Abstract
Purpose
The aim of this study was to identify the association of normal bacterial flora with vernal keratoconjunctivitis (VKC) occurrence in VKC and non-VKC groups.
Methods
Conjunctival specimens were collected from 18 VKC patients and 22 healthy controls, cultured and identified following standard methods. The association between the presence of bacteria and occurrence of VKC was analyzed using Chi square statistic.
Results
Comparable bacterial growth was observed in VKC (77.8%) as well as control group (77.2%) (p = 0.970). Analysis of individual bacterial revealed that Staphylococcus aureus was detected more frequently in VKC (27.78% vs. 4.55% in control, p = 0.041) and Staphylococcus epidermidis was found much more commonly in the control eyes (45.45% in control vs. 5.56% in VKC, p = 0.005).
Conclusions
An aggravating role of S. aureus colonization in the occurrence of VKC, and a possible role of S. epidermidis against the occurrence of VKC were concluded.
Keywords: Allergy, Conjunctivitis, Eyes, Staphylococci, Vernal keratoconjunctivitis
Introduction
Vernal keratoconjunctivitis (VKC) is a bilateral allergic conjunctivitis, most commonly observed in young patients living in warm dry climate as in Saudi Arabia.1 It is characterized by recurrent seasonal episodes of itching, tearing, burning, mucous stringy discharge, severe photophobia, blepharospasm and foreign body sensation.2, 3 Although in early phase of the allergic reaction, antihistamines are effective treatment options, and severe allergic reaction necessitates cytotoxic and immunosuppressant treatment for a long time.4
The pathophysiology of VKC seems to be multifactorial, as several different mechanisms involving immune, nervous, and endocrine systems have been proposed. Earlier, it was believed that the expression of a classical type I IgE-mediated hypersensitivity reaction at the conjunctival level is involved in the immunopathogenesis of VKC.5 However, since nearly half of VKC cases are not associated with positive RAST or skin prick test, it is unlikely to be solely an IgE-mediated disease.6 Other studies demonstrated the involvement of neural factors such as substance P (a neuropeptide) and nerve growth factor in the pathogenesis of VKC. The over-expression of estrogen and progesterone receptors in the conjunctiva of VKC patients points toward the possible involvement of endocrine system.5 Detection of Toll-like receptor (TLR) expression at the ocular surface during VKC led to the suggestions about possible role of TLRs in VKC.7 As such, the etiology and pathophysiology of VKC remain unclear.
Conjunctival sac is colonized by different species of gram positive as well as gram negative normal bacterial flora,8 of which staphylococci form the majority.8, 9 There is evidence to indicate the possible role of normal bacterial flora, particularly Staphylococcus aureus in triggering and exacerbation of different forms of allergies. Staphylococcal enterotoxin A (SEA) and B (SEB)-specific IgE antibodies have been detected in the tears of patients with allergic conjunctival disorders, particularly during exacerbation.10, 11 Staphylococcal enterotoxins have also been linked to disease severity of skin allergy and asthma as well.12, 13 In this study, we aim to find out the association between the presence of normal bacterial flora (particularly S. aureus and S. epidermidis) and occurrence of VKC.
Materials and methods
The study included 18 VKC patients (13 males, 5 females) and 22 healthy volunteers (13 males and 9 females) as controls. Healthy volunteers were selected from subjects who came to have refractive examinations to receive spectacles and contact lenses and who consented to cultures and to the protocol of the study. The study was approved by Institutional Review Board and written informed consent was obtained from all adult subjects and in the case of children from their parents.
Sample collection and transportation
Upper tarsal conjunctiva, lower conjunctival sac, and upper lid margin skin were swabbed for bacterial cultures in all subjects using sterile swabs under strict aseptic conditions. Each swab was immediately inserted in a tube containing Brain Heart infusion broth (Oxoid, Basingstoke, Hampshire, England) which served as a transport medium. Inoculated specimens were transported to the laboratory for bacteriological analysis.
Isolation of bacterial agents
A swab from each specimen was streaked onto a blood agar plate and MacConkey agar plates. Inoculated plates were incubated aerobically at 37 °C for 24 h. Plates were examined for bacterial growth. Number and types of colonies were labeled and recorded till further identification.
Identification of bacterial agents
Bacterial growth was subjected to an identification scheme using morphological and biochemical tests following standard procedures.14 Isolates were first examined microscopically using Gram stained slides to look for gram positive cocci that usually arranged in clusters. Isolates were then examined for hemolysis after overnight incubation at 37 °C on sheep blood agar. DNase production was tested on DNase test agar as per the manufacturer’s recommendations (Difco). Coagulase test was done with rabbit plasma following the procedure described by the manufacturer (Bio-Merieux). Catalase test was done by transferring a small amount of bacterial colony to a surface of clean, dry glass slide using a loop or sterile wooden stick. A drop of 3% H2O2 was paced on to the slide and the preparation was mixed. A positive result is the rapid evolution of oxygen (within 5–10 s.) as evidenced by bubbling. A negative result is shown when no bubbles are evident. Oxidase test was done by soaking a small piece of filter paper in 1% Kovács oxidase reagent which was left for to dry. A loopful of a well-isolated bacterial colony from a fresh bacterial plate was picked and rubbed onto the filter paper. Oxidase positive organisms produced show color changes to dark purple within 5–10 s.
Identities of organism were then confirmed using Vitek II automated identification system following procedures described by manufacturer (BioMérieux SA, Marcy, France). The VITEK card contains 64 wells which comprise many fluorescent biochemical tests: 20 of which are carbohydrate assimilation; 4 are phosphatase, urea, nitrate, and actidione tests. When a test outcome is documented as “low discrimination,” this indicates that the result is doubtful. In these cases, the previously mentioned morphological and biochemical tests were repeated to come to a decision on such uncertain results. The VITEK 2 scheme managed card automatically from filling, sealing and then transferring them into the connected incubator (35 °C). The cards are filled automatically every 15 min by a fluorescence system. Each resulting profile is decoded according to a precise algorithm. The acquired results were compared to the ID-GP (identification of gram positive bacteria) database. In the majority of the cases the recognized gram positive bacteria are identified with high percentages of certainties.
Statistical analysis
Collected patients’ data and results of cultured samples were analyzed, using Statistical Package for Social Sciences program (SPSS; Version 16). The association between the presence of bacteria and occurrence of VKC was analyzed using Chi square statistic. P-values < 0.05 were considered statistically significant.
Results
Table 1 demonstrates the bacterial growth of samples from both vernal keratoconjunctivitis (VKC) group and control group. Fourteen out of 18 VKC samples (77.8%) vs. 17 out of 22 control samples (77.27%) revealed bacterial growth (p = 0.970). Staphylococcus species were the most common bacterial isolates representing 50% (9/18) of the VKC samples and 54.55% (12/22) of the control samples (p = 0.775). Streptococcus species were the next most common bacteria constituting 11.11% (2/18) of the bacterial isolates in VKC samples and 18.18% (4/22) in controls (p = 0.533).
Table 1.
Bacteria isolates recovered from vernal keratoconjunctivitis (VKC) cases (n = 18) compared to control cases without VKC (n = 22).
| VKC patient (n = 18) | % | VKC negative individuals (n = 22) | % | |
|---|---|---|---|---|
| No growth | 4 | 22.22 | 5 | 22.73 |
| Klebsiella pneumoniae | 1 | 5.56 | 1 | 4.55 |
| Pseudomonas fluorescens | 1 | 5.56 | 0 | 0.00 |
| Staphylococcus epidermidis | 1 | 5.56 | 10 | 45.45 |
| Staphylococcus aureus | 5 | 27.78 | 1 | 4.55 |
| Staphylococcus hominis | 1 | 5.56 | 0 | 0.00 |
| Staphylococcus warneri | 1 | 5.56 | 0 | 0.00 |
| Staphylococcus sp. | 1 | 5.56 | 1 | 4.55 |
| Streptococcus spp. | 2 | 11.11 | 4 | 18.18 |
Staphylococci detected in this study (50%) grew aerobically on blood agar. All strains produced catalase, which differentiated them from the catalase-negative streptococci. S. aureus was coagulase positive; this test distinguished it from the other staphylococci. S. aureus produced opaque, smooth, circular, colonies that were yellow (golden) to white with beta hemolysis whereas S. epidermidis were white in color with alpha- or no hemolysis.
Among VKC samples, S. aureus (27.8%, 5/18) was the most common staphylococci isolate and among the controls, S. epidermidis (45.5%, 10/22) was the most common staphylococci isolate. Analysis of individual bacterial revealed that S. aureus represented 27.78% (5/18) of the VKC samples compared to 4.55% (1/22) of the controls (p = 0.041) (Fig. 1). S. epidermidis was isolated in 5.56% (1/18) of the VKC samples and 45.45% (10/22) of the controls (p = 0.005).
Figure 1.
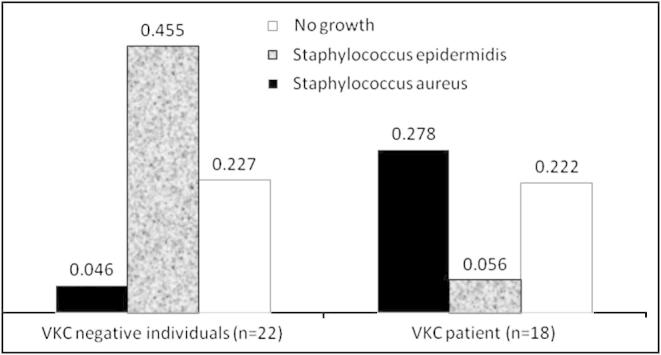
Association of Staphylococcus epidermidis with VKC negative control and Staphylococcus aureus with increased VKC rates.
Discussion
Vernal keratoconjunctivitis (VKC) is a potentially serious seasonal illness that is characterized by bilateral inflammation of the conjunctiva. It is frequently encountered in the hot and dry climates such as in Saudi Arabia.1 It is logical to make attempts at identifying the trigging factors involved and prevent them, if possible. Data from the previous literature seem to suggest the possible role of S. aureus in causing exacerbation of VKC and AKC. This study was aimed to evaluate the presence of any association between the type of conjunctival bacterial flora and occurrence of VKC.
Overall, the growth of any bacterial flora between the VKC and controls was similar (p = 0.970). Similarly, there was no statistically significant difference in the number of Staphylococcal and Streptococcal species isolated from VKC vs. control eyes. S. aureus, which has previously been linked to exacerbation of VKC was isolated more frequently in VKC eyes compared to the controls (27.78% vs. 4.55%), the difference in frequencies being statistically significant (p = 0.041). In contrast, S. epidermidis, the most common bacterial isolate in our study, was found much more frequently in the controls compared to the VKC (45.45% vs. 5.56%), the difference in frequencies being statistically highly significant (p = 0.005).
A review of literature reveals the potential role of S. aureus and its secretions staphylococcal enterotoxins A and B in the exacerbation of VKC. Shoji et al. investigated the presence of staphylococcal enterotoxin A (SEA) and B (SEB)-specific IgE antibodies in tears from patients with allergic conjunctival disorders and found high frequency of VKC eyes to be positive for SEA-specific IgE antibodies and high frequency of AKC eyes to be positive for SEB-specific IgE antibodies.10 Titers for antibodies were higher in patients with more severe clinical findings. In contrast, the normal controls were negative for both antibodies.10 Similarly, Tabuchi et al. found that IgE antibodies to staphylococcal enterotoxin A and/or B were present in the serum of almost 60% (13/22) of the VKC/AKC patients that had exacerbation in the form of atopic dermatitis; in contrast none of eyes in the control group (allergic conjunctivitis without AD) had IgE antibodies to SEA/SEB detected in their tears.11 The statistically significant higher frequency of S. aureus isolates in VKC eyes (compared to the controls) complements the findings by Shoji et al. and Tabuchi et al. As such, the findings of our study point toward the potentially aggravating role of S. aureus colonization in the occurrence of VKC.
Another important finding in our study was the statistically significant higher detection of S. epidermidis in the controls. To the best of our knowledge, such a finding has never been reported earlier in VKC patients. However, in dermatology, higher frequency of S. epidermidis has been reported from the skin of healthy persons in contrast to atopic dermatitis patients who had high frequency of S. aureus detection from their skin.15 Apparently, there could be a protective role of S. epidermidis against the growth of S. aureus and potentially, the occurrence of VKC. Likewise, studies investigating bacterial interference have found that a serine protease Esp, produced by S. epidermidis inhibits the growth of S. aureus.16
The findings of this study seem to point toward the possible association of S. aureus and VKC occurrence. Data of the study also suggest possible association between the presence of S. epidermidis and defense against VKC. It is important to observe that high frequency of S. aureus in VKC patients may also be due to higher potential of infection in such patients, possibly due to transmittance of infecting bacteria due to frequent touch or rubbing the eyes.15 Alternatively, compromised immunity due to treatment with steroids may explain the higher frequency of S. aureus in VKC patients; however, most of the VKC patients in our study were not using steroids. In addition, contrary to the expected, a previous paper studying S. aureus colonization in the skin of atopic dermatitis patients found a decline in S. aureus colonization from 100% at the time of exacerbation to 30% following 4–12 weeks of treatment with antihistamines and steroids (and no antibiotics), ruling out steroid induced compromised immunity as a cause of S. aureus colonization.17
Identifying triggers, especially the modifiable ones is an important aspect of the research aimed at improving the management of VKC. Future studies with larger sample size along with experimental animal models would confirm the role of S. aureus colonization and its secreted staphylococcal enterotoxins A and B in the occurrence and exacerbation of VKC and at determining ways to modify such triggers.
Conflict of interest
The authors report no conflicts of interest.
Acknowledgments
The research was funded by the Deanship of Scientific Research, King Khalid University, Abha, Saudi Arabia (project number: 1433/235).
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Kumar S. Vernal keratoconjunctivitis: a major review. Acta Ophthalmol. 2009;87:133–147. doi: 10.1111/j.1755-3768.2008.01347.x. [DOI] [PubMed] [Google Scholar]
- 2.Al-Akily S.A., Bamashmus M.A. Ocular complications of severe vernal keratoconjunctivitis (VKC) in Yemen. Saudi J Ophthalmol. 2011;25:291–294. doi: 10.1016/j.sjopt.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabbara K.F. Ocular complications of vernal keratoconjunctivitis. Can J Ophthalmol. 1999;34:88–92. [PubMed] [Google Scholar]
- 4.Pucci N., Caputo R., Mori F. Long-term safety and efficacy of topical cyclosporine in 156 children with vernal keratoconjunctivitis. Int J Immunopathol Pharmacol. 2010;23:865–871. doi: 10.1177/039463201002300322. [DOI] [PubMed] [Google Scholar]
- 5.Bonini S., Coassin M., Aronni S. Vernal keratoconjunctivitis. Eye (Lond) 2004;18:345–351. doi: 10.1038/sj.eye.6700675. [DOI] [PubMed] [Google Scholar]
- 6.Stahl J.L., Barney N.P. Ocular allergic disease. Curr Opin Allergy Clin Immunol. 2004;4:455–459. doi: 10.1097/00130832-200410000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Bonini S., Micera A., Iovieno A. Expression of Toll-like receptors in healthy and allergic conjunctiva. Ophthalmology. 2005;112:1528. doi: 10.1016/j.ophtha.2005.04.009. discussion 48–9. [DOI] [PubMed] [Google Scholar]
- 8.Hsu H.Y., Lind J.T., Tseng L. Ocular flora and their antibiotic resistance patterns in the midwest: a prospective study of patients undergoing cataract surgery. Am J Ophthalmol. 2013;155(36–44):e2. doi: 10.1016/j.ajo.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Capriotti J.A., Pelletier J.S., Shah M. Normal ocular flora in healthy eyes from a rural population in Sierra Leone. Int Ophthalmol. 2009;29:81–84. doi: 10.1007/s10792-008-9196-4. [DOI] [PubMed] [Google Scholar]
- 10.Shoji J., Kato H., Kitazawa M. Evaluation of staphylococcal enterotoxin-specific IgE antibody in tears in allergic keratoconjunctival disorders. Jpn J Ophthalmol. 2003;47:609–611. doi: 10.1016/j.jjo.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Tabuchi K., Inada N., Shoji J. The relationship between Staphylococcus aureus and atopic keratoconjunctivitis. Nihon Ganka Gakkai Zasshi. 2004;108:397–400. [PubMed] [Google Scholar]
- 12.Bachert C., van Steen K., Zhang N. Specific IgE against Staphylococcus aureus enterotoxins: an independent risk factor for asthma. J Allergy Clin Immunol. 2012;130(376–81):e8. doi: 10.1016/j.jaci.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Nomura I., Tanaka K., Tomita H. Evaluation of the staphylococcal exotoxins and their specific IgE in childhood atopic dermatitis. J Allergy Clin Immunol. 1999;104:441–446. doi: 10.1016/s0091-6749(99)70390-8. [DOI] [PubMed] [Google Scholar]
- 14.Forbes B., Sahm D.F., Weissfeld A.S. 11 ed. St. Louis; Mosby, Toronto: 2002. Bailey and Scott’s diagnostic microbiology. [Google Scholar]
- 15.Nakata K., Inoue Y., Harada J. A high incidence of Staphylococcus aureus colonization in the external eyes of patients with atopic dermatitis. Ophthalmology. 2000;107:2167–2171. doi: 10.1016/s0161-6420(00)00406-1. [DOI] [PubMed] [Google Scholar]
- 16.Vandecandelaere I., Depuydt P., Nelis H.J. Protease production by Staphylococcus epidermidis and its effect on Staphylococcus aureus biofilms. Pathog Dis. 2014 doi: 10.1111/2049-632X.12133. [DOI] [PubMed] [Google Scholar]
- 17.Adamek-Guzik T., Guzik T., Czerniawska-Mysik G. Effects of combined therapy with oral antihistamines and topical corticosteroids on Staphylococcus aureus colonization in atopic dermatitis. Alergia Astma Immunologia. 2002;7:33–43. [Google Scholar]


