Abstract
In this study, the effect of purified quercetin-3-O-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranosid (QCGG) on melanogenesis was investigated. QCGG was isolated from the calyx of a traditional Korean medicinal herb, Persimmon (Diospyros kaki). The hypopigmentation effects of QCGG were determined by examination of cellular melanin contents, tyrosinase activity assay, cAMP assay, and Western blotting of α-MSH-stimulated B16F10 mouse melanoma cells. Our results showed that QCGG inhibited both melanin synthesis and tyrosinase activity in a concentration-dependent manner as well as significantly reduced the expression of melanogenic proteins such as microphthalmia-associated transcription factor (MITF), tyrosinase-related protein-1, tyrosinase-related protein-2, and tyrosinase. Moreover, QCGG inhibited intracellular cAMP levels, cAMP response element-binding protein (CREB), and p38 MAPK expression in α-MSH-stimulated B16F10 cells. Taken together, the suppressive effects of QCGG on melanogenesis may involve down-regulation of MITF and its downstream signaling pathway via phosphorylation of p38 MAPK and CREB along with reduced cAMP levels. These results indicate that QCGG reduced melanin synthesis by reducing expression of tyrosine and tyrosine-related proteins via extracellular signal-related protein kinase (ERK) activation, followed by down-regulation of CREB, p38, and MITF.
Keywords: Persimmon calyx, Melanogenesis, TRPs, p38 MAPK, CREB, cAMP
1. Introduction
Mammalian melanocytes synthesize melanins in melanosomes, which exist in the skin, eyes, and hair. There are four chromophores that determine the skin color (carotenoids, haemoglobin, oxyhaemoglobin, and melanins), of which melanins are the predominant component (Kameyama et al., 1995, Hearing, 2005). In humans, hyperpigmentation occurs after stimulation by ultraviolet radiation or solar light, confirming that the function of melanin is to protect the skin from ultraviolet radiation (Tsatmali et al., 2002). Melanogenesis, or melanin biosynthesis within melanosomes, is mainly regulated by enzymes such as tyrosinase-related protein-1 (TRP-1), tyrosinase-related protein-2 (TRP-2), and tyrosinase (Kameyama et al., 1995). Among them, tyrosinase plays two important rate-limiting steps during melanin biosynthesis (Hearing and Jimenez, 1987). The tyrosinase family genes TRP-1 and TRP-2 are transcriptionally regulated by microphatalmia-associated transcription factor (MITF), which suggests that MITF is an important transcription regulator during melanogenesis (Tachibana, 2001, Levy et al., 2006, Ye et al., 2010, Tsang et al., 2012). Previously, researchers have documented that mitogen-activated protein kinases (MAPKs) consist of a family of serine/threonine kinases, including p38 MAPK, extracellular response kinase (ERK), and c-Jun N-terminalkinase (JNK), which play important roles in melanogenesis (Seger and Krebs, 1995, Cohen, 1997, Jiang et al., 2009). Recent investigations have reported that p38 MAPK is particularly involved in MITF regulation (Saha et al., 2006). In addition, activation of the p38 MAPK signaling pathway increases the transcription of tyrosinase, which activates melanin synthesis (Corre and Galibert, 2005, Singh et al., 2005). The second messenger cAMP derived from ATP plays an important function in intracellular signal transduction (Park et al., 2006). Increased cAMP level affects protein kinase A (PKA), cAMP response element-binding protein (CREB), and cAMP response element (CRE) located in M promoter of MITF (Tachibana, 2000). cAMP-related biological effects depend on PKA, which has a direct influence on melanogenesis. PKA activation can cause MITF expression via phosphorylation of CREB, which increases melanin synthesis (Busc and Ballotti, 2000).
Tyrosinase inhibitors have gained attention due to the major role of tyrosinase in melanogenesis (Chang, 2009). Some tyrosinase inhibitors such as kojic acid and arbutin are involved in cosmetic products (Smit et al., 2009). Although kojic acid has no carcinogenic activity such as the commonly used whitening agent hydroquinone, dermatologists have reported that long and persistent use of kojic acid can produce severe dermal problems such as itchiness, red rash, and irritation. Herbal medicines are a largely unexplored material for developing new and safe ingredients that can antagonize tyrosinase activity (Kiken and Cohen, 2002, Pieroni et al., 2004).
Persimmon (Diospyros kaki), belonging to Ebenaceae, is a famous conventional Korean medicinal herb. Persimmon calyx has been used to prepare herbal drinks in Korea, China, and Japan due to its abundance of fructose, glucose, and triterpenoid. Persimmon calyx is also generally used as a traditional medicine in Korea to relieve asthma, chronic bronchitis, and cough symptoms (Bei et al., 2009, Sa et al., 2005, Sakanaka et al., 2005). Persimmon leaves have been reported to have various biological effects such as microbial inhibition, radical scavenging, neuroprotection, blood pressure reduction, and thrombosis inhibition (Bei et al., 2009, Sakanaka et al., 2005, An et al., 2005, Byun et al., 2010). Previously, we reported that flavonoid constituents from leaves of persimmon have collagen biosynthesis activity in human skin fibroblasts (Duthie and Dobson, 1999). Since most studies have focused on the leaves and fruits of persimmon, there are limited data available on its chemical components and biological potential. Therefore, we focused on the isolation and characterization of the active components of persimmon calyx. Quercetin (QC), which is found in fruits, vegetables, and grains, is one of the most common dietary flavonoids (Olszewska, 2008). Previous reports showed that QC induces apoptosis of cancer cells, inhibits protein-kinase C (Murota and Terao, 2003), and modulates redox status (Garcia-Mediavilla et al., 2007). Here, another derivative of QC, quercetin-3-O-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranoside (QCGG), was isolated from the calyx of persimmon for the first time. Therefore, we aimed to determine the effect of QCGG on melanin synthesis and its underlying signaling pathways in alpha-melanocyte-stimulating hormone (α-MSH)-stimulated B16F10 cells.
2. Materials and methods
2.1. Reagents
Sephadex LH-20 column of 25–10 μm was purchased from Pharmacia (Stockholm, Sweden), MCI-gel CHP 20P column of 75–150 μm was purchased from Mitsubishi (Tokyo, Japan), and YMC-gel ODS-A column of 230–70 and 500–400 mesh were purchased from YMC Co (PA, USA). Dimethylsulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), L-DOPA, alpha-melanocyte stimulating hormone (α-MSH), and phosphate-buffered saline (PBS) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were obtained from Invitrogen–Gibco (Grand Island, NY, USA). Antibodies against MITF, tyrosinase, TRP-1, TRP-2, phospho-p38 MAPK, p38 MAPK, phospho-ERK, ERK, phospho-JNK, JNK, phospho-CREB, and CREB purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-mouse and anti-goat IgG antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). The cAMP assay kit was purchased from Cayman Chemical (Ann Arbor, MI). All other reagents were purchased from Sigma–Aldrich Chemical Co. (St. Louis, MO, USA).
2.2. Plant materials
Persimmon calyx was collected from Dae-Bo Korea Traditional Parmacy in Yeongcheon, Gyeungbuk, Korea in October 2009. A voucher specimen (PC 2010-02) has been deposited at the herbarium of the Department of Cosmeceutical Science, Daegu Haany University.
2.3. Extraction and isolation
The air-dried calyx (4.0 kg) was extracted three times with 70% aqueous acetone at room temperature. After removing acetone under vacuum, the aqueous solution was filtered. The filtrate was then concentrated and applied to a Sephadex LH-20 column (2 kg, 10 × 70 cm) containing increasing proportions of EtOH, yielding five fractions. Repeated column chromatography of fraction 2 (1.9 g) on MCI-gel CHP 20P (75–150 nm, 5 × 60 cm) with a H2O-100% MeOH gradient produced Fr. 2–2 (0.98 g), which was subjected to final purification using YMC-ODS gel (12 nm, 600 g, 5 × 60 cm) with a 20–100% MeOH gradient to produce compound 1 (500 mg).
2.4. Thin layer chromatography (TLC) and nuclear magnetic resonance (NMR)
TLC was carried out on a pre-coated silica gel 60 F254 plate (Merck). The spots were detected under UV radiation and by spraying with FeCl3 and 10% H2SO4, followed by heating. The 1H- and 13C NMR spectra were recorded on a Varian Unity NMR at 500 MHz and 125 MHz (13C- NMR). The chemical shifts are shown in δ (ppm) relative to TMS. The structure of 1 was identified as quercetin-3-O-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranoside by comparison of its physicochemical and spectroscopic data (1H, and 13C NMR) with literature values.
2.5. Cell culture
B16F10 mouse melanoma cells (CRL 6323) were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco Life Technologies, France) supplemented with 10% fetal calf serum and 1% penicillin/streptomycin in a 5% CO2 humidified incubator at 37 °C.
2.6. Cell viability assay
The general viability of cultured cells was determined through reduction of MTT to formazan. After QCGG treatment, cells were incubated for 48 h at 37 °C in an atmosphere containing 5% CO2. MTT (1 mg/ml in phosphate-buffered saline; PBS) was then added to each well at 10% of the medium volume. Cells were incubated at 37 °C for 3 h, and dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals. The absorbance was measured at 570 nm using a Spectra MAX 190 spectrophotometer (Molecular Devices, Sunnyvale, CA, USA).
2.7. Measurement of cellular melanin contents
The melanin content was measured by slight modification of a previously described method (Tsuboi et al., 1998). Briefly, cells were washed with PBS and then dissolved in 1 N NaOH in 10% DMSO at 80 °C for 1 h. The relative melanin content was determined by measuring the absorbance at 475 nm in an enzyme-linked immunosorbent assay (ELISA) reader. The value of each measurement was expressed as percentage changes from the control.
2.8. Tyrosinase activity assay
Tyrosinase activity was estimated by measuring the rate of l-DOPA oxidation (Tomita et al., 1992). Briefly, cells were lysed by incubation at 37 °C for 30 min in RIPA buffer (0.1 M sodium phosphate, pH 7.0, 1% Triton X-100, 0.1 mM phenylmethanesulfonylfluoride (PMSF), 1 mM NaF). The lysates were then centrifuged at 10,000×g for 20 min to obtain the supernatant as crude tyrosinase extract for the activity assay. The reaction mixture contained 0.1 M sodium phosphate (pH 7.0), 0.05% l-DOPA, and the supernatant (tyrosinase source). After incubation at 37 °C for 1 h, dopachrome was monitored by measuring the absorbance at 405 nm in an ELISA reader. The value of each measurement was expressed as percentage changes from the control.
2.9. Western blotting
B16F10 cells were treated with QCGG, and cells were cultured and harvested using RIPA buffer containing 20 mM Tris–HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 2 mM EDTA, 1 mM PMSF, 10 mM NaF, 1 mM Na3VO4, and protease inhibitors (Sigma, USA). Protein concentrations were then determined using Bradford Assay (Bio-Rad, Richmond, CA, USA), after which 30 g of protein was separated by electrophoresis on a 10% SDS–polyacrylamide gel and then transferred to a nitrocellulose membrane (Whatman, Germany). The membrane was blocked with 5% skim milk and incubated with MITF, tyrosinase, TRP-1, TRP-2, phospho-p38 MAPK, p38 MAPK, phospho-ERK, ERK, phospho-JNK, JNK, phospho-CREB, CREB, and GAPDH antibodies (diluted 1:1000). All bands were visualized using horseradish peroxidase-conjugated secondary antibodies (1:1000, Santa Cruz Biotech, CA, USA) using an enhanced chemiluminescence system (Pierce Biotech, Rockford, IL, USA). Western blotting results reported here are representative of at least three experiments.
2.10. Measurement of cAMP levels
Cells were serum-starved overnight and then treated with QCGG (10–100 μg/ml). After treatment, cells were lysed in 0.1 M HCl. Cell debris was then removed by centrifugation (1000 g, 15 min), after which the supernatant was subjected to determination of cAMP levels using a commercially available cAMP EIA kit (Cayman Chemical, Ann Arbor, MI). cAMP levels were normalized to total protein content.
2.11. Statistical analysis
Data are presented as mean ± standard deviation. One-way ANOVA followed by Tukey’s post hoc test was performed using SPSS software (version19) to determine the statistical significance between the groups. The results were considered statistically significant at ∗P < 0.05 and ∗∗P < 0.01.
3. Results
3.1. Identification of quercetin-3-O-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranoside
Yellow amorphous powder; 1H-NMR (500 MHz, CD3OD) δ: 7.91 (1H, d, J = 1.8 Hz, H-2′), 7.62 (1H, dd, J = 8.4, 1.8 Hz, H-6′), 6.98 (1H, d, J = 8.4 Hz, H-5′), 6.53 (1H, d, J = 1.8 Hz, H-8), 6.29 (1H, d, J = 1.8 Hz, H-6), 5.22 (1H, d, J = 8.4 Hz, H-1″), 3.93 (1H, d, J = 7.2 Hz, H-1″′); 13C-NMR (CD3OD, 125 MHz) δ: 178.6 (C-4), 165.7 (C-7), 161.9 (C-5), 157.8 (C-9), 157.5 (C-2), 149.2 (C-4′), 145.0 (C-3′), 134.9 (C-3), 122.1 (C-6′), 121.9 (C-1′), 117.4 (C-2′), 115.5 (C-5′), 104.8 (C-10), 104.6 (C-1″), 103.9 (C-1″′), 99.5 (C-6), 94.5 (C-8), 77.4 (C-3″), 77.3 (C-5″′), 76.2 (C-3″′), 74.9 (C-5″), 74.2 (C-2″), 72.4 (C-2″′), 70.1 (C-4″), 68.7 (C-4″′), 61.7 (C-6″), 60.8 (C-6″′) (Figure 1).
Figure 1.
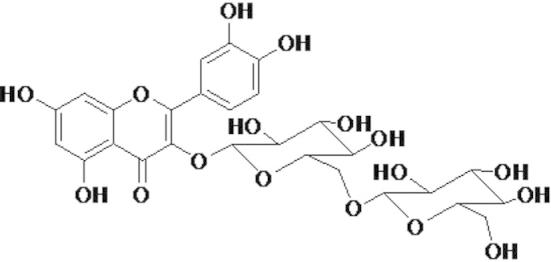
Chemical structure of quercetin-3-O-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranoside (QCGG).
3.2. Effect of QCGG on B16F10 cell viability
To assess the effect of QCGG on cell viability, B16F10 melanoma cells were treated with various concentrations of QCGG (10–100 μg/ml) for 48 h (Figure 2A). MTT assay is a colorimetric assay that measures enzyme activity related to reduction of MTT to formazan dyes, which are purple in color. Results are expressed as percent viability relative to control (0 μg/ml). According to our results, QCGG had no significant effect on cell mortality (101% at 10 μg/ml, 100% at 50 μg/ml, and 104% at 100 μg/ml).
Figure 2.
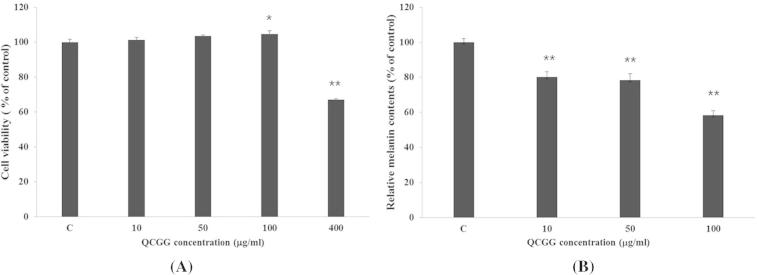
Effect of QCGG on (A) B16F0 cell viability. B16F0 melanoma cells were treated with various concentrations of QCGG for 48 h. Cell viability was determined using MTT assay. Each percentage value in treated cells was calculated with respect to that in control cells; (B) cellular melanin synthesis in B16F0 cells. Cells were exposed to α-MSH (100 nM) alone or plus with QCGG (0–100 μg/ml) for 48 h. Each percentage value for treated cells was reported relative to that of control cells. Values are the mean ± SD of duplicate determinations from three separate experiments. ∗P < 0.05, ∗∗P < 0.01 compared with the control.
3.3. Effect of QCGG on melanin content of B16F10 cells
To determine the effect of QCGG on melanogenesis, B16F10 melanoma cells were exposed to α-MSH (100 nM) alone or with QCGG (0–100 μg/ml) for 48 h. Treatment with QCGG at 10–100 μg/ml had a significant inhibitory effect on melanin synthesis in a dose-dependent manner in B16F10 melanoma cells pretreated with α-MSH. Melanin content was represented as a percentage of control (0 μg/ml of QCGG). After treatment, melanin content decreased gradually to 80.11% at 10 μg/ml, 78.46% at 50 μg/ml, and 58.41% at 100 μg/ml (Figure 2B). This result indicates that QCGG has a potent inhibitory effect on melanin formation in B16F10 melanoma cells.
3.4. Inhibitory effect of QCGG on tyrosinase activity in B16F10 cells
To more precisely examine the possible mechanism of the inhibitory effect of QCGG on melanogenesis, we assessed intracellular tyrosinase activity in B16F10 melanoma cells. Cells were exposed to α-MSH (100 nM) alone or with QCGG (0–100 μg/ml) for 48 h. QCGG at 10–100 μg/ml inhibited α-MSH-induced tyrosinase activity in a dose-dependent pattern (Figure 4). After treatments, remaining B16F16 intracellular tyrosinase activity was 94.91% at 10 μg/ml, 89.54% at 50 μg/ml, and 87.26% at 100 μg/ml. The results shown in Figure 3A were in accordance with those indicated in Figure 2B, which means that QCGG inhibited intracellular tyrosinase activity and then reduced melanin content in a dose-dependent manner in B16F10 melanoma cells.
Figure 4.
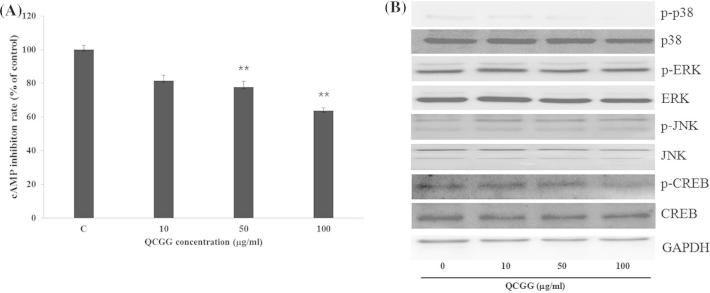
Effect of QCGG on (A) intracellular cAMP accumulation in B16F10 cells. Cells were exposed to α-MSH (100 nM) alone or with QCGG (0–100 μg/ml) for 48 h. Intracellular cAMP levels were measured using an enzyme immunoassay protocol; (B) expression levels of MAPKs, phosphorylated-MAPKs, CREB, and phosphorylated-CREB in B16 cells. Cells were exposed to α-MSH (100 nM) alone or with QCGG (0–100 μg/ml) for 48 h. Protein expression was determined by Western blotting. Equal protein loading was checked by GAPDH. Representatives of three independent experiments are shown. Results shown are means ± SEM and are representative of three independent experiments. ∗P < 0.05, ∗∗P < 0.01 compared with the control.
Figure 3.
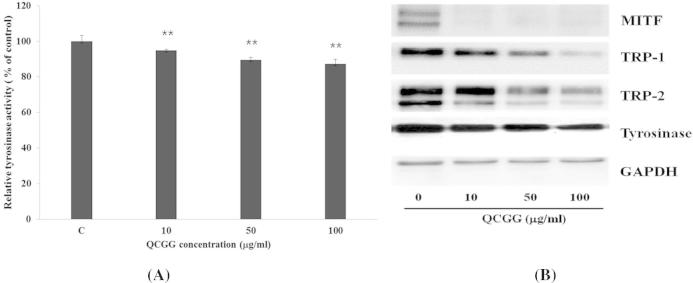
Effect of QCGG on (A) tyrosinase in B16F0 cells exposed to α-MSH (100 nM) alone or with QCGG (0–100 μg/ml) for 48 h. Each percentage value for treated cells was reported relative to that of control cells; (B) expression of melanogenesis-related proteins in B16F0 cells. B16F0 cells were exposed to α-MSH (100 nM) alone or with QCGG (0–100 μg/ml) for 48 h. Expression levels of MITF, TRP-1, TRP-2, and tyrosinase were measured by Western blotting using specific antibodies. Equal protein loading was checked by GAPDH. Representatives of three independent experiments are shown. Values are the mean ± SD of duplicate determinations from three separate experiments. ∗P < 0.05, ∗∗P < 0.01 compared with the control.
3.5. Effect of QCGG on cellular melanogenesis-related protein expression
Many proteins are involved in tyrosinase expression, including microphthalmia-associated transcription factor (MITF), tyrosinase-related protein-1 (TRP-1), and tyrosinase-related protein-2 (TRP-2). To test whether or not QCGG regulates expression of these proteins, B16F10 melanoma cells were exposed to α-MSH (100 nM) alone or with QCGG (0–100 μg/ml) for 48 h. MITF, tyrosinase, TRP-1, and TRP-2 levels were assayed by Western blotting (Figure 3B). QCGG treatment dramatically reduced MITF, TRP-1, TRP-2, and tyrosinase levels in α-MSH-stimulated B16F10 melanoma cells (Figure 3B).
3.6. QCGG suppresses melanin synthesis through cAMP signaling down-regulation and melanogenesis-related signaling pathways
In our results, QCGG induced down-regulation of MITF, tyrosinase, TRP-1, and TRP-2 expression in α-MSH-stimulated B16F10 cells. α-MSH, which is an adenyl cyclase activator, can increase intracellular cAMP levels and consequently activate tyrosinase regulation and melanin synthesis (Abdel-Malek et al., 1999, Bertolotto et al., 1998, Costin and Hearing, 2007). Hence, we investigated whether or not the inhibitory mechanism of QCGG is involved in cAMP-dependent signaling in α-MSH-stimulated B16F10 cells. B16F10 cells were exposed to α-MSH (100 nM) alone or with QCGG (0–100 μg/ml) for 48 h prior to measurement of intracellular cAMP levels. As shown in Figure 4A, QCGG treatment dose-dependently reduced cAMP levels in α-MSH-stimulated B16F10 cells. These results suggest that the inhibitory effect of QCGG during melanogenesis may be related to down-regulation of α-MSH-induced cAMP signaling. To further investigate the relationship between QCGG-induced melonogenic inhibitory activity and cAMP-related signaling pathway, we performed Western blotting to assess phosphorylation levels of p38 MAPK, ERK, JNK, and CREB. As shown in Figure 4B, phosphorylation of p38 MAPK and CREB were dose-dependently inhibited in α-MSH-stimulated B16F10 cells at 48 h. These results suggest that the inhibitory effect of QCGG may be related to inhibition by p38 MAPK and CREB phosphorylation.
4. Discussion
Melanin plays an important role in protecting human skin from unwanted UV radiation from sunlight as well as toxic effects of drugs and chemicals. The accumulation of unwanted melanin in a specific body parts (melasma and freckles) may become problems with age. In Asian countries, many women dislike hyperpigmentation such as melasma and freckles, as light skin tone is a traditional and cultural requisite for maintaining beauty. Many whitening agents have been reported and are used in cosmetics and medical applications to treat skin and pigment abnormalities. However, some of these agents such as hydroquinone and mercury have cytotoxicity or undesirable side effects on the skin. Hydroquinone is known to induce allergic reactions, redness of the skin, and skin lightening cream containing mercury is known to be associated with kidney damage, skin rashes, discoloration, as well as reduced skin resistance capabilities against bacterial and fungal infection. These adverse effects of commonly used skin lightening agents have forced researchers to search for safe, effective, and natural whitening agents (Ladizinski et al., 2011). In this study, we demonstrated the inhibitory mechanism of QCGG isolated from persimmon calyx in α-MSH-stimulated B16F10 melanoma cells.
In humans, variations in skin color occur due to skin melanin. Melanin content correlates directly with tyrosinase activity and tyrosinase-related protein levels (Maeda et al., 1997, Shibahara et al., 2000). Therefore, as a first step to determining the effects of QCGG on α-MSH-induced melanogenesis, we investigated whether or not QCGG could inhibit melanin synthesis in the presence of α-MSH. We found that QCGG significantly inhibited melanin synthesis, as tyrosinase and tyrosinase-related protein levels were down-regulated in a dose-dependent manner (Figs. 2B and 3A). Cell viability assay of QCGG showed a lack of cytotoxic effects on B16F10 cells (Figure 2A). These results suggest that QCGG may be a safe alternative skin lightener agent for down-regulation of tyrosinase activity and inhibition of cellular melanin synthesis in B16F10 cells.
Generally, α-MSH potently influences MITF expression via activation of TRP-1, TRP-2, and tyrosinase activity, resulting in increased melanin synthesis (Abdel-Malek et al., 1999, Bertolotto et al., 1998, Costin and Hearing, 2007). Therefore, down-regulation of MITF via TRP-1, TRP-2, and tyrosinase is the most important factor affecting the anti-melanogenic ability of any compound. To elucidate the mechanisms underlying the anti-tyrosinase and anti-melanogenic activities of QCGG, we first examined the effects of QCGG on expression of melanogenic enzymes (TRP-1, TRP-2, and tyrosinase) by Western blotting. As shown in Figure 3B, QCGG at 10–100 μg/ml dose-dependently reduced expression of TRP-1, TRP-2, and tyrosinase at 48 h. Since these three enzymes are transcriptionally regulated by MITF, we next examined the effect of QCGG on MITF expression. Results showed that QCGG dramatically and dose-dependently inhibited MITF expression in B16F10 cells (Figure 3B). These results suggest that the inhibitory effects of QCGG coordinated MITF, TRP-1, TRP-2, and tyrosinase down-regulation in B16F10 cells. Thus, the inhibitory effect of QCGG is accompanied by corresponding down-regulation of MITF expression, which consequently reduces melanogenic protein expression.
For improved understanding of the inhibitory effect of QCGG on α-MSH-induced melanogenesis, we explored whether or not QCGG could affect the intracellular cAMP level induced by α-MSH, as promotion of cAMP up-regulates MITF expression. MITF has been shown to specifically bind to the M and E box motifs as well as up-regulate tyrosinase, TRP-1, and TRP-2 promoter activities, eventually leading to enhanced melanin synthesis (Abdel-Malek et al., 1999, Bertolotto et al., 1998, Costin and Hearing, 2007). Therefore, the regulation of α-MSH-induced cAMP signaling is thought to be potentially important for regulating hyperpigmentation. In accordance with this view, QCGG dose-dependently reduced the intracellular cAMP level in α-MSH-stimulated B16F10 melanoma cells (Figure 4A). This observation suggests that QCGG profoundly suppresses expression of MITF through down-regulation of cAMP levels, resulting in reduced melanin content and tyrosinase activity in α-MSH-stimulated B16F10 melanoma cells.cAMP has been reported to inhibit the MAP kinase pathway in numerous cell types. However, it has been shown that cAMP up-regulation activates MAPK in B16 melanoma cells as well as normal human melanocytes (Buscà et al., 1999). The cAMP signaling pathway is modulated through a PI3 K-dependent mechanism via both the MAPK and PKA pathways during melanin synthesis. Intracellular cAMP binds to two regulatory subunits of protein kinase A (PKA). After phosphorylation of its substrate by PKA, translocates to nucleus where it phosphorylates cAMP response element (CRE), there by phosphorylate CREB (Khaled et al., 2003). To better understand the inhibitory mechanism of QCGG in α-MSH-induced cAMP-dependent MITF signaling, we investigated the effect of QCGG treatment on activation of p38 MAPK, JNK, ERK, and CREB using Western blotting. Results showed that although phosphorylation of ERK and JNK were unaffected, phosphorylation of p38 MAPK and CREB was dose-dependently reduced by QCGG at 48 h. In addition, QCGG treatment did not significantly alter expression levels of p38 MAPK, ERK, JNK, and CREB (Figure 4B). Negative results for the effect of QCGG treatment on ERK and JNK phosphorylation suggest that ERK and JNK do not participate in the anti-melanogenic activity of QCGG.
5. Conclusion
In conclusion, QCGG primarily inhibits cellular melanin production and tyrosinase activity in B16F10 cells by suppressing the expression of MITF, TRP-1, TRP-2, and tyrosinase through p38 MAPK and CREB. Moreover, we found that the QCGG-induced inhibitory effect on melanogenesis could be attributed to suppression of intracellular cAMP production. Therefore, QCGG may be a useful therapeutic agent for the treatment of hyperpigmentation, and could be used as an ingredient in whitening and lightening cosmetics. However, it should be noted that safety is a primary consideration before practical use in humans.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Sun Chul Kang, Email: sckang@daegu.ac.kr.
Byung Ho Bang, Email: gunnerbh@eulgi.ac.kr.
References
- Abdel-Malek Z., Suzuki I., Tada A., Im S., Akcali C. The melanocortin-1 receptor and human pigmentation. Ann. NY Acad. Sci. 1999;885:117–133. doi: 10.1111/j.1749-6632.1999.tb08669.x. [DOI] [PubMed] [Google Scholar]
- An B.J., Kwak J.H., Park J.M., Lee J.Y., Park T.S., Lee J.T. Inhibition of enzyme activities and the antiwrinkle effect of polyphenol isolated from the Persimmon leaf (Diospyros kaki folium) on human skin. Dermatol. Surg. 2005;31:848–854. doi: 10.1111/j.1524-4725.2005.31730. [DOI] [PubMed] [Google Scholar]
- Bei W., Zang L., Guo J., Peng W., Xu A., Good D.A. Neuroprotective effects of a standardized flavonoid extract from Diospyros kaki leaves. J. Ethnopharmacol. 2009;126:134–142. doi: 10.1016/j.jep.2009.07.034. [DOI] [PubMed] [Google Scholar]
- Bertolotto C., Abbe P., Hemesath T.J., Bille K., Fisher D.E., Ortonne J.P., Ballotti R. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J. Cell Biol. 1998;142:827–835. doi: 10.1083/jcb.142.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busc A.R., Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000;13:60–69. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- Buscà R., Abbe P., Mantoux F., Aberdam E., Eychene A., Ortonne J.P., Ballotti R. B-Raf mediates the cAMP activation of MAPK in B16 melanoma cells. Pigment Cell Res. Suppl. 1999;7:106. [Google Scholar]
- Byun E., Jeong G.S., An R.B., Min T.S., Kim Y.C. Tribuli fructus constituents protect against tacrine-induced cytotoxicity in HepG2 cells. Arch. Pharm. Res. 2010;33:67–70. doi: 10.1007/s12272-010-2226-6. [DOI] [PubMed] [Google Scholar]
- Chang T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 1997;7:353–361. doi: 10.1016/S0962-8924(97)01105-7. [DOI] [PubMed] [Google Scholar]
- Corre S., Galibert M.D. Upstream stimulating factors: highly versatile stress-responsive transcription factors. Pigment Cell Melanoma Res. 2005;18:337–348. doi: 10.1111/j.1600-0749.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- Costin G.E., Hearing V.J. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- Duthie S.J., Dobson V.L. Dietary flavonoids protect human colonocyte DNA from oxidative attack in vitro. Eur. J. Nutr. 1999;38:28–34. doi: 10.1007/s003940050043. [DOI] [PubMed] [Google Scholar]
- Garcia-Mediavilla V., Crespo I., Collado P.S., Esteller A., Sanchez-Campos S., Tunon M.J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in chang liver cells. Eur. J. Pharmacol. 2007;557:221–229. doi: 10.1016/j.ejphar.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Hearing V.J. Biogenesis of pigment granules: a sensitive way to regulate melanocyte function. J. Dermatol. Sci. 2005;37:3–14. doi: 10.1016/j.jdermsci.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Hearing V.J., Jimenez M. Mammalian tyrosinase – the critical regulatory control point in melanocyte pigmentation. Int. J. Biochem. Cell B. 1987;19:1141–1147. doi: 10.1016/0020-711x(87)90095-4. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Xu J., Long M., Tu Z., Yang G., He G. 2,3,5 40-Tetrahydroxystilbene-2-O-b-d-glucoside (THSG) induces melanogenesis in B16 cells by MAP kinase activation and tyrosinase up regulation. Life Sci. 2009;85:345–350. doi: 10.1016/j.lfs.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Kameyama K., Sakai C., Kuge S., Nishiyama S., Tomita Y., Ito S., Wakamatsu K., Hearing V.J. The expression of tyrosinase, tyrosinase-related proteins 1 and 2 (TRP1 and TRP2), the silver protein, and a melanogenic inhibitor in human melanoma cells of differing melanogenic activities. Pigment Cell Res. 1995;8:97–104. doi: 10.1111/j.1600-0749.1995.tb00648.x. [DOI] [PubMed] [Google Scholar]
- Khaled M., Larribere L., Bille K., Ortonne J.P., Ballotti R., Bertolotto C. Microphthalmia associated transcription factor is a target of the phosphatidylinositol-3-kinase pathway. J. Invest. Dermatol. 2003;121:831–836. doi: 10.1046/j.1523-1747.2003.12420.x. [DOI] [PubMed] [Google Scholar]
- Kiken D.A., Cohen D.E. Contact dermatitis to botanical extracts. Am. J. Contact Dermatol. 2002;13:148–152. [PubMed] [Google Scholar]
- Ladizinski B., Mistry N., Kundu R.V. Widespread use of toxic skin lightening compounds: medical and psychosocial aspects. Dermatol. Clin. 2011;29:111–123. doi: 10.1016/j.det.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Levy C., Khaled M., Fisher D.E. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006;12:406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Maeda K., Yokokawa Y., Hatao M., Naganuma M., Tomita Y. Comparison of the melanogenesis in human black and light brown melanocytes. J. Dermatol. Sci. 1997;14:199–206. doi: 10.1016/s0923-1811(96)00575-0. [DOI] [PubMed] [Google Scholar]
- Murota K., Terao J. Antioxidative flavonoid Q: implication of its intestinal absorption and metabolism. Arch. Biochem. Biophys. 2003;417:12–17. doi: 10.1016/s0003-9861(03)00284-4. [DOI] [PubMed] [Google Scholar]
- Olszewska M. Separation of quercetin, sexangularetin, kaempferol and isorhamnetin for simultaneous HPLC determination of flavonoid aglycones in inflorescences, leaves and fruits of three Sorbus species. J. Pharm. Biomed. Anal. 2008;48:629–635. doi: 10.1016/j.jpba.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Park H.Y., Wu C., Yonemoto L., Murphy-Smith M., Wu H., Starchur C.M., Gilchrest B.A. MITF mediates cAMP-induced protein kinase C-beta expression in human melanocytes. Biochem. J. 2006;395:571–578. doi: 10.1042/BJ20051388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieroni A., Quave C.L., Villanelli M.L., Mangino P. Ethnopharmacognostic survey on the natural ingredients used in folk cosmetics, cosmeceuticals and remedies for healing skin diseases in the inland Marches, Central-Eastern Italy. J. Ethnopharmacol. 2004;91:331–344. doi: 10.1016/j.jep.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Sa Y.S., Kim S.J., Choi H.S. The anticoagulant fraction from the leaves of Diospyros kaki L. has an antithrombotic activity. Arch. Pharm. Res. 2005;28:667–674. doi: 10.1007/BF02969356. [DOI] [PubMed] [Google Scholar]
- Saha B., Singh S.K., Sarkar C., Bera R., Ratha J., Tobin D.J., Bhadra R. Activation of the Mitf promoter by lipid-stimulated activation of p38-stress signalling to CREB. Pigment Cell Melanoma Res. 2006;19:595–605. doi: 10.1111/j.1600-0749.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- Sakanaka S., Tachibana Y., Okada Y. Preparation and antioxidant properties of extracts of Japanese Persimmon leaf tea (kakinoha-cha) Food Chem. 2005;89:4569–4575. [Google Scholar]
- Seger R., Krebs E.G. MAPK signalling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- Shibahara S., Yasumoto K., Amae S., Udono T., Watanabe K., Saito H., Takeda K. Regulation of pigment cell-specific gene expression by MITF. Pigment Cell Res. 2000;13(Suppl. 8):98–102. doi: 10.1034/j.1600-0749.13.s8.18.x. [DOI] [PubMed] [Google Scholar]
- Singh S.K., Sarkar C., Mallick S., Saha B., Bera R., Bhadra R. Human placental lipid induces melanogenesis through p38 MAPK in B16F10 mouse melanoma. Pigment Cell Melanoma Res. 2005;18:113–121. doi: 10.1111/j.1600-0749.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- Smit N., Vicanova J., Pavel S. The hunt for natural skin whitening agents. Int. J. Mol. Sci. 2009;10:5326–5349. doi: 10.3390/ijms10125326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. MITF: a stream flowing for pigment cells. Pigment Cell Res. 2000;13(4):230–240. doi: 10.1034/j.1600-0749.2000.130404.x. [DOI] [PubMed] [Google Scholar]
- Tachibana M. Cochlear melanocyte sand MITF signaling. J. Invest. Dermatol. Symp. Proc. 2001;6:95–98. doi: 10.1046/j.0022-202x.2001.00017.x. [DOI] [PubMed] [Google Scholar]
- Tomita Y., Maeda K., Tagami H. Melanocyte-stimulating properties of arachidonic acid metabolites: possible role in post-inflammatory pigmentation. Pigment Cell Res. 1992;5:357–361. doi: 10.1111/j.1600-0749.1992.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Tsang T.F., Ye Y., Tai W.C.S., Chou G.X., Leung A.K.M., Yu Z.L., Hsiao W.L.W. Inhibition of the p38 and PKA signaling pathways is associated with the anti-melanogenic activity of Qian-wang-hong-bai-san, a Chinese herbal formula, in B16 cells. J. Ethnopharmacol. 2012;141:622–628. doi: 10.1016/j.jep.2011.08.043. [DOI] [PubMed] [Google Scholar]
- Tsatmali M., Ancans J., Thody A.J. Melanocyte function and its control by melanocortin peptides. J. Histochem. Cytochem. 2002;50:125–133. doi: 10.1177/002215540205000201. [DOI] [PubMed] [Google Scholar]
- Tsuboi T., Kondoh H., Hiratsuka J., Mishima Y. Enhanced melanogenesis induced by tyrosinase gene-transfer increases boron-uptake and killing effect of boron neutron capture therapy for amelanotic melanoma. Pigment Cell Res. 1998;11:275–282. doi: 10.1111/j.1600-0749.1998.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Ye Y., Chu J.H., Wang H., Xu H., Chou G.X., Leung A.K.M., Fong W.F., Yu Z.L. Involvement of p38 MAPK signaling pathway in the anti-melanogenic effect of San-bai-tang, a Chinese herbal formula, in B16 cells. J. Ethnopharmacol. 2010;132:533–535. doi: 10.1016/j.jep.2010.09.007. [DOI] [PubMed] [Google Scholar]


