Abstract
The mammalian kidney develops from reciprocal interactions between the metanephric mesenchyme and ureteric bud, the former of which contains nephron progenitors. The third lineage, the stroma, fills up the interstitial space and is derived from distinct progenitors that express the transcription factor Foxd1. We showed previously that deletion of the nuclear factor Sall1 in nephron progenitors leads to their depletion in mice. However, Sall1 is expressed not only in nephron progenitors but also in stromal progenitors. Here we report that specific Sall1 deletion in stromal progenitors leads to aberrant expansion of nephron progenitors, which is in sharp contrast with a nephron progenitor-specific deletion. The mutant mice also exhibited cystic kidneys after birth and died before adulthood. We found that Decorin, which inhibits Bmp-mediated nephron differentiation, was upregulated in the mutant stroma. In contrast, the expression of Fat4, which restricts nephron progenitor expansion, was reduced mildly. Furthermore, the Sall1 protein binds to many stroma-related gene loci, including Decorin and Fat4. Thus, the expression of Sall1 in stromal progenitors restricts the excessive expansion of nephron progenitors in a non-cell autonomous manner, and Sall1-mediated regulation of Decorin and Fat4 might at least partially underlie the pathogenesis.
A typical mammalian kidney contains approximately one million nephrons, which are functional units consisting of glomeruli, proximal and distal renal tubules, and collecting ducts. During development, the nephron is formed by reciprocally inductive interactions between two precursor tissues: the metanephric mesenchyme and the ureteric bud. The former contains nephron progenitors that express the transcription factor Six2, and give rise to most components of the nephron epithelia, including those in glomeruli (podocytes and parietal cells) and renal tubules1. In contrast, the collecting duct epithelium is derived from the ureteric bud. However, these two lineages are not sufficient to generate the complete kidney structure. A third lineage—the stroma—is required, which is derived from a distinct progenitor population that expresses the transcription factor Foxd12,3. The stromal progenitors surround the Six2-positive nephron progenitors, and are maintained in the outermost cortical region of the kidney during development. The interstitial tissue between the nephron’s epithelia is filled with differentiated stromal cells, such as fibroblasts, pericytes, and glomerular mesangial cells, the latter two being closely associated with vasculature.
The stromal cells not only fill up the interstitial space but also regulate the development of the nephron. Several genes for transcription factors are expressed in the cortical stroma, including Foxd1, Pbx1, and Tcf21 (also known as Pod1). Deletion of these genes in mice results in nephron progenitor expansion4,5,6,7,8, suggesting an interaction between the stroma and the nephron. It has been reported that Foxd1 deletion leads to Decorin up-regulation9. Decorin encodes a proteoglycan that functions as a sequestering receptor for the Tgf-β family of ligands, thus the increase in Decorin expression inhibits Bmp-mediated nephron differentiation resulting in progenitor expansion. Foxd1Cre-mediated cell ablation also leads to nephron progenitor expansion10,11, and this phenotype is explained by the loss of Fat4 in the stroma10. Fat4 belongs to an atypical cadherin family conserved between Drosophila and mammals, and stroma-specific Fat4 deletion leads to nephron progenitor expansion12. Whereas the Fat proteins in Drosophila activate the Hippo pathway, it remains controversial whether Fat4 deletion in the mammalian kidney stroma leads to a reduction of the Hippo pathway and subsequent nuclear localisation of Yap/Taz in nephron progenitors10,12,13. Fat4 is also involved in planar cell polarity and ubiquitous Fat4 deletion disrupts oriented cell division, leading to dilatation and impaired elongation of nephrons14. However, it remains unclear in which lineage a Fat4 deletion is responsible for the phenotype.
The region-specific homeotic gene spalt (sal) was first isolated from Drosophila and encodes a nuclear protein characterised by multiple double zinc finger motifs15. Humans and mice each have four known sal-like genes. Mutations in human SALL1 and SALL4 have been associated with Townes–Brocks and Okihiro syndromes, respectively, both of which are autosomal dominant diseases that involve abnormalities in various organs including ears, limbs, heart, and kidneys16,17. We have shown that conventional Sall1 knockout mice exhibit kidney agenesis resulting from failure of ureteric bud attraction toward the mesenchyme18. Sall1-expressing cells in the metanephric mesenchyme represent multipotent nephron progenitors19, and Sall1 deletion in Six2-positive nephron progenitors results in kidney hypoplasia and severe progenitor depletion20. Thus, Sall1 is essential for the maintenance of nephron progenitors. However, Sall1 is not only expressed in nephron progenitors but also in stromal progenitors20. To show the role of Sall1 in the latter cell population, we deleted Sall1 in stromal progenitors by utilizing the Foxd1Cre strain2,3. Although Foxd1 is also expressed in developing podocytes, Foxd1Cre is detected only in a subset of maturing-stage podocytes2,21. In addition, Sall1 expression in podocytes is minimal. Therefore, we reasoned that we could address the role of Sall1 in stromal progenitors through the analysis of Foxd1Cre:Sall1flox/flox mice.
Results
Mice lacking Sall1 in the stromal progenitors exhibit dilatation of all nephron components and die before adulthood
To examine the roles of Sall1 in the stroma, we crossed the floxed allele of Sall1 with Foxd1Cre mice expressing Cre recombinase in their stromal progenitors3,20. The Foxd1Cre; Sall1flox/flox mice were born alive but most of them had died before 5 weeks of age (n = 8 out of 10). The mutant kidney was reduced in size, and contained multiple cysts (Fig. 1A,B). Staining of the markers for various nephron lineages showed that all the nephron components were dilated, including Bowman’s space of the glomeruli, Lotus tetragonolobus lectin (LTL)-positive proximal and Slc12a3-positive distal tubules, and cytokeratin-positive collecting ducts (Fig. 1C–F). The surviving mutants also appeared sick and showed similar kidney phenotypes, when euthanized at 8 weeks after birth (n = 2). Consistent with the histology, the renal functions of these two mice were worse than their littermates, as shown by the elevated levels of blood urea nitrogen (290.0 and 43.8 mg/dl in the mutants vs. 30.7 and 26.2 mg/dl in the controls), and of serum creatinine (1.00 and 0.18 mg/dl in the mutants vs. 0.11 and 0.12 mg/dl in the controls). Because we never observed ureteric dilatations, these phenotypes were unlikely to be caused by obstruction of the lower urinary tract.
Figure 1. Mice lacking Sall1 in the stromal progenitors exhibit dilatation of all nephron components.
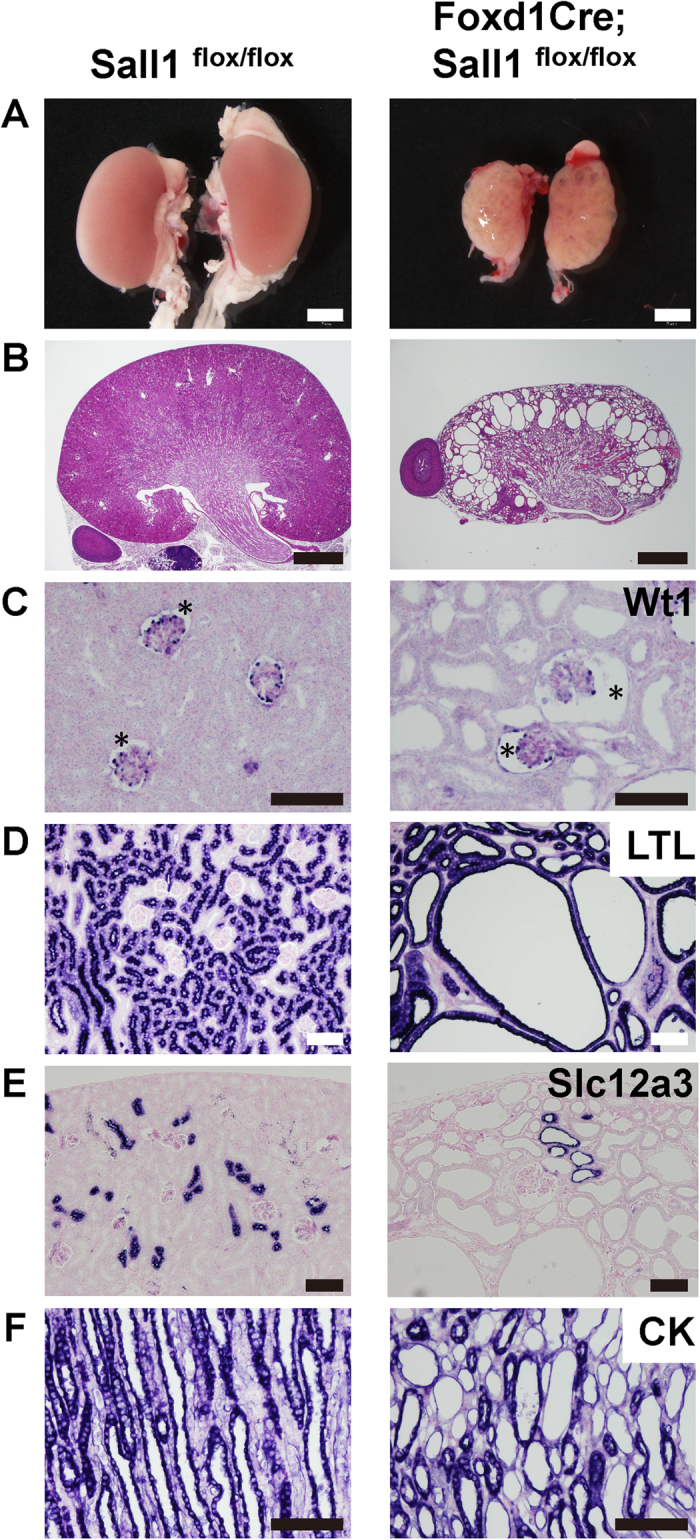
(A) Macroscopic views of Sall1flox/flox and Foxd1Cre; Sall1flox/flox kidneys at 8 weeks of age, showing a significant reduction in size of the mutant kidney. (B) Haematoxylin and eosin (HE) staining of control and mutant kidneys at 8 weeks of age. (C) Expansion of Bowman’s space (asterisks). Glomerular podocytes have been stained with an anti-Wt1 antibody. (D–F) Dilatation of proximal tubules (LTL immunostaining), distal tubules (Slc12a3), and collecting ducts (cytokeratin, CK). Scale bars: (A) = 2 mm; (B) = 1 mm; (C–F) = 100 μm.
Nephron progenitors are excessively expanded in the Sall1-deficient kidney
When analysed at birth, stromal cells, which are positive for platelet-derived growth factor receptor β (Pdgfrβ)2, were detected throughout the interstitial space in the mutants, as in the controls. Pdgfrβ-positive cells in the glomeruli—corresponding to mesangial cells—were also detectable in the mutants (Fig. 2A). Vascular endothelial cells were distributed in the interstitial space and in glomeruli (Fig. 2B). Thus, the development of stromal cells and vascular tissues were apparently unaffected in the Sall1 mutant neonatal mice. The dilatations of the nephron were not yet prominent: the proximal tubules were mildly dilated, while no dilatations were observed in the other parts of the nephron, including Bowman’s space of the glomeruli (Fig. 2C–E). More remarkably, areas of Six2-positive nephron progenitors at birth were expanded and thickened around the ureteric bud tips (Fig. 2F). Therefore, the major abnormalities in the stroma-specific Sall1 deletion were non-cell autonomously observed in the nephrons: namely nephron dilatation and progenitor expansion.
Figure 2. Nephrons start to dilate in Sall1-deficient neonatal mice.
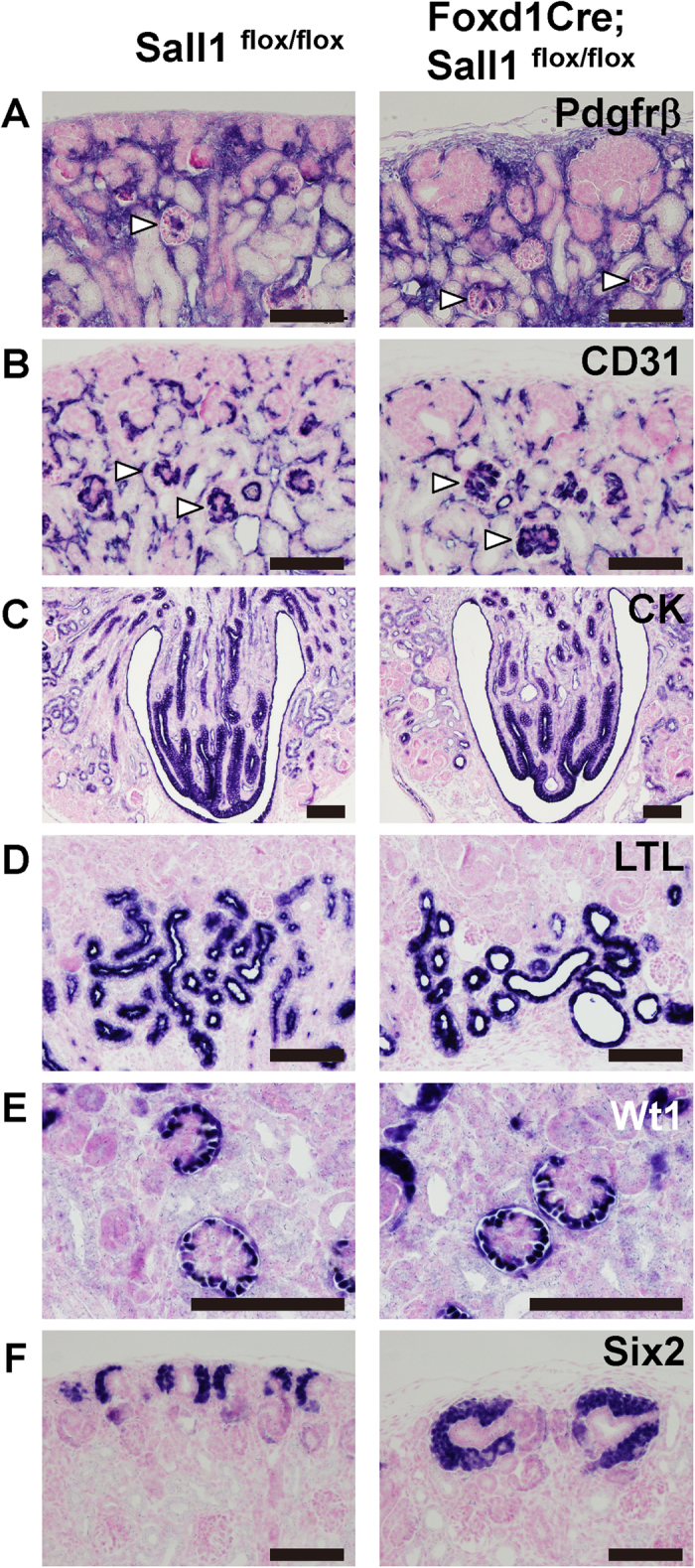
(A,B) Formation of stromal cells and vasculature is unaffected at birth. Immunostaining of Pdgfrβ (A) and CD31 (B), respectively. Arrowheads: glomeruli. (C) Collecting ducts in the medulla shown by immunostaining for cytokeratin. (D) Some proximal tubules are dilated, as shown by LTL immunostaining. (E) Bowman’s space of the glomeruli is not dilated yet. Glomerular podocytes are immunostained with an anti-Wt1 antibody. (F) Immunostaining for Six2 shows expansion of nephron progenitors at birth. Scale bars = 100 μm.
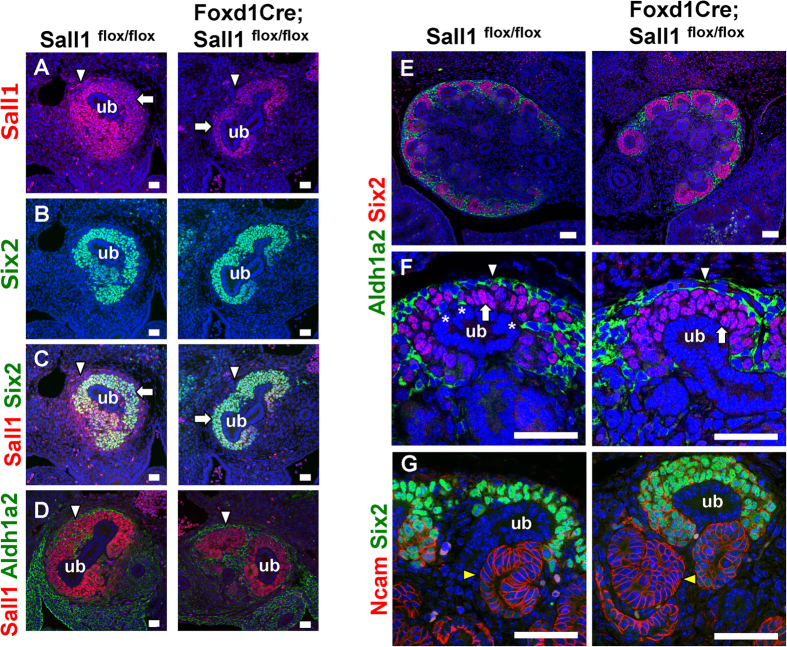
Next, we analysed the mutant mice during gestation. At embryonic day (E) 12.5, Sall1 was expressed not only in Six2-positive nephron progenitors but also in the surrounding stroma in the controls (Fig. 3A–C, Supplementary Fig. S1), as we reported previously20. Sall1 expression in the stroma disappeared in the Foxd1Cre; Sall1flox/flox kidney, and was restricted to the Six2-positive domain (Fig. 3A–C). Co-immunostaining for the stromal marker Aldh1a2 (also known as retinoic acid dehydrogenase 2: Raldh2)22 also showed the absence of Sall1 in the stromal cells (Fig. 3D). Because Foxd1Cre mice express a fusion protein of Green Fluorescent Protein (GFP) and Cre recombinase, GFP was detected in the stromal progenitors surrounding the nephron progenitors, and Sall1 was absent in the former population (Supplementary Fig. S1).
Figure 3. Nephron progenitors are expanded in the Sall1 mutant kidney.
(A–C) Dual immunostaining for Sall1 (red) and Six2 (green) in Sall1flox/flox and Foxd1Cre; Sall1flox/flox kidneys at E12.5. Sall1 expression in the stroma (arrowheads) is absent but is retained in the nephron progenitors (arrows) that are positive for Six2. ub: ureteric bud. (D) Immunostaining for Sall1 (red) and Aldh1a2 (green) of Sall1flox/flox and Foxd1GFPCre; Sall1flox/flox kidneys at E12.5. Note the absence of Sall1 in the Aldh1a2-positive stromal cells (arrows). (E,F) Dual immunostaining for Six2 (red) and Aldh1a2 (green) at E14.5. Six2-positive nephron progenitors (arrows) are expanded, while Aldh1a2-positive stromal cells (arrowheads) are retained in the mutant kidney. Some stromal cells (asterisks) reach the ureteric bud (ub) tip epithelium in the control. (G) Dual immunostaining for Six2 (green) and Ncam (red) at E14.5. Nephron progenitors are expanded, but the Ncam-positive nascent nephrons (yellow arrowheads) are formed. Scale bars = 20 μm.
Thus, Sall1 was deleted specifically in the stroma by E12.5. At this stage, nephron progenitor expansion was not apparent (Fig. 3B). The progenitor numbers per ureteric bud (the mean ± standard deviation) were 138.7 ± 25.8 in the mutants, while they were 124.4 ± 25.3 in the controls (p = 0.24). However, at E14.5, expansion of nephron progenitors was evident (Fig. 3E–G, Supplementary Fig. S1). The progenitor numbers per ureteric bud were 63.7 ± 18.5 in the mutants, while they were 41.7 ± 12.2 in the controls (p < 0.01). In the controls, Six2-positive nephron progenitors had accumulated around the ureteric bud tips, and Aldh1a2-positive stromal cells surrounded the nephron progenitors (Fig. 3E). Notably, some stromal cells penetrated the Six2-positive areas and directly attached to the ureteric bud epithelia (Fig. 3F), which might explain Ret regulation in the ureteric bud by retinoic acid supplied from the stroma22. In the Sall1 mutants, the layers of Six2-positive cells were thickened, and the stromal cells rarely reached the ureteric buds. Differentiation of the nephrons was not affected significantly, because the morphology of Ncam-positive nascent nephrons was similar to that in the controls (Fig. 3G). Thus, nephron progenitor expansion started at mid-gestation upon stroma-specific Sall1 deletion, which suggests that the non-cell autonomous restriction of nephron progenitors by the stroma is affected in the absence of Sall1.
In contrast, we did not detect any significant differences in cell deaths between the control and mutant stromal progenitors at E13.5 or E14.5 (Supplementary Fig. S2), although the mutant kidneys showed mild increases. Cell proliferation, as shown by staining for phosphorylated histone H3 (PHH3), also exhibited no differences (Supplementary Fig. S2). We then introduced a tdTomato lineage reporter into the Sall1 mutant mice. In the controls, all the stromal cells in the interstitial space, as well as glomerular mesangial cells, were labelled with tdTomato (Supplementary Fig. S2), which is consistent with reports that these cells are derived from Foxd1-positive stromal progenitors2,3. In the Sall1 mutants, the same population was labelled, and the signals were absent from nephron progenitors and the ureteric buds, as in the controls, suggesting that aberrant differentiation from the stroma toward the other lineages did not occur in the absence of Sall1.
Fat4 is reduced and Decorin is increased in Sall1 mutant kidneys
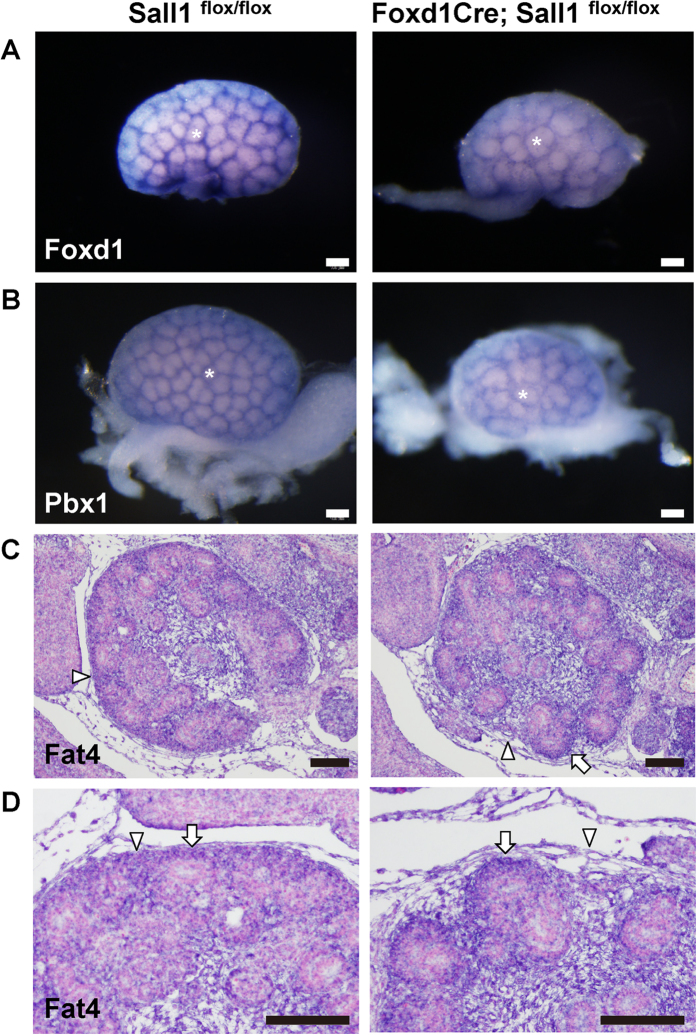
Nephron progenitor expansion has been reported in several mutant mice lacking Foxd1 or Pbx14,5,6. These genes, as well as Meis1, produced mesh-like patterns in control embryonic kidneys as shown by whole mount in situ hybridisation (Fig. 4A,B; Supplementary Fig. S3). This was because they were more abundantly expressed in the cortical stroma filling the gaps between the clusters of nephron progenitors. None of these gene expression levels was decreased in the Sall1 mutants. However, the meshes were larger than in the controls, which is consistent with the expansion of nephron progenitor clusters, as shown in Fig. 3. Hoxd10, expressed both in nephron progenitors and cortical stroma23, and Tcf21, expressed more abundantly in interstitial stroma8, were also unaffected in the Sall1 mutants (Supplementary Fig. S3).
Figure 4. Fat4 expression is reduced mildly in the Sall1 mutant kidney.
(A,B) Whole-mount in situ hybridisation of the control and Sall1 mutant kidneys at E14.5. The expression levels of Foxd1 (A) and Pbx1 (B) are unaffected. The size of each nephron progenitor cluster (white asterisks) is increased in the mutant kidneys. (C,D) Section in situ hybridisation of Fat4 at E14.5. Expression in the cortical stroma (arrowheads) is slightly reduced, but that in the nephron progenitors (arrows) is retained. (D) magnified images of panel (C). Scale bars = 100 μm.
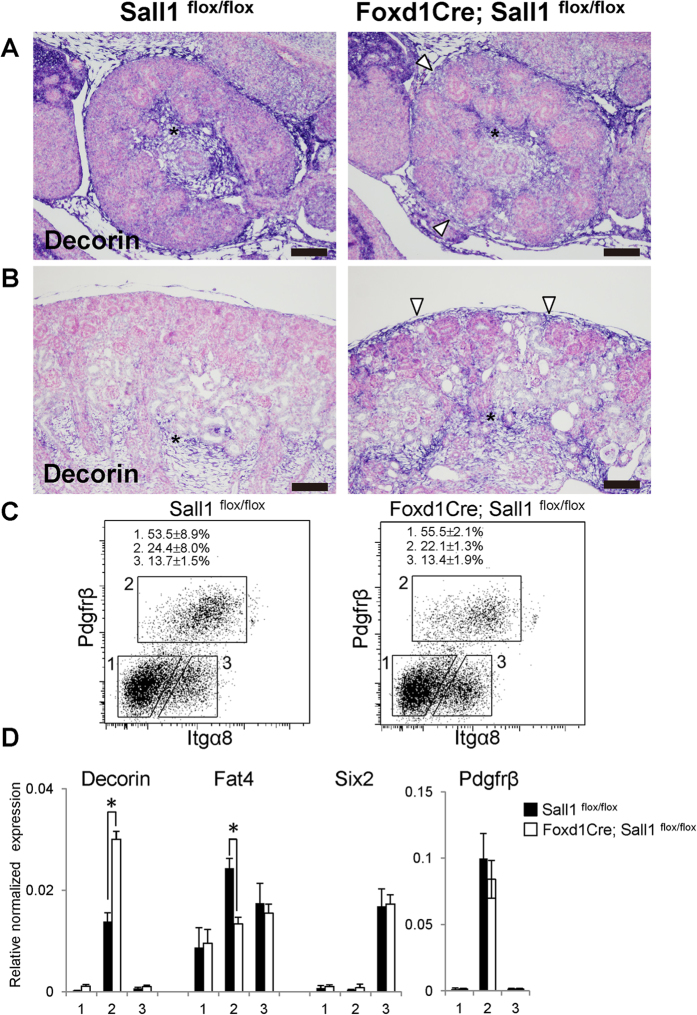
Conventional and cortical stroma-specific deletion of Fat4 in mice leads to nephron progenitor expansion10,11,12,13. It has also been reported that Foxd1 deletion leads to Decorin up-regulation, which inhibits Bmp-mediated differentiation resulting in nephron progenitor expansion9. In the Sall1 mutants, in situ hybridisation on sections showed that Fat4 was slightly reduced in the cortical stroma but was retained in the nephron progenitors (Fig. 4C,D). Whereas Decorin was mainly expressed in the medullary stroma in the controls, its expression domain began to expand to the cortical region in the mutants at E14.5 (Fig. 5A), and became apparent at P0 (Fig. 5B).
Figure 5. Decorin is increased in the Sall1 mutant stromal cells.
(A,B) Section in situ hybridisation of Decorin at E14.5 (A) and P0 (B). Expression domain is expanded from the medulla (asterisks) to the cortical region (arrowheads) in the mutant kidneys. (C) FACS analysis of control and mutant new-born kidneys. Fraction 1: Pdgfrβ−/integrin α8− cells, Fraction 2: Pdgfrβ+/integrin α8− stromal cells, Fraction 3: Pdgfrβ−/integrin α8+ nephron progenitors. The percentages of each fraction represent the average and standard deviation of Sall1flox/flox controls and Sall1-deficient (Foxd1GFPCre; Sall1flox/flox) mutants (3 embryos each). (D) Quantitative RT-PCR analysis of the three fractions. The means and standard deviations of three controls and three mutants are shown. *p < 0.01 by Student’s t test. Scale bars = 100 μm.
To quantify the expression levels of Decorin and Fat4, we further sorted the Pdgfrβ-positive stromal cells from newborn kidneys (Fig. 5C). Whereas the percentages of stromal cells in the kidneys were similar between the controls and the Sall1 mutants, our data showed that Decorin was upregulated in the mutant stroma, while Fat4 expression was reduced mildly (Fig. 5D). Thus, misregulation of Decorin and Fat4 might be responsible—at least partially—for nephron progenitor expansion in the Sall1 mutants. Six2 expression in the nephron progenitor fraction, which was sorted based on integrin α8 expression24, was unaltered in the mutants, which is consistent with the immunostaining data in Fig. 3.
Sall1 binds directly to many stroma-related gene loci, including Decorin and Fat4
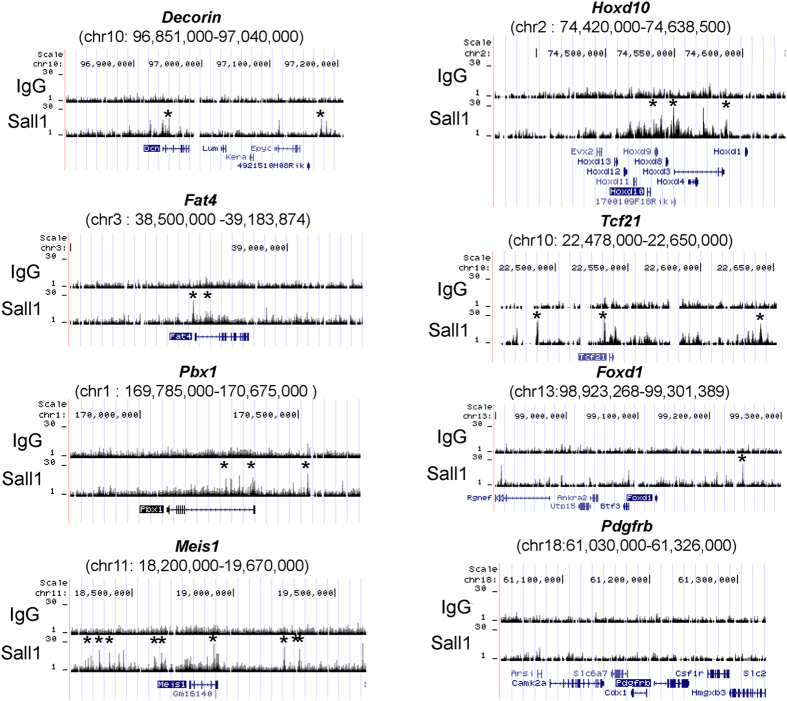
We previously performed chromatin immunoprecipitation-sequencing (ChIP-seq) analysis by using the whole embryonic kidney, and identified Sall1 binding sites throughout the genome20. We re-analysed these data and found that the Sall1 protein bound to the upstream regions of the Decorin and Fat4 loci (Fig. 6). Sall1 also bound to many stroma-related gene loci, including Pbx1, Meis1, Hoxd10, and Tcf21, but not to Foxd1 or Pdgfrβ (Fig. 6). Despite these Sall1 bindings, the expression levels of these stroma-related genes were largely unaffected, except for Decorin and Fat4 (Fig. 4, Supplementary Fig. S3). These findings suggest that Decorin and Fat4 are likely to be direct targets of Sall1 in the kidney.
Figure 6. Sall1 binds directly to stroma-related gene loci, including Decorin and Fat4.
ChIP-seq analysis of Sall1 within the stromal progenitor-related loci (mm9 coordinates). Sall1 binds to Decorin, Fat4, Pbx1, Meis1, Hoxd10, and Tcf21 loci, but not to Foxd1 or Pdgfrβ loci. Asterisks show peaks occupied by Sall1. Peak calling was carried out as described20.
Discussion
The epithelial component of the nephron is formed through interactions between nephron progenitors in the metanephric mesenchyme and the ureteric bud. However, the stroma also plays important roles in regulating the development of the other two lineages. We showed previously that Sall1 is essential for the maintenance of nephron progenitors20, but found here that Sall1 in the stromal progenitors restricted nephron progenitor expansion in a non-cell autonomous manner. Therefore, Sall1 is essential for both nephron and stromal progenitors, but exerts opposite effects against nephron progenitors. Given that the Sall1 protein binds to the Mi2/NuRD repressor complex in the developing kidney20,25, Sall1 may directly inhibit Decorin expression in the stroma. Decorin is de-repressed upon Sall1 deletion in the stroma, which in turn inhibits Bmp-mediated nephron differentiation resulting in progenitor expansion9. Because the Sall1-binding peak in the proximal promoter of Decorin was within 5 kb from the reported Foxd1-binding site9, it would be interesting to examine the relationship between Sall1 and Foxd1 in the regulation of Decorin. Sall1 can also function as an activator20, therefore Fat4 might be activated directly by Sall1 and restrict nephron progenitor expansion. To prove these hypotheses and examine the relative importance of the two target genes, reducing Decorin or increasing Fat4 expressions in vivo in the Sall1 mutant kidneys should ameliorate the phenotypes.
However, the changes in expression levels of Decorin and Fat4 were mild even when the phenotype became apparent at E14.5. Sall1 binds to many stroma-related gene loci, which is reminiscent of the situation in nephron progenitors where Sall1 binds to many key loci, such as Six2, Osr1, Eya1, and Pax220. Perhaps Sall1 forms a network with other nuclear factors, both by protein–protein interaction and mutual transcriptional activation/repression, thereby maintaining respective progenitors. In this type of network, deletion of Sall1 alone would lead to minor changes in many targets, which still lead to characteristic phenotypes. Indeed this holds true for Sall1 in the maintenance of nephron progenitors and for Sall4 in embryonic stem cells20,26,27. Alternatively, Fat4 and Decorin might play only subsidiary roles in enhancing the phenotype caused by other yet-to-be-identified Sall1 targets. Comprehensive microarray analysis of the mutant stromal cells would help in identifying such targets.
The mechanism underlying the dilatation of nephrons in Sall1 mutants remains unclear. Because proliferation or survival of the E-cadherin-positive nephron epithelia were not significantly affected at birth (Supplementary Fig. S2), other mechanisms, including impaired planar cell polarity, might be involved. Nephron epithelial cells usually divide along the elongating axis, but alteration of the dividing orientation could lead to increased diameter. Because Foxd1 deletion accompanied by Decorin up-regulation does not produce this phenotype, Decorin might not be involved primarily in nephron dilatation in the Sall1 mutants. Ubiquitous Fat4 deletion produces a similar phenotype caused by impaired planar cell polarity14. However, it needs to be determined whether nephron dilatation is caused by the absence of Fat4 in the nephron or in the stroma, because Fat4 expression in the Sall1 mutants was reduced only in the stroma, but not in the nephron. A collecting duct-specific Wnt9b deletion also produces a similar phenotype of nephron dilatation28, but it remains unclear whether Wnt9b exerts its planar polarity effect to the nephron epithelia in an autocrine manner or by way of the stroma. Therefore, it is possible that stromal genes regulated by Sall1 mediate the planar polarity effect evoked by Fat4 or Wnt9. Identification of such Sall1 targets will be important to reveal the unappreciated role of the stroma.
In summary, Sall1 expression in stromal progenitors non-cell autonomously restricted excessive nephron progenitor expansion and regulated nephron diameter. The former function might be mediated by Decorin and Fat4, at least partially. Because Sall1 mutations are found in human hereditary diseases16, our finding will also be useful for clinical paediatrics. Furthermore, we have recently succeeded in inducing Six2/Sall1-positve nephron progenitors and immature nephron tissues from mouse embryonic stem cells and human induced pluripotent cells24. However, stromal cells are required to generate the fully organised kidney. Further studies on the stromal cells, as well as their interactions with nephrons, would help advance our understanding toward the generation of the complex three-dimensional structures of the kidney.
Methods
Generation of conditional Sall1 mutant mice
Sall1flox mice were produced as described20,27. Foxd1GFPCre (012463) and R26R-tdTomato (007905) mice were obtained from the Jackson Laboratory3,29. The primers used for genotyping were as follows: Cre1 (5′–AGGTTCGTTCACTCATGGA–3′) and Cre2 (5′–TCGACCAGTTTAGTTACCC–3′) for the Cre allele (250 bp); Sall1 flox2 (5′–CCTCTGCCCGAGAGATCG–3′), Sall1flox3 (5′–GGCGCGTCTGATTTTATTTC–3′) for the Sall1 allele (wild-type: 220 bp; mutant: 280 bp). Polymerase chain reaction (PCR) amplifications were performed using GoTaq DNA polymerase (Promega) by denaturation at 95 °C for 2.5 min, followed by 35 cycles of 95 °C for 30 s, 58 °C for 60 s, and 72 °C for 30 s, and a final extension at 72 °C for 7 min. All animal experiments were performed in accordance with the institutional guidelines and approved by the licencing committee of Kumamoto University (#A27-018).
Immunohistochemistry
Tissues were fixed in 10% formalin, embedded in paraffin wax and cut into 6-μm sections. Immunostaining was carried out automatically using a BlueMap kit and the Discovery System (Roche) or manually for immunofluorescence staining. The following primary antibodies were used: anti-Sall126 (PPMX Perseus Proteomics: PP-K9814-00); anti-Six2 (Proteintech); anti-Six2 (Abnova); anti-Wt1 (Santa Cruz); LTL (Vector); anti-Slc12a3 (Millipore); anti-cytokeratin (Sigma-Aldrich); anti-Ncam (Developmental Studies Hybridoma Bank #5B8); anti-E-cadherin (BD Biosciences: mouse-derived, Cell Signaling: rabbit derived), anti-CD31 (Abcam); anti-Pdgfrβ (Cell Signaling); anti-Aldh1a2 (Abcam); anti-PHH3 (Millipore; rabbit-derived, Abcam; mouse-derived); anti-Red Fluorescent Protein (RFP; Rockland); and anti-GFP (Abcam; chicken-derived). In paraffin sections, no GFP or tdTomato signals were detected unless the respective antibodies were used. TUNEL assays were performed using an ApopTag Plus fluorescein in situ apoptosis detection kit (Millipore), and the signal was enhanced using the biotin-conjugated anti-digoxigenin antibody (Sigma-Aldrich) and Alexa 594-conjugated streptavidin (Life Technologies). Immunofluorescence was visualised with an LSM780 confocal microscope (Zeiss).
Quantification of immunostained samples
Six2-positive nephron progenitors surrounding the ureteric bud were counted from the sections of four control and six mutant kidneys at E12.5, and from six kidneys for each genotype at E14.5. The Six2 images in green colour channel were incorporated into Image J30, trimmed, and subjected to smoothing for noise elimination, before cell counting. Numbers of PHH3- or TUNEL-positive cells out of Aldh1a2-positive stromal cells or E-cadherin-positive nephron epithelia were counted manually using high-resolution images from sections of six kidneys for each genotype at E13.5, 14.5, and P0. Data were evaluated for statistical significance using Student’s t-test.
In situ hybridisation
For whole-mount in situ hybridisation, embryonic kidneys were fixed with 4% paraformaldehyde and processed using an automated InsituPro VS (Intavis AG) according to the manufacturer’s protocol. Templates for the probes were generated by reverse transcription (RT)-PCR and sequenced. Multiple pairs of control and mutant kidneys (for example, 4 pairs for Foxd1) were examined. In situ hybridisation was performed using paraffin sections and an automated Discovery System (Roche) according to the manufacturer’s protocols.
Quantitative RT–PCR from sorted stromal cells
The new-born kidneys were dissociated into single cells by using a mixture of collagenase XI (Sigma-Aldrich), dispase (Life Technologies), and DNase (Roche) at 37 °C for 10 min and subsequent treatment with 0.25% trypsin-EDTA at 37 °C for 5min. Cells were stained with an Allophycocyanin-conjugated anti-Pdgfrβ antibody (Biolegend) and a biotin-conjugated anti-integrin α8 antibody (R&D Systems), followed by staining with the Phycoerythrin-conjugated streptavidin. Pdgfrβ+/integrin α8− stromal cells, Pdgfrβ−/integrin α8+ nephron progenitors, and the remaining Pdgfrβ−/integrin α8− cells were sorted using a FACSAria SORP (BD Biosciences). RNA was isolated using an RNeasy Plus Micro Kit (Qiagen) and reverse-transcribed with random primers using the Superscript VILO cDNA Synthesis Kit (Life Technologies). Quantitative PCR was carried out using the Dice Real Time System Thermal Cycler (Takara Bio) and Thunderbird SYBR qPCR Mix (Toyobo). Primer sequences are shown in Supplementary Table. All the samples were normalized against β-actin expression.
ChIP-Seq analysis
ChIP-Seq analysis of the whole kidneys at E16.5 was performed as described previously20, and the data have been deposited to Genbank/DNA Data Bank of Japan (accession no. DRA000957). The sequences were mapped to the mouse genome (mm9).
Additional Information
How to cite this article: Ohmori, T. et al. Sall1 in renal stromal progenitors non-cell autonomously restricts the excessive expansion of nephron progenitors. Sci. Rep. 5, 15676; doi: 10.1038/srep15676 (2015).
Supplementary Material
Acknowledgments
We thank Shingo Usuki and the members of the Liaison Laboratory Research Promotion Centre in Kumamoto University for technical assistance. This study was supported in part by a Grant-in-Aid (KAKENHI 26253051) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Footnotes
Author Contributions T.O. generated the mice, T.O. and S.F. performed the histological analyses, and S.T. performed sorting experiments and obtained the ChIP-seq data. Y.K. prepared the whole mount in situ hybridisation data and contributed to the quantitative analyses. R.N. designed the experiments and wrote the paper. All authors reviewed the manuscript.
References
- Kobayashi A. et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3, 169–181 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A. et al. Identification of a multipotent self-renewing stromal progenitor population during mammalian kidney organogenesis. Stem Cell Reports 3, 650–662 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys B. D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176, 85–97 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatini V., Huh S. O., Herzlinger D., Soares V. C. & Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of winged helix transcription factor BF-2. Genes Dev 10, 1467–1478 (1996). [DOI] [PubMed] [Google Scholar]
- Levinson R. S. et al. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development 132, 529–539 (2005). [DOI] [PubMed] [Google Scholar]
- Schnabel C. A., Godin R. E. & Cleary M. L. Pbx1 regulates nephrogenesis and ureteric branching in the developing kidney. Dev Biol 254, 262–276 (2003). [DOI] [PubMed] [Google Scholar]
- Quaggin S. E. et al. The basic-helix-loop-helix protein Pod1 is critically important for kidney and lung organogenesis. Development 126, 5771–5783 (1999). [DOI] [PubMed] [Google Scholar]
- Cui S., Schwartz L. & Quaggin S. E. Pod1 is required in stromal cells for glomerulogenesis. Dev Dyn 226, 512–522 (2003). [DOI] [PubMed] [Google Scholar]
- Fetting J. L. et al. FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney. Development 141, 17–27 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A. et al. Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat Cell Biol 15, 1035–1044 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hum S., Rymer C., Schaefer C., Bushnell D. & Sims-Lucas S. Ablation of the renal stroma defines its critical role in nephron progenitor and vasculature patterning. PLoS One 9, e88400 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagherie-Lachidan M. et al. Stromal Fat4 acts non-autonomously with Dachsous1/2 to restrict the nephron progenitor pool. Development 142, 2564–2573 (2015). [DOI] [PubMed] [Google Scholar]
- Mao Y., Francis-West P. & Irvine K. D. A Fat4-Dchs1 signal between stromal and cap mesenchyme cells influences nephrogenesis and ureteric bud branching. Development 142, 2574–2585 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saburi S. et al. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40, 1010–1015 (2008). [DOI] [PubMed] [Google Scholar]
- Kühnlein R. P. et al. spalt encodes an evolutionarily conserved zinc finger protein of novel structure which provides homeotic gene function in the head and tail region of the Drosophila embryo. EMBO J 13, 168–179 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhase J., Wischermann A., Reichenbach H., Froster U. & Engel W. Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nat Genet 18, 81–83 (1998). [DOI] [PubMed] [Google Scholar]
- Kohlhase J. et al. Okihiro syndrome is caused by SALL4 mutations. Hum Mol Genet 11, 2979–2987 (2002). [DOI] [PubMed] [Google Scholar]
- Nishinakamura R. et al. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development 128, 3105–3115 (2001). [DOI] [PubMed] [Google Scholar]
- Osafune K., Takasato M., Kispert A., Asashima M. & Nishinakamura R. Identification of multipotent progenitors in the embryonic mouse kidney by a novel colony-forming assay. Development 133, 151–161 (2006). [DOI] [PubMed] [Google Scholar]
- Kanda S. et al. Sall1 maintains nephron progenitors and nascent nephrons by acting as both an activator and a repressor. J Am Soc Nephrol 25, 2584–2595 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunskill E. W., Georgas K., Rumballe B., Little M. H. & Potter S. S. Defining the molecular character of the developing and adult kidney podocyte. PLoS One 6, e24640 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosselot C. et al. Non-cell-autonomous retinoid signaling is crucial for renal development. Development 137, 283–292 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yallowitz A. R., Hrycaj S. M., Short K. M., Smyth I. M. & Wellik D. M. Hox10 genes function in kidney development in the differentiation and integration of the cortical stroma. PLoS One 6, e23410 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A. et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53–67 (2014). [DOI] [PubMed] [Google Scholar]
- Denner D. R. & Rauchman M. Mi-2/NuRD is required in renal progenitor cells during embryonic kidney development. Dev Biol 375, 105–116 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki-Yumoto M. et al. The murine homolog of SALL4, a causative gene in Okihiro syndrome, is essential for embryonic stem cell proliferation, and cooperates with Sall1 in anorectal, heart, brain and kidney development. Development 133, 3005–3013 (2006). [DOI] [PubMed] [Google Scholar]
- Yuri S. et al. Sall4 is essential for stabilization, but not for pluripotency, of embryonic stem cells by repressing aberrant trophectoderm gene expression. Stem Cells 27, 796–805 (2009). [DOI] [PubMed] [Google Scholar]
- Karner C. M. et al. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet 41, 793–799 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13, 133–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S. & Eliceiri K. W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9, 671–675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.