Abstract
Background
To report on five patients with decreased visual acuity due to glistening and severe sub-surface nano-glistening (SSNG) formation within their intraocular lenses (IOLs).
Design
Case reports and analysis of extracted IOLs.
Participants and samples
We report improved visual acuity when IOLs with severe glistening and SSNG were exchanged for clear IOLs in five patients.
Methods
Case reports.
Main outcome measures
The main outcome measure was visual acuity. The secondary outcome measure was light transmission. Explanted IOLs were subjected to investigation. Pre- and postoperative slit lamp images of the anterior eye and microscopic images of the extracted IOLs were taken and compared. Light transmission of the IOL was measured using a double beam type spectrophotometer. An integrated value of the percentage light transmittance in the visible light spectrum was calculated.
Results
We report on five patients whose visual acuity improved when IOLs were exchanged because of severe glistening and SSNG. All of the affected IOLs were MA60BM (Alcon, Forth Wroth Texas, USA) and the original implantation had occurred over a range of 6–15 years prior to the IOL exchange. Light transmission was decreased in all affected lenses compared to a similar control IOL.
Conclusions
Although only a few reports of cases in which glistening and SSNG have progressed to the level of decreased visual function have been published, the likelihood is that this phenomena will increase as the severity and incidence of these inclusions have been shown to increase with time. Appropriate evaluations of visual function in such patients are needed and consideration should be given to IOL exchange in symptomatic patients.
Keywords: Glistening, Sub-surface nano-glistening, Whitening, Hydrophobic acrylic IOL, Microvacuole
Introduction
Visual function in ophthalmology can be assessed using a number of methods, including Snellen acuity, contrast sensitivity, disability glare testing, visual field analysis, accommodative amplitude, and reading speed. However, the most commonly used simple method of assessing visual outcome in intraocular surgery is still visual acuity. Causes of decreased visual function due to IOLs, confirmed both in vivo and in vitro, are glistenings1, 2, 3 and whitening.4 The former term is given to fluid-filled microvacuoles within the IOL optic which appear to “glisten” as light passes through them. The latter refers to the clinical appearance from subsurface nanoglistenings (SSNG) of reflected white light due to light scattering as light encounters nanosized fluid filled vacuoles that occur at the anterior and posterior IOL surface. Whitening is widely recognized and reported in Japan.4, 5
Considerable controversy exits regarding the extent of impact on visual function due to glistening and SSNG. The majority of papers in the literature have reported that these changes did not influence the visual function.6, 7, 8 However, there are also reports that argue that glistenings and SSNG have led to such significant symptoms and/or visual function deterioration in selected cases which necessitated IOL explantation and replacement.9, 10, 11
In this paper, we report improved visual acuity when IOLs with severe glistening and SSNG were exchanged for clear IOLs in five patients.
Methods
A retrospective chart review was undertaken to identify patients who had undergone prior IOL exchange for visually significant glistenings and SSNGs. The study adhered to the Tenets of the Declaration of Helsinki. Each subject was asked to provide informed consent before undergoing the IOL exchange procedure. Ethics Review Board approval for this retrospective study was obtained from the Bioethics Committee of Dokkyo Medical University in Japan.
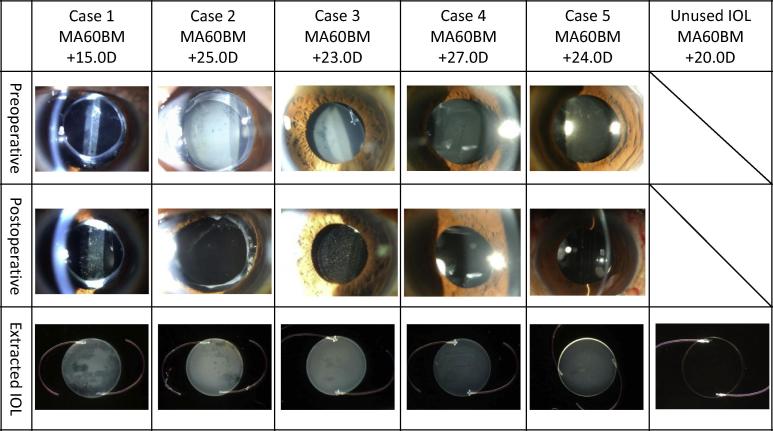
Explanted IOLs were subjected to investigation. Care was taken to explant the IOL with the optic intact so as to allow for measurement of light transmission. Only cases with intact optics were included in this review. Pre- and postoperative slit lamp images of the anterior eye and microscopic images of the extracted IOLs were taken and compared (Fig. 1; upper row is of preoperative photos, middle row is of postoperative photos, and lower row is of the explanted IOL). The extracted IOLs were immediately placed in physiological saline at 33 °C to avoid any change in the severity of glistening and whitening.12 Any lens capsule and tissue attached to the extracted IOL were removed while it was submerged in physiological saline at 33 °C, and the IOL surface was examined under light microscopy (F23PL20WK; Optron, Kanagawa, Japan). Next, using a double beam type spectrophotometer (U-3000 or U-4100; Hitachi, Tokyo, Japan), light transmission of the IOL in physiological saline at 33 °C was measured. All observations and analyses included an unimplanted MA60BM (Alcon) for comparison. An integrated value of the percentage light transmittance in the visible light spectrum (800–360 nm) was calculated (see Fig. 2).
Figure 1.
Anterior segment images before and after IOL replacement and the extracted IOLs. The upper row shows anterior eye photographs before IOL extraction. The middle row shows photographs of the anterior segment after IOL extraction. The bottom row shows optical microscope images of the extracted IOLs with the lens capsule and other tissue removed.
Figure 2.
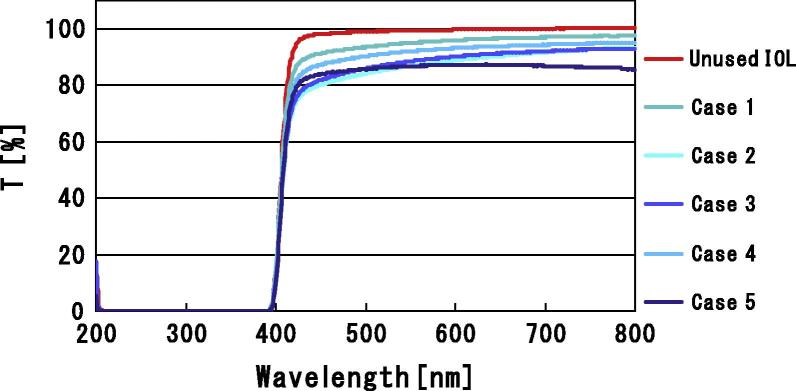
Light transmittance of extracted IOLs. Light transmittance is shown for Cases 1–5. Compared with light transmittance through an unused +20.0D IOL, light transmittance is decreased in the extracted IOLs.
Results
Case 1
A 29-year-old man with a history of uveitis related cataract had right cataract extraction with IOL insertion (Alcon MA60BM, +15.0D) in 1999. Postoperatively, corrected distance visual acuity (CDVA) was 20/25. In 2009, the patient presented with complaints of decreased right visual acuity; examination revealed a CDVA of 20/50. Severe glistening, severe SSNG, and minor secondary cataract were diagnosed on slit lamp examination. As the secondary cataract was considered to be minor and IOL exchange was recommended for the microvacuole inclusions, no capsulotomy was performed. The IOL was removed through a 6.0 mm incision and exchanged for a sulcus fixated IOL (UY-60SB, +14.0D; HOYA, Tokyo, Japan) at Miyake Eye Hospital (Nagoya, Japan) on April 21, 2009. One week after surgery, the visual acuity had improved as CDVA was 20/30. Slit lamp examination several months post-operatively revealed progression of the secondary cataract and a transparent IOL. On September 9, 2009, a YAG posterior capsulotomy was performed. Following this surgery, the right CDVA was 20/25. Light transmission of the explanted IOL was 85.0%, compared with 88.9% in the control unimplanted Alcon MA60BM IOL.
Case 2
A 77-year-old woman had right cataract extraction with IOL insertion (Alcon MA60BM, +25.0D) and vitrectomy for a macular hole in 2001. The patient complained of decreased right visual acuity in 2009, and the CDVA was found to be 20/100. Glistening and SSNG were observed in the optic on slit lamp observation. The IOL was extracted through a 6.0 mm incision on April 14, 2009. During the surgery, although a rent occurred in the posterior capsule, the IOL could be safely placed in the bag; a VA-70AD (+24.5D; HOYA, Tokyo, Japan) was inserted at Yoshida Eye Hospital (Hakodate, Japan). Right CDVA improved to 20/50 after surgery. Visible light transmission in the explanted IOL was decreased at 78.2% (this case had the greatest amount of decreased light transmittance measured).
Case 3
A 53-year-old man, undergoing hemodialysis for chronic renal failure, had right cataract extraction with IOL insertion (Alcon MA60BM, +23.0D) and vitreoretinal surgeries for diabetic retinopathy in 1995. The patient complained of decreased right visual acuity in 2010; his CDVA was 20/25. Glistening and SSNG were seen on biomicroscopy but the posterior capsule was not apparent on slit lamp examination. The IOL was explanted through a 6.0 mm incision and replaced with a sulcus fixated IOL (UY-60SB, +21.5D, HOYA, Tokyo, Japan) at Yoshida Eye Hospital (Hakodate, Japan) on June 24, 2010. Two weeks after surgery, right CDVA was found to be improved to 20/20. Visible light transmission in the extracted IOL was diminished to 79.1%.
Case 4
A 62-year-old man, with glaucoma, had left cataract extraction with IOL insertion (Alcon MA60BM, +27.0D) and vitreoretinal surgeries for diabetic retinopathy in 1997. Several years after surgery, the patient complained of decreased visual acuity and CDVA was found to be 20/50. Glistening and SSNG were observed on examination, as well as a posterior capsulotomy. The IOL was explanted through a 6.0 mm incision and replaced with a sulcus fixated IOL (VA-70AD, +25.5D, HOYA, Tokyo, Japan), at Dokkyo Medical University (Tochigi, Japan) on March 25, 2010. One month after surgery, the left CDVA was improved at 20/32. Visible light transmission in the extracted IOL was decreased to 80.1%.
Case 5
A 63-year-old man had left cataract extraction with IOL insertion (Alcon MA60BM, +24.0D) and vitreoretinal surgeries for diabetic retinopathy in 2004. The patient began complaining of decreased left visual acuity in 2010. The left CDVA was measured at 20/32; glistening, SSNG, and a slight secondary cataract were diagnosed. The IOL was explanted through a 6.0 mm incision and replaced with an IOL in the bag (VA-70AD, +21.5D; HOYA, Tokyo, Japan) at Dokkyo Medical University (Tochigi, Japan) on June 3, 2010. One month after surgery, the left CDVA was 20/20. Visible light transmission in the extracted IOL was 76.7%.
Discussion
Although glistenings have been reported in IOL optic manufactured from all materials (silicone, hydrophilic acrylic and hydrophobic acrylic), they are most frequently seen in hydrophobic acrylic IOLs1, 2, 3, in particular those manufactured by Alcon. Globally, the Alcon hydrophobic acrylic IOL is one of most commonly implanted lenses; over 70 million Acrysof lenses have been implanted (source, Alcon Fort Worth, USA). This preference is due to the reported low rate of posterior capsular opacification (PCO) formation.13 Whitening due to SSNG has only been reported in Alcon’s hydrophobic acrylic IOLs.4, 5, 6 All of the cases in our series were implanted with the MA60BM IOL (Alcon, Fort Worth, Texas); this lens is made of hydrophobic acrylic material, and has a sharp edge to the posterior optic surface.
Medical conditions have been reported to have an influence on the severity of the microvacuole inclusions (glistenings and SSNG’s)14, 15, 16, in particular ocular inflammation, diabetes mellitus and glaucoma. Four of five of our patients were afflicted with one or both of these conditions.
Several reports have described the effects of glistening and SSNG on visual function. Miyata6 performed analysis 3 years postoperatively on Alcon hydrophobic acrylic lenses; he found that while surface scattering intensified with time in the MA60BM and SA60AT lenses, no effect was seen on visual acuity or contrast sensitivity. Similarly, Mönestam7 and Hayashi8 analyzed glistening by clinical grade classification and light scattering; they reported that although microvacuolar inclusions increased in frequency and intensity over the course of 10 years following AcrySof implantation, no effect on visual acuity or contrast visual acuity could be found, even in the most severe cases. Conversely, cases have been reported in which glistening formation occurs, fundus visualization becomes difficult, and extraction is necessary9; Cases in whom YAG posterior capsulotomy was difficult because of glistening10, and cases in whom visual function is decreased due to pronounced SSNG have also been reported.11
In our series, we have encountered five patients complaining of decreased vision in whom the IOLs contained severe SSNG and glistening formation. Light transmittance of the intraocular lenses explanted from cases 1, 2, 3, 4 and 5 was 85.0%, 78.2%, 79.1% 80.1% and 76.7% (respectively). Compared with the light transmittance of a control unused IOL of 88.9%; these values represent a decrease of from 4.4% to 13.7% in our patients. Previous published studies of light transmittance in IOLs have been performed on opacified IOLs. Light transmittance of PMMA IOLs with snowflake degeneration and calcification was found to range from 81.08% to 97.1%, and from 81.48% to 98.66% respectively.17 When compared with these previous reports, the decrease in light transmittance in our cases is significant.
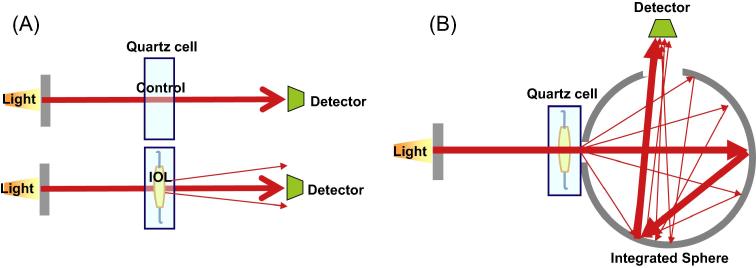
Interestingly, previous reports on light transmittance using AcrySof lenses with glistening and SSNG did not find any decrease18, 19 and seem to contradict our findings. In their studies, an integrated sphere type spectrophotometer (Lambda 35 UV/Vis spectrophotometer, PerkinElmer Inc.) was used; however, we used a double beam spectrophotometer and this may explain the difference in findings. The integrated sphere method detects all the light transmitted through the IOL optic; thus, it measures both transmitted and scattered light (see Fig 3). The double beam method detects only the light passing through the IOL optic which is convergent on the detector; thus, only convergent light is measured while scattered light is lost to measurement. The integrated sphere spectrophotometer measurements tend to be higher.
Figure 3.
Differences in 2 types of spectrophotometer. (A) Double Beam Spectrophotometer. The transmittance was measured from the difference between the control and the sample IOL light. This method detected only the light converged to the detector passes through the IOL optic. The scattered light is not detected. So the result becomes lower than the result using integrated sphere spectrophotometer. (B) Integrated Sphere Spectrophotometer. This integrated sphere detects all the light transmitted through the IOL optic. And, the scattered light is also detected. So the result becomes higher than the result using double beam spectrophotometer.
In our case series, the IOL with glistenings and SSNG was explanted and replaced with a new IOL. Visual acuity, visual function and patient satisfaction improved in all cases. As the only significant procedure performed was IOL exchange, we deduce that the decreased visual function in these five cases was caused by SSNG and glistening.
Of note is that all these cases were associated with other ocular conditions including uveitis, macular hole, or diabetic retinopathy, representing eye diseases that could affect retinal function. It can be argued that the decreased visual function may have been due to the other ocular pathologies present in these patients. Only an IOL exchange was performed and this resulted in an improvement in visual symptoms and visual performance; thus, confirming our suspicion that severe glistening and SSNG of the IOL was the main cause of the decreased visual acuity. It is possible that retinal pathology may have been the cause of the decreased visual function in our series of patients; however, as only an IOL exchanged was performed, then the vision should have not been improved.
Our case series of patients with Alcon lenses highlight the deterioration in visual function and, in particular visual acuity, which glistening and SSNG produce in patients. Our clinical experience supports the position that glistenings and SSNGs decrease visual function, since light transmission and visual acuity were found to be affected. This case series would support the role of IOL exchange for improvement of vision in similar cases, and would give cause for thought for IOL exchange in healthy eyes with severe glistenings or SSNGs that are associated with symptoms of poor vision and/or decreased visual acuity.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
Light transmittance studies were performed on explanted IOLs.
References
- 1.Werner L. Glistening and surface light scattering in intraocular lens. J Cataract Refract Surg. 2010;36:1398–1420. doi: 10.1016/j.jcrs.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Kato K., Nishida M., Yamane H. Glistening formation in an AcrySof lens initiated by spinodal decomposition of the polymer network by temperature change. J Cataract Refract Surg. 2001;27:1493–1498. doi: 10.1016/s0886-3350(01)00895-1. [DOI] [PubMed] [Google Scholar]
- 3.Miyata A., Uchida N., Nakajima K. Clinical and experimental observation of glistening in acrylic intraocular lenses. Jpn J Ophthalmol. 2001;45:564–569. doi: 10.1016/s0021-5155(01)00429-4. [DOI] [PubMed] [Google Scholar]
- 4.Matsushima H., Mukai K., Nagata M. Analysis of surface “whitening” of extracted AcrySof intraocular lenses. J Cataract Refract Surg. 2009;35:1927–1934. doi: 10.1016/j.jcrs.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Nishihara H., Yaguchi S., Onishi T. Surface scattering in implanted hydrophobic intraocular lenses. J Cataract Refract Surg. 2003;29:1385–1388. doi: 10.1016/s0886-3350(02)01994-6. [DOI] [PubMed] [Google Scholar]
- 6.Miyata K., Otani S., Nejima R. Comparison of postoperative surface light scattering of different intraocular lenses. Br J Ophthalmol. 2009;93:684–687. doi: 10.1136/bjo.2008.144691. [DOI] [PubMed] [Google Scholar]
- 7.Mönestam E., Behndig A. Impact on visual function from light scattering and glistenings in intraocular lenses, a long-term study. Acta Ophthalmol. 2010;88:1–5. doi: 10.1111/j.1755-3768.2009.01833.x. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi K., Hirata A., Yoshida M. Long-term effect of surface light scattering and glistening of intraocular lenses on visual function. Am J Ophthalmol. 2012;154:240–250. doi: 10.1016/j.ajo.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Werner L., Storsberg J., Mauger O. Unusual pattern of glistening formation on a 3-piece hydrophobic acrylic intraocular lens. J Cataract Refract Surg. 2008;34:1604–1609. doi: 10.1016/j.jcrs.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 10.Mitooka K., Shiba T., Tsuneoka H. A case of IOL eye with decrease of visual function by glistening. Ganka. 1998;40:1501–1504. [Japanese] [Google Scholar]
- 11.Yoshida S., Matsushima H., Nagata M. Decreased visual function due to high-level light scattering in a hydrophobic acrylic intraocular lens. Jpn J Ophthalmol. 2011;55:62–66. doi: 10.1007/s10384-010-0901-2. [DOI] [PubMed] [Google Scholar]
- 12.Oshika T., Shiokawa Y., Amano S. Influence of glistenings on the optical quality of acrylic foldable intraocular lens. Br J Ophthal. 2001;9:1034–1037. doi: 10.1136/bjo.85.9.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwase T., Nishi Y., Oveson B.C. Hydrophobic versus double-square-edged hydrophilic foldable acrylic intraocular lens: effect on posterior capsule opacification. J Cataract Refract Surg. 2011;37:1060–1068. doi: 10.1016/j.jcrs.2010.12.059. [DOI] [PubMed] [Google Scholar]
- 14.Dick H.B., Olson R.J., Augustin A.J. Vacuoles in the AcrySof intraocular lens as factor of the presence of serum in aqueous humor. Ophthalmic Res. 2001;33:61–67. doi: 10.1159/000055645. [DOI] [PubMed] [Google Scholar]
- 15.Moreno-Montanés J., Alvarez A., Rodríguez-Conde R. Clinical factors related to the frequency and intensity of glistenings in AcrySof intraocular lenses. J CataractRefract Surg. 2003;29:1980–1984. doi: 10.1016/s0886-3350(03)00136-6. [DOI] [PubMed] [Google Scholar]
- 16.Colin J., Orignac I., Touboul D. Glistenings in a large series of hydrophobic acrylic intraocular lenses. J Cataract Refract Surg. 2009;35:2121–2212. doi: 10.1016/j.jcrs.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Michelson J., Werner L., Ollerton A. Light scattering and light transmittance in intraocular lenses explanted because of optic opacification. J Cataract Refract Surg. 2012;8:1476–1485. doi: 10.1016/j.jcrs.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 18.Morris C., Werner L., Barra D. Light scattering and light transmittance of cadaver eye-explanted intraocular lenses of different materials. J Cataract Refract Surg. 2014;40:129–137. doi: 10.1016/j.jcrs.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Werner L., Morris C., Liu E. Light transmittance of 1-piece hydrophobic acrylic intraocular lenses with surface light scattering removed from cadaver eyes. J Cataract Refract Surg. 2014;40:114–120. doi: 10.1016/j.jcrs.2013.05.050. [DOI] [PubMed] [Google Scholar]