Abstract
Acanthopanax sessiliflorus, a small woody shrub has traditionally been referred to have anticancer activity, but it has not been scientifically explored so far. Therefore, to investigate the anticancer effects of A. sessiliflorus stem bark extracts (ASSBE), MDA-MB-231 and MCF-7 human breast cancer cells were treated with one of its bioactive fractions, n-hexane (ASSBE-nHF). Cytotoxicity (24 h) was determined by MTT assay and antiproliferative effect was assessed by counting cell numbers after 72 h treatment using hemocytometer. The role of ASSBE-nHF on apoptosis was analysed by annexin V-FITC/PI, Hoechst 33342 staining, DNA fragmentation pattern and immunoblotting of apoptosis markers. For the assay of enhanced production of ROS and mitochondrial membrane depolarization, specific stains such as DCFH-DA and JC-1 were used, respectively. To understand the mode of action of ASSBE-nHF on MCF-7 cells, cells were pre-treated with antioxidant, n-acetylcysteine. The hexane fraction of ASSBE showed maximum activity towards human breast cancer cells compared to other two fractions at a minimal concentration of 50 μg/ml. The annexin V-FITC/PI, Hoechst 33342 staining, DNA fragmentation and immunoblotting assays showed that ASSBE-nHF induces non-apoptotic cell death in MCF-7 and MDA-MB-231 cells. ASSBE-nHF significantly increased the production of ROS and decreased the mitochondrial membrane potential (MMP) in MCF-7 cells. Similarly, it decreased the MMP in MDA-MB-231 cells, but had no effect on ROS production. Further, the cytotoxic effect of ASSBE-nHF in MCF-7 cells was not significantly reversed even in the presence of n-acetylcysteine, an antioxidant. These findings revealed that ASSBE-nHF induces non-apoptotic cell death via mitochondria associated with both ROS dependent and independent pathways in human breast cancer cells.
Abbreviations: ASSBE, Acanthopanax sessiliflorus stem bark extracts; ASSBE-nHF, normal hexane fraction of Acanthopanax sessiliflorus stem bark extracts; ROS, reactive oxygen species; MMP, mitochondrial membrane potential; JC-1, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide; DCFH-DA, 2′,7′-dichlorodihydrofluorescein diacetate
Keywords: Traditional medicinal plants, Acanthopanax sessiliflorus, Breast cancer, Cytotoxicity
1. Introduction
For many decades, plants have not only been used as dietary supplements but also as traditional medicines for various diseases worldwide. This clearly shows the potential of plants as a rich source of bioactive compounds. Acanthopanax sessiliflorus, belonging to the Araliaceae family, is a smaller woody deciduous shrub widely distributed in Asian countries like Korea, China, and Japan, has been used as traditional medicines for various ailments, including cancer. Recently, the extracts and compounds from various parts of this plant have been reported to possess promising antimetastatic effect against breast cancer cells, immunomodulatory, anti-inflammatory, proapoptotic activity against colon cancer cells and antiplatelet aggregation properties (Lee et al., 2012a, Lee et al., 2012b, Lee et al., 2013, Kim et al., 2013). However, the anticancer effects of this plant extracts against human breast cancer cells have not yet been studied.
Breast cancer is a serious public health concern being the second most common of all cancers and by far the most frequent death reason of cancer amongst women throughout the world (Ferlay et al., 2015). Although chemotherapies have been found to effectively control the progression of breast cancer, the adverse effects and the development of resistance to anticancer drugs are a common clinical problem in the treatment of various cancers including breast cancer (Brouckaert et al., 2012). Therefore, identification of effective chemotherapy for breast cancer is highly desirable. According to the American Cancer Society, breast cancer is classified into two types based on the presence or absence of hormone receptors. MCF-7 cells express receptors for the ovarian hormones, oestrogen and progesterone, whereas MDA-MB-231 cells lack the expression of these hormone receptors. However, hormone receptor positive breast cancer cells can generally be targeted by receptor antagonists, the occasional loss of receptors due to genetic lesions in tumour cells needs an alternate treatment strategy. Hence, these two different cell types provide an ideal in vitro model system to identify the potential anticancer drugs. Since, breast cancer has become the most diagnosed cancer amongst Korean women and generally worldwide (Jung et al., 2014), the present study was aimed to investigate the cytotoxic potential of A. sessiliflorus stem bark extracts (ASSBE) against MCF-7 and MDA-MB-231 human breast cancer cell lines.
2. Materials and methods
2.1. Plant source
A. sessiliflorus stem bark (ASSB) was collected from Hongcheon-gun, a local city in the Republic of Korea. The plant was identified and authenticated in Kookmin University, College of Forest Science by the corresponding author Prof. Young-Kyoon Kim. A voucher specimen (YKAS-013) was deposited in the herbarium of the College of Forest Science, Kookmin University, Korea.
2.2. Extraction and fractionation
Fresh ASSB was washed with water, cut into small pieces, air dried at room temperature and powdered. About 1.5 kg of powdered material was subjected to extraction with analytical grade solvent, methanol (30 L) at 50 °C, three times for 5 h each. The crude extracts were filtered through filter paper and then concentrated under reduced pressure using rotary evaporator. Further, the crude extracts (107.01 g) were suspended in distilled water (1 L) and partitioned with n-hexane (1 L) and then the remaining was partitioned with butane (1 L). These two partitions and the remaining portion in distilled water were concentrated under reduced pressure (n-hexane, 23.6 g; butanol, 24.56 g; water, 52.82 g) and stored at −20 °C until further use.
2.3. Cell culture
The human breast cancer cell lines MDA-MB-231 and MCF-7 were obtained from the Korean Cell Line Bank, Korea. Cells were grown in DMEM supplemented with 10% FBS and antibiotics under 5% CO2 at 37 °C. Cells were seeded at the density of 2.5 × 104 to 1 × 105 per millilitre for the designed experimental assays.
2.4. Cytotoxicity assay
The cytotoxic effect of fractions of ASSBE against human breast cancer cells was determined according to the method of Mosmann, with slight modifications (Mosmann, 1983). Briefly, cells were seeded into 96-well plates at the density of 5 × 103 cells/well. After 24 h of culture, the medium was removed and replaced with a fresh medium containing DMSO (vehicle control) or various concentrations (25, 50, 100, 250 and 500 μg/ml) of n-hexane, butanol and aqueous fractions of ASSBE. Following 24 h incubation, the media was discarded, cells were washed with PBS once and 100 μl of MTT solution (0.5 mg/ml in serum and phenol red free DMEM) was added to each well, incubated for 4 h at 37 °C. Afterwards, the solution was discarded and 100 μl of DMSO was added to each well and absorbance was measured at 570 nm. Three independent experiments were carried out for each fraction. The results were expressed as the percentage of viable cells in comparison with the control cells.
2.5. Anti-proliferative assay
Cells were seeded into 35 mm dish at the density of 2.5 × 104 cells/ml and incubated overnight. Subsequently, the cells were treated with ASSB-nHF (25, 50 and 100 μg/ml) in complete growth medium for 72 h. Afterwards, cells were washed with PBS, harvested and cell numbers were counted by Trypan blue dye exclusion using hemocytometer.
2.6. Morphological assessment
Cells were seeded into 60 mm dish at the density of 1 × 105 cells/ml and allowed to adhere overnight. After 24 h treatment with ASSB-nHF (25, 50 and 100 μg/ml), the morphological characteristics were observed and the images were captured under an inverted phase-contrast microscope.
2.7. Determination of necrosis/apoptosis by annexin V/PI staining
Apoptosis/necrosis was analysed using annexin V-FITC and propidium iodide (PI) stain according to the manufacturer’s instructions. Following 24 h treatment with ASSB-nHF (25, 50 and 100 μg/ml), the floating cells were collected and the adherent cells were detached with accutase and centrifuged at 100g. The pellets were pooled, washed with PBS, and resuspended in 1× binding buffer (1 × 106 cells/ml). 100 μl of cell suspension was incubated with 5 μl of annexin V-FITC and 5 μl of PI for 15 min in the dark at room temperature. Then, 400 μl of 1× binding buffer was added and the samples were analysed immediately by a flow cytometer. For each sample, 10,000 events were collected and the results were analysed by using BD accuri C6 software.
2.8. Nuclear staining with Hoechst 33342
Following 12 h treatment with ASSB-nHF (25, 50 and 100 μg/ml), cells were fixed with 4% paraformaldehyde, stained with 5 μg/ml of Hoechst 33342 for 20 min at 37 °C. Then cells were washed with PBS, fluorescent nuclei were screened for normal, necrotic and apoptotic characteristics using the Ti eclipse fluorescence microscope.
2.9. Visualization of mitochondrial membrane potential (MMP)
Cells were treated with ASSB-nHF (25, 50 and 100 μg/ml) for 12 h, following this cells were incubated with 1 μg/ml of JC-1 in fresh medium for 30 min. Subsequently, cells were washed with PBS twice, MMP was visualized by detecting fluorescence and images were captured at the Ti eclipse fluorescence microscope.
2.10. Detection of intracellular reactive oxygen species
The intracellular ROS level was determined based on the conversion of non-fluorescent substance, DCFH-DA into a highly fluorescent product DCF, as described previously. Briefly, cells were seeded into 35 mm dish at a density of 1 × 105 cells/ml and incubated overnight. Following 12 h treatment with ASSB-nHF (25, 50 and 100 μg/ml), the cells were incubated with DCFH-DA (20 μM) for 30 min. After the removal of excessive DCFH-DA, the cells were washed with PBS and images were captured under fluorescence microscope.
2.11. DNA fragmentation assay
Cells were treated with ASSB-nHF for 24 h and DNA fragmentation analysis was carried out according to the method of Kasibhatla et al. (2006). Briefly, 5 × 105 cells were transferred to sterile eppendorf tubes and lysed with 20 μl TES (Tris, EDTA, SDS) lysis buffer by stirring with a wide-bore pipette tip. Lysates were mixed with RNase A and RNase T1 (10 μl), incubated for 60 min at 37 °C, followed by incubation with proteinase K (10 μl) for 90 min at 50 °C. Then 5 μl of 6× DNA loading buffer was added and electrophoresed on 1.5% agarose gel in TAE buffer at 50 V until loading dye has reached two-third of the way down the gel. DNA ladders were visualized by a UV light source after staining with ethidium bromide and documented by photography.
2.12. Automated western analysis
Cells were lysed in RIPA buffer (50 mM Tris–HCl pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS and supplemented with complete mini protease inhibitor (Roche Life Science) and clarified by centrifugation. Protein concentration was determined using BCA kit (Sigma Aldrich). Samples were adjusted to equal protein concentration with lysis buffer and western analysis was performed according to the ProteinSimple (San Jose, California, 95134 USA) user manual. In brief, 4 parts of cell lysates (equal amount of protein) were mixed with 1 part of master mix (ProteinSimple) containing fluorescent molecular weight markers and DTT (40 mM) and then heated at 95 °C for 5 min. The samples, biotinylated ladder and its respective secondary conjugate (ProteinSimple), blocking reagent, primary antibodies (Caspase 8 from Novusbio, NB100-56116, 1:500 dilution; Caspase 9 from CST, 9502, 1:100 dilution; Caspase 7 from CST, 9492, 1:50 dilution; Caspase 3 from CST, 9662, 1:50 dilution; GAPDH from Santa cruz, sc-25778, 1:300 dilution), HRP-conjugated secondary antibody (ProteinSimple), chemiluminescent substrate, and wash buffer were loaded into the prefilled microplates containing separation and stacking matrices. After plate loading, the separation electrophoresis and immunodetection steps took place in the capillary system and were fully automated (Chen et al., 2013).
2.13. Statistical analysis
Data are presented as the mean ± SD of six values in each group. Data were analysed using SPSS 16.0 student software (SPSS Inc, Chicago, IL). The hypothesis-testing method included one-way analysis of variance (ANOVA) followed by post hoc testing with a least significance difference test. P < 0.05 was considered significant.
3. Results and discussion
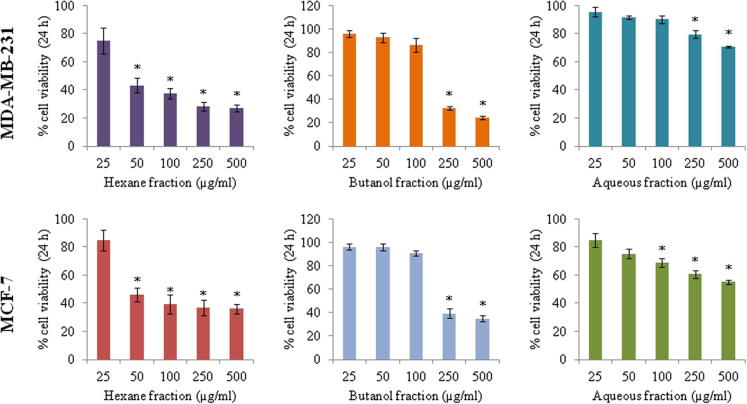
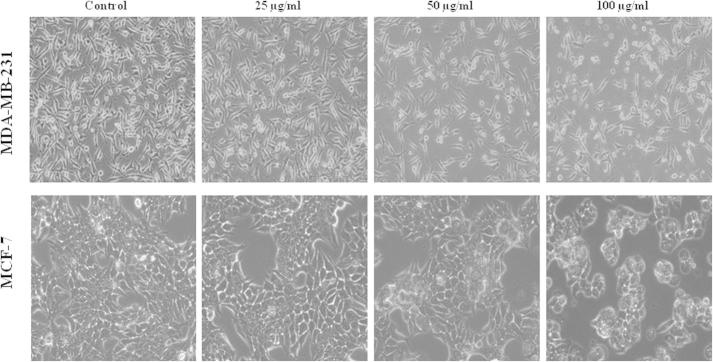
In search for new drug from traditional medicinal plants for breast cancer, we investigated the effects of three different fractions from methanolic extracts of A. sessiliflorus stem bark using human breast cancer cell lines, MCF-7 and MDA-MB-231. Treatment of cells with fractions at various concentrations inhibited cell viability in dose dependent manner. Compared to other two fractions, the hexane fraction (ASSBE-nHF) showed greater cytotoxic effect towards both the MCF-7 (54.69% cell death) and MDA-MB-231 (53.78% cell death) breast cancer cell lines at a minimum concentration of 50 μg/ml (Fig. 1). The IC50 values of the active fraction, ASSBE-nHF were 53.46 μg/ml for MDA-MB-231 cells and 58.40 μg/ml for MCF-7 cells. Further, ASSBE-nHF significantly suppressed the proliferation of MCF-7 as well as MDA-MB-231 cells (Fig. 2). In order to assess whether ASSBE-nHF induce any morphological changes, cells were treated for 24 h and then observed under a microscope. As shown in Fig. 3 ASSBE-nHF treatment changed the cellular morphology. To elucidate the ASSBE-nHF induced mode of action on breast cancer cells, further experimental assays were performed.
Figure 1.
Cytotoxic effects of different fractions of Acanthopanax sessiliflorus stem bark extracts (ASSBE) on human breast cancer cells. Cells were treated with n-hexane, butanol and aqueous fractions (25, 50, 100, 250 and 500 μg/ml) or with 0.5% DMSO as the vehicle control for 24 h. Then, the cell viability was determined by the colorimetric MTT assay. The results are expressed as percentage of the corresponding control. Results are represented as the mean ± SD from three independent experiments. ∗Significant compared to control cells.
Figure 2.
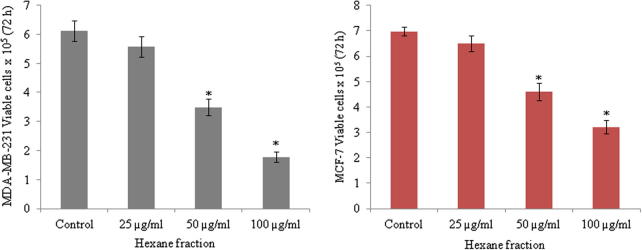
Anti-proliferative effect of Acanthopanax sessiliflorus stem bark extracts against human breast cancer cells. Cells were treated with ASSBE-nHF in complete growth medium for 72 h and then cells were harvested and cell numbers were counted by trypan blue dye exclusion method using a hemocytometer. Results are represented as the mean ± SD from three independent experiments. ∗Significant compared to control cells.
Figure 3.
Effect of Acanthopanax sessiliflorus stem bark extracts on the morphology of human breast cancer cells. The morphological characteristics were observed after 24 h treatment with ASSBE-nHF and the images were captured under an inverted phase-contrast microscope (200× magnification).
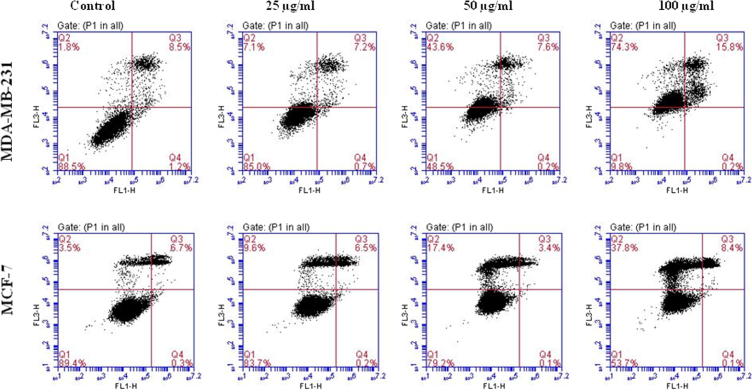
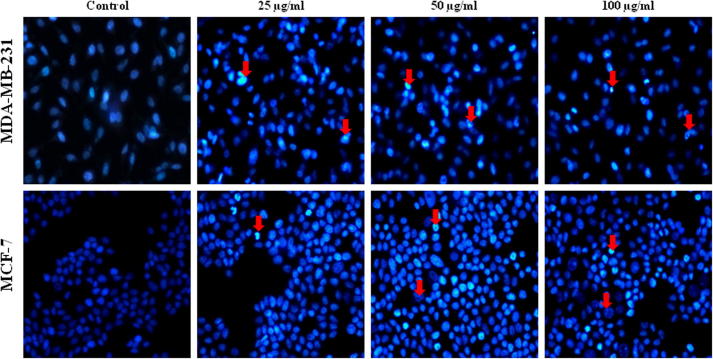
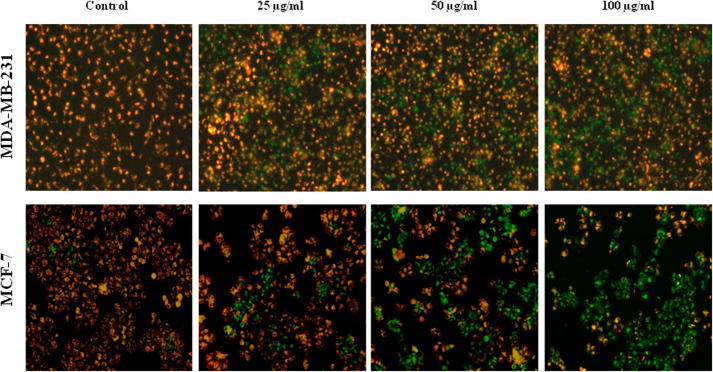
More often cell death occurs by two alternate modes, necrosis or apoptosis. Necrosis is characterized as passive, accidental form of cell death resulting from environmental perturbations with uncontrolled release of inflammatory cellular contents. Whereas, Apoptosis is a programmed, managed form of cell death, occurs without eliciting local inflammatory response. Apoptosis is an important physiological process for the maintenance of tissue homeostasis and plays a pivotal role in the pathogenesis of various disorders (Agostini et al., 2011). In some pathological conditions, the crisis is due to abnormal apoptosis, while in others, defective apoptosis is the cause. Cancer is one of the scenarios where, defective apoptosis is also a major causative factor, in addition to uncontrolled proliferation, resulting in tumour cells that will not die (Hipfner and Cohen, 2004). Therefore, the unrestrained growth of malignant cells and deregulation of apoptosis has become the major targets for anticancer strategies (Sun et al., 2004, Chan et al., 2012). Nevertheless, ASSBE-nHF induces cell death, to discriminate whether it induces apoptosis in breast cancer cells, the appearance of the early apoptosis biomarker phosphatidylserine at the cell surface was analysed by flow cytometry using annexin V-FITC and propidium iodide. It was observed that ASSBE-nHF induces non-apoptotic cell death in both MCF-7 and MDA-MB-231 breast cancer cells (Fig. 4). This was also confirmed by observing the nuclear characteristics using Hoechst 33342 fluorescence stain (Fig. 5), absence of apoptotic DNA fragments and cleaved fragments of caspases (Figure 6, Figure 7).
Figure 4.
Necrosis like effect of Acanthopanax sessiliflorus stem bark extracts on human breast cancer cells. Following 24 h treatment with ASSBE-nHF, both floating and adherent cells were harvested, stained with annexin V-FITC and propidium iodide and then the percentage of necrotic and apoptotic cells in each group was analysed by flow cytometry. The representative fluorescent histogram showing percentage distribution of viable (Q1), early apoptotic (Q4), late apoptotic (Q3) and dead (Q2) cells in control and ASSBE-nHF treated groups.
Figure 5.
Effect of Acanthopanax sessiliflorus stem bark extracts on the nuclear morphology of human breast cancer cells. Cells were treated with ASSBE-nHF for 12 h. To assess the nuclear morphology, cells were fixed with 4% formaldehyde and stained with Hoechst 33342, and then the images were captured using fluorescence microscopy (400× magnification). The arrows red point to the necrotic nucleus.
Figure 6.
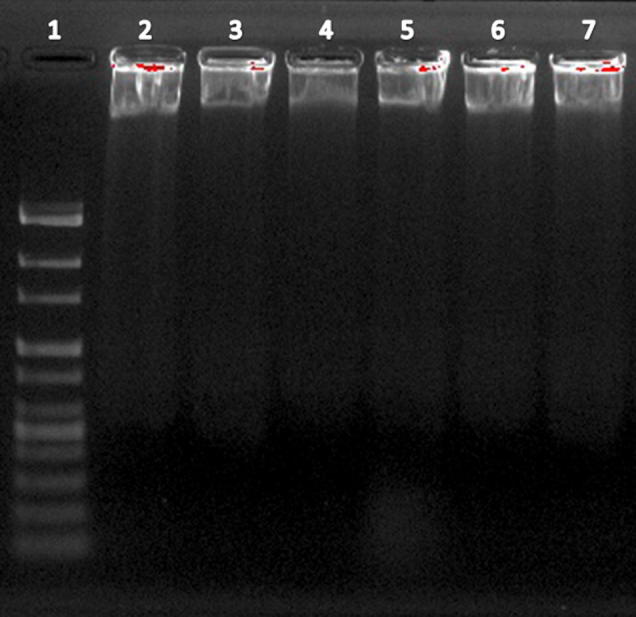
Effect of Acanthopanax sessiliflorus stem bark extracts on DNA fragmentation in human breast cancer cells. Lane 1: 100 bp Ladder, Lane 2–4 treated MCF-7 cells with ASSBE-nHF (0, 50, 100 μg/ml) for 24 h, Lane 5–7 treated MDA-MB-231 cells with ASSBE-nHF (0, 50, 100 μg/ml) for 24 h. ASSBE-nHF treatment didn’t induce apoptotic DNA fragmentation in both the breast cancer cells.
Figure 7.

Effect of Acanthopanax sessiliflorus stem bark extracts on the levels of apoptosis markers in human breast cancer cells. Representative western blotting data show the effect of ASSBE-nHF on the levels of caspases and GAPDH proteins in MCF-7 and MDA-MB-231 cells. The cells were treated with ASSBE-nHF (100 μg/ml) for 0, 12 and 24 h, and then immunoblotting for caspases was performed using specific antibodies (Novusbio and Cell Signalling Technology) in automated WES Proteinsimple instrument with GAPDH (Santa cruz, sc-25778) as a loading control.
In addition to its metabolic functions, mitochondria play a major role in the management of other cellular functions like cell survival and death (Galluzzi et al., 2012). Intriguingly, mitochondria have several components that function as cell death regulators. Following intrinsic or extrinsic excessive stress beyond the recovery state associated with mitochondrial membrane depolarization, cells die either via apoptosis or other modes of cell death like necrosis (Zong and Thompson, 2006, Parlato et al., 2014). Hence, to assess the involvement of mitochondria in ASSBE-nHF induced cell death, the mitochondrial membrane potential (MMP) was studied using a cationic fluorescent probe JC-1. It can selectively enter into the mitochondrion of cells, and reversibly change from red to green fluorescence as the mitochondrial membrane potential decreases. By measuring such a shift in the fluorescence emission by fluorescence microscopy, MMP was readily detected. As shown in Fig. 8 ASSBE-nHF dose dependently increased the ratio of green fluorescence in both cell lines which evidences the ASSBE-nHF induces cell death in human breast cancer cells with the involvement of mitochondrial membrane depolarization.
Figure 8.
Effect of Acanthopanax sessiliflorus stem bark extracts on mitochondrial membrane potential in human breast cancer cells. Cells were treated with ASSBE-nHF for 12 h and then, cells were stained with a fluorescent dye JC-1 for 30 min, washed with PBS, visualized under fluorescence microscopy and images were captured at 100× magnification.
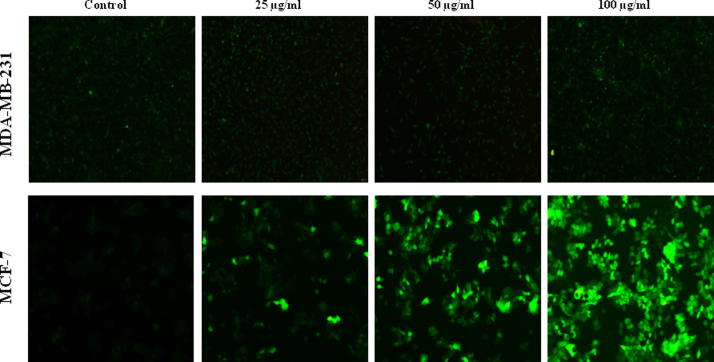
Reactive oxygen species (ROS) are known to play dual role either that may be beneficial or harmful depending on their accumulation levels. Apart from its importance in physiological functions, ROS generally contributes to cell death either by apoptosis or necrosis at the levels beyond the cellular antioxidant defence mechanisms (Fiers et al., 1999, Matés and Sánchez-Jiménez, 2000). So, we determined the intracellular ROS levels by measuring the intensity of a highly fluorescent derivative 2′,7′-dichlorofluorescein (DCF) which is produced from externally applied non-fluorescent substance, DCFH-DA by the cellular redox reactions. As shown in Fig. 9, ASSBE-nHF dose dependently increased the ratio of green fluorescence in MCF-7 cells suggesting that ASSBE-nHF stimulates production of intracellular ROS in MCF-7 cells. On the other hand it had no effect on ROS production in MDA-MB-231 breast cancer cells. The antioxidant, n-acetylcysteine (NAC) failed to reverse the cytotoxic effect of ASSBE-nHF in MCF-7 cells (Fig. 10). This indicates that ASSBE-nHF treatment led to ROS dependent and independent cell death in human breast cancer cells.
Figure 9.
Effect of Acanthopanax sessiliflorus stem bark extracts on the generation of ROS in breast cancer cells. After treatment with ASSBE-nHF for 12 h, cells were incubated with DCFH-DA for 30 min and after the removal of excessive DCFH-DA, the cells were washed twice with PBS and images were captured under fluorescence microscope (100× magnification).
Figure 10.
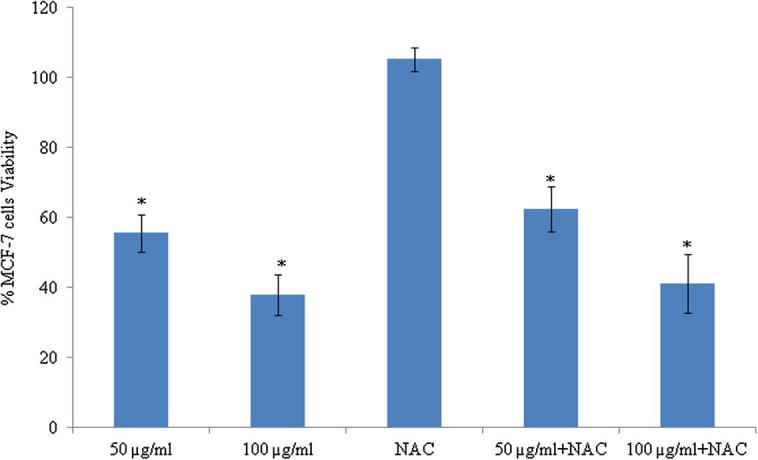
Effect of n-acetylcysteine, an antioxidant on the cytotoxicity of Acanthopanax sessiliflorus stem bark extracts in the human breast cancer cells. MCF-7 cells were pre-treated with or without n-acetylcysteine (2 mM) for 1 h and then n-hexane fraction (50 and 100 μg/ml) for 24 h. Afterwards, the cell viability was determined by the colorimetric MTT assay. The results are expressed as percentage of the corresponding control. Results are represented as the mean ± SD from three independent experiments. ∗Significant compared to control cells.
4. Conclusion
In the present study, we were provided evidence that the hexane fraction, a part of A. sessiliflorus stem bark methanolic extracts induces cell death like necrosis in MCF-7 and MDA-MB-231, human breast cancer cells. However, the result of immunoblotting assay showed that ASSBE-nHF significantly reduced the levels of caspases including the housekeeping protein GAPDH (Fig. 7). Since necrotic dying cells retain the process of protein synthesis, A. sessiliflorus stem bark extracts induced cell death in human breast cancer cells needs further investigation (Saelens et al., 2005).
Conflict of interest
The authors declare that no conflicts of interest exist.
Acknowledgments
The financial support of the present study was from Forest Science & Technology projects (Project No. S211314L010110), Forest Service, Republic of Korea and Kookmin University Research grant (2013).
Footnotes
Peer review under responsibility of King Saud University.
References
- Agostini M., Tucci P., Melino G. Cell death pathology: perspective for human diseases. Biochem. Biophys. Res. Commun. 2011;414:451–455. doi: 10.1016/j.bbrc.2011.09.081. [DOI] [PubMed] [Google Scholar]
- Brouckaert O., Wildiers H., Floris G., Neven P. Update on triple-negative breast cancer: prognosis and management strategies. Int. J. Women Health. 2012;4:511–520. doi: 10.2147/IJWH.S18541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.S., Koh C.G., Li H.Y. Mitosis-targeted anti-cancer therapies: where they stand. Cell Death Dis. 2012;3:e411. doi: 10.1038/cddis.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.Q., Heldman M.R., Herrmann M.A., Kedei N., Woo W., Blumberg P.M., Goldsmith P.K. Absolute quantitation of endogenous proteins with precision and accuracy using a capillary Western system. Anal. Biochem. 2013;442:97–103. doi: 10.1016/j.ab.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Fiers W., Beyaert R., Declercq W., Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–7730. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- Galluzzi L., Kepp O., Trojel-Hansen C., Kroemer G. Mitochondrial control of cellular life, stress, and death. Circ. Res. 2012;111:1198–1207. doi: 10.1161/CIRCRESAHA.112.268946. [DOI] [PubMed] [Google Scholar]
- Hipfner D.R., Cohen S.M. Connecting proliferation and apoptosis in development and disease. Nat. Rev. Mol. Cell Biol. 2004;5:805–815. doi: 10.1038/nrm1491. [DOI] [PubMed] [Google Scholar]
- Jung K.W., Won Y.J., Kong H.J., Oh C.M., Lee D.H., Lee J.S. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res. Treat. 2014;46:109–123. doi: 10.4143/crt.2014.46.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasibhatla S., Amarante-Mendes G.P., Finucane D., Brunner T., Bossy-Wetzel E., Green D.R. Analysis of DNA fragmentation using agarose gel electrophoresis. CSH Protoc. 2006;2006 doi: 10.1101/pdb.prot4429. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Shin E.H., Lee H.Y., Lee B.G., Park S.H., Moon D.I., Goo G.C., Kwon D.Y., Yang H.J., Kim O.J., Oh H.G. Immunostimulating effects of extract of Acanthopanax sessiliflorus. Exp. Anim. 2013;62:247–253. doi: 10.1538/expanim.62.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Lee D.Y., Yoo K.H., Baek N.I., Park J.H., Chung I.S. Calenduloside E 6′-methyl ester induces apoptosis in CT-26 mouse colon carcinoma cells and inhibits tumor growth in a CT-26 xenograft animal model. Oncol. Lett. 2012;4:22–28. doi: 10.3892/ol.2012.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.Y., Seo K.H., Jeong R.H., Lee S.M., Kim G.S., Noh H.J., Kim S.Y., Kim G.W., Kim J.Y., Baek N.I. Anti-inflammatory lignans from the fruits of Acanthopanax sessiliflorus. Molecules. 2012;18:41–49. doi: 10.3390/molecules18010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J., Hong S., Yoo S.H., Kim G.W. Cyanidin-3-O-sambubioside from Acanthopanax sessiliflorus fruit inhibits metastasis by downregulating MMP-9 in breast cancer cells MDA-MB-231. Planta Med. 2013;79:1636–1640. doi: 10.1055/s-0033-1350954. [DOI] [PubMed] [Google Scholar]
- Matés J.M., Sánchez-Jiménez F.M. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int. J. Biochem. Cell Biol. 2000;32:157–170. doi: 10.1016/s1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Parlato M., Souza-Fonseca-Guimaraes F., Philippart F., Misset B., Captain Study Group. Adib-Conquy M., Cavaillon J.M. CD24-triggered caspase-dependent apoptosis via mitochondrial membrane depolarization and reactive oxygen species production of human neutrophils is impaired in sepsis. J. Immunol. 2014;192:2449–2459. doi: 10.4049/jimmunol.1301055. [DOI] [PubMed] [Google Scholar]
- Saelens X., Festjens N., Parthoens E., Vanoverberghe I., Kalai M., van Kuppeveld F., Vandenabeele P. Protein synthesis persists during necrotic cell death. J. Cell. Biol. 2005;168:545–551. doi: 10.1083/jcb.200407162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.Y., Hail N., Jr., Lotan R. Apoptosis as a novel target for cancer chemoprevention. J. Natl. Cancer Inst. 2004;96:662–672. doi: 10.1093/jnci/djh123. [DOI] [PubMed] [Google Scholar]
- Zong W.X., Thompson C.B. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]