Abstract
Citronellal, a well-known plant-derived mosquito repellent, was previously reported to repel Drosophila melanogaster via olfactory pathways involving but not directly activating Transient Receptor Potential Ankyrin 1 (TRPA1). Here, we show that citronellal is a direct agonist for Drosophila and human TRPA1s (dTRPA1 and hTRPA1) as well as Anopheles gambiae TRPA1 (agTRPA1). Citronellal-induced activity is isoform-dependent for Drosophila and Anopheles gambiae TRPA1s. The recently identified dTRPA1(A) and ag-TRPA1(A) isoforms showed citronellal-provoked currents with EC50s of 1.0 ± 0.2 and 0.1 ± 0.03 mM, respectively, in Xenopus oocytes, while the sensitivities of TRPA1(B)s were much inferior to those of TRPA1(A)s. Citronellal dramatically enhanced the feeding-inhibitory effect of the TRPA1 agonist N-methylmaleimide (NMM) in Drosophila at an NMM concentration that barely repels flies. Thus, citronellal can promote feeding deterrence of fruit flies through direct action on gustatory dTRPA1, revealing the first isoform-specific function for TRPA1(A).
Keywords: Anopheles gambiae, citronellal, Drosophila melanogaster, human TRPA1, insect repellent, TRPA1
INTRODUCTION
Although citronellal is a major component of the essential oil distilled from Cymbopogon plants, it is present in plants at relatively low concentrations (Carlson et al., 2001). The airborne vapor of citronellal is well known to repel mosquitoes (Sakulku et al., 2009). A molecular genetics study in Drosophila melanogaster proposed that two distinct olfactory pathways are responsible for the aversion of flies to citronellal when high concentrations of citronellal in dimethylsulfoxide (DMSO) were allowed to evaporate (Kwon et al., 2010). One pathway is mediated by insect-specific olfactory receptors, since loss of Orco, the gene encoding the essential coreceptor for the function of other olfactory receptors (Larsson et al., 2004), abolished citronellal-induced spikings in olfactory receptor neurons (ORNs) and lowered aversive behavioral responses (Kwon et al., 2010). The second pathway involving Drosophila transient receptor potential A1 (dTrpA1) is complex; while dTrpA1 is required in Orco–expressing ORNs for citronellal avoidance, ORNs lacking dTrpA1 showed an increase in spiking rather than a decrease upon citronellal application, which briefly persists after ceasing citronellal puffing (Kwon et al., 2010). As dTRPA1 was unresponsive to citronellal in Xenopus oocytes, it appeared that dTrpA1 functions downstream of a GPCR acting as a citronellal receptor (Kwon et al., 2010).
With the recent discovery of TRPA1 isoform diversity in mammals (Zhou et al., 2013) and insects (Kang et al., 2012; Kwon et al., 2010), we suspected that the lack of dTRPA1 response to citronellal was due to the use of the thermosensory Drosophila TRPA1 isoform TRPA1(B). Expression of the dTrpA1(B) transcript in AC neurons in the brain rescued the thermotaxis defect of dTrpA1-deficient flies (Hamada et al., 2008; Kang et al., 2012; Ni et al., 2013), while dTrpA1(A) was found to be predominantly expressed in the bitter gustatory receptor neurons (GRNs) of the labral sense organ (Kang et al., 2010) and labellum (Kang et al., 2012). This isoform expression suggested that dTRPA1(A) would be the isoform relevant to citronellal perception. To test this possibility, we examined the dTRPA1(A) isoform for citronellal sensing through behavioral and physiological approaches. To mimic the conditions associated with the natural occurrence of citronellal, low concentrations of citronellal solubilized or emulsified in water-based liquid food were used in our physiological experiments and gustatory behavioral capillary feeder (Café) assay (Ja et al., 2007). We find that the dTRPA1(A) isoform can be directly activated by citronellal, and citronellal enhances dTRPA1-dependent feeding deterrence, demonstrating that citronellal is capable of affecting insects through gustation in addition to the previously reported olfaction.
MATERIALS AND METHODS
Fly strains
UAS-TrpA1(A) and UAS-TrpA1(B) are inserted in the attP16 site (Kang et al., 2012). Gr66a-Gal4 and TrpA1ins were previously described (Dunipace et al., 2001; Kang et al., 2012).
Capillary feeder (Café) assay
Café assays were conducted as described (Ja et al., 2007) with modifications. For avoidance, 30 mM sucrose was placed in both tubes, with one tube containing citronellal (27470, Sigma Aldrich, USA) and the other citronellal/NMM (389412, Sigma Aldrich). Flies starved overnight were offered food-containing calibrated capillaries (#2920107, Marienfeld, Germany) for 30 min. Ingestion lowers the meniscus in the capillaries and was measured by converting the distance to consumed volume (15 mm/μl). To eliminate the evaporation effect, empty vials with capillary tubes were assayed side by side. An avoidance index (AI) was obtained in the following manner: AI = [consumption amount of sucrose-only − consumption amount of sucrose with aversive chemicals] / [total consumption amount]. The strongest avoidance index would be 1, neutral 0, and the strongest preference −1.
Characterization of TRPA1 channels in Xenopus oocytes
Citronellal-evoked TRPA1 currents were recorded in oocytes by two-electrode voltage clamping as described (Choi et al., 2014; Kang et al., 2010; 2012). Briefly, surgically isolated ovaries were treated with collagenase to obtain free oocytes. One day after cRNA microinjection, oocytes were perfused in the recording solution (96 NaCl, 1 KCl, 1 MgCl2, 5 HEPES, pH 7.6 in mM). The membrane potential was initially held at −60 mV, and a 300-ms voltage ramp from −60 to +60 mV was applied every second by the GeneClamp 500B amplifier (Molecular Devices, USA) communicating with Digidata 1440A (Molecular Devices).
In vivo recording of GRNs
Extracellular recordings in taste bristles were conducted as described (Kang et al., 2012). Tricholine citrate (TCC, 30 mM) was used as electrolyte. Citronellal in 30 mM TCC was placed in direct contact with the tip of I-type sensilla with a fine manipulator. Data were sampled at 20 kb/sec and band-passed between 100–20,000 Hz. Hemolymph-like solution 3.1 was used in the reference electrode which penetrates the fly from the back of thorax to the vicinity of labella taste cell bodies. Tasteprobe from Syntech (Netherlands) was used to record from GRNs. Occasionally, spikes with low amplitudes were observed as in Figs. 3A and 3C, which strongly correlated with the mechanical stimuli from physical contact between the electrolyte and the bristle, independent of citronellal sensitivity.
Fig. 3.
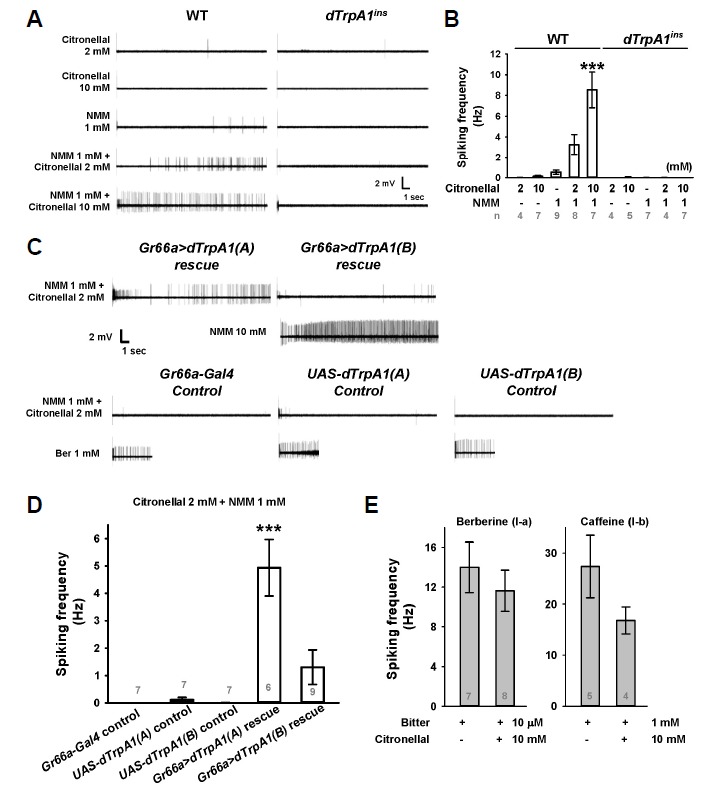
Citronellal facilitates activation of TrpA1-positive gustatory neurons by NMM at the suboptimal concentration of 1 mM. (A) Typical examples of extracellular action potential recordings of taste neurons in I-type sensilla. The viability of non-responding neurons in WT and TrpA1ins was examined by exposure to 1 mM berberine that stimulates TrpA1-independent bitter signaling in the same neuron as shown in (C). (B) Bar graphs represent averages of spiking frequencies from in (A). (C) Reintroduction of Drosophila TrpA1 cDNAs differentially restored the neuronal responses to a mixture of 2 mM citronellal and 1 mM NMM. (D) A summary of experiments in (C). (E) Neuronal responses to berberine and caffeine were not enhanced by citronellal, unlike NMM. The number of experiments is presented in grey on each bar (B, D, and E). p < 0.001, ANOVA Tukey test.
Statistics
Student’s t-test and ANOVA Tukey multiple comparison, were performed with Sigmaplot12. Error bars indicate standard error of mean (SEM). Normality was tested by the Shapiro-Wilk method. When data failed to pass either normality or equal variance test, they are analyzed by rank sum tests such as Mann-Whitney U and ANOVA Dunn’s tests.
RESULTS AND DISCUSSION
To test if TRPA1(A) can respond to citronellal, the two TRPA1 isoforms were heterologously expressed in Xenopus oocytes, and subjected to electrophysiological evaluation with increasing concentrations of citronellal (Fig. 1). Since the TRPA1 isoforms showed similar sensitivity to N-methyl maleimide (NMM) (Kang et al., 2012), responses to citronellal were normalized to responses to 0.1 mM NMM (Fig. 1D). Citronellal was emulsified by vortexing to a concentration of 10 mM, since the water solubility of citronellal is saturated at about 2 mM. Cells expressing dTRPA1(A) showed responses with an EC50 of 1.0 ± 0.2 mM (Figs. 1A and 1D), which were almost saturated at 10 mM and produced current amplitudes that were roughly half of NMM-provoked current sizes. In contrast, TRPA1(B) barely responded to 10 mM citronellal, while being robustly activated by 0.1 mM NMM (Fig. 1B). Thus, citronellal-elicited dTRPA1 activity is highly isoform-dependent. TRPA1 is a reactive chemical receptor conserved in bilaterians (Kang et al., 2010), and electrophiles activate TRPA1 by covalently modifying key cysteines in the N-terminal ankyrin repeat domain (ARD) (Macpherson et al., 2007). To examine whether citronellal stimulates TRPA1 by the ARD cysteine pathway, we generated the TRPA1(A)2C mutant in which serines replace Cys704 and Cys724 that are widely conserved in bilateria and known to be important for the normal reactive chemical sensitivity of TRPA1(B) (Kang et al., 2010). TRPA1(A)2C expressed in oocytes responded to citronellal at similar levels to TRPA1(A)WT, indicating that citronellal does not stimulate TRPA1 via cysteine modification (Figs. 1C and 1D).
Fig. 1.
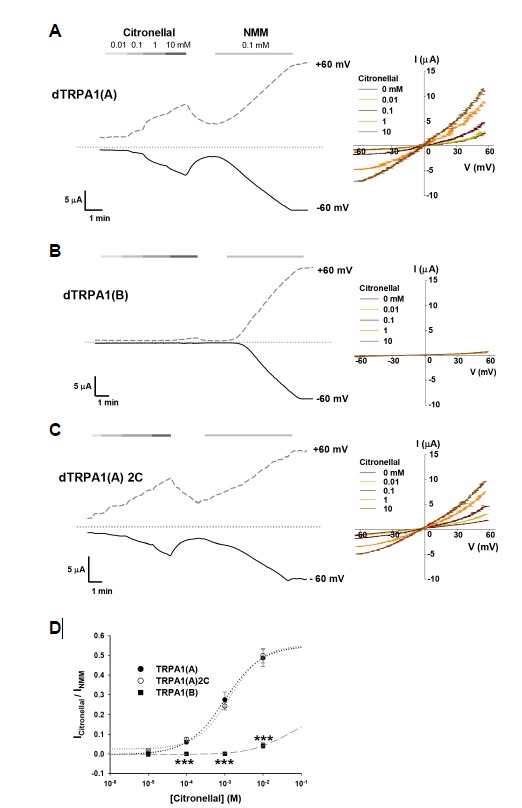
dTRPA1(A), and not dTRPA1(B), heterologously expressed in Xenopus oocytes is activated by citronellal. (A–C) Left, Representative current recordings at −60 and +60 mV from (A) dTRPA1(A)-, (B) dTRPA1(B)-, or (C) dTRPA1(A)2C-expressing oocytes. Right, Current-voltage (IV) relationship observed at the indicated citronellal concentrations ranging from 0 to 10 mM. (D) Dose dependence of indicated Drosophila TRPA1 variants of TRPA1(A), TRPA1(A)2C and TRPA1(B) in Xenopus oocytes (n = 5–6). Mean±standard error of mean (SEM) is shown. ***: p < 0.001, Tukey tests.
Although TRPA1 is conserved between insects and mammals as a key receptor for noxious chemical reactivity, insect TRPA1s are not activated by chemicals that non-covalently activate mammalian TRPA1s (Kang et al., 2010) and insect TRPA1-activating chemicals independent of the ARD cysteines are as yet unknown. Since dTRPA1(A) sensitivity to citronellal did not require the ARD cysteines (Figs. 1C and 1D), citronellal may be the first example of a non-covalent activator for TRPA1 channels in insects. To test if citronellal activation of TRPA1 is conserved, we ectopically expressed human TRPA1 (hTRPA1) in Xenopus oocytes as well as agTRPA1(A) and agTRPA1(B) from the malaria-transmitting mosquito (Anopheles gambiae) that have not been quantitatively appraised for citronellal responsiveness. Dose dependence experiments revealed that the human and mosquito TRPA1 channels are citronellal receptors with better functional characteristics than dTRPA1(A) (Fig. 2). Citronellal sensitivity and the NMM-normalized maximum current amplitude of agTRPA1(A) were an order of magnitude higher (EC50 = 0.10 ± 0.03 mM) and ∼3x higher, respectively (Figs. 2A and 2E), than dTRPA1(A). These functional parameters of agTRPA1(A) confirms that citronellal is a highly potent and selective repellent to mosquitoes considering the chemical-nociceptive role of TRPA1 in insects. Mosquito TRPA1(B) also performed better than Drosophila TRPA1(B) at a similar level to Drosophila TRPA1(A) (Figs. 2C, 2E, and 2F). As observed with Drosophila TRPA1(A)2C, the ARD cysteines in agTRPA1(A) do not appear to be involved in citronellal detection (Figs. 2B and 2E). Interestingly, hTRPA1 responded to citronellal with a combination of the functional properties of the two insect TRPA1(A)s (Figs. 2D, 2E, and 2F). The sensitivity of hTRPA1 was close to Drosophila TRPA1(A), with similar EC50s. However, maximal relative amplitudes normalized to NMM response were similar to agTRPA1(A) (Fig. 2F). These results indicate that the ability to sense citronellal is a function of TRPA1 conserved between insects and humans with some evolutionary divergence, probably to meet the needs of different environmental niches.
Fig. 2.
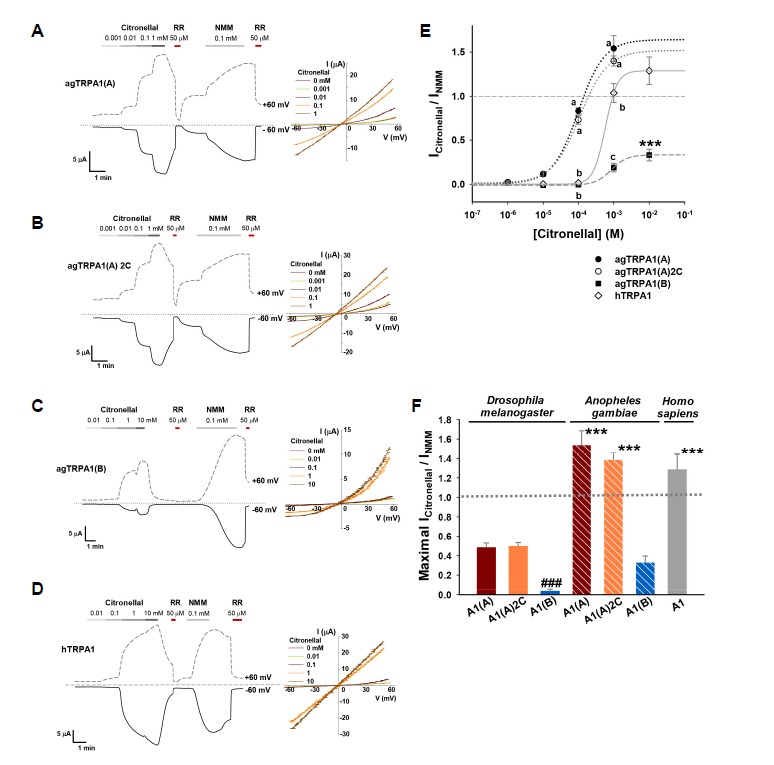
hTRPA1 exhibits functional parameters intermediate to agTRPA1(A) and dTRPA1(A) when heterologously expressed in Xenopus oocytes. (A–D) Indicated TRPA1 channels were heterologously expressed in oocytes and subjected to electrophysiological characterization upon exposure to different doses of citronellal. Responses at +60 and −60 mV are shown in the left panels and IV relationships in the right panels. RR: 0.05 mM ruthenium red, a TRPA1 inhibitor. (E) Citronellal dose-dependence of Anopheles gambiae TRPA1 (agTRPA1) isoforms and human TRPA1 (hTRPA1) in oocytes. Letters indicate statistically distinct groups (Tukey, p < 0.01, n = 6–7). ***: p < 0.001, Student’s t-test. (F) Maximal citronellal responses normalized to those of NMM. Citronellal concentrations used for maximal responses: 10 mM for dTRPA1s, agTRPA1(B), hTRPA1, and 1 mM for agTRPA1(A) channels (n = 6–7). ***: p < 0.001, all comparisons. ###: p < 0.001, comparisons between dTRPA1s and agTRPA1(B) that show means lower than 1.0. ANOVA Tukey test.
Although dTrpA1 expression in GRNs is well-documented in previous studies (Kang et al., 2010; 2012), the role of gustatory dTrpA1 in citronellal detection remains unexplored. To evaluate the functional implications of citronellal sensitivity in vivo, action potential responses of dTrpA1-expressing bitter gustatory neurons in the I-a sensilla in the labellum were monitored by the extracellular tip recording technique (Meunier et al., 2003; Weiss et al., 2011). To our surprise, 2 and 10 mM citronellal applied in 30 mM tricholine citrate (TCC) rarely provoked firing of the bitter gustatory neurons that endogenously express dTrpA1(A) (Kang et al., 2012), in contrast to the observations in oocytes (Figs. 3A and 3B). The well-known TRPA1 agonist NMM was previously found to require a concentration 100 times higher (10 mM) than the EC50 of dTRPA1 activation (∼0.1 mM) observed in heterologous expression for behavioral efficacy (Kang et al., 2010). Given the low sensitivity to citronellal that dTRPA1(A) showed in oocytes (EC50 = 1.0 ± 0.2 mM), we suspected that the concentrations of citronellal solubilizable in aqueous solution (< 10 mM) might be subthreshold for the activation of in vivo gustatory neurons. Next, we hypothesized that the in vivo subthreshold concentrations of citronellal may be able to potentiate the action of dTRPA1 agonists. To test this possibility, the response of dTrpA1-expressing gustatory neurons was monitored upon application of citronellal mixed with 1 mM NMM. NMM at 1 mM alone was insufficient for robust induction of action potential responses in the bitter neurons (Figs. 3A and 3B). Increasing concentrations of citronellal dramatically elevated the spiking responses to 1 mM NMM, which was not observed in the same neurons deficient of TrpA1 in TrpA1ins mutant flies (Figs. 3A and 3B). The lack of response to the mixtures of citronellal and NMM in TrpA1ins was rescued by reintroduction of the TrpA1(A) transcript using the Gr66a-Gal4 transgene (Figs. 3C and 3D). Consistent with the results in oocytes, expression of TrpA1(B) failed to confer citronellal-potentiated NMM sensitivity on the TrpA1ins I-a sensilla bitter neurons, while the TrpA1(B)-expressing neurons robustly responded to 10 mM NMM (Figs. 3C and 3D). To test if the potentiating action of citronellal is specific for TrpA1 as anticipated from the direct action on TRPA1 observed in oocytes, citronellal was co-applied with the bitter compounds berberine and caffeine. Bitter chemical detection is mediated by gustatory receptors, and thought to be largely independent of TRPA1. Berberine is a major stimulant for I-a sensilla, while caffeine stimulates the I-b sensilla in the labellum (Weiss et al., 2011). Consistent with specific potentiation of dTRPA1 by citronellal, spiking responses to the bitter compounds were not enhanced by co-application with citronellal (Fig. 3E). Thus, citronellal specifically acts on TRPA1 to enhance sensitivity to the TRPA1 agonist NMM, without positively affecting bitter chemical.
Excitation or inhibition of neurons often results in behavioral consequences. We examined whether the TRPA1 potentiation by citronellal that enhances NMM detection in the gustatory neurons significantly affects 1 mM NMM-induced feeding deterrence. The Café assay, which offers a choice between two flavored foods in separate capillary tubes (Ja et al., 2007), was used for behavioral experiments (See “Materials and Methods” for detail). Citronellal at 2 and 10 mM in 30 mM sucrose solution did not significantly repel or attract flies for ingestion when offered with a tube containing only 30 mM sucrose (Fig. 4A), as expected from the absence of robust action potential induction by citronellal in the dTrpA1–positive bitter neurons. However, TrpA1-deficient TrpA1ins flies preferred ingestion of 10 mM citronellal-containing sucrose solution, implying that the TrpA1-dependent avoidance pathway that Kwon et al. (2010) found neutralizes attraction to citronellal through an unknown mechanism in this behavioral paradigm (Fig. 4A). To assess citronellal as an enhancer for food aversion by the dTRPA1 agonist NMM, 1 mM NMM was included to the tube containing either 2 or 10 mM citronellal, while 30 mM sucrose was offered in both tubes (Fig. 4A). One mM NMM did not inhibit 30 mM sucrose ingestion (Fig. 4A) as expected based on the absence of robust action potential induction in I type sensilla observed above (Figs. 3A and 3B). Co-application of 1 mM NMM with citronellal significantly increased the avoidance index (AI) depending on citronellal concentration, and this was dependent on dTrpA1 based on experiments with the TrpA1ins mutant (Fig. 4A). Thus, citronellal serves as an enhancer for feeding deterrence mediated by dTRPA1, although the low sensitivity of TRPA1 to citronellal did not guarantee activation of dTrpA1-expressing bitter neurons by sole application of citronellal. Gr66a-Gal4, which has been successfully used for RNAi-knockdown and rescue of dTrpA1-dependent feeding avoidance, was used to express TrpA1(A) or TrpA1(B) in the GRNs of TrpA1ins mutant flies to test for rescue of defects in citronellal enhancement of avoidance to1 mM NMM. Consistent with the physiological data acquired in Xenopus oocytes and Drosophila in vivo bitter gustatory neurons, only dTrpA1(A) could restore the citronellal-enhanced feeding aversion to 1 mM NMM (Fig. 4B). In contrast, olfactory expression using Orco-Gal4 failed to fully rescue the avoidance defect observed in the TrpA1ins animals (Fig. 4C), indicating that citronellal facilitates avoidance by acting on the gustatory neurons, and not the olfactory neurons, despite the volatility of citronellal. Therefore, citronellal facilitation of dTrpA1-dependent firing in bitter neurons promotes gustatory feeding aversion to a subthreshold concentration of the dTRPA1 agonist NMM. Given that the dTrpA1(B) transcript successfully rescued the olfactory avoidance defect in the previous study by Kwon et al. (2010), the olfactory pathway may require GPCRs upstream of dTRPA1(B), but likely is not involved in the direct sensitivity of dTRPA1 to citronellal. The gustatory avoidance to citronellal observed in our Café assays was conferred by expression of only dTrpA1(A) that encodes the isoform which directly responds to citronellal. Although olfactory stimulation by citronellal previously caused negative chemotaxis, ingestion deterrence of WT animals was not significantly affected by the naturally occurring level of citronellal in our study. Rather, NMM-assisted gustatory aversion by citronellal requires expression of dTRPA1(A) in bitter gustatory cells to show effects such as actual feeding suppression, thus accentuating the importance of the ability of dTRPA1 to directly respond to citronellal. Nevertheless, the sensitivity of dTRPA1 is much attenuated compared to agTRPA1s, suggesting that citronellal sensitivity is associated with adaptation to the ecological niches of different species. Indeed, Drosophila live in a close relationship with plants, which probably necessitates repression of aversion to plant-derived chemicals (Goldman-Huertas et al., 2015). On the other hand, hematophagous mosquitoes might benefit from avoiding plants by maintaining the high sensitivity of agTRPA1s to citronellal or other phytochemicals. By efficiently avoiding plants, mosquitoes might have better chances of encountering blood-bearing organisms. It would be of further intrigue to determine which structural features of TRPA1(A) are evolutionary hot spots that bolster or correlate with phytochemical avoidance. These putative domains might be common target sites of various natural compounds that selectively repel blood-drinking insects. A thorough structure-function study identifying primary sequences critical for citronellal sensitivity of insect and human TRPA1s may identify such domains, thereby providing a molecular basis for development of potent hematophagy-selective insect repellents.
Fig. 4.
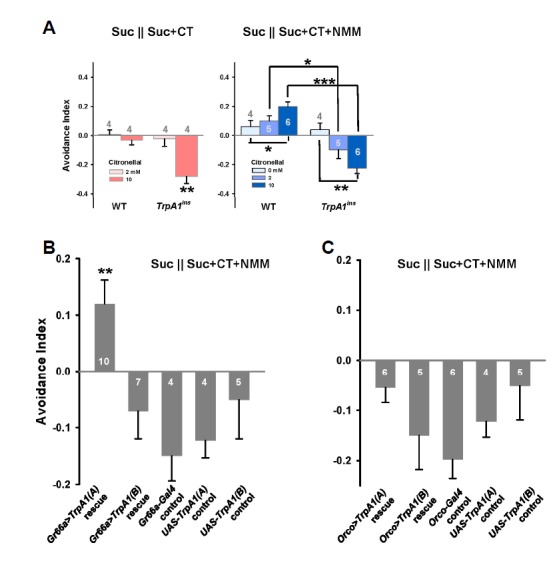
Citronellal enhances TrpA1-dependent feeding avoidance to 1 mM NMM. (A) Feeding choice tests using the capillary feeder (Café) assay. Left, The behavioral responses of WT and TrpA1ins to indicated concentrations of citronellal (CT) only in 30 mM sucrose (Suc). Right, Feeding avoidance to 1 mM NMM with or without citronellal. (B, C), Reintroduction of dTRPA1(A) in TrpA1ins bitter gustatory neurons using Gr66a-Gal4 restored the citronellal-enhanced NMM avoidance (B), while that in olfactory receptor neurons did not (C). The numbers of experiments are presented in grey or white on bars. *: p < 0.05, **: p < 0.01, ***: p < 0.001, Tukey tests.
Acknowledgments
This work was supported by funds to KJK from Sungkyunkwan University (S-2012-1188-000) and from the Samsung Biomedical Research Institute (SMX1150561), as well as grants to JYK and MSC from the Basic Science Research Programs through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, Republic of Korea [NRF-2011-0017239 and NRF-2013R1A1A2061120] and by the Ministry of Science, ICT and Future Planning [NRF-2014R1A1A1037477], respectively.
REFERENCES
- Carlson L.H.C., Machado R., aF. S, pricigo C.B., Pereira L.K., Bolzan A. Extraction of lemongrass essential oil with dense carbon dioxide. J. Supercrit. Fluids. 2001;21:33–39. [Google Scholar]
- Choi S.H., Lee B.H., Kim H.J., Jung S.W., Kim H.S., Shin H.C., Lee J.H., Kim H.C., Rhim H., Hwang S.H., et al. Ginseng gintonin activates the human cardiac delayed rectifier K+ channel: involvement of Ca2+/calmodulin binding sites. Mol. Cells. 2014;37:656–663. doi: 10.14348/molcells.2014.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunipace L., Meister S., McNealy C., Amrein H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr. Biol. 2001;11:822–835. doi: 10.1016/s0960-9822(01)00258-5. [DOI] [PubMed] [Google Scholar]
- Goldman-Huertas B., Mitchell R.F., Lapoint R.T., Faucher C.P., Hildebrand J.G., Whiteman N.K. Evolution of herbivory in Drosophilidae linked to loss of behaviors, antennal responses, odorant receptors, and ancestral diet. Proc. Natl. Acad. Sci. USA. 2015;112:201424656. doi: 10.1073/pnas.1424656112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada F.N., Rosenzweig M., Kang K., Pulver S.R., Ghezzi A., Jegla T.J., Garrity P.A. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ja W.W., Carvalho G.B., Mak E.M., de la Rosa N.N., Fang A.Y., Liong J.C., Brummel T., Benzer S. Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. USA. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K., Pulver S.R., Panzano V.C., Chang E.C., Griffith L.C., Theobald D.L., Garrity P.A. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K., Panzano V.C., Chang E.C., Ni L., Dainis A.M., Jenkins A.M., Regna K., Muskavitch M.A.T., Garrity P.A. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature. 2012;481:76–80. doi: 10.1038/nature10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y., Kim S.H., Ronderos D.S., Lee Y., Akitake B., Woodward O.M., Guggino W.B., Smith D.P., Montell C. Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. Curr. Biol. 2010;20:1672–1678. doi: 10.1016/j.cub.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M.C., Domingos A.I., Jones W.D., Chiappe M.E., Amrein H., Vosshall L.B. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Macpherson L.J., Dubin A.E., Evans M.J., Marr F., Schultz P.G., Cravatt B.F., Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Meunier N., Rospars J., Tanimura T., Marion-Poll F. Peripheral coding of bitter taste in Drosophila. J. Neurobiol. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]
- Ni L., Bronk P., Chang E.C., Lowell A.M., Flam J.O., Panzano V.C., Theobald D.L., Griffith L.C., Garrity P.A. A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila. Nature. 2013;500:580–584. doi: 10.1038/nature12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakulku U., Nuchuchua O., Uawongyart N., Puttipipatkhachorn S., Soottitantawat A., Ruktanonchai U. Characterization and mosquito repellent activity of citronella oil nanoemulsion. Int. J. Pharm. 2009;372:105–111. doi: 10.1016/j.ijpharm.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Weiss L.a, Dahanukar A., Kwon J.Y., Banerjee D., Carlson J.R. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Suzuki Y., Uchida K., Tominaga M. Identification of a splice variant of mouse TRPA1 that regulates TRPA1 activity. Nat. Commun. 2013;4:2399. doi: 10.1038/ncomms3399. [DOI] [PMC free article] [PubMed] [Google Scholar]


