H), 1.25–1.27 (m, 8H), 1.78–1.84 (m, 2H), 1.88 (s, 3H,), 2.94–2.98 (m, 2H), 3.26–3.27 (m, 2H), 3.41–3.44 (t, J=7.4 Hz, 1H); 13C NMR: (101 MHz, MeOD-d4) =14.4, 22.6, 23.6, 28.8, 29.2, 30.2, 31.7, 32.8, 40.1, 65.9, 173.4, 175.6, 199.8; HRMS: calc.: 290.14206 C13H24O4NS [M+H]+, 312.12400 C13H23O4NaS [M+Na]+; found: 290.14226 C13H24O4NS [M+H]+, 312.12417 C13H23O4NaS [M+Na]+; Rf: 0.23 (DCM/MeOH 9:1, KMnO4).
3-((2-Acetamidoethyl)thio)-3-oxo-2-phenylpropanoic acid (7): 1H NMR: (400 MHz, MeOD-d4) =1.88 (s, 3H), 2.58–2.61 (m, 2H), 2.98–3.01 (m, 2H), 3.35 (s, 1H), 7.24–7.33 (m, 5H); HRMS: calc.: 282.07946 C13H16O4NS[M+H]+; found: 282.07936 C13H16O4NS[M+H]+; Rf: 0.2 (DCM/MeOH: 9:1, KMnO4).
Mutagenesis of DEBS3 for an enzyme-directed mutasynthesisin S. erythraea
S. erythraea NRRL-B-24071, S. erythraeaΔAT6hygR [1] and S. erythraea AT6* were used for fermentation.
The alterations of the selected residues in the YASH motif [1] were accomplished by oligonucleotide-mediated mutagenesis and overlap-extension PCR using the Phusion Flash Master Mix (Thermo Fisher). Briefly, mutagenesis was achieved by performing PCR with designed oligonucleotide primers (Table 1) that include the desired mutation in their sequence (oligonucleotides 3 and 4) and flanking oligonucleotides (1 and 2) in a Piko™ Thermocycler with the following program: 3 min denaturation at 99 °C, 5 cycles of 15 s at 99 °C, annealing for 15 s at 65 °C and 40 s extension at 72 °C, 25 cycles of 15 s 99 °C, 40 s at 72 °C, and a final extension of 60 s at 72 °C. The EcoRV digested plasmid pKSSU89 was used as template [1].The PCR products were DpnI digested, purified and precipitated using SureClean (Bioline, German) and redissolved in water. The two overlapping fragments were fused together in a subsequent extension reaction. The inclusion of flanking primers 1 and 2 in the extension reaction allowed the amplification of the fused product by PCR: 3 min at 99 °C, 25 cycles of 15 s at 99 °C and 40 s of 72 °C, 60 s of 72 °C. The final PCR products were gel-purified and cloned into ScaI linerarized pKSSU96 via SLIC-MIX [7]. Insert-containing clones were identified by colony PCR and analysis of isolated plasmids. Identity of the plasmids was confirmed by DNA sequencing. The DEBS3-encoding plasmids carrying the desired mutations were transformed into E. coli ET12567/pUZ8002 and then conjugated into S. erythraea ΔAT6hygR. Conjugation and propagation of resulting clones was performed as in reference [8].
Table 1.
Oligonucleotides used in this study.
| No. | Sequence |
|---|---|
| 1 | TTACGGCAAGTCGCGCGGGTCGTCGGGCCCGGTGCTGCTGGGTTCGGTG |
| 2 | AAGCCGCCTTCCAGGTCCACCGGCGTGGTGGCCAGCGGTCGCCAGTCG |
| 3 | CCAAGACGCTCCCGGCCGACGGCGCCGGCCACTCCCGCCACGTCGAGGAG |
| 4 | CTCCTCGACGTGGCGGGAGTGGCCGGCGCCGTCGGCCGGGAGCGTCTTGG |
Acknowledgements
E.S.-G acknowledges a Liebig-stipend from the Fonds der Chemischen Industrie. This work was supported by the Cluster of Excellence RESOLV (EXC 1069) and the Collaborative Research Center SFB1093, both funded by the Deutsche Forschungsgemeinschaft.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dib.2015.09.052.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- 1.Sundermann U., Bravo-Rodriguez K., Klopries S., Kushnir S., Gomez H., Sanchez-Garcia E. Enzyme-directed mutasynthesis: a combined experimental and theoretical approach to substrate recognition of a polyketide synthase. ACS Chem. Biol. 2013;8(2):443–450. doi: 10.1021/cb300505w. [DOI] [PubMed] [Google Scholar]
- 2.Bravo-Rodriguez K., Klopries S., Koopmans K.R.M., Sundermann U., Yahiaoui S., Arens J., Kushnir S., Schulz F., Sanchez-Garcia E. Substrate flexibility of a mutated acyltransferase domain and implications for polyketide biosynthesis. Chem. Biol. 2015 doi: 10.1016/j.chembiol.2015.02.008. in press. [DOI] [PubMed] [Google Scholar]
- 3.Armarego W.L.F., Chai C.L.L. Purification of organic chemicals. In: Armarego W.L.F., CHai C.L.L., editors. Purification of Laboratory Chemicals. Sixth ed. Butterworth-Heinemann; Oxford: 2009. pp. 88–444. (Chapter 4) [Google Scholar]
- 4.Koryakina I., Williams G.J. Mutant malonyl-CoA synthetases with altered specificity for polyketide synthase extender unit generation. ChemBioChem. 2011;12(15):2289–2293. doi: 10.1002/cbic.201100383. [DOI] [PubMed] [Google Scholar]
- 5.Singh R.K., Danishefsky S. Preparation of activated cyclopropanes by phase transfer alkylation. J. Org. Chem. 1975;40(20):2969–2970. [Google Scholar]
- 6.Hrubowchak D.M., Smith F.X. The reductive alkylation of meldrum׳s acid. Tetrahedron Lett. 1983;24(45):4951–4954. [Google Scholar]
- 7.Kushnir S., Sundermann U., Yahiaoui S., Brockmeyer A., Janning P., Schulz F. Minimally invasive mutagenesis gives rise to a biosynthetic polyketide library. Angew. Chem. Int. Ed. 2012;51(42):10664–10669. doi: 10.1002/anie.201202438. [DOI] [PubMed] [Google Scholar]
- 8.Kieser T.B., Buttner M.J., Bibb M.J., Chater K.F., Hopwood D.A. John Innes Foundation; Norwich Research Park, UK: 2000. Practical streptomyces genetics. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material