Abstract
We developed a novel bath-immersion technology to produce large numbers of infertile fish. As seafood consumption shifts from fishery harvests towards artificially propagated species, optimization of aquaculture practices will be necessary to maximize food production and minimize ecological impact. Farming infertile fish is the most effective genetic-containment strategy to support the development of environmentally-responsible aquaculture. We discovered that a molecular transporter, Vivo, can effectively carry the Morpholino oligomer (MO) across the chorion, enter the embryo and reach target cells. Vivo-conjugated MO against zebrafish deadend (dnd-MO-Vivo) effectively caused primordial germ cell mis-migration and differentiation into somatic cells, which resulted in generation of infertile fish. Optimal conditions were achieved when embryos, immediately after fertilization, were immersed with dnd-MO-Vivo at the initial concentration of either 60 or 40 μM followed by a lower serially diluted concentration. Under these conditions, 100% induced sterility was achieved even when the total immersion time was reduced from 24 to 5 hours. In 8 independent experiments, 736 adults developed from these conditions were all found to be infertile fish that possessed minimally-developed gonads that lacked any gametes. The results demonstrate that dnd-MO-Vivo bath immersion is an effective strategy to produce infertile fish without introducing transgenic modifications.
Aquaculture is progressively becoming more prevalent and vital to resolve the current and projected shortages in aquatic food availability. While the shift in reliance from fishery harvests to artificially propagated aquatic species continues, the increase in aquaculture activities poses a great threat to our ecosystem and environment. Non-native, selectively bred and, eventually, genetically modified farmed fish may escape from aquaculture containments, propagate and/or interbreed with wild stock, subsequently changing the genetic composition of populations or causing species extinction1,2. The use of reproductively sterile farmed fish will be the most effective strategy for genetic-containment, particularly in large scale operations, thereby achieving environmentally-responsible aquaculture practices. Infertile fish are desirable for a number of reasons. First, sterility carries environmental significance, as the escape of cultured sterile fish alleviates the threat to ecological balance and genetic contamination of wild populations1,2. Second, sterilization enhances muscle development by minimizing energy input toward gonadal growth and prevents sexual maturation that can cause deterioration of flesh quality and an increase in susceptibility to stress and disease3. Third, sterility is a means for producers to safeguard against unauthorized propagation of valuable fish strains.
In this study, we developed a bath immersion technology to produce infertile fish. We discovered that a molecular transporter comprised of a dendrimeric oligoguanidine with a triazine core, also known as Vivo4, can effectively traverse the Morpholino oligomer (MO) across the chorion and reach embryos. Vivo-conjugated MO against zebrafish deadend (dnd-MO-Vivo), an essential gene for zebrafish primordial germ cell (PGC) development5,6, effectively disrupted PGC development that led to the elimination of germ cells and resulted in the development of reproductive sterile fish. Our technology offers the aquaculture industry a convenient approach to maintain a fertile broodstock and generate infertile farmed fish without the introduction of transgenic modifications to foodfish.
Results
Vivo, a molecular transporter, effectively carries zebrafish dnd-MO across the chorion, transports it into the embryos and delivers it to target cells, resulting in disruption of PGC development
Dnd has been proven to be an essential protein for germ cell development during embryogenesis in zebrafish5,6, loach7 and goldfish8. Knockdown of Dnd expression by microinjecting embryos with dnd-MO disrupted PGC development and resulted in reproductively sterile fish. These results indicate that blocking Dnd function is a viable approach to produce reproductively sterile fish. However, microinjection is not a technically or economically effective method for large-scale commercial operations. Hence, a bath immersion technology was developed to administer dnd-MO into embryos. In order to visualize and monitor germ cell development, Tg (kop:DsRed-nanos3) transgenic embryos that express DsRed specifically in the PGCs under the control of the germ-cell-specific kop promoter and nanos3 3′UTR9 were used to develop and optimize the bath immersion technology, utilizing zebrafish dnd-MO or a molecular transporter (Vivo) conjugated to dnd-MO (dnd-MO-Vivo). When embryos were immersed with 20 μM of dnd-MO, they developed normally and reached the 1K cell stage at 3 hours post-fertilization (hpf) and the 30–50% epiboly stage at 5 hpf (Fig. 1A, B) with no observable difference from the water-only controls. When embryos were immersed with 20 μM of dnd-MO-Vivo, their development was slightly delayed and only reached the 256–512 cell stage at 3 hpf (Fig. 1C) and the sphere stage at 5 hpf (Fig. 1D) with uncharacterized aggregates found around the inner part of the chorion or the surface of the blastodisc of embryos. These aggregates were not seen in the control groups that were immersed with water or 20 μM of dnd-MO (Fig. 1A,B). The results indicated that Vivo was able to act on and traverse the chorion, a thick acellular multi-layer coat, which resulted in the presence of the uncharacterized aggregates. In order to evaluate whether the Vivo-conjugated dnd-MO can enter embryos and eventually reach PGCs and knock down Dnd expression, the bath immersion protocol was optimized. A series titration in a 24-hour (total time) immersion bath was developed by starting the immersion at either 60, 40, 20 or 10 μM and ending at 5 μM of dnd-MO-Vivo. Under these immersion conditions, most embryos survived after treatment (Tables S1–4). The immersed embryos were examined using a fluorescence microscope at 2 to 3 days post-fertilization (dpf). In the control groups that were immersed in dnd-MO, control-MO-Vivo or water, PGCs migrated to the gonadal region and maintain their morphology as round-shaped cells (Fig. 2A). When embryos were initially treated with either 60 or 40 μM dnd-MO-Vivo, disruption of PGC development was found in all embryos examined, in which the PGCs were found to be at ectopic areas and some of them have differentiated into other cell types that can be clearly seen by the change of their morphology (Fig. 2B).
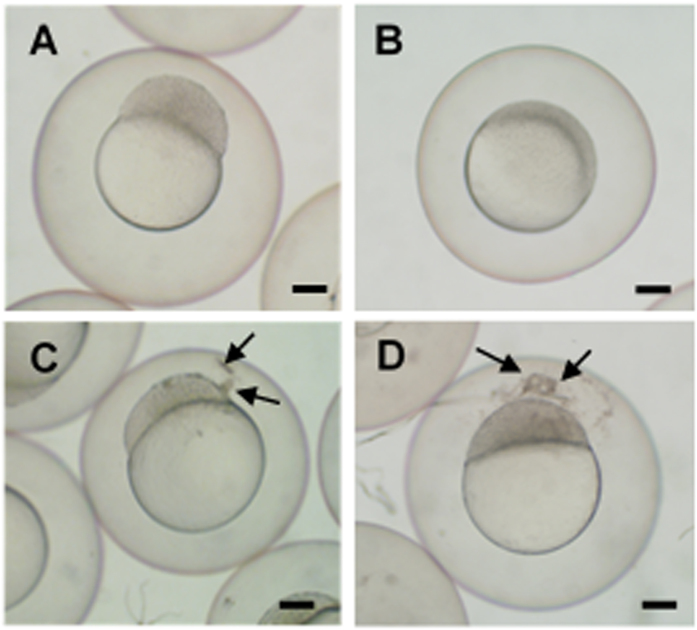
Figure 1. Vivo conjugated Morpholino oligomer (MO) caused developmental delays and uncharacterized aggregates in zebrafish embryos.
When treated with 20 μM dnd-MO, embryos developed normally and reached (A) 1K cell stage after a 3 hour immersion and (B) 30-50% epiboly stage after a 5 hour immersion. When treated with 20 μM Vivo conjugated dnd-MO (dnd-MO-Vivo), embryo development was slightly delayed and only reached (C) 256-512 cell stage with aggregates found between the chorion and blastodisc (arrows) after a 3 hour immersion, and only reached (D) sphere stage with more aggregates (arrows) after a 5 hour immersion. Scale bar = 200 μm.
Figure 2. Zebrafish dnd-MO-Vivo disrupted germ cell development in zebrafish.
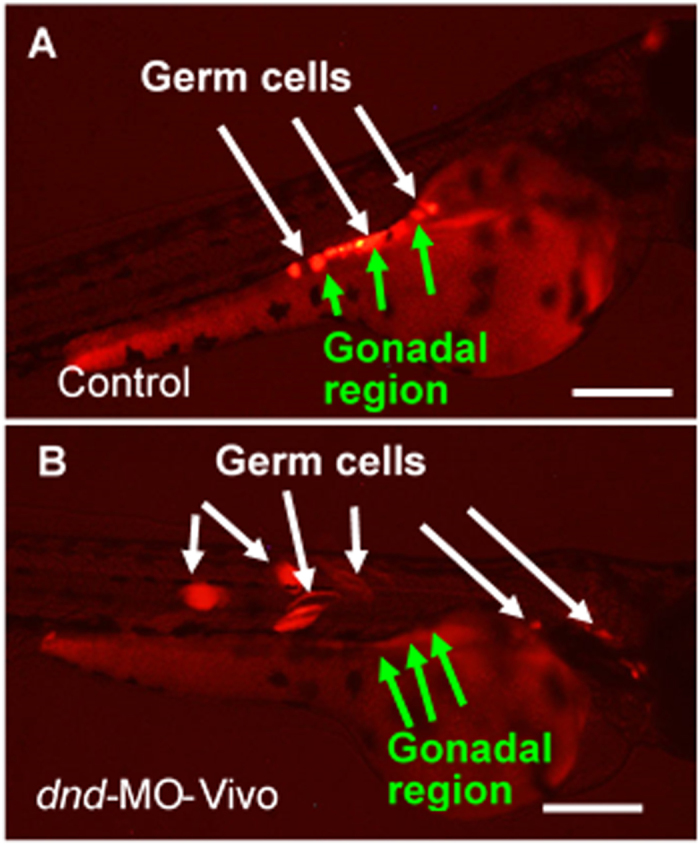
(A) In control embryos immersed in only water or dnd-MO solution, germ cells migrated to the gonadal region and maintained their morphology as round-shaped cells. (B) Treatment of dnd-MO-Vivo caused germ cell mis-migration and eventually differentiation into other cell types that can be clearly seen by the change of their morphology. Scale bar = 200 μm.
Embryos immersed with dnd-MO-vivo developed into reproductively sterile adults
The bath-immersed embryos were raised to adults and the development of the fish was examined. A total of 1,201 adult fish were obtained from 4 separate 24-hour immersion experiments in which all of the 301 adult fish developed from embryos that were initially immersed with dnd-MO-Vivo at 60 μM for 0.5–1.5 hours, or at 40 μM for 3 hours, immediately after fertilization were found to be infertile. When embryos were initially immersed with 20 μM dnd-MO-Vivo, a total of 132 out of 255 fish were infertile. None of the control fish that were immersed with either dnd-MO, standard control MO-Vivo or water were found to be infertile (Table S1–4). The infertile adult fish have a male-like presence and exhibited no observable difference in survival rate, appearance (normality) and body weight from control males (Fig. 3A,B). Unlike control males, no sperm could be expressed from these male-like fish by gently pressing on their abdomen. Dissections were performed to examine gonad development in dnd-MO-Vivo immersed fish and control fish. The results revealed that testes (Fig. 3C) and ovary (Fig. 3D) development was normal in the control fish while gonad development was absent, except for a thin filament of connective tissue surrounded by fat tissue in all individuals that were initially treated with 60 or 40 μM dnd-MO-Vivo (Fig. 3E). Histological examination revealed that control male and female fish possessed fully formed gonads and active gametogenesis (Fig. 3F,G). In contrast, no gametogenesis was found in the filament-like gonad from dnd-MO-Vivo immersed fish (Fig. 3H). The treatment conditions and sterility results of 4 independent experiments that were immersed with dnd-MO-Vivo initially at either 60, 40, 20 or 10 μM, followed by a serially diluted concentration to final 5 μM for a total 24 hour immersion (Tables S1–4) are summarized in Fig. 4A.
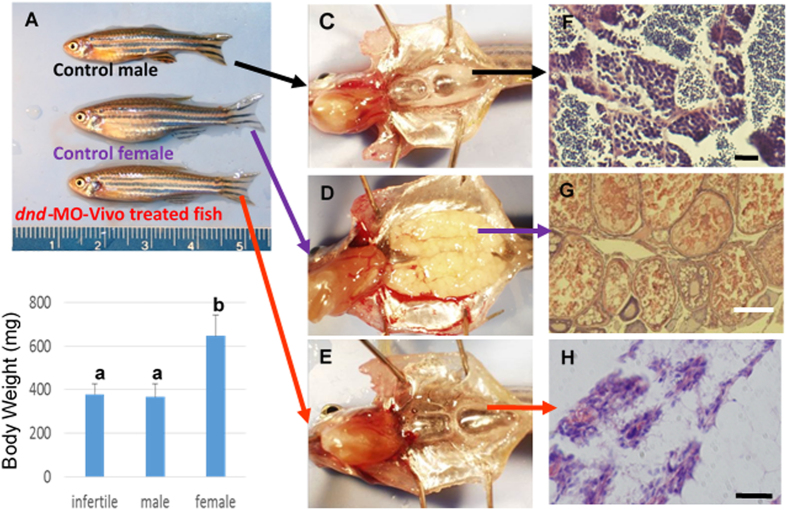
Figure 3. Zebrafish dnd-MO-Vivo induced sterility in zebrafish.
Embryos initially treated with 60 or 40 μM of dnd-MO-Vivo developed into male-like adults. (A) No difference in appearance or overall size was observed between treated adult fish and control males. (B) No significant difference in body-weight (Mean ± SD) of 3-month-old fish (N = 12 by random sampling) was noted among dnd-MO-Vivo treated fish and control males (Data that share the same letter are not significantly different from each other). Examination of gonadal tissue showing (C) a fully-developed testis of a control male fish, (D) a fully-developed ovary of a control female fish, (E) the gonads of dnd-MO-Vivo treated fish that developed into a thin filament-like tissue. Photomicrographs (F–H) show (F) advanced spermatogenesis in the testis of a control male fish, (G) a well-developed ovary of a control female fish with oocytes at advanced stages of gametogenesis, (H) the gonad of dnd-MO-Vivo treated fish appears to be under-developed and surrounded with a large amount of adipocytes, without advanced gonadal structure or germ cells. Scale bar: white = 200 μm, black = 20 μm.
Figure 4. Zebrafish dnd-MO-Vivo treated embryos developed into infertile adults.
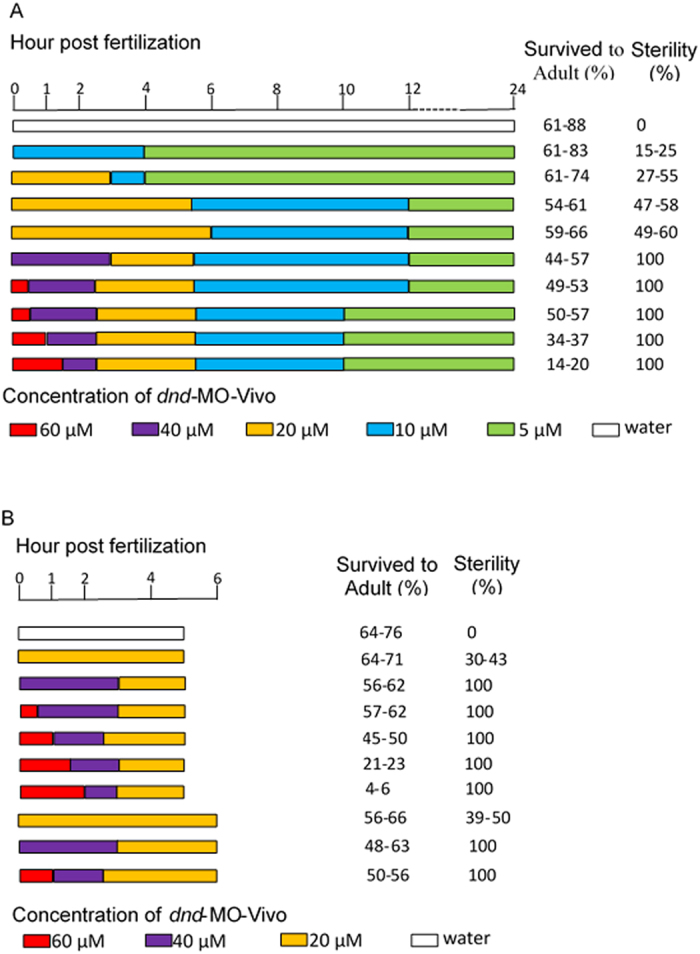
In both (A) 24 hour immersions (data summarized from Tables S1–4) and (B) 5 to 6 hour immersions (data summarized from Tables S5–8), all the embryos that were initially immersed, immediately after fertilization, with 60 or 40 μM of zebrafish dnd-MO-Vivo developed into infertile fish. Data includes the water-only controls. Control-MO-Vivo and dnd-MO controls are presented in Table S1–8.
Treatment conditions affected survival rate and efficiency of sterility
The above results demonstrated that reproductively sterile fish can be obtained when fish embryos were initially immersed with either 60 or 40 μM dnd-MO-Vivo (Tables S2–4). However, 100% sterility could be achieved only when the embryos were immersed immediately after in vitro fertilization. If the naturally spawned embryos (about 30–60 minutes post-fertilization) were collected and used for immersion, only 52–68% of the embryos initially treated with 60 μM of dnd-MO-Vivo developed into infertile fish. Similar data were obtained when the immersion of in vitro fertilized embryos were started at 1 hpf, which resulted in only 44–59% of infertile fish (Table S4). These results indicated that treatment during the first hour after fertilization has an important effect on the efficiency of bath immersion. It also suggested that the total time of immersion could be shortened. As such, we optimized the protocol by reducing the length of bath immersion. In 4 separate 5 to 6-hour immersions, all 435 fish that were initially immersed with 60 or 40 μM of dnd-MO-Vivo developed into infertile adults. When embryos were immersed with 20 μM of dnd-MO-Vivo, only 30–50% of them developed into infertile adults (Tables S5–8). Furthermore, when embryos were immersed with 60 μM of dnd-MO-Vivo for longer than 1 hour the survival rate of treated individuals decreased dramatically. Only 4–6% of embryos survived to adults when they were initially treated with 60 μM of dnd-MO-Vivo for 2 hours (Table S6). When the total length of immersion was shortened to 4 hours, 100% sterility was not achieved even when embryos were initially treated with either 60 or 40 μM (data not shown). The treatment conditions and sterility results of 4 independent experiments that were immersed with dnd-MO-Vivo initially at either 60, 40 or 20 μM, followed by a serially diluted concentration for a total 5–6 hour immersion (Tables S5–8) are summarized in Fig. 4B. Optimal conditions resulting in 100% sterility induction were achieved when embryos, immediately after fertilization, were immersed with dnd-MO-Vivo at an initial concentration of either 60 or 40 μM.
Discussion
To meet the demand of growing seafood consumption and compensate for the global decline of wild fishery stocks, it is imperative that highly efficient aquaculture practices are developed to enhance fishery production. However, the expansion of aquaculture operations also creates a great risk to our ecosystems and environment. Effective and practical fish sterilization technologies are crucial to resolve current and predicted threats posed by escapees from fish farms. Manipulating chromosome set normality by triploidization or interspecies hybridization is the most common method used to produce infertile fish10,11. However, some hybrids and triploids were found to be fertile10,11,12,13 and/or under-performing14,15,16. Another approach is the inhibition of gonadotropin-releasing hormone (Gnrh), a decapeptide required to develop and maintain a normal reproductive system in vertebrates, by transgenically expressing Gnrh antisense RNA to block Gnrh expression. Yet, low levels of Gnrh expression or development persist in some fish, resulting in a failure to completely induce sterility17. Recently, genetic germ-cell ablation by inducing germ cell death18,19,20 or disruption of germ cell development9 has been successfully developed to generate sterile fish21. The downside of this approach is that it requires the generation of transgenic fish in each species, thus introducing genetic modification into the fish and leading to long delays for regulatory approval as well as potential consumer resistance22,23. To overcome this hurdle, non-transgenic approaches to produce infertile fish are urgently needed to ease food safety concerns and propel aquaculture operations toward more environmentally responsible practices.
In this study, we developed a non-transgenic bath immersion method to induce fish sterility. Our technology is based on the administration of an antisense MO to block Dnd synthesis, which has been shown to be effective for inducing sterility in several fish species. To date, all the successful approaches were through the microinjection of dnd-MO into early-stage embryos5,6,7,8, which is not practical in commercial aquaculture. Immersion treatment can be used to administer bio-active or beneficial compounds to fish eggs or early-stage embryos. However, due to limited permeability of the chorion24, larger molecular compounds are not able to traverse the chorion and reach the embryo. Our innovation is based on the use of a molecular transporter that is able to effectively carry the compounds conjugated to it across the chorion, a thick acellular multi-layer envelope24,25, transport it into the egg or embryo, and deliver it to the target cells. This molecular transporter, also known as Vivo, was originally designed to induce endocytosis by transporting conjugated molecules into cells4. Similarly, after crossing the chorion, Vivo also promotes up-take of conjugated molecules into embryonic cells through endocytosis. Although it is not known how Vivo-conjugated compounds cross the chorion, and in particular whether they traverse through miniscule channels and pores, the aggregates found around the inner part of the chorion after the embryos were immersed with dnd-MO-Vivo and the ultimate disruption of PGC development indicated that Vivo is able to act on the chorion and transport the conjugated compound (dnd-MO-Vivo) across the barrier, thereby reaching the PGCs. These results were not seen in the control group of embryos that were immersed with dnd-MO. Our findings also indicated that Dnd protein may play an essential role in blocking PGCs from differentiating to other cell types, thus maintaining their germ cell characteristics. Knockdown of Dnd caused the differentiation of PGCs to fiber-like and other somatic cell types. This kind of cell morphology change and differentiation were not seen in other mis-migrated PGCs that were caused by over-expression of leukemia inhibitory factor26 or stromal-derived factor 1a9.
The identification of novel functionality of Vivo might also provide a method to efficiently deliver other larger molecules such as proteins, antibodies, DNAs and RNAs into fish eggs by conjugating them to Vivo, which has never been achieved without using micro-injection. Our results demonstrated that effective uptake of dnd-MO-Vivo and 100% sterility can be achieved under optimal treatment conditions. However, they also indicated that higher concentration and longer duration of immersion could increase mortality. When 100% sterility was achieved under optimized conditions, there were about 15–20% less fish that survived to adulthood (compared to control groups). Our results have shown that a slight delay of embryonic development occurred when embryos were immersed with dnd-MO-Vivo. In high concentration and longer duration of dnd-MO-Vivo immersion, the continuously high endocytosis activity during earlier embryonic stages may have slowed down and, eventually, irreversibly disrupted embryonic development, which led to the death of the embryos. Because these deaths happen at very early stages of development, it causes less destruction to aquaculture operations than deaths occurring at later stages of development. That being said, the complete pharmacology and animal/environmental toxicology of Vivo-MO will need to be studied before this technology is utilized by the aquaculture industry to produce sterile foodfish. It is also worth emphasizing that immersion delivery needs to be introduced shortly after fertilization. The delay of the initiation of immersion has been shown to reduce the efficiency of sterility induction. After fish eggs are fertilized and water-activated, the chorion permeability continues to decrease due to water hardening24,27,28, which may affect the up-take of dnd-MO-Vivo. As such, the first several hours after fertilization are the critical window for bath immersion effectiveness. Additionally, when the MO is taken up earlier, it may be distributed more efficiently to target cells and begin its knockdown function earlier, which also contributes to its effectiveness. MO antisense technology provides a promising approach to knock down gene expression and study gene function in vivo. However, due to unexpected non-specific interactions of MO with proteins and non-target mRNA29, and given that the addition of Vivo that may cause other non-specific effects, it is imperative that each species-specific dnd-MO-Vivo be evaluated carefully with the necessary controls.
Zebrafish were selected for initial development of the technical methodology, due to their short generation time and the large numbers of embryos that can be readily produced per pair (on a daily basis, if desired)30,31. Additionally, the transparent embryos and the availability of the transgenic Tg (kop:DsRed-nanos3) zebrafish line9 enables the visual observation of the early germ cells. Dnd is highly conserved in fish and its essential role in PGC development has been demonstrated in multiple species5,6,7,8. Moreover, development of PGCs and gonads within the embryo is an evolutionarily conserved mechanism in fish32. As such, it is expected that the technology can be successfully applied to other fish including a wide variety of aquaculture species. The success of our approach potentially provides an inducible method to generate reproductively sterile fish for aquaculture production without introduction of any genetic modifications into the foodfish. Additionally, the use of bath immersion makes it convenient to maintain a fertile broodstock population by simply omitting the treatment of the embryos. Our technology can thus be used for genetic containment and cost-effective aquaculture operations, which will contribute to the development of environmentally and economically sustainable production to meet the growing global demand for seafood.
Methods
Animals and Ethics
Zebrafish were maintained and staged as previously described33. All of the experimental procedures and protocols described in this study were approved by the University of Maryland Animal Care and Use Committee and adhered to the National Research Council’s Guide for Care and Use of Laboratory Animals.
Bath immersion
To visualize PGC development, embryos of Tg (kop:DsRed-nanos3) zebrafish that carry maternal DsRed-labeled PGCs were generated by in vitro fertilization and 50 eggs in duplication were set aside for fertilization rate measurement when embryos developed to the 16 to 32 cell stage. To perform in vitro fertilization, sperm was collected from 4–8 males and suspended in 0.1 ml of ice-cold Hank’s solution, and eggs from 4–8 females were collected in a 10 cm Petri dish following a published protocol33. 30–50 μl of the sperm/Hank’s solution was added to the eggs and mixed gently, followed by the addition of 2 ml of fresh tank system water for 1 minute. An additional 10 ml of fresh tank system water was added to the Petri dish for another 2-minute incubation and then the fertilization solution was replaced with15–20 ml of fresh tank system water. 50–80 eggs were transferred into each well of 48-well plates that contained 300 μl of fresh tank system water. After the allocation of eggs, system water in each well was replaced with 200–300 μl of water that initially contained 0–60 μM of zebrafish dnd-MO (5′-GCTGGGCATCCATGTCTCCGACCAT-3), dnd-MO-Vivo or standard control MO-Vivo (5′-CCTCTTACCTCAGTTACAATTTATA-3′) (Gene Tools LLC. Philomath, OR, USA). After 0.5–3 hours of incubation, the initial concentration of dnd-MO, dnd-MO-Vivo or control-MO-Vivo was gradually decreased by adding 1-fold of water each time to the desired final incubation concentration. To prevent rapid change of the dnd-MO-Vivo concentration during the dilution, one half of the immersion solution of each treatment was transferred and mixed with 1 volume of water and then the solution was gradually transferred back to each well. The total immersion time was 4 to 24 hours, depending on each immersion condition. After the treatment, the immersion solution was gradually replaced with fresh water and embryos were transferred to 10 cm Petri dishes and incubated in a 28–29 °C incubator. At 2–3 dpf, embryos were examined using a MZ12 stereomicroscope (Leica, Buffalo Grove, USA), or an Axioplan2 fluorescence microscope (ZEISS, Thornwood, NY, USA). Both microscopes were equipped with a DP70 digital camera (Olympus, Center Valley, PA, USA). All the embryos were raised to adults to determine their fertility.
Histology
Zebrafish were euthanized in 0.016% tricaine (ethyl-3-aminobenzoate methanesulfonic acid; Sigma-Aldrich) solution in water, and gonads were removed and fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) at 4 °C overnight. After two rinses in PBS, the fixed gonads were processed through successive ethanol treatments (50%, 70%, 95%, and 100%), followed by two xylene treatments, and embedded in paraffin. The serial 5 μm paraffin sections were prepared using a HM340 microtome (Leica). For histology, the sections were stained with hematoxylin-eosin and examined via light microscopy.
Statistical Analysis
Data are presented as the mean and standard deviation. For statistical analysis one-way ANOVA was applied followed by a Bonferroni–Dunn test using the SAS program. Significance was accepted at p < 0.05.
Additional Information
How to cite this article: Wong, T.-T. and Zohar, Y. Production of reproductively sterile fish by a non-transgenic gene silencing technology. Sci. Rep. 5, 15822; doi: 10.1038/srep15822 (2015).
Supplementary Material
Acknowledgments
The authors thank EuroPharma LLC and the Gudelsky Foundation for supporting this research and publication of the results, the Aquaculture Research Center staff for daily maintenance and health monitoring of experimental animals, and John Stubblefield for editing this manuscript.
Footnotes
Author Contributions T.T.W. and Y.Z. designed the experiments. T.T.W. performed the experiments and analyzed the results. T.T.W. and Y.Z. wrote and reviewed the manuscript.
References
- Muir W. M. & Howard R. D. Possible ecological risks of transgenic organism release when transgenes affect mating success: sexual selection and the Trojan gene hypothesis. Proc Natl Acad Sci USA 96, 13853–13856 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigebrandt A., Aure J., Ervik A. & Hansen P. K. Regulating the local environmental impact of intensive marine fish farming: III. A model for estimation of the holding capacity in the Modelling-Ongrowing fish farm-Monitoring system. Aquaculture 234, 239–261 (2004). [Google Scholar]
- Zohar Y. Endocrinology and fish farming: Aspects in reproduction, growth, and smoltification. Fish Physiol Biochem 7, 395–405 (1989). [DOI] [PubMed] [Google Scholar]
- Li Y. F. & Morcos P. A. Design and synthesis of dendritic molecular transporter that achieves efficient in vivo delivery of morpholino antisense oligo. Bioconjug Chem 19, 1464–1470 (2008). [DOI] [PubMed] [Google Scholar]
- Weidinger G. et al. dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr Biol 13, 1429–1434 (2003). [DOI] [PubMed] [Google Scholar]
- Slanchev K., Stebler J., de la Cueva-Mendez G. & Raz E. Development without germ cells: the role of the germ line in zebrafish sex differentiation. Proc Natl Acad Sci USA 102, 4074–4079 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T. et al. Sexual dimorphism of gonadal structure and gene expression in germ cell-deficient loach, a teleost fish. Proc Natl Acad Sci USA 107, 17211–17216 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto R. et al. Germ cells are not the primary factor for sexual fate determination in goldfish. Dev Biol 370, 98–109 (2012). [DOI] [PubMed] [Google Scholar]
- Wong T. T. & Collodi P. Inducible sterilization of zebrafish by disruption of primordial germ cell migration. PLoS One 8, e68455 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K. Genetic improvement of aquaculture finfish species by chromosome manipulation techniques in Japan. Aquaculture 197, 205–228 (2001). [Google Scholar]
- Donaldson E. M. & Benfey T. J. in Proceedings of the Third International Symposium on Reproductive Physiology of Fish (eds. Idler D. R., Crim L. W. & Walsh J. M.) 108–119 1987). [Google Scholar]
- Johnstone R. in: Recent Advances in Aquaculture. (eds. Muir J. F. & Roberts R. J.) 69–77 (Blackwell Scientific Publications, London; 1993). [Google Scholar]
- Feindel N. J., Benfey T. J. & Trippel E. A. Gonadal development of triploid Atlantic cod Gadus morhua. Journal of Fish Biology 78, 1900–1912 (2011). [DOI] [PubMed] [Google Scholar]
- Wagner E. J., Arndt R. E., Routledge M. D., Latremouille D. & Mellenthin R. F. Comparison of hatchery performance, agonistic behavior, and poststocking survival between diploid and triploid rainbow trout of three different Utah strains. North American Journal of Aquaculture 68, 63–73 (2006). [Google Scholar]
- Piferrer F. et al. Polyploid fish and shellfish: Production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture 293, 125–156 (2009). [Google Scholar]
- Kerby J. H. et al. Performance comparisons between diploid and triploid sunshine bass in fresh water ponds. Aquaculture 211, 91–108 (2002). [Google Scholar]
- Uzbekova S. et al. Transgenic rainbow trout expressed sGnRH-antisense RNA under the control of sGnRH promoter of Atlantic salmon. J Mol Endocrinol 25, 337–350 (2000). [DOI] [PubMed] [Google Scholar]
- Hu S. Y. et al. Nitroreductase-mediated gonadal dysgenesis for infertility control of genetically modified zebrafish. Mar Biotechnol (NY) 12, 569–578 (2010). [DOI] [PubMed] [Google Scholar]
- Hsu C. C., Hou M. F., Hong J. R., Wu J. L. & Her G. M. Inducible male infertility by targeted cell ablation in zebrafish testis. Mar Biotechnol (NY) 12, 466–478 (2010). [DOI] [PubMed] [Google Scholar]
- Lauth X. & Buchanan J. T. in US Patent & Trademark Office, Patent Full Text and Image Database. (ed. U.P.T. Office) (AquaBounty Technologies, Inc. (Waltham, MA) United State; 2012). [Google Scholar]
- Wong T. T. & Zohar Y. Production of reproductively sterile fish: A mini-review of germ cell elimination technologies. Gen Comp Endocrinol, in press (2015). [DOI] [PubMed] [Google Scholar]
- Van Eenennaam A. L. & Muir W. M. Transgenic salmon: a final leap to the grocery shelf? Nat Biotechnol 29, 706–710 (2011). [DOI] [PubMed] [Google Scholar]
- Ledford H. Transgenic salmon nears approval. Nature 497, 17–18 (2013). [DOI] [PubMed] [Google Scholar]
- Hagenmaier H. E. The hatching process in fish embryos. 3. The structure, polysaccharide and protein cytochemistry of the chorion of the trout egg, Salmo gairdneri (Rich.). Acta Histochem 47, 61–69 (1973). [PubMed] [Google Scholar]
- Cotelli F., Andronico F., Brivio M. & Lamia C. L. Structure and composition of the fish egg chorion (Carassius auratus). Journal of Ultrastructure and Molecular Structure Research 99, 70–78 (1988). [Google Scholar]
- Wong T. T. & Collodi P. Effects of specific and prolonged expression of zebrafish growth factors, Fgf2 and Lif in primordial germ cells in vivo. Biochem Biophys Res Commun 430, 347–351 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. M. Effect of activation on the water permeability of salmon eggs. Journal of Cellular and Comparative Physiology 45, 1–12 (1955). [DOI] [PubMed] [Google Scholar]
- Harvey B. & Chamberlain J. B. Water permeability in the developing embryo of the zebrafish, Brachydanio rerio. Canadian Journal of Zoology 60, 268–270 (1982). [Google Scholar]
- Corey D. R. & Abrams J. M. Morpholino antisense oligonucleotides: tools for investigating vertebrate development. Genome Biol 2, REVIEWS1015 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever W., Stemple D., Schier A. & Solnica-Krezel L. Zebrafish: genetic tools for studying vertebrate development. Trends Genet 10, 152–159 (1994). [DOI] [PubMed] [Google Scholar]
- Roush W. Zebrafish embryology builds better model vertebrate. Science 272, 1103 (1996). [DOI] [PubMed] [Google Scholar]
- Braat A. K., Speksnijder J. E. & Zivkovic D. Germ line development in fishes. Int J Dev Biol 43, 745–760 (1999). [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio), Edn. Ed. 4. (Westerfield M., [Eugene, Or.]; 2000). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.