Abstract
The manufacture and use of explosives throughout the past century has resulted in the extensive pollution of soils and groundwater, and the widespread interment of landmines imposes a major humanitarian risk and prevents civil development of large areas. As most current landmine detection technologies require actual presence at the surveyed areas, thus posing a significant risk to personnel, diverse research efforts are aimed at the development of remote detection solutions. One possible means proposed to fulfill this objective is the use of microbial bioreporters: genetically engineered microorganisms “tailored” to generate an optical signal in the presence of explosives’ vapors. The use of such sensor bacteria will allow to pinpoint the locations of explosive devices in a minefield. While no study has yet resulted in a commercially operational system, significant progress has been made in the design and construction of explosives-sensing bacterial strains. In this article we review the attempts to construct microbial bioreporters for the detection of explosives, and analyze the steps that need to be undertaken for this strategy to be applicable for landmine detection.
Keywords: explosives; landmines; microbial bioreporters; biosensors; bioluminescence; 2,4,6- trinitrotoluene; 2,4-dinitrotoluene
Introduction
The extensive production and use of explosives for both civilian and military purposes throughout the past century has created diverse environmental problems, including the contamination of soil and groundwater with explosive residues. Evidence for the toxic and mutagenic effects of explosive contaminants, such as 2,4,6-trinitrotoluene (TNT), hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX), is well established (Berthe-Corti et al., 1998; Schäfer and Achazi, 1999; Frische, 2002; Rosen and Lotufo, 2007). The international Agency for Research on Cancer (IARC) lists 2,4-dinitrotoluene (2,4-DNT) and 2,6-dinitrotoluene (2,6-DNT) as possible carcinogens in humans (IARC, 1996), and has determined that evidence regarding the carcinogenic potential of TNT is still inadequate. Substantial evidence for the contamination of groundwater reservoirs with explosives has been found in proximity to explosive manufacturing facilities (Funk et al., 1993; Schmelling et al., 1997; Spain et al., 2000; Bernstein et al., 2008).
Current detection and quantification methods for trace explosives in soil and groundwater mostly rely on analytical devices such as gas or liquid chromatography coupled with mass spectrometry. Although highly accurate and extremely sensitive, such analytical methodologies depend upon expensive equipment that is restricted to specialized laboratories and requires a high degree of expertise. A potential complementary approach, which provides information also on the bioavailability and toxicity of the target compounds is based on the use of live cell sensors (Belkin, 2003; Van der Meer and Belkin, 2010). Such bioreporters have also been proposed (Burlage, 2003; Garmendia et al., 2008; Yagur-Kroll et al., 2014) as a tool for the remote detection of buried landmines, out of which traces of explosives’ vapors have been demonstrated to leak and accumulate in the soil around them (Jenkins et al., 2001).
The most common explosive material present in both antipersonnel and antitank landmines is TNT, sometimes in combination with RDX (Jenkins et al., 2001; MacDonald et al., 2003). Two volatile impurities that accompany TNT are 1,3-dinitrobenzene (1,3-DNB) and – more prominently – 2,4-DNT. Although the latter accounts for less than 1% of the explosive material, its vapor pressure is much higher than that of TNT, resulting in higher concentrations of 2,4-DNT at ground level (MacDonald et al., 2003); this compound is therefore considered the most reliable landmine “signature” chemical (Jenkins et al., 2001).
Microbial Bioreporters
Bioreporters are microbial strains genetically engineered to produce a dose-dependent quantifiable signal in response to the presence of pre-determined specific chemicals, groups of chemicals or stress factors. Numerous bioreporters have been described over the last two decades, mostly in the context of environmental monitoring, targeting either specific compounds such as heavy metals (Holmes et al., 1994; Corbisier et al., 1996; Belkin et al., 1997; Magrisso et al., 2008) or hydrocarbons (Applegate et al., 1998; Tecon et al., 2009), or global biological effects such as toxicity or genotoxicity (Vollmer et al., 1997; Biran et al., 2010). General and specific aspects of bacterial bioreporters’ construction and characterization have been described in numerous review articles over the last few years (Van der Meer and Jaspers, 2004; Marqués et al., 2006; Yagi, 2007; Van der Meer and Belkin, 2010; Choffnes et al., 2011; Eltzov and Marks, 2011; Roda et al., 2011; Su et al., 2011; Michelini et al., 2013; Cevenini et al., 2015) and will thus not be discussed in the present communication, which focuses only on microbial bioreporters tailored to sense and report upon the presence of trace explosives.
Whole-Cell Bioreporters for the Detection of Explosives
Bacteria
A list of microbial sensor strains previously reported to detect explosive-related chemicals is presented in Table 1.
Table 1.
Reported attempts to construct microbial bioreporters for explosives’ detection.
| Organism | Reporting element | Target analytes(A) | Reference |
|---|---|---|---|
| Unspecified bacterium | GFP | TNT | Burlage et al., 1999 |
| Vibrio fischeri(B) | Intrinsic lux operon | TNT, 4A-DNT, 2A-DNT | Frische, 2002 |
| Escherichia coli | GFP | TNT, L-lactate, serotonin | Looger et al., 2003(C) |
| Dictyosphaerium chlorelloides | Intrinsic chlorophyll A fluorescence | TNT | Altamirano et al., 2004 |
| Saccharomyces cerevisiae | GFP | DNT | Radhika et al., 2007 |
| Pseudomonas putida | luxAB, GFP | DNT | Garmendia et al., 2008 |
| Escherichia coli | Flagellar motion | nitrite, nitrate | Kim et al., 2008 |
| Escherichia coli | GFP | DNT | Lönneborg et al., 2012 |
| Escherichia coli | GFP | DNT | Davidson et al., 2012 |
| Escherichia coli | GFPmut2, luxCDABE | DNT, TNT | Yagur-Kroll et al., 2014 |
| Escherichia coli | GFP | DNT, TNT, DNB | Tan et al., 2015 |
(A) TNT: 2,4,6-trinitrotoluene; DNT: 2,4-dinitrotoluene; 4A-DNT: 4-amino-2,6-dinitrotoluene; 2A-DNT: 2-amino-4,6-dinitrotoluene; DNB: dinitrobenzene; (B) Now Aliivibrio fischeri; (C) Results were contested by Reimer et al. (2014).
Burlage et al. (1999) were the first to suggest the use of recombinant bacteria as bioreporters for this purpose. The proposed scheme was simple: bacteria, genetically engineered to fluoresce upon exposure to TNT and 2,4-DNT (Figure 1), are sprayed on the area targeted for landmine clearance. The bacteria are then allowed to rest for two hours, in the course of which cells in the proximity of buried explosives will be exposed to TNT vapors, resulting in the activation of the reporter gene. By scanning the area with a UV source, the locations of buried explosives are revealed. A mild irradiation of the area with electromagnetic energy in order to increase vapor concentration in the vicinity of buried explosives was also suggested.
FIGURE 1.
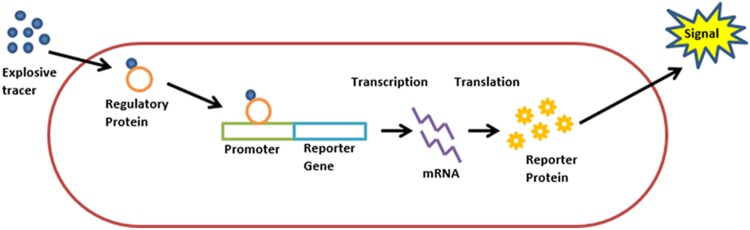
Schematic description of a “lights on” bioreporter design. A target analyte molecule enters the cell. The analyte, or its metabolite, is identified by a regulatory protein, which then activates the promoter attached to the reporter gene. Transcription is initiated, resulting in the synthesis of a reporter protein and the production of a measurable signal.
Burlage did not describe the promoter used for inducing the reporter gene, nor did he specify the host organism used (both Pseudomonas putida and Bacillus subtilis are mentioned). Although partially successful preliminary field tests were reported (MacDonald et al., 2003), we are not aware of any reports describing further developments of this system.
One of the difficulties reported in the concept described above is that direct dispersion of the bacteria on dry soils resulted in the immediate absorbance of the bacteria to the soil, leading to rapid signal loss (Burlage, 2003). One manner by which this could be at least partially circumvented is by encapsulating the bacteria in a water and nutrient-retaining polymeric matrix (Bjerketorp et al., 2006). Several polymers have been reported over the years to be suitable for such purposes including alginate (Zohar-Perez et al., 2002), agar–agar (Kar et al., 2009), or gelatin (de las Heras and de Lorenzo, 2011). For mechanical dispersion of such immobilized bacteria over large areas, it is likely that encapsulation will need to be in a micro-bead format.
Yagur-Kroll et al. (2014) described an Escherichia coli bioreporter for the detection of TNT, DNT, and DNB (Figure 2A-C). A library containing approximately 2,000 E. coli clones, each bearing a plasmid with the GFPmut2 gene fused to a different gene promoter, was screened for response to 2,4-DNT. Two gene promoters that exhibited the strongest response, yqjF (encoding a predicted quinol oxidase subunit) and ybiJ (encoding a protein of unknown function), were cloned into a low copy plasmid expressing the P. luminescens luxCDABE genes. These strains displayed a distinct dose-dependent response to 2,4-DNT, TNT, and 1,3-DNB. Interestingly, the reporter strain harboring the yqjF gene promoter as the sensing element was not induced directly by 2,4-DNT or TNT, but rather by metabolites of these compounds.
FIGURE 2.
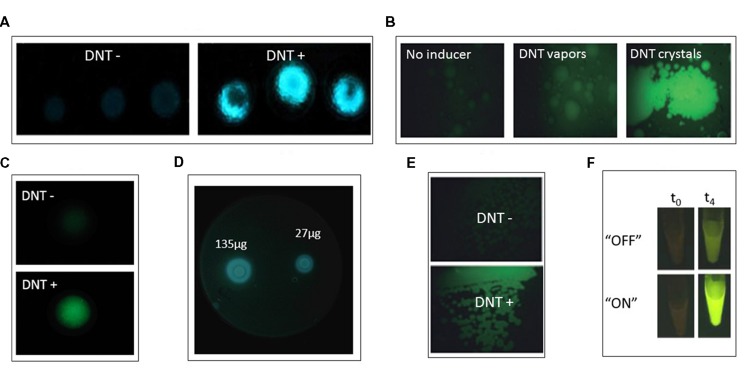
(A,B) yqjF-based bioreporter response when immobilized in agar and exposed to DNT buried in soil; (A) bioluminescent lux-based reporter; (B) fluorescent gfp-based reportet (from Yagur-Kroll et al., 2014, by permission; copyright (2013) Springer-Verlag Berlin Heidelberg). (C) yqjF-based bioluminescent bioreporter response when spread on LB agar plates and exposed to disks soaked with varying amounts of DNT (Yagur-Kroll et al., unpublished). (D) xylR5-based bioreporter response to DNT vapors and DNT crystals (from Garmendia et al., 2008, by permission). (E) xylR5-based bioreporter fluorescent response when spread on LB agar plates supplemented with DNT (from Garmendia et al., 2008, by permission; copyright (2008) Society for Applied Microbiology and Blackwell Publishing Ltd.). (F) Riboswitch-based bioreporer response to DNT when riboswitch is in “ON” or “OFF” mode at time zero and after 4 h of exposure. Reprinted (adapted) with permission from Davidson et al. (2012). Copyright (2012) American Chemical Society.
In an attempt to improve the capabilities of the constructed reporter in terms of detection threshold, signal intensity and time of detection, Yagur-Kroll et al. (2015) employed a “directed evolution” approach, involving four rounds of random mutagenesis of the yqjF promoter region by error prone PCR. The process yielded a variant that exhibited an over 3000-fold increase in luminescent signal intensity in the presence of 2,4-DNT, a 50-fold increase in the response ratio, a 75% reduction in the detection threshold and a response time that was cut down to half. An analysis of the point mutations accumulated in the course of this process indicated that the major contributors to these effects were manipulations of the -35 element of the yqjF gene promoter.
Tan et al. (2015) applied a similar approach to construct a bioreporter for the detection of nitroaromatic explosives, but instead of using a single gene promoter as the sensing element, five promoters found to respond to TNT, DNT, and DNB were fused to GFP. With this design, a detection threshold of 20.9 μM for TNT was obtained.
A non-specific use of a bacterial reporter was described by Frische (2002), who assessed the suitability of the luminescent bacterium Aliivibrio fischeri (formerly Vibrio fischeri) as a tool to detect the toxicity of TNT and its metabolites in soil samples. By combining chemical analysis that determined the concentrations of TNT and its degradation products, and the A. fischeri “lights off” assay for assessing the toxic effects, Frische (2002) was able to determine whether TNT is the main toxicant in a soil sample.
A principle common to most reports describing the design and construction of bacterial bioreporters is that the selected sensing element is nearly always based on a gene promoter activated in the presence of the target compounds. When such a gene has not been identified, an alternative approach may be to genetically manipulate a gene encoding a protein that binds similar molecules, thus modifying its binding site to recognize a new target. This has been the strategy employed by Galvão and De Lorenzo (2006), who made use of the P. putida XylR protein, which contains a domain that interacts directly with toluene and controls the activity of the σ54-dependent Pu promoter of the TOL plasmid for biodegradation of toluene and xylene (Figure 2D-E). By changing this domain through shuffling of its DNA sequence with a similar domain of the homologous protein DmpR, a XylR mutant that selectively binds 2,4-DNT was found (Garmendia et al., 2001). This mutant was expressed in a plasmid and inserted into P. putida strain Pu:GFP, in which GFP is expressed under the control of the Pu promoter. This bioreporter was induced by 2,4-DNT in a model soil setup (Figure 2D). The fact that P. putida is a soil bacterium renders it favorable as a bioreporter host for landmines detection (Garmendia et al., 2008).
A different approach for modifying regulatory protein specificity so that it responds to compounds it initially had little or no affinity to was attempted by Looger et al. (2003), who used a computational method to redesign ligand-binding-site specificity in proteins. The algorithm used high resolution three-dimensional structures to identify amino acid sequences predicted to form a complimentary surface between the protein and the target ligand. This algorithm was employed to engineer a new binding site for TNT that replaced the wild-type binding site for a ribose binding protein, a member of the E. coli periplasmic binding protein superfamily. It was claimed that the redesigned protein acquired an affinity to TNT, and that the receptors could distinguish the absence of a single nitro or methyl group. When integrated into a synthetic two-component signal transduction pathway in E. coli, the system was reported to have been activated by TNT. This report, however, was contested by Reimer et al. (2014) who demonstrated that there was no binding of TNT to the purified protein, nor was there an induction of the signal transduction pathway.
Two existing transcriptional factors in bacteria that were found to harbor the ability to bind 2,4-DNT were DntR from Burkholderia sp. (Suen and Spain, 1993) and NtdR in Acidovorax sp. strain JS42 (Lessner et al., 2003). Both are LysR-type transcription regulators and their amino acid sequence is 97% identical. NtdR, which activates the expression of genes involved in 2-nitrotoluene degradation, responds to 2,4-DNT and several other nitroaromatic compounds (Ju et al., 2009). Since NtdR has a broader range of ligands than DntR, it was selected as a starting point for a directed evolution process, initially by mutagenic PCR and then by recombination of the mutated sequences using staggered PCR. A mutant was obtained which displayed a 2,4-DNT detection limit of 10 μM, a 25-fold improvement compared with the WT strain. A combination of two mutations affecting the ligand-protein conformation was instrumental in the improvement of the binding (Lönneborg et al., 2012).
Employing a different approach, Kim et al. (2008) proposed to make use of the E. coli flagellar motor sensitivity to the presence of nitrate and nitrite; A glass-tethered E. coli strain KAF95, which carries a cheY gene deletion and is thus capable only of counterclockwise flagellar rotation, almost instantaneously stops its rotation in the presence of nitrate and nitrite. Flagellar motion was monitored by a microscope equipped with a CCD camera, and detection limits were reported to be 2.5 mM and 12 mM for nitrate and nitrite, respectively. However, responses to nitroaromatics were not demonstrated, and the obvious problem of false positives due to natural nitrates was not addressed.
Detection of DNT by a bioreporter that was designed outside the promoter-reporter fusion concept was reported by Davidson et al. (2012), based on riboswitch engineering (Figures 2D-E). A riboswitch is an element found in the 5′ untranslated region of some RNAs that has the ability to bind specific target molecules. The binding changes the secondary structure of the RNA, thus leading to a change in gene and protein expression. The riboswitch is composed of two components: a binding component (an aptamer) and an expression platform. A TNT binding aptamer (Ehrentreich-Förster et al., 2008), coupled with a PCR-generated expression platform placed upstream of the gene encoding tobacco etch virus protease, comprised the sensing component of the biosensor. Upon binding of the target DNT to the aptamer the expressed protease cleaved the binding between a GFP molecule and a yellow fluorescence protein that – when bound – quenched its fluorescence. The ensuing green fluorescence, when the system was expressed in E. coli, allowed detection of 0.5 mM DNT.
Yeast
Although the study of yeast strains as bioreporters for environmental contaminants is quite extensive (Belkin, 2003; Sanseverino et al., 2005; Xu et al., 2013) and degradation mechanisms of certain explosives by yeast strains have been characterized (Zaripov et al., 2002), reports of yeasts as explosives bioreporters are very limited. One notable attempt was reported by Radhika et al. (2007), who inserted the primary components of the rat olfactory system to the yeast Saccharomyces cerevisiae.
The olfactory receptors (ORs) in mammalian organisms are activated by very specific odorants, resulting in the stimulation of the G protein Golf (Jones and Reed, 1989). This eventually leads to the synthesis of cyclic AMP (cAMP) which stimulates a Ca2+ channel and increases Na+ and Ca2+ influx, thus creating an action potential which eventually reaches the central nervous system and is translated to an odor sensation. Screening of various ORs and their coupled G-proteins (GPCRs) for response toward 2,4-DNT resulted in the identification of the Olfr226 OR.
In Radhika’s olfactory yeast strain WIF-1α, GFP expression is coupled to cAMP synthesis. Upon exposure to 2,4-DNT the Olfr225 OR and GPCR are stimulated, cAMP is synthesized, GFP is expressed and a dose-dependent fluorescent signal is produced. This bioreporter responded to 25 μM of 2,4-DNT; no detection limit was reported by the authors.
Microalgae
Measuring chlorophyll a fluorescence can provide an indication of photosynthetic activity, which is directly linked to the organism’s well-being. Thus, when a photosynthetic cell is stressed, inhibition in chlorophyll a fluorescence may be observed (Schreiber et al., 1995). This response, however, is very unspecific; Sanders et al. (2001), for example, immobilized Chlorella vulgaris and showed that by measuring the relative fluorescence of the cells it is possible to detect airborne chemical warfare agents. To make use of this phenomenon in a more specific manner, Altamirano et al. (2004) compared the inhibition of chlorophyll a fluorescence in the WT and a TNT-resistant strain of the green microalga Dictyosphaerium chlorelloides. The reportedly TNT-specific difference in fluorescence between the two strains allowed the detection of TNT concentrations as low as 0.5 mg/L.
Enhancing Bioreporters Performance
A common denominator to all reports of cell-based sensing of explosives is the fact that the detection thresholds displayed are not sufficiently low to detect the very low concentrations expected to exist above buried landmines and other explosives-containing military hardware. Equilibrium headspace concentrations of DNT and TNT vapors above TNT based landmines can be as low as 0.28 pg/mL and 0.077 pg/mL, respectively (Jenkins et al., 2001).
If the detection is based on a promoter element induced by an explosive metabolite rather than the explosive itself, as in the case described by Yagur-Kroll et al. (2014), understanding the degradation process can be critical for enhancing the bioreporter’s performance. Knockout mutations in selected downstream genes or overexpression of selected upstream genes, for example, may be used to increase accumulation or production, respectively, of the inducing metabolite.
Another possible approach is the use of mixed cultures; Páca et al. (2008) showed that a mixed microbial culture could degrade 2,4-DNT 15–20 fold faster than single strain cultures of the same consortia. Applying such consortia in which one strain is used as the reporting element, while others degrade the parent material, could result in higher concentrations of the inducing metabolite released to the environment, eventually permeating to the bioreporter cell and increasing the response.
Among the practical problems that may be involved in the use of existing bioreporters are the shelf life prior to field application and the expected difficulties in coping with environment factors such as extreme temperatures and water availability once in the field. One attractive manner by which these difficulties may be addressed is the use of alternative resistant hosts, including spore-forming ones, such as those reviewed by Knecht et al. (2011). Another very important issue that has not been sufficiently addressed in many of the publications reviewed herein is the reporters’ specificity. For a viable field application it will be essential that the bioreporters used will respond only to a very limited range of target chemicals and their degradation products, as has been demonstrated by Yagur-Kroll et al. (2014). This specificity will be mostly dictated by the molecular elements selected as the sensing entities in the reporter construction, the manipulation of which should ensure minimal occurrence of false positives without compromising detection sensitivity.
Conclusion and Outlook
The last two decades have witnessed significant progress in design and construction of bioreporters for the detection and monitoring of diverse environmental contaminants. As highlighted in the present review, this was not accompanied by parallel advances in the development cell-based sensors for the detection of explosives, a field which has received only a limited attention. While each of the bacterial reporters described to date may be a promising candidate for a future scheme for the detection of buried explosives, significant progress has yet to be made before such a scheme may be deemed practical.
The use of bioreporters for the detection of explosives, or other pollutants with environmental significance, is not without limitations. First and foremost, significant enhancement of currently reported detection sensitivity needs to be obtained. At the moment, detection thresholds appear to be inferior to analytical detection methods such as GC/MS or LC/MS, able to detect concentrations in the nM range, while most bioreporters do not perform well below 0.1 μM (Van der Meer and Jaspers, 2004). Some of the potential research avenues by which this objective may be achieved have been outlined above; other directions to be pursued include the use of additional microbial hosts, as well as additional basic studies of the molecular mechanisms and biochemical pathways by which microbes metabolize explosives’ molecules or respond to their presence.
Another point that should be considered is the nature of the steps that need to be taken to ensure that the released bacteria survive in the field for periods that are sufficiently long to allow them to respond to their inducers and generate a readable signal, but also prevent their subsequent proliferation. The latter issue is essential in view of the genetically engineered nature of the bioreporters, even if by themselves they do not constitute any environmental or human safety risk. Relevant regulations will need to be adhered to, and public opinion issues considered. Possible precaution steps that may be taken include an engineered auxotrophy to nutrients unavailable in the environment, or the introduction of a “suicide circuit” that will not allow environmental survival (García and Díaz, 2014).
It should also be remembered that in many cases bacterial bioreporters may display detection thresholds that are inferior compared to chemical analysis. Their main advantage may lay in their ability, once released in the field, to act as independent agents and generate a dose-dependent signal that may be monitored from a distance. Thus, in parallel to the continuous development of better microbial sensors, attention should be devoted to the engineering of the hardware required for the remote detection of their optical signals and for pinpointing their activity hotspots.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to the Minerva Center for Bio-Hybrid Complex Systems for its support.
References
- Altamirano M., García-Villada L., Agrelo M., Sánchez-Martín L., Martín-Otero L., Flores-Moya A., et al. (2004). A novel approach to improve specificity of algal biosensors using wild-type and resistant mutants: an application to detect TNT. Biosens. Bioelectron. 19 1319–1323. 10.1016/j.bios.2003.4460.11.001 [DOI] [PubMed] [Google Scholar]
- Applegate B., Kehrmeyer S., Sayler G. (1998). A Chromosomally based tod-luxCDABE whole-cell reporter for benzene, toluene, ethybenzene, and xylene (BTEX) sensing. Appl. Environ. Microbiol. 64 2730–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkin S. (2003). Microbial whole-cell sensing systems of environmental pollutants. Curr. Opin. Microbiol. 6 206–212. 10.1016/S1369-5274(03)00059-6 [DOI] [PubMed] [Google Scholar]
- Belkin S., Smulski D. R., Dadon S., Vollmer A. C., Van Dyk T. K., Larossa R. A. (1997). A panel of stress-responsive luminous bacteria for the detection of selected classes of toxicants. Water Res. 31 3009–3016. 10.1016/S0043-1354(97)00169-3 [DOI] [Google Scholar]
- Bernstein A., Ronen Z., Adar E., Nativ R., Lowag H., Stichler W., et al. (2008). Compound-specific isotope analysis of RDX and stable isotope fractionation during aerobic and anaerobic biodegradation. Environ. Sci. Technol. 42 7772–7777. 10.1021/es8005942 [DOI] [PubMed] [Google Scholar]
- Berthe-Corti L., Jacobi H., Kleihauer S., Witte I. (1998). Cytotoxicity and mutagenicity of a 2,4,6-trinitrotoluene (TNT) and hexogen contaminated soil in S. typhimurium and mammalian cells. Chemosphere 37 209–218. 10.1016/S0045-6535(98)00039-3 [DOI] [PubMed] [Google Scholar]
- Biran A., Yagur-Kroll S., Pedahzur R., Buchinger S., Reifferscheid G., Ben-Yoav H., et al. (2010). Bacterial genotoxicity bioreporters. Microb. Biotechnol. 3 412–427. 10.1111/j.1751-7915.2009.00160.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerketorp J., Håkansson S., Belkin S., Jansson J. K. (2006). Advances in preservation methods: keeping biosensor microorganisms alive and active. Curr. Opin. Biotechnol. 17 43–49. 10.1016/j.copbio.2005.12.005 [DOI] [PubMed] [Google Scholar]
- Burlage R. S. (2003). “Biological systems (Paper I),” in Alternatives for Landmine Detection eds MacDonald J., Lockwood J. R., McFee J., Altshuler T., Broach T., Carin L., et al. (Pittsburg, PA: RAND; ) 265–272. [Google Scholar]
- Burlage R. S., Patek D. R., Everman K. R. (1999). Spraying recombinant bacteria on surface of ground, examining ground to detect visible signal formed after period of time. U.S. Patent No. 5972638 Washington, DC: U.S. Patent and Trademark Office. [Google Scholar]
- Cevenini L., Calabretta M., Calabria D., Roda A., Michelini E. (2015). “Luciferase genes as reporter reactions: how to use them in molecular biology?,” in Bioluminescence: Fundamentals and Applications in Biotechnology; Advances in Biochemical Engineering/Biotechnology eds Thouand E., Marks R. (Berlin: Springer; ). [DOI] [PubMed] [Google Scholar]
- Choffnes E. R., Relman D. A., Pray L. (2011). The Science and Applications of Synthetic and Systems Biology: Workshop Summary. Washington, D.C: National Academies Press. [PubMed] [Google Scholar]
- Corbisier P., Thiry E., Diels L. (1996). Bacterial biosensors for the toxicity assessment of solid wastes. Environ. Toxicol. Water Qual. 11 171–177. [Google Scholar]
- Davidson M. E., Harbaugh S. V., Chushak Y. G., Stone M. O., Kelley-Loughnane N. (2012). Development of a 2, 4-dinitrotoluene-responsive synthetic riboswitch in E. coli cells. ACS Chem. Biol. 8 234–241. 10.1021/cb300274g [DOI] [PubMed] [Google Scholar]
- de las Heras A., de Lorenzo V. (2011). In situ detection of aromatic compounds with biosensor Pseudomonas putida cells preserved and delivered to soil in water-soluble gelatin capsules. Anal. Bioanal. Chem. 400 1093–1104. 10.1007/s00216-010-4558-y [DOI] [PubMed] [Google Scholar]
- Ehrentreich-Förster E., Orgel D., Krause-Griep A., Cech B., Erdmann V. A., Bier F., et al. (2008). Biosensor-based on-site explosives detection using aptamers as recognition elements. Anal. Bioanal. Chem. 391 1793–1800. 10.1007/s00216-008-2150-5 [DOI] [PubMed] [Google Scholar]
- Eltzov E., Marks R. S. (2011). Whole-cell aquatic biosensors. Anal. Bioanal. Chem. 400 895–913. 10.1007/s00216-010-4084-y [DOI] [PubMed] [Google Scholar]
- Frische T. (2002). Screening for soil toxicity and mutagenicity using luminescent bacteria—a case study of the explosive 2, 4, 6-trinitrotoluene (TNT). Ecotoxicol. Environ. Saf. 51 133–144. 10.1006/eesa.2001.2124 [DOI] [PubMed] [Google Scholar]
- Funk S. B., Roberts D., Crawford D., Crawford R. (1993). Initial-phase optimization for bioremediation of munition compound-contaminated soils. Appl. Environ. Microbiol. 59 2171–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão T. C., De Lorenzo V. (2006). Transcriptional regulators a la carte: engineering new effector specificities in bacterial regulatory proteins. Curr. Opin. Biotechnol. 17 34–42. 10.1016/j.copbio.2005.12.002 [DOI] [PubMed] [Google Scholar]
- García J. L., Díaz E. (2014). Plasmids as tools for containment. Microbiol. Spectr. 2 10.1128/microbiolspec.PLAS-0011-2013 [DOI] [PubMed] [Google Scholar]
- Garmendia J., De Las Heras A., Galvão T. C., De Lorenzo V. (2008). Tracing explosives in soil with transcriptional regulators of Pseudomonas putida evolved for responding to nitrotoluenes. Microb. Biotechnol. 1 236–246. 10.1111/j.1751-7915.2008.00027.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmendia J., Devos D., Valencia A., De Lorenzo V. (2001). A la carte transcriptional regulators: unlocking responses of the prokaryotic enhancer-binding protein XylR to non-natural effectors. Mol. Microbiol. 42 47–59. 10.1046/j.1365-2958.2001.02633.x [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Dubey S. K., Gangolli S. (1994). Development of biosensors for the detection of mercury and copper ions. Environ. Geochem. Health 16 229–233. 10.1007/BF01747919 [DOI] [PubMed] [Google Scholar]
- IARC (1996). Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon: International Agency for Research on Cancer. Available at: http://monographs.iarc.fr/ENG/Monographs/vol65/mono65.pdf [Accessed March 12, 2014]. [Google Scholar]
- Jenkins T. F., Leggett D. C., Miyares P. H., Walsh M. E., Ranney T. A., Cragin J. H., et al. (2001). Chemical signatures of TNT-filled land mines. Talanta 54 501–513. 10.1016/S0039-9140(00)00547-6 [DOI] [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. (1989). Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science 244 790–795. 10.1126/science.2499043 [DOI] [PubMed] [Google Scholar]
- Ju K., Parales J. V., Parales R. E. (2009). Reconstructing the evolutionary history of nitrotoluene detection in the transcriptional regulator NtdR. Mol. Microbiol. 74 826–843. 10.1111/j.1365-2958.2009.06904.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar S., Swain M. R., Ray R. C. (2009). Statistical optimization of alpha-amylase production with immobilized cells of Streptomyces erumpens MTCC 7317 in Luffa cylindrica L. sponge discs. Appl. Biochem. Biotechnol. 152 177–188. 10.1007/s12010-008-8248-6 [DOI] [PubMed] [Google Scholar]
- Kim J.-W., Kim J.-H., Tung S. (2008). “Nanoscale flagellar-motor based MEMS biosensor for explosive detection,” in Proceedings of the Conference on Nano/Micro Engineered and Molecular Systems, NEMS 2008 (Sanya: IEEE; ) 630–632. [Google Scholar]
- Knecht L. D., Pasini P., Daunert S. (2011). Bacterial spores as platforms for bioanalytical and biomedical applications. Anal. Bioanal. Chem. 400 977–989. 10.1007/s00216-011-4835-4 [DOI] [PubMed] [Google Scholar]
- Lessner D. J., Parales R. E., Narayan S., Gibson D. T. (2003). Expression of the nitroarene dioxygenase genes in Comamonas sp. strain JS765 and Acidovorax sp. strain JS42 is induced by multiple aromatic compounds. J. Bacteriol. 185 3895–3904. 10.1128/JB.185.13.3895-3904.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönneborg R., Varga E., Brzezinski P. (2012). Directed evolution of the transcriptional regulator DntR: isolation of mutants with improved DNT-response. PLoS ONE 7:e29994 10.1371/journal.pone.0029994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looger L. L., Dwyer M. A., Smith J. J., Hellinga H. W. (2003). Computational design of receptor and sensor proteins with novel functions. Nature 423 185–190. 10.1038/nature01556 [DOI] [PubMed] [Google Scholar]
- MacDonald J., Lockwood J., McFee J., Altshuler T., Broach T. (2003). Alternatives for Landmine Detection. Santa Monica, CA: RAND Corporation. [Google Scholar]
- Magrisso S., Erel Y., Belkin S. (2008). Microbial reporters of metal bioavailability. Microb. Biotechnol. 1 320–330. 10.1111/j.1751-7915.2008.00022.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqués S., Aranda-Olmedo I., Ramos J. L. (2006). Controlling bacterial physiology for optimal expression of gene reporter constructs. Curr. Opin. Biotechnol. 17 50–56. 10.1016/j.copbio.2005.12.001 [DOI] [PubMed] [Google Scholar]
- Michelini E., Cevenini L., Calabretta M. M., Spinozzi S., Camborata C., Roda A. (2013). Field-deployable whole-cell bioluminescent biosensors: so near and yet so far. Anal. Bioanal. Chem. 405 6155–6163. 10.1007/s00216-013-7043-6 [DOI] [PubMed] [Google Scholar]
- Páca J., Halecký M., Hudcová T., Bajpai R. (2008). Aerobic biodegradation of dinitrotoluenes in batch systems by pure and mixed cultures. Folia Microbiol. (Praha) 53 105–109. 10.1007/s12223-008-0015-0 [DOI] [PubMed] [Google Scholar]
- Radhika V., Proikas-Cezanne T., Jayaraman M., Onesime D., Ha J. H., Dhanasekaran D. N. (2007). Chemical sensing of DNT by engineered olfactory yeast strain. Nat. Chem. Biol. 3 325–330. 10.1038/nchembio882 [DOI] [PubMed] [Google Scholar]
- Reimer A., Yagur-Kroll S., Belkin S., Roy S., van der Meer J. R. (2014). Escherchia coli ribose binding protein based bioreporters revisited. Sci. Rep. 4:5626 10.1038/srep05626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roda A., Roda B., Cevenini L., Michelini E., Mezzanotte L., Reschiglian P., et al. (2011). Analytical strategies for improving the robustness and reproducibility of bioluminescent microbial bioreporters. Anal. Bioanal. Chem. 401 201–211. 10.1007/s00216-011-5091-3 [DOI] [PubMed] [Google Scholar]
- Rosen G., Lotufo G. R. (2007). Toxicity of explosive compounds to the marine mussel, Mytilus galloprovincialis, in aqueous exposures. Ecotoxicol. Environ. Saf. 68 228–236. 10.1016/j.ecoenv.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Sanders C. A., Rodriguez M., Jr., Greenbaum E. (2001). Stand-off tissue-based biosensors for the detection of chemical warfare agents using photosynthetic fluorescence induction. Biosens. Bioelectron. 16 439–446. 10.1016/S0956-5663(01)00158-0 [DOI] [PubMed] [Google Scholar]
- Sanseverino J., Gupta R. K., Layton A. C., Patterson S. S., Ripp S. A., Saidak L., et al. (2005). Use of Saccharomyces cerevisiae BLYES expressing bacterial bioluminescence for rapid, sensitive detection of estrogenic compounds. Appl. Environ. Microbiol. 71 4455–4460. 10.1128/AEM.71.8.4455-4460.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer R., Achazi R. (1999). The toxicity of soil samples containing TNT and other ammunition derived compounds in the enchytraeid and collembola-biotest. Environ. Sci. Pollut. Res. 6 213–219. 10.1007/BF02987330 [DOI] [PubMed] [Google Scholar]
- Schmelling D. C., Gray K. A., Kamat P. V. (1997). The influence of solution matrix on the photocatalytic degradation of TNT in TiO2 slurries. Water Res. 31 1439–1447. 10.1016/S0043-1354(96)00358-2 [DOI] [Google Scholar]
- Schreiber U., Bilger W., Neubauer C. (1995). “Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis,” in Ecophysiology of Photosynthesis eds Schulze E.-D., Caldwell M. M. (Berlin: Springer; ) 49–70. [Google Scholar]
- Spain J. C., Hughes J. B., Knackmuss H.-J. (2000). Biodegradation of Nitroaromatic Compounds and Explosives. Boca Raton, FL: CRC Press. [Google Scholar]
- Su L., Jia W., Hou C., Lei Y. (2011). Microbial biosensors: a review. Biosens. Bioelectron. 26 1788–1799. 10.1016/j.bios.2010.09.005 [DOI] [PubMed] [Google Scholar]
- Suen W., Spain J. (1993). Cloning and characterization of Pseudomonas sp. strain DNT genes for 2, 4-dinitrotoluene degradation. J. Bacteriol. 175 1831–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J., Kan N., Wang W., Ling J., Qu G., Jin J., et al. (2015). Construction of 2, 4, 6-trinitrotoluene biosensors with novel sensing elements from Escherichia coli K-12 MG1655. Cell Biochem. Biophys. 10.1007/s12013-014-0481-8 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Tecon R., Beggah S., Czechowska K., Sentchilo V., Chronopoulou P.-M., McGenity T. J., et al. (2009). Development of a multistrain bacterial bioreporter platform for the monitoring of hydrocarbon contaminants in marine environments. Environ. Sci. Technol. 44 1049–1055. 10.1021/es902849w [DOI] [PubMed] [Google Scholar]
- Van der Meer J. R., Belkin S. (2010). Where microbiology meets microengineering: design and applications of reporter bacteria. Nat. Rev. Microbiol. 8 511–522. 10.1038/nrmicro2392 [DOI] [PubMed] [Google Scholar]
- Van der Meer J. R., Jaspers M. (2004). Illuminating the detection chain of bacterial bioreporters. Environ. Microbiol. 6 1005–1020. 10.1111/j.1462-2920.2004.00655.x [DOI] [PubMed] [Google Scholar]
- Vollmer A. C., Belkin S., Smulski D. R., Van Dyk T. K., LaRossa R. A. (1997). Detection of DNA damage by use of Escherichia coli carrying recA’:: lux, uvrA’:: lux, or alkA’:: lux reporter plasmids. Appl. Environ. Microbiol. 63 2566–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Close D. M., Sayler G. S., Ripp S. (2013). Genetically modified whole-cell bioreporters for environmental assessment. Ecol. Indic. 28 125–141. 10.1016/j.ecolind.2012.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi K. (2007). Applications of whole-cell bacterial sensors in biotechnology and environmental science. Appl. Microbiol. Biotechnol. 73 1251–1258. 10.1007/s00253-006-0718-6 [DOI] [PubMed] [Google Scholar]
- Yagur-Kroll S., Amiel E., Rosen E., Belkin S. (2015). Detection of 2,4-dinitrotoluene and 2,4,6-trinitrotoluene by an Escherichia coli bioreporter: performance enhancement by directed evolution. Appl. Microbiol. Biotechnol. 99 7177–7188. 10.1007/s00253-015-6607-0 [DOI] [PubMed] [Google Scholar]
- Yagur-Kroll S., Lalush C., Rosen R., Bachar N., Moskovitz Y., Belkin S. (2014). Escherichia coli bioreporters for the detection of 2, 4-dinitrotoluene and 2,4,6-trinitrotoluene. Appl. Microbiol. Biotechnol. 98 885–895. 10.1007/s00253-013-4888-8 [DOI] [PubMed] [Google Scholar]
- Zaripov S., Naumov A., Abdrakhmanova J., Garusov A., Naumova R. (2002). Models of 2,4,6-trinitrotoluene (TNT) initial conversion by yeasts. FEMS Microbiol. Lett. 217 213–217. 10.1111/j.1574-6968.2002.tb11477.x [DOI] [PubMed] [Google Scholar]
- Zohar-Perez C., Ritte E., Chernin L., Chet I., Nussinovitch A. (2002). Preservation of chitinolytic Pantoae agglomerans in a viable form by cellular dried alginate-based carriers. Biotechnol. Prog. 18 1133–1140. 10.1021/bp025532t [DOI] [PubMed] [Google Scholar]


