Abstract
Continuous gas-phase synthesis of nanoparticles is associated with rapid agglomeration, which can be a limiting factor for numerous applications. In this report, we challenge this paradigm by providing experimental evidence to support that gas-phase methods can be used to produce ultrapure non-agglomerated “singlet” nanoparticles having tunable sizes at room temperature. By controlling the temperature in the particle growth zone to guarantee complete coalescence of colliding entities, the size of singlets in principle can be regulated from that of single atoms to any desired value. We assess our results in the context of a simple analytical model to explore the dependence of singlet size on the operating conditions. Agreement of the model with experimental measurements shows that these methods can be effectively used for producing singlets that can be processed further by many alternative approaches. Combined with the capabilities of up-scaling and unlimited mixing that spark ablation enables, this study provides an easy-to-use concept for producing the key building blocks for low-cost industrial-scale nanofabrication of advanced materials.
The ability to tailor engineering nanoparticles (ENPs) is a key to designing novel nanostructured materials for applications in printable electronics1, energy conversion and storage2,3, catalysis4,5, sensor technology6,7,8, as well as products in medicine9. The size and composition of ENPs are the two most important variables determining the properties of the resulting materials and products. Gas-phase methods for ENP synthesis exhibit fast kinetics because the relevant diffusion coefficients are three orders of magnitude larger compared to those encountered in wet-chemistry techniques. Although the fast kinetics enable effective continuous processes, which are gaining significant ground in view of industrial applications over the recent years10,11, they commonly lead to agglomerated particles which are undesirable in many cases.
Here we challenge the paradigm that “nanoparticle synthesis in the gas-phase leads to agglomerates”12 by coining a scalable concept of “singlet” particle production. Using spark ablation as an example of the vapour point source, we show that the concept can lead to singlets ranging from clusters of a few atoms to particles of any desired size by tuning the operating conditions. In addition, using first principles we derive an analytical model for predicting the size of the singlet particles under different operating conditions. The proposed approach exhibits enormous flexibility for high-throughput and ultrapure production13, thereby advancing ENP synthesis and enabling low-cost fabrication of nanomaterials on an industrial scale.
Traditionally, generation of ENPs is performed by wet-chemistry techniques, which offer unique possibilities of controlling particle shape14. These techniques, however, employ precursor solutions which commonly result in impurities on the synthesized ENPs as well as in hazardous wastes. In contrast to these classical paths, dry gas-phase methods provide more versatile and more environmentally friendly alternatives15, involving a very limited number of preparation steps, producing ENPs in a continuous manner, allowing for simple and continuous conditioning and deposition/immobilization, and generating very little wastes. Clean and simple gas-phase processes such as laser ablation16 and electric discharges17,18, can directly and locally vaporize bulk materials to form nanoparticles, thereby avoiding involvement of any additional compounds and guaranteeing high purity. The resulting ultrapure nanoparticless can be further processed in the gas-phase19,20,21,22, before being deposited and immobilized onto various (flat or porous) substrates (e.g., silicon wafers, glass slides, polymeric, filter membranes, ceramics), thereby opening a land of new possibilities for producing hierarchically patterned coatings and membranes23. Alternatively, nanoparticle synthesized in the gas-phase can be suspended into a liquid for coulpling to the wet-chemsitry routes, enabling other innovative methodes for novel material synthesis20,24,25,26.
Due to fast kinetics, continuous gas-phase methods generally yield agglomerates consisting of primary particles (typically regarded as the smallest size the particles can have) that are difficult to take apart12. A number of previous works have therefore focused on ways to avoid the collisions of these particles before deposition or immobilization. In spark discharges, for example, agglomeration can be reduced by using the high space charge density27, but this can be a limiting factor for the scalability of the technique. For flame aerosol synthesis, it has been shown that the associated elevated temperatures lead to non-agglomerated particles having sizes in the micron range28. Modifying the operating conditions in the gas-phase processes can also lead to the production of atomic clusters29,30,31.
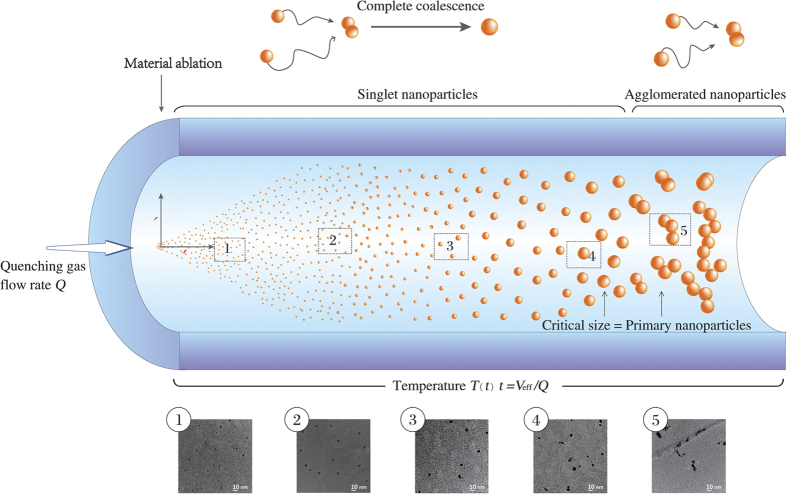
In this report, we provide a general concept of continuous gas-phase synthesis of ultrapure singlet particles ranging from single atoms to particles in the nanometre range. The essence of this concept is illustrated in Fig. 1. Vapours are produced by localized material ablation using lasers or electric discharges. The vapours are strongly quenched by an inert gas flow of variable temperature, thereby producing particles by condensation. As the supersaturations reached in the rapidly quenched vapour cloud are extremely high, the critical nucleus size is pushed down to the atomic scale. As a result, the growth governed by particle-particle collisions can be considered to start from the atomic scale, and therefore particle-particle collisional growth represents a valid model for the description of the size distribution evolution30,32,33,34,35. Note that this simplification is only valid in the case of rapidly quenched vapours emitted from point sources. If the quenching flows are low (and thus the cooling rates are substantially low) as in most nanoparticle production methods in the gas phase, more sophisticated models will be required to describe particle formation and growth36. The atomic clusters and smallest nanoparticles that are formed at the early stages of the process are liquid-like even at room temperature37, and therefore fully coalesce into singlets when colliding with each other. Growth of singlets to a critical size above which coalescence only partly occurs or ceases for the selected operating temperature (see below), signals the onset of agglomeration which leads to non-spherical/agglomerated particles. For drastically quenched processes, the temperature in the particle growth phase can be decoupled from the localized vaporization, and can be set to a value guaranteeing complete coalescence (cf. Fig. 1). In contrast to other high temperature aerosol synthesis methods28,38, this feature provides great flexibility in controlling the size of the resulting nanoparticles.
Figure 1. Schematic representation of the formation of singlet and agglomerated aerosol nanoparticles resulting from material ablation at atmospheric pressure.
Results
Theoretical framework
In order to exclusively produce singlet particles, the process must be controlled in a way that particle growth does not exceed the critical size, which in turn depends on the material of the particles and temperature38,39, and is relatively insensitive to other process parameters. In practice, singlets of any diameter can be achieved by controlling the temperature of the aerosol (i.e., particles dispersed within the carrier gas), so that coalescence is guaranteed up to the desired size. To further ensure that coalescence is not hindered by unwanted oxidation of particles due to the presence of trace amounts of oxygen and/or water in the carrier gas, extremely clean conditions are required throughout the production line. Such conditions can be achieved using suitable absorbers (i.e., molecular sieves and catalysts) to purify the carrier gas upstream the particle generator40.
In the next paragraphs we develop a simple model to predict the evolution of singlet nanoparticles produced by material ablation in the gas-phase under conditions that guarantee complete coalescence (see Supplementary Section S1). According to Smoluchoswski’s theory32, the decay rate of particle concentration dN(t)/dt is proportional to the square of N(t). Any particle losses by diffusional transport to the walls, where van der Waals forces normally guarantee sticking, can be approximated by a linear term in N(t)41,42. For sufficiently high concentrations during particle evolution, losses by diffusion to the walls as well as rapid turbulent dilution (cf. Supplementary Fig. S1 for additional details) can be neglected compared to the vigorous coagulation. N(t) can therefore be described by:
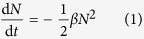 |
where 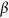 is the coagulation kernel, which changes with time depending on the momentary particle size distribution and temperature, gas flow conditions, and inter-particle forces43,44. For the early stages of atomic cluster formation,
is the coagulation kernel, which changes with time depending on the momentary particle size distribution and temperature, gas flow conditions, and inter-particle forces43,44. For the early stages of atomic cluster formation, 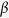 is unknown because the sticking probability after each collision depends largely on the stability of the atomic clusters45. Turbulent flow conditions that are not well enough defined in practical cases of particle coagulational growth, lead to yet another intractable problem in deriving any rigorous model for predicting the evolution of the particles. For tackling these problems, it is therefore instructive to evaluate the general behaviour of coagulating systems, governed by equation (1).
is unknown because the sticking probability after each collision depends largely on the stability of the atomic clusters45. Turbulent flow conditions that are not well enough defined in practical cases of particle coagulational growth, lead to yet another intractable problem in deriving any rigorous model for predicting the evolution of the particles. For tackling these problems, it is therefore instructive to evaluate the general behaviour of coagulating systems, governed by equation (1).
As the initial concentration N0 of the vapour atoms produced by material ablation is many orders of magnitude larger than the final concentration N(tf) of the particles (i.e., N0 >> N(tf)), equation (1) implies that N(tf) is independent of N0. In fact, N(tf) is only determined by the evolution of N(t) during approximately the last decade concentration reduction (cf. Supplementary Fig. S2)46. This stage, referred to as the “the final coagulation stage” in the following, covers most of the total coagulation time. Therefore, the complex turbulent flow conditions and the uncertainties in using an appropriate value for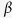 (associated with the early stages of atomic clusters growth) can be reasonably excluded as explained further in the supplement (cf. Supplementary Section S1 and S2 therein). For suitable ablation methods, the temperature in the particle growth region can be decoupled from that in the vaporization stage, and set to a well-defined value during the final coagulation stage. For a specific mass ablation rate, the particles grow approximately by a factor of two during the final coagulation stage due to the proportionality of particle size dp to
(associated with the early stages of atomic clusters growth) can be reasonably excluded as explained further in the supplement (cf. Supplementary Section S1 and S2 therein). For suitable ablation methods, the temperature in the particle growth region can be decoupled from that in the vaporization stage, and set to a well-defined value during the final coagulation stage. For a specific mass ablation rate, the particles grow approximately by a factor of two during the final coagulation stage due to the proportionality of particle size dp to 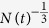 . Coagulation models show that β does not vary by more than a factor of two when particle size doubles, and its dominating value corresponds to the final size. It is therefore reasonable to assume that β is constant and solve equation (1) considering N0 >> N(tf) to yield:
. Coagulation models show that β does not vary by more than a factor of two when particle size doubles, and its dominating value corresponds to the final size. It is therefore reasonable to assume that β is constant and solve equation (1) considering N0 >> N(tf) to yield:
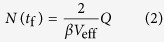 |
where Q is the gas volumetric flow rate and Veff the effective volume corresponding to the volume incorporating most of the flow and occupied by the coagulating aerosol. In a continuous flow arrangement, the residence time of the particles is then tf = Veff/Q and represents the duration for an aerosol parcel to travel from the vapour source to the point of measurement or further particle processing, where coagulation is inhibited by immobilization or adequate dilution. A good estimation for the relevant β can be based on the final particle size as explained above.
The increase of particle size due to coagulation is related to the decrease in number concentration. As the average particle size increased, the measured particle concentration drops while the total particle mass concentration 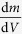 remains approximately constant47. Considering that the mass production rate
remains approximately constant47. Considering that the mass production rate 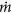 can be expressed as
can be expressed as 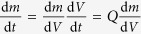 , the particle size dp can be calculated by:
, the particle size dp can be calculated by:
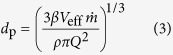 |
where 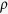 is the material density. This expression can be used to determine the particle size as a function of gas flow rate Q and particle mass production rate
is the material density. This expression can be used to determine the particle size as a function of gas flow rate Q and particle mass production rate 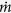 . Equation (3) is generally valid for any material ablation process or similar processes where vaporization is localized enough for the coagulation temperature, defining
. Equation (3) is generally valid for any material ablation process or similar processes where vaporization is localized enough for the coagulation temperature, defining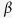 , to be decoupled from the vaporization temperature, at least during the final coagulation stage. Evidently, rapid dilution (i.e., dilution time td << tf; cf. Supplementary Fig. S1) and high enough temperature for complete coalescence as mentioned above, must also be ensured.
, to be decoupled from the vaporization temperature, at least during the final coagulation stage. Evidently, rapid dilution (i.e., dilution time td << tf; cf. Supplementary Fig. S1) and high enough temperature for complete coalescence as mentioned above, must also be ensured.
Considering that the particle losses are neglected, equation (3) will generally lead to an overestimation of particle size. We expect, however, the effect of losses on particle size to be moderate for most systems because 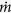 enters equation (3) as the cube root. We will show below that the model can be applied to a microsecond-pulsed particle source where in practice the system is mixed well enough to provide a uniform concentration during the final coagulation stage. The particle size predicted by equation (3) remains an approximation, but the elegance of the approach lies in the fact that uncertainties are circumvented because the final size depends on the final stage of the process, where the system is well defined.
enters equation (3) as the cube root. We will show below that the model can be applied to a microsecond-pulsed particle source where in practice the system is mixed well enough to provide a uniform concentration during the final coagulation stage. The particle size predicted by equation (3) remains an approximation, but the elegance of the approach lies in the fact that uncertainties are circumvented because the final size depends on the final stage of the process, where the system is well defined.
From this point onwards we will consider spark ablation as the source of vapours for particle formation. As a scalable technique, spark ablation has, to the best of our knowledge, the greatest versatility of all nanoparticle production methods. Being applicable to many inorganic materials13, and virtually allowing unlimited mixing combinations at atomic and nanometre scale48,49, it represents a powerful approach for synthesizing advanced materials with multiple fuctionalities50,51.
Details of the spark ablation setup are described in the experimental section (cf. Supplementary Section S3 and Supplementary Fig. S3 for additional information). In brief, repeated microsecond-pulsed sparks initiated between two electrodes ablate electrode materials to produce vapour clouds. These vapours are subsequently quenched by a high-purity gas flow and condensed to form atomic clusters and nanoparticles. Depending on the process variables (i.e., quenching gas flow rate Q, spark energy E, and spark repetition frequencies f), the resulting particles can have sizes that range from clusters of a few atoms up to any desired size. For the sake of completeness, it must be noted here that if agglomerates are produced by spark ablation, or by any other similar gas-phase method, they can be converted to singlet particles by heating in gas suspension after growth has essentially ceased38,52,53. This technique has been applied in diluted laboratory setups with particle mass production rates in the range of mg h−1. By contrast, the concept presented in this work, where coalescence is induced in the particle growth phase, is only limited by the vapour mass production rate from the spark ablated electrodes. Considering that the measurements of ablated mass per spark indicate that a production rate of the order of 1 g h−1 is feasible (cf. Supplementary Section S4), the concept can lead up to three orders of magnitude higher singlet particle production rate than other commonly used techniques13. Additionally, the possibility of numbering up the generators can further increase the production rate of the desired particles to meet industrial demands10.
The mass produced by a single spark is given by:
 |
here C is a material-dependent constant (cf. Supplementary equation (S6)), E the spark energy, and E0 is the minimum spark energy for producing particles (cf. Supplementary equation (S7)). C and E0 can be calculated by the evaporation model, which is derived by the energy balance of the evaporation process (cf. Supplementary equation (S5))54. The mass production rate is given by 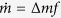 (cf. Supplementary Fig. S4), where f is the spark repetition frequency. Pulsed sparks can be regarded as a continuous particle source given that sufficient mixing guarantees a uniform concentration before the final coagulation stage is reached. Combining equations (3) and (4) yields:
(cf. Supplementary Fig. S4), where f is the spark repetition frequency. Pulsed sparks can be regarded as a continuous particle source given that sufficient mixing guarantees a uniform concentration before the final coagulation stage is reached. Combining equations (3) and (4) yields:
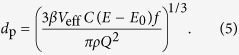 |
Recipes and sources for determining sufficiently accurate values for the required quantities of C, E0, β, and Veff are given in the supplement (cf. Supplementary Section S4 and S5). In the following, we justify the proposed model (i.e., equations (2) and (3) that give the evolution of particle concentration and size) with the measurements.
Experimental validation
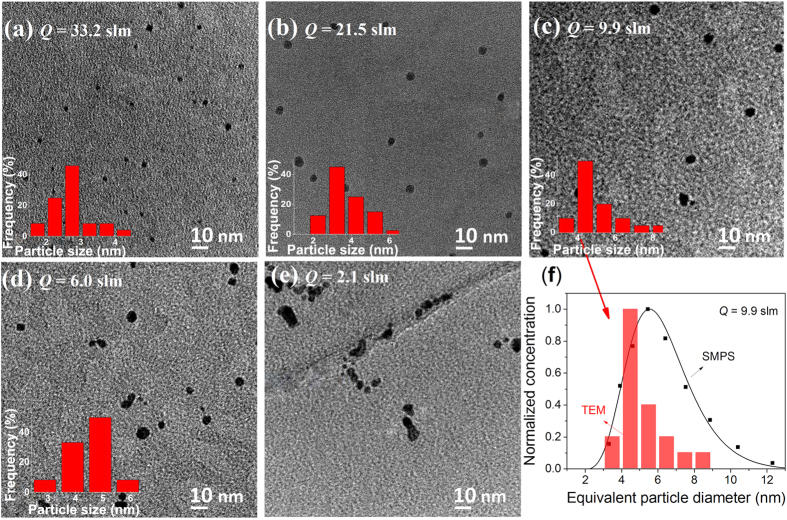
Figure 2(a–e) shows TEM images of particles collected using our experimental setup (cf. Supplementary Section S3 and Supplementary Fig. S3) under different quenching gas flow rates at a fixed spark energy E and frequency f, which allows variation of the singlet particle size from ca. 3 nm upwards. Evidently, the coalescence is complete up to sizes between 5 and 6 nm (cf. Supplementary Fig. S6 for more details). Larger particles are agglomerates, indicating that the selected coagulation temperature of ca. 20 °C is adequate for generation of Au singlet particles up to ca. 6 nm.
Figure 2. Electron Micrographs of Au singlets(a–c) and partly agglomerated particles (d and e) produced by spark ablation, and particle size distributions determined by the SMPS and TEM image at Q = 9.9 slm (f). (d,e) show that the size of primary particle is ca. 6 nm, which corresponds to the largest singelt particles of Au.
In coagulation with full coalescence, the size distribution approaches a lognormal self-preserving distribution with geometric standard deviation (GSD) ranging from ca. 1.33 to 1.3555. Figure 2f shows that the geometrical mean diameter (GMD) of the singlet particles measured by the scanning mobility particle sizer (SMPS) is ca.10% larger than that determined by the TEM images when Q = 9.9 standard litres per minute (slm). This discrepancy can be explained by the larger representation of the small particles (having large diffusion coefficients) collected by diffusion on the TEM grids. The limited number of particles counted in TEM images also adds a minor statistical error in the microscopy observations.
Figure 3 shows how the total number concentration of the particles at the exit of the particle generator varies with quenching gas flow rate. It should be noted here that the concentration is derived from the particle sizes measured by the SMPS with the assumption that there are no diffusional losses, i.e., mass of ablated material per unit volume is conserved (cf. Supplementary Fig. S2 and Supplementary equation (S4)). It should be pointed out that in order to derive the particle size distributions at the exit of the particle generator, the space charge effect in the differential mobility analyser (DMA)56 is neglected as it affects the particle size measurements by less than 4% assuming the highest possible concentration of charged particles we observed (ca. 107 cm−3; cf. Supplementary Section S7). Moreover, for particle concentrations of the order of 1010 cm−3, coagulation within the DMA can also be neglected during the short residence time of 0.15 s, as discussed in Supplementary Section S2 (cf. Supplementary Fig. S2).
Figure 3. Concentration of Au singlet particles as a function of quenching gas flow rate at a spark energy of 16 mJ and a spark repetition frequency of 60 Hz.
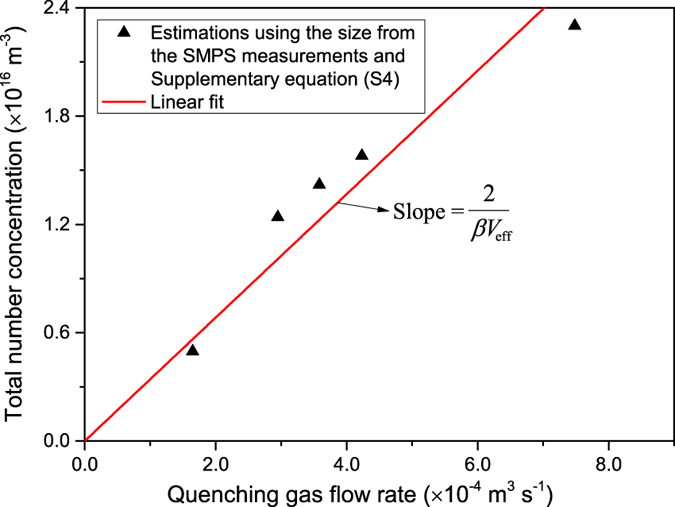
The estimation of singlet particle concentration confirms the dependence of N(tf) on Q predicted by equation (2), which has been derived using a constant 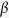 and assuming that N0 >> N(tf). The slope (i.e.,
and assuming that N0 >> N(tf). The slope (i.e., 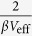 ) of the best-fitted straight line through the measurements indicates that the product
) of the best-fitted straight line through the measurements indicates that the product 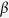 Veff is 5.93 × 10−20 m6 s−1. This value is in line with a theoretical value for
Veff is 5.93 × 10−20 m6 s−1. This value is in line with a theoretical value for 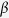 that considers poly-dispersity as well as van der Waals forces between the particles (cf. Supplementary Section S5.1 and S5.2)44,57. Note that the image potential described by Ouyang et al.44, can be neglected for our low concentration of charged particles (cf. Supplementary Section S5.3). The value of
that considers poly-dispersity as well as van der Waals forces between the particles (cf. Supplementary Section S5.1 and S5.2)44,57. Note that the image potential described by Ouyang et al.44, can be neglected for our low concentration of charged particles (cf. Supplementary Section S5.3). The value of 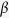 is in the order of 10−16 m3 s−1 (cf. Supplementary equation (S8)) based on the final (desired) size and an estimated effective volume Veff of 10−4 m3 corresponding to an adequate fraction of the volume of our spark chamber (cf. Supplementary Fig. S5).
is in the order of 10−16 m3 s−1 (cf. Supplementary equation (S8)) based on the final (desired) size and an estimated effective volume Veff of 10−4 m3 corresponding to an adequate fraction of the volume of our spark chamber (cf. Supplementary Fig. S5).
Figure 4 shows the variation of the GMD with the term 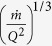 , which is linear according to equation (3). The slope (i.e.,
, which is linear according to equation (3). The slope (i.e., 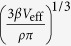 ) of the best-fitted solid line through the experiments is 1.42 × 10−9 kg−1/3 s−1/3 m3. For a density of 19.3 g cm−3 for Au, the product
) of the best-fitted solid line through the experiments is 1.42 × 10−9 kg−1/3 s−1/3 m3. For a density of 19.3 g cm−3 for Au, the product 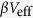 derived from this slope is 5.78 × 10−20 m6 s−1, which is in good agreement with the value derived from Fig. 3, and thus also with the theoretical value of
derived from this slope is 5.78 × 10−20 m6 s−1, which is in good agreement with the value derived from Fig. 3, and thus also with the theoretical value of 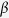 and an estimate of
and an estimate of 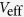 from the geometry of the confinement (see details in Supplementary Section S5). The value of
from the geometry of the confinement (see details in Supplementary Section S5). The value of 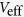 has the highest uncertainty. For systems of similar geometry to that used in this work we recommend
has the highest uncertainty. For systems of similar geometry to that used in this work we recommend 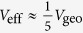 , where
, where 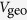 is the geometric volume of the confinement (details are provided in Supplementary Fig. S5). Note that an error in
is the geometric volume of the confinement (details are provided in Supplementary Fig. S5). Note that an error in 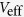 within a factor of two would only alter the predicted dp by 25%. Only the experimental values for particles having sizes below 7 nm are plotted in Fig. 4, since larger particles are agglomerates (cf. Fig. 2e) due to incomplete coalescence.
within a factor of two would only alter the predicted dp by 25%. Only the experimental values for particles having sizes below 7 nm are plotted in Fig. 4, since larger particles are agglomerates (cf. Fig. 2e) due to incomplete coalescence.
Figure 4. Mean mobility diameter of particles produce by spark ablation as a function of mass production rate and quenching gas flow rate.
Discussion
In principle, the GMD of singlet particles produced via spark ablation (and any other similar gas-phase process) can be tuned from atomic clusters to any desired size by carefully varying the gas flow rate Q, and the mass production rate 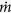 , which in turn is defined by the spark energy E and the spark repetition frequency f. Of course, full coalescence must be guaranteed by choosing a sufficiently high operating temperature and a high-purity carrier gas. In order to up-scale the singlet ENP production process, one needs to increase the production rate
, which in turn is defined by the spark energy E and the spark repetition frequency f. Of course, full coalescence must be guaranteed by choosing a sufficiently high operating temperature and a high-purity carrier gas. In order to up-scale the singlet ENP production process, one needs to increase the production rate 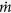 . This can be achieved by numbering up the particle generators, with each of them delivering a mass rate in the order of 1 g h−1.
. This can be achieved by numbering up the particle generators, with each of them delivering a mass rate in the order of 1 g h−1.
Our model can estimate the singlet size distribution at the outlet of the nanoparticle generator used in this work. The GMD is given by equation (3) while the lognormal size distribution has a self-preserving GSD of ca. 1.3555. Mobility size classification in the gas-phase can easily be applied for applications requiring narrower size distributions58, although it should be noted that doing so will lead to substantial particle losses and will therefore limit scalability.
Deposition of well-defined singlets on a substrate can yield films and materials of unique properties. Avoiding coalescence of the colliding particles on the substrate is a prerequisite to retain these properties. An elegant way to hinder coalescence is by coating the particles (e.g., with an oxidized layer) while they are still suspended in the gas (i.e., before deposition)59,60. For deposited uncoated particles, coalescence on the substrate can be avoided by keeping the surface coverage low enough (cf. Supplementary Fig. S8) or by manipulating charge effects between particles27,61. Low surface coverages are sufficient to improve the conversion efficiency of solar cells and photo catalysts for water splitting62,63. In the case of high surface coverage, where the particles are in contact, the substrate temperature should be controlled below the threshold temperature to avoid the coalescence of the arriving singlets64,65. Integrating gas-phase synthesis of singlets to wet-chemistry routes can also be used for supressing particle collisions, since the collision rate is decreased by three orders of magnitude in the liquid phase, and opens numerous possibilities of further processing20.
In summary, we introduce a general concept for continuous gas-phase synthesis of well-defined singlet particles in the nanometre size regime and even below that. The concept of promoting coalescence by using an ultrapure carrier gas and a sufficiently high temperature in the particle growth zone, has been tested on the example of synthesizing Au nanoparticles smaller than ca. 6 nm using spark ablation. In addition, we derive an analytical model that can be used to determine the combination of process parameters required to obtain singlet nanoparticles of a desired size. The model can be applied to predict the size (ranging from that of single atoms to any value) of singlet nanoparticles (consisting of any material; cf. Supplementary Fig. S9 of the Ag singlets) produced by rapidly quenched gas-phase processes.
Combined with the various advantages of continuous gas-phase processes, including their scalability, high particle purity and high versatility (i.e., particles of virtually any inorganic composition or mixture that spark ablation enables), the method used as an example here (spark ablation) exhibits enormous flexibility for high-throughput production of ultrapure singlets, especially in the size regime below 10 nm where other continuous scalable methods hardly exist. Consequently, the technique enables the advancment of ENP synthesis and paves the way towards cost-effective fabrication of novel nanomaterials for numerous applications (cf. Supplementary Section S8) on the industrial scale.
Methods
Spark Discharge Generator
A spark discharge generator consists of a pair of electrodes with a gap of ca. 1 mm between them, connected to an electric circuit (see Supplementary Fig. S3). The circuit induces microsecond pulsed discharges in a typical range of energy per spark from 0.3 to 200 mJ and a repetition frequency ranging from 0.1 to 25 kHz13. An inert gas flow continuously flushes the inter-electrode gap carrying away the produced vapors and particles to the point where they can be processed.
Experimental Set-up
The system consists of units for the generation (I), collection (II), and online size distribution measurement (III) of nanoparticles (cf. Supplementary Fig. S3). The size distributions of the resulting aerosols are measured with a Scanning Mobility Particle Sizer (SMPS) system, consisting of a differential mobility analyzer (DMA), and a Faraday cup aerosol electrometer (AEM), but avoiding an aerosol neutralizer. The singlet nanoparticless produced by the spark ablation were collected on TEM grids, using a custom-made electrostatic precipitator (ESP) placed at the DMA outlet. The DMA classifies the particles according to their mobility. The resulting nanoparticles are led to the SMPS through a ca. 0.4-m long stainless steel tube with an inner diameter of 4 mm.
Additional Information
How to cite this article: Feng, J. et al. Toward industrial scale synthesis of ultrapure singlet nanoparticles with controllable sizes in a continuous gas-phase process. Sci. Rep. 5, 15788; doi: 10.1038/srep15788 (2015).
Supplementary Material
Acknowledgments
The research leading to these results has received funding from the European Union’s Seventh Framework Program under Grant Agreement No. 280765 (BUONAPART-E).
Footnotes
Author Contributions J.F. designed and conducted all the experiments and measurements and prepared the manuscript. G.B. and A.S. contributed to the design of the experiments, and the revising of the manuscript.
References
- Cole J. J., Lin E. C., Barry C. R. & Jacobs H. O. Mimicking electrodeposition in the gas phase: a programmable concept for selected-area fabrication of multimaterial nanostructures. Small 6, 1117–1124 (2010). [DOI] [PubMed] [Google Scholar]
- Clavero C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 8, 95–103 (2014). [Google Scholar]
- Vons V. A., Leegwater H., Legerstee W. J., Eijt S. W. H. & Schmidt-Ott A. Hydrogen storage properties of spark generated palladium nanoparticles. Int. J. Hydrogen Energy 35, 5479–5489 (2010). [Google Scholar]
- Tedsree K. et al. Hydrogen production from formic acid decomposition at room temperature using a Ag-Pd core-shell nanocatalyst. Nat. Nanotechnol. 6, 302–307 (2011). [DOI] [PubMed] [Google Scholar]
- Decan M. R., Impellizzeri S., Marin M. L. & Scaiano J. C. Copper nanoparticle heterogeneous catalytic ‘click’ cycloaddition confirmed by single-molecule spectroscopy. Nat. Commun. 5, 4612 (2014). [DOI] [PubMed] [Google Scholar]
- Han X., Liu Y. & Yin Y. Colorimetric stress memory sensor based on disassembly of gold nanoparticle chains. Nano Lett. 14, 2466–2470 (2014). [DOI] [PubMed] [Google Scholar]
- Schmidt M. A., Lei D. Y., Wondraczek L., Nazabal V. & Maier S. A. Hybrid nanoparticle-microcavity-based plasmonic nanosensors with improved detection resolution and extended remote-sensing ability. Nat. Commun. 3, 1108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac N. A. et al. Optical hydrogen sensing with nanoparticulate Pd–Au films produced by spark ablation. Sensors Actuators B Chem. 221, 290–296 (2015). [Google Scholar]
- Fan K. et al. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol. 7, 459–464 (2012). [DOI] [PubMed] [Google Scholar]
- Charitidis C. A., Georgiou P., Koklioti M. A., Trompeta A.-F. & Markakis V. Manufacturing nanomaterials: from research to industry. Manuf. Rev. 1, 11 (2014). [Google Scholar]
- Mädler L., Kammler H. K., Mueller R. & Pratsinis S. E. Controlled synthesis of nanostructured particles by flame spray pyrolysis. J. Aerosol Sci. 33, 369–389 (2002). [Google Scholar]
- Gutsch A., Mühlenweg H. & Krämer M. Tailor-made nanoparticles via gas-phase synthesis. Small 1, 30–46 (2005). [DOI] [PubMed] [Google Scholar]
- Pfeiffer T. V., Feng J. & Schmidt-Ott A. New developments in spark production of nanoparticles. Adv. Powder Technol. 25, 56–70 (2014). [Google Scholar]
- Swihart M. T. Vapor-phase synthesis of nanoparticles. Curr. Opin. Colloid Interface Sci. 8, 127–133 (2003). [Google Scholar]
- Mcmillin B. K., Biswas P. & Zachariah M. R. In situ characterization of vapor phase growth of iron oxide-silica nanocomposites: Part I. 2-D planar laser-induced fluorescence and Mie imaging. J. Mater. Res. 11, 1552–1561 (1996). [Google Scholar]
- Morales A. M. & Lieber C. M. A laser ablation method for the synthesis of crystalline semiconductor nanowires. Science 279, 208–211 (1998). [DOI] [PubMed] [Google Scholar]
- Schwyn S., Garwin E. & Schmidt-Ott A. Aerosol generation by spark discharge. J. Aerosol Sci. 19, 639–642 (1988). [Google Scholar]
- Tabrizi N. S., Ullmann M., Vons V. A., Lafont U. & Schmidt-Ott A. Generation of nanoparticles by spark discharge. J. Nanoparticle Res. 11, 315–332 (2009). [Google Scholar]
- King D. M., Liang X. & Weimer A. W. Functionalization of fine particles using atomic and molecular layer deposition. Powder Technol. 221, 13–25 (2012). [Google Scholar]
- Byeon J. H. & Kim Y.-W. An aerosol-seed-assisted hybrid chemical route to synthesize anisotropic bimetallic nanoparticles. Nanoscale 4, 6726–6729 (2012). [DOI] [PubMed] [Google Scholar]
- Sengar S. K., Mehta B. R., Kumar R. & Singh V. In-flight gas phase growth of metal/multi layer graphene core shell nanoparticles with controllable sizes. Sci. Rep. 3, 2814 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurlin M. et al. Continuous gas-phase synthesis of nanowires with tunable properties. Nature 492, 90–94 (2012). [DOI] [PubMed] [Google Scholar]
- Peineke C. Production and Deposition of Well Defined Aerosol Nanoparticles for Studies of Basic Properties, Ch. 4, 97–112 (TU Delft, 2008).
- Sanedrin R. G., Georganopoulou D. G., Park S. & Mirkin C. A. Seed-Mediated Growth of Bimetallic Prisms. Adv. Mater. 17, 1027–1031 (2005). [Google Scholar]
- Tour J. M. Materials chemistry: Seeds of selective nanotube growth. Nature 512, 30–31 (2014). [DOI] [PubMed] [Google Scholar]
- Boissiere C., Grosso D., Chaumonnot A., Nicole L. & Sanchez C. Aerosol route to functional nanostructured inorganic and hybrid porous materials. Adv. Mater. 23, 599–623 (2011). [DOI] [PubMed] [Google Scholar]
- Park K.-T., Farid M. M. & Hwang J. Anti-agglomeration of spark discharge-generated aerosols via unipolar air ions. J. Aerosol Sci. 67, 144–156 (2014). [Google Scholar]
- Pratsinis S. E. Flame aerosol synthesis of ceramic powders. Prog. Energy Combust. Sci. 24, 197–219 (1998). [Google Scholar]
- Wang Y. et al. Application of Half Mini DMA for sub-2 nm particle size distribution measurement in an electrospray and a flame aerosol reactor. J. Aerosol Sci. 71, 52–64 (2014). [Google Scholar]
- Fang J. et al. Measurement of sub-2 nm clusters of pristine and composite metal oxides during nanomaterial synthesis in flame aerosol reactors. Anal. Chem. 86, 7523–7529 (2014). [DOI] [PubMed] [Google Scholar]
- Peineke C. et al. Production of equal sized atomic clusters by a hot wire. J. Aerosol Sci. 40, 423–430 (2009). [Google Scholar]
- Smoluchowski M. von. Versuch einer mathematischen theorie der koagulationskinetik kolloider lösungen. Z. phys. Chem 92, 129–168 (1917). [Google Scholar]
- Thajudeen T., Gopalakrishnan R. & Hogan C. J. The collision rate of nonspherical particles and aggregates for all diffusive Knudsen numbers. Aerosol Sci. Technol. 46, 1174–1186 (2012). [Google Scholar]
- Gopalakrishnan R., Thajudeen T. & Hogan C. J. Collision limited reaction rates for arbitrarily shaped particles across the entire diffusive Knudsen number range. J. Chem. Phys. 135, 054302 (2011). [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan R. & Hogan C. J. Determination of the transition regime collision kernel from mean first passage times. Aerosol Sci. Technol. 45, 1499–1509 (2011). [Google Scholar]
- Biswas P., Wu C. Y., Zachariah M. R. & McMillin B. Characterization of iron oxide-silica nanocomposites in flames: Part II. Comparison of discrete-sectional model predictions to experimental data. J. Mater. Res. 12, 714–723 (1997). [Google Scholar]
- Bourlinos A. B. et al. Surface-functionalized nanoparticles with liquid-like behavior. Adv. Mater. 17, 234–237 (2005). [Google Scholar]
- Kruis F. E., Fissan H. & Rellinghaus B. Sintering and evaporation characteristics of gas-phase synthesis of size-selected PbS nanoparticles. Mater. Sci. Eng. B 69–70, 329–334 (2000). [Google Scholar]
- Lehtinen K. E. J. & Zachariah M. R. Energy accumulation in nanoparticle collision and coalescence processes. J. Aerosol Sci. 33, 357–368 (2002). [Google Scholar]
- Vons V. A. et al. Silicon nanoparticles produced by spark discharge. J. Nanoparticle Res. 13, 4867–4879 (2011). [Google Scholar]
- Alonso M. Effect of diffusion losses on the size growth of nanoparticles by coagulation. Rev. Metal. 34, 413–415 (1998). [Google Scholar]
- Uhrner U. et al. Dilution and aerosol dynamics within a diesel car exhaust plume-CFD simulations of on-road measurement conditions. Atmos. Environ. 41, 7440–7461 (2007). [Google Scholar]
- Seinfeld J. H. & Pandis S. N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. (John Wiley & Sons, 2006). [Google Scholar]
- Ouyang H., Gopalakrishnan R. & Hogan C. J. Nanoparticle collisions in the gas phase in the presence of singular contact potentials. J. Chem. Phys. 137, 064316 (2012). [DOI] [PubMed] [Google Scholar]
- Schmidt M., Kusche R., von Issendorff B. & Haberland H. Irregular variations in the melting point of size-selected atomic clusters. Nature 393, 238–240 (1998). [Google Scholar]
- Hinds W. C. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. (John Wiley & Sons, 1999). [Google Scholar]
- Ravi L. & Girshick S. L. Coagulation of nanoparticles in a plasma. Phys. Rev. E 79, 026408 (2009). [DOI] [PubMed] [Google Scholar]
- Tabrizi N. S., Xu Q., Pers N. M. & Schmidt-Ott A. Generation of mixed metallic nanoparticles from immiscible metals by spark discharge. J. Nanoparticle Res. 12, 247–259 (2010). [Google Scholar]
- Anastasopol A. et al. Reduced enthalpy of metal hydride formation for Mg-Ti nanocomposites produced by spark discharge generation. J. Am. Chem. Soc. 135, 7891–7900 (2013). [DOI] [PubMed] [Google Scholar]
- Banin U., Ben-Shahar Y. & Vinokurov K. Hybrid semiconductor–metal nanoparticles: from architecture to function. Chem. Mater. 26, 97–110 (2014). [Google Scholar]
- Chung H. T., Won J. H. & Zelenay P. Active and stable carbon nanotube/nanoparticle composite electrocatalyst for oxygen reduction. Nat. Commun. 4, 1922 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ott A. In situ measurement of the fractal dimensionality of ultrafine aerosol particles. Appl. Phys. Lett. 52, 954–956 (1988). [Google Scholar]
- Schmidt-Ott A. New approaches to in situ characterization of ultrafine agglomerates. J. Aerosol Sci. 19, 553–563 (1988). [Google Scholar]
- Llewellyn Jones F. Electrode erosion by spark discharges. Br. J. Appl. Phys. 1, 60–65 (1950). [Google Scholar]
- Vemury S. & Pratsinis S. E. Self-preserving size distributions of agglomerates. J. Aerosol Sci. 26, 175–185 (1995). [Google Scholar]
- Camata P. R., Atwater A. H. & Flagan C. R. Space-charge effects in nanoparticle processing using the differential mobility analyzer. J. Aerosol Sci. 32, 583–599 (2001). [Google Scholar]
- Lee K. W. & Chen H. Coagulation rate of polydisperse particles. Aerosol Sci. Technol. 3, 327–334 (1984). [Google Scholar]
- Biskos G., Vons V. A., Yurteri C. U. & Schmidt-Ott A. Generation and sizing of particles for aerosol-based nanotechnology. KONA Powder Part. J. 26, 13–35 (2008). [Google Scholar]
- Pfeiffer T., Kedia P., Messing M. Valvo M.& Schmidt-Ott A. Precursor-less coating of nanoparticles in the gas phase. Materials 8, 1027–1042 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-T. & Chang J.-S. Generation of metal oxide aerosol particles by a pulsed spark discharge technique. J. Electrostat. 63, 911–916 (2005). [Google Scholar]
- Zonnevylle A. C., Hagen C. W., Kruit P. & Schmidt-Ott A. Directed assembly of nano-particles with the help of charge patterns created with scanning electron microscope. Microelectron. Eng. 86, 803–805 (2009). [Google Scholar]
- Pfeiffer T. V. et al. Plasmonic nanoparticle films for solar cell applications fabricated by size-selective aerosol deposition. Energy Procedia 60, 3–12 (2014). [Google Scholar]
- Valenti M., Dolat D., Biskos G., Schmidt-ott A. & Smith W. A. Enhancement of the photoelectrochemical performance of CuWO4 thin films for solar water splitting by plasmonic nanoparticle functionalization. J. Phys. Chem. C 119, 2096–2104 (2015). [Google Scholar]
- Thimsen E. & Biswas P. Nanostructured photoactive films synthesized by a flame aerosol reactor. AIChE J. 53, 1727–1735 (2007). [Google Scholar]
- Biswas P. & Elijah T. Aerosol Measurement: Principles, Techniques, and Applications 3rd edn, (eds Kulkarni P. et al.) Ch. 33, 723–738 (John Wiley & Sons, 2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.