Abstract
Objectives
Conventional magnetic resonance imaging (MRI) is used to diagnose and monitor inflammatory disease in relapsing remitting (RR) multiple sclerosis (MS). In the less common primary progressive (PP) form of MS, in which focal inflammation is less evident, biomarkers are still needed to enable evaluation of novel therapies in clinical trials. Our objective was to characterize the association — across the brain and cervical spinal cord — between clinical disability measures in PPMS and two potential biomarkers (one for myelin, and one for atrophy, both resulting from the same imaging technique).
Methods
Multi-component driven equilibrium single pulse observation of T1 and T2 (mcDESPOT) MRI of the brain and cervical spinal cord were obtained for 15 PPMS patients and 11 matched controls. Data were analysed to estimate the signal related to myelin water (VFM), as well as volume measurements. MS disability was assessed using the Multiple Sclerosis Functional Composite score, which includes measures of cognitive processing (Paced Auditory Serial Addition Test), manual dexterity (9-Hole Peg Test) and ambulatory function (Timed 25-Foot Walk); and the Expanded Disability Status Scale.
Results
Brain and spinal cord volumes were different in PPMS compared to controls, particularly ventricular (+ 46%, p = 0.0006) and cervical spinal cord volume (− 16%, p = 0.0001). Brain and spinal cord myelin (VFM) were also reduced in PPMS (brain: − 11%, p = 0.01; spine: − 19%, p = 0.000004). Cognitive processing correlated with brain ventricular volume (p = 0.009). Manual dexterity correlated with brain ventricular volume (p = 0.007), and both brain and spinal cord VFM (p = 0.01 and 0.06, respectively). Ambulation correlated with spinal cord volume (p = 0.04) and spinal cord VFM (p = 0.04).
Interpretation
In this study we demonstrated that mcDESPOT can be used to measure myelin and atrophy in the brain and spinal cord. Results correlate well with clinical disability scores in PPMS representing cognitive, fine motor and ambulatory disability.
Abbreviations: CCV, cervical cord volume; CSF, cerebrospinal fluid; EDSS, Expanded Disability Status Scale; FOV, field of view; mcDESPOT, Multi-component driven equilibrium single pulse observation of T1 & T2; MR, magnetic resonance; MRI, magnetic resonance imaging; MS, multiple sclerosis; MSFC, Multiple Sclerosis Functional Composite; PASAT, Paced Auditory Serial Addition Test; PP, primary progressive; RR, relapsing remitting; SPGR, spoiled gradient echo; SSFP, steady state free precession; TE, echo time; TR, repetition time; T25FW, Timed 25-Foot Walk; vCSF, ventricular cerebrospinal fluid; VFM, myelin water volume fraction; 9HPT, 9-Hole Peg Test
Keywords: Myelin water imaging, Primary progressive multiple sclerosis, Spinal cord, Atrophy, Myelin
Highlights
-
•
A biomarker is needed to evaluate therapies for primary progressive MS (PPMS).
-
•
We used novel MRI providing both general atrophy and specific myelin measures.
-
•
We studied brain and cervical spinal cord in PPMS and healthy controls.
-
•
Brain and spinal cord volume and myelin measures were reduced in PPMS vs controls.
-
•
MRI measures described cognitive, fine motor and ambulatory disability scores.
1. Introduction
In 10–20% of cases, multiple sclerosis (MS) presents with progressive development of disability from onset (primary progressive (PP) MS), differentiating it from the more common relapsing–remitting (RR) form of the disease. Although it is not believed to be a separate disorder (Antel et al., 2012), none of the current therapies used to treat RRMS have been found to be effective in PPMS (Kantarci, 2013). The identification of an effective therapy for PPMS has been hindered by the lack of a sufficiently sensitive biomarker to evaluate a therapeutic effect in early phase clinical trials. There is therefore an urgent need for methods that can better monitor PPMS disease progression to assist in the development of disease modifying drugs and for incorporation into clinical trials (Rice et al., 2013).
Atrophy occurs early in MS (De Stefano et al., 2001, Sastre-Garriga et al., 2005a, Tiberio et al., 2005) and has been measured in all MS phenotypes (De Stefano et al., 2010, Grassiot et al., 2009, Pagani et al., 2005, Tedeschi et al., 2005), with PPMS showing greater and earlier spinal cord atrophy than RRMS (Bieniek et al., 2006, Lukas et al., 2013). Atrophy of the brain and cervical spinal cord correlate with clinical scores and appear to be good biomarkers for disability; they have also been demonstrated to be reliable measures for assessing disease progression (Bieniek et al., 2006, Chard et al., 2004, Grassiot et al., 2009, Lukas et al., 2013, Sastre-Garriga et al., 2005b, Tiberio et al., 2005). Cervical cord volume (CCV) has been shown to decrease significantly over two years in PPMS, demonstrating potential utility within the timeframe of most clinical trials (Laule et al., 2010).
While atrophy measurements are clearly sensitive and relevant to MS progression, they are unspecific. It is also important to differentiate between contributing pathological processes, including inflammation, axonal damage, and particularly, myelin damage, as these may distinguish between different MS subtypes and provide a greater understanding of disease pathogenesis and treatment effect.
Multi-component relaxation imaging is a sensitive and specific MRI technique for measuring changes in myelin. This approach separates MR (magnetic resonance) signal into that originating from different water environments, based on relaxation characteristics; water in intra/extracellular spaces relaxes more slowly than water trapped between myelin sheaths (MacKay et al., 1994). Multi-component driven equilibrium single pulse observation of T1 & T2 (mcDESPOT) consists of a series of spoiled gradient echo (SPGR) and balanced steady state free precession (SSFP) images, each acquired over a range of flip angles. The various flip angles provide an assortment of different contrasts with varying degrees of T1- and T2-weighting. It can be used to estimate the fraction of signal from each water pool, in particular, the fraction of faster-relaxing signal of water associated with myelin, the myelin water volume fraction (VFM) (Deoni, 2011, Deoni et al., 2008). Previous studies have demonstrated reduced VFM in PPMS brain (Kolind et al., 2012), and robust results in healthy cervical spinal cord (Kolind and Deoni, 2011). Beneficially, the images that make up the mcDESPOT acquisition can also be assessed as standard structural images. An SPGR scan with a flip angle of 18° provides excellent contrast for assessment of brain and spinal cord volume. Thus our protocol can be used to simultaneously assess atrophy and VFM, both of which appear to be important in PPMS.
In this study, we applied a mcDESPOT protocol to measure atrophy and VFM in PPMS brain and spinal cord, comparing results with those from matched healthy controls as well as with clinical measures of disability. The goal was to demonstrate the applicability of mcDESPOT to the study of PPMS and its potential for use in future clinical trials of disease modifying therapies.
2. Materials and methods
2.1. Subject information
The study was ethically reviewed and given a favourable opinion, under the UK's Health Research Authority, by the South East Coast—Surrey Research Ethics Committee (REC reference: 11/LO/0739). Appropriate approvals were also obtained from the King's College NHS Foundation Trust, through which patients were recruited. Informed consent was obtained from all participants. Fifteen subjects with clinically defined PPMS fulfilling the 2005 revised McDonald criteria for diagnosis (Polman et al., 2005): 11 males, mean age 52 (range 41–67) years; median Expanded Disability Status Scale (EDSS) = 5.0 (2.5–6.5); mean disease duration = 6 (2-17) years; mean brain lesion volume = 19 (0–61) cm3; mean number of spinal cord lesions = 4 (2-6) were recruited and compared to 11 age and gender-matched healthy controls (9 males, mean age 49 (range 37–64) years).
Disability was assessed for the MS subjects using EDSS (Kurtzke, 1983) and the Multiple Sclerosis Functional Composite (MSFC) score (Fischer et al., 1999), which includes measures of cognitive processing (Paced Auditory Serial Addition Test; PASAT), manual dexterity (9-Hole Peg Test; 9HPT) and ambulatory function (Timed 25-Foot Walk; T25FW).
2.2. Image acquisition
Brain: MR image acquisition was performed on a 1.5 T GE Signa system (General Electric, Waukesha, USA). mcDESPOT data were acquired sagittally over the whole brain, with a 1.7 mm isotropic voxel size; SPGR: TE/TR = 1.9/5 ms, 8 optimised flip angles up to 18°; SSFP: TE/TR = 1.8/3.6 ms, 8 optimised flip angles up to 70°, phase-cycling pattern = 0° and 180° (for correction of off-resonance effects); total scan time 10 min (Deoni et al., 2008). Axial FLAIR and PD/T2-weighted images were also collected for lesion identification (total 7 min).
Spinal Cord: mcDESPOT data were acquired sagittally over the entire cervical spinal cord (field of view (FOV) = 20 cm) with 0.78(A/P) × 0.78(A/P) × 1 mm(S/I) voxel size; SPGR: TE/TR = 2.2/4.9 ms, 8 optimised flip angles up to 18°; inversion recovery prepared SPGR (IRSPGR): TE/TR = 2.05/4.9 ms, α = 5°, TI = 350 ms (for correction of B1 inhomogeneity effects (Deoni, 2011)); SSFP: TE/TR = 1.6 ms/3.2 ms, 8 optimised flip angles up to 70°, phase-cycling pattern = 0° and 180°; total scan time 24 min (Kolind and Deoni, 2011). Sagittal T1-weighted and T2-weighted and axial multiple-echo recombined gradient echo (“MERGE”) images were also collected for lesion identification (total 15.5 min).
2.3. Image analysis
Volume measurements: Both brain and cervical spinal cord volume measurements were calculated using one of the 3D T1-weighted SPGR scans from the mcDESPOT data (flip angle = 18°).
Brain volume measurements: Whole-brain, peripheral and total grey matter, white matter and ventricular cerebrospinal fluid (vCSF) volume were calculated using the FSL tool Structural Image Evaluation using Normalisation of Atrophy (SIENAX) (Smith et al., 2002). First, lesions were manually delineated by an experienced neuroradiologist on the PD images and linearly registered to the SPGR. Within SIENAX, brain and skull images are extracted and affine registered to the Montreal Neurological Institute 152 standard image. Tissue-type segmentation is then carried out, taking the lesion masks as input to ensure correct classification of white matter voxels with altered intensities. A back-normalised standard space mask is used to isolate peripheral (i.e. cortical) from total GM, which also includes subcortical structures. Finally, SIENAX produces tissue-class-specific volumes normalised for head size, using a scaling factor derived from the registration.
Spinal cord volume measurements: Cervical cord area was computed from 13 slices at the C2/C3 intervertebral disc using a modified version of the semi-automatic method by Tench et al. (Tench et al., 2005). The algorithm is a region-growing technique that uses edge detection, partial volume estimation and cord angle correction to ensure accuracy.
VFM measurements: VFM maps were calculated voxelwise using a three-pool mcDESPOT analysis approach (Deoni et al., 2013). White matter was extracted using FSL-FAST. Regions of interest in the corpus callosum and minor forceps were selected based on previous results showing correlation between VFM and the mental functional system EDSS score in these regions (Kolind et al., 2012). These regions of interest were generated from the JHU atlases in FSL, manually edited to remove regions of partial volume, and analysed in native space. Spinal cord tissue was extracted (over the whole cervical cord from C1 to C7) using FSL-FAST, and manually edited to remove regions of partial volume. Mean VFM values were calculated for all white matter, the corpus callosum, minor forceps, and whole cervical cord. Two controls were excluded from the VFM spinal cord analysis. One could not fit comfortably into the neck coil, and the other had substantial motion artefact; there was still no significant age difference between groups after excluding these participants.
Statistics: Non-parametric statistics were performed to compare volume measurements and VFM values between PPMS and controls (Mann–Whitney U-test), and to correlate volume measurements and VFM values with clinical scores (Spearman's rank test). As this was an exploratory study applying a new method, and because many of our measures may be correlated with each other, we did not apply a strict correction for multiple comparisons. Instead, we carefully selected (and minimized) the tests to be performed, and report uncorrected p-values for these tests so that readers can appreciate the likely degree of significance whether or not corrections for multiple comparisons were performed.
3. Results
3.1. Volume differences
Brain and spinal cord volume was significantly reduced in PPMS subjects in comparison to controls (an example is shown in Fig. 1, Fig. 2a–e).
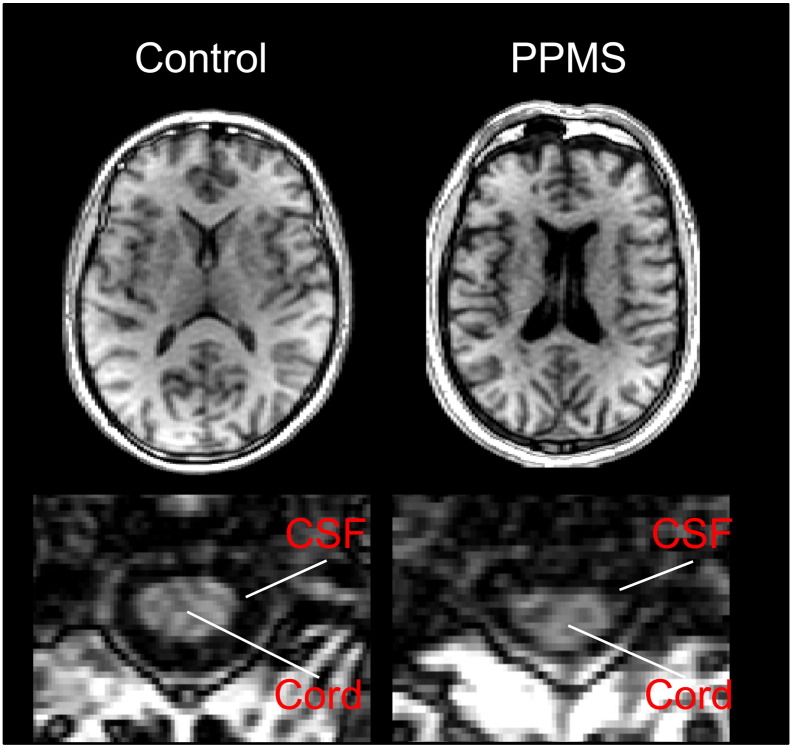
Fig. 1.
Illustrative case of atrophy in both brain and spinal cord for a subject with PPMS (age 54, EDSS 6.0) compared to a control (age 58).
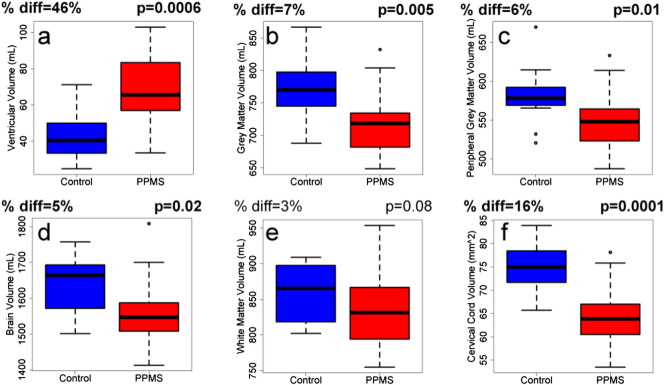
Fig. 2.
Bar charts illustrating the difference in volume between control (blue) and PPMS (red) brain and spinal cord; specifically (a) ventricular volume, vCSF, (b) grey matter volume, (c) peripheral grey matter volume, (d) total brain volume, (e) white matter volume and (f) cervical spinal cord volume, CCV. The mean percent difference and p-value are noted, with significant differences (p < 0.05) indicated in bold. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Compared to controls, PPMS subjects had larger CSF volumes (vCSF; on average + 46%, p = 0.0006, Fig. 2a), and smaller total grey matter (− 7%, p = 0.005, Fig. 2b), peripheral grey matter (− 6%, p = 0.01, Fig. 2c), whole-brain (− 5%, p = 0.02, Fig. 2d) and white matter (− 3%, p = 0.07, Fig. 2e) volume. Cervical spinal cord volume was also 16% lower than healthy controls (p = 0.0001, Fig. 2f).
3.2. Myelin water volume fraction differences
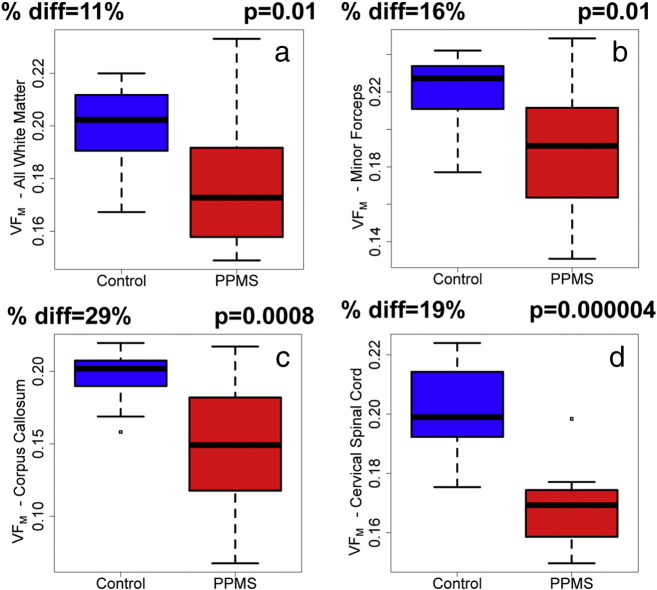
Global normal appearing white matter VFM was reduced in PPMS subjects compared to control normal white matter (− 11%, p = 0.01, Fig. 3a) as well as for the individual structures of the minor forceps (− 16%, p = 0.01, Fig. 3b) and corpus callosum (− 29%, p = 0.0008, Fig. 3c). VFM was also decreased by 19% in the cervical spinal cord of PPMS subjects compared to controls (p = 0.000004, Fig. 3d).
Fig. 3.
Bar charts illustrating the difference in mcDESPOT myelin water volume fraction, VFM, between control (blue) and PPMS (red) brain and spinal cord regions; specifically (a) all (normal-appearing) white matter, (b) minor forceps, (c) corpus callosum and (d) all cervical spinal cord. The mean percent difference and p-value are noted (all were significant, p < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Comparison with clinical scores
Table 1 reports Spearman rank correlation coefficients (R) and p-values for correlations between the MR metrics and clinical scores. VFM for total white matter, and for the minor forceps, were excluded from the table as no significant relationships were found.
Table 1.
Spearman rank correlation coefficients between MR measures and clinical scores.
| Brain Volume |
Brain VFM |
Spinal cord Volume |
Spinal cord VFM |
|
|---|---|---|---|---|
| vCSF | Corpus callosum | CCV | Whole cervical cord | |
| EDSS | R = 0.23, p = 0.4 | R = − 0.50, p = 0.06 | R = − 0.47, p = 0.08 | R = − 0.53, p = 0.04 |
| MSFC | R = − 0.73, p = 0.002 | R = 0.46, p = 0.09 | R = 0.45, p = 0.09 | R = 0.57, p = 0.03 |
| PASAT | R = − 0.65, p = 0.009 | R = 0.11, p = 0.7 | R = 0.16, p = 0.6 | R = 0.33, p = 0.2 |
| 9HPT | R = − 0.67, p = 0.007 | R = 0.64, p = 0.01 | R = 0.11, p = 0.7 | R = 0.49, p = 0.06 |
| T25FW | R = 0.08, p = 0.8 | R = − 0.40, p = 0.1 | R = − 0.54, p = 0.04 | R = − 0.53, p = 0.04 |
Significant relationships (p < 0.05) indicated in bold, and trends (p = 0.06) in italics.
9HPT was measured as the average of the inverse of the time for the dominant hand and the inverse of the time for the non-dominant hand (thus 9HPT score decreases for longer times), and T25FW was measured as the average time over 2 attempts (thus T25FW score increases for longer times).
The brain atrophy measure, vCSF, correlated with the overall MSFC score (R = − 0.73, p = 0.002) as well as with the cognitive subtest, PASAT (R = − 0.65, p = 0.009) and the manual dexterity test, 9HPT (R = − 0.67, p = 0.007), but not with the ambulatory function, T25FW (R = 0.075, p = 0.8) nor EDSS (R = 0.231, p = 0.4). PPMS CCV correlated with the T25FW (R = − 0.54, p = 0.04) but did not reach significance with EDSS (R = − 0.47, p = 0.08).
The corpus callosum VFM correlated with the 9HPT (R = 0.64, p = 0.01) with a trend towards significance with EDSS (R = − 0.50, p = 0.06). The VFM of the cervical spinal cord was related to the most clinical scores, showing correlations with EDSS (R = − 0.53, p = 0.04), MSFC (R = 0.57, p = 0.03) and T25FW (R = − 0.53, p = 0.04) with a trend towards significance with 9HPT (R = 0.49, p = 0.06).
3.4. Comparison between volume and VFM
Across all participants, there was a correlation between CCV and VFM (R = 0.56, p = 0.005). When separated into controls or PPMS, there were no correlations (controls: R = − 0.08, p = 0.8; PPMS: R = 0.21, p = 0.5). Similarly in brain, there was a weak correlation between vCSF and global normal appearing white matter VFM (R = − 0.45, p = 0.02) that was not present for controls alone (R = − 0.34, p = 0.3) nor PPMS alone (R = − 0.27, p = 0.3).
4. Discussion
There is currently no effective treatment for PPMS, as detailed in the recent review by Ontaneda et al. (Ontaneda et al., 2015). Development of therapies has been hindered by the lack of an effective imaging outcome for clinical trials; in RRMS, gadolinium enhancing and new T2 lesions on conventional MRI can be used. Advanced MRI may provide sensitive and specific measures to meet this challenge in PPMS. While atrophy measurements demonstrate sensitivity to volume loss over time in PPMS, they are unspecific. The relative contributions of water, myelin or axon loss to volume changes is not equal across all types of MS, all patients, or even all lesions. For example, in a pathological study of spinal cord, Tallantyre et al. (Tallantyre et al., 2009) demonstrated more extensive reduction of axonal density in demyelinated regions in PPMS than in secondary progressive MS, with less inflammatory activity. Myelin loss in MS could be due to inflammatory demyelination, or Wallerian degeneration leading to loss of axons and their myelin sheaths. While demyelination itself may not be the primary driver of chronic disease progression or disability, being able to differentiate between areas of inflammation, edema, demyelination, axonal loss and remyelination would be invaluable to determining such important factors as the likelihood of repair of a region, or the relative axon loss to myelin loss. Thus a measurement providing sensitive measures of volume combined with a specific marker for myelin would be extremely valuable.
The most commonly used advanced MRI measurements related to myelin are magnetization transfer imaging and diffusion tensor imaging, both of which have revealed differences between PPMS and healthy controls in the brain and spinal cord. Magnetization transfer (MT) imaging provides estimates of macromolecular-bound water, which have been shown to be associated with myelin in histopathological studies, albeit with mixed results (reported R2 values with myelin stain include 0.2 (van Waesberghe et al., 1997), 0.42 (Mottershead et al., 2003), 0.58 (Bot et al., 2004) and 0.80 (Schmierer et al., 2007)). It should be noted that MT effects have been shown to be strongly influenced by inflammation in animal models (Gareau et al., 2000, Serres et al., 2009). In postmortem MS brain, no significant difference between active and inactive lesions was found in MT nor any other quantitative MRI or histology indices, suggesting inflammation may interfere to a lesser degree in the association between myelin and MT than in animal models (Schmierer et al., 2007). However, in vivo studies have demonstrated a strong link between MT and total water content, advocating that results should be interpreted with caution (Fox et al., 2005, van Waesberghe et al., 1997, Vavasour et al., 2011).
Diffusion tensor imaging, while exquisitely sensitive to the microstructural architecture, reflects not only myelin, but also fibre coherence, axonal density, and membrane permeability (Beaulieu, 2002, Harsan et al., 2006).
Multi-component relaxation imaging may be the most sensitive and specific MRI technique for measuring changes in myelin. Preclinical (Kozlowski et al., 2008, McCreary et al., 2009, Odrobina et al., 2005, Stanisz et al., 2004, Webb et al., 2003) and postmortem human studies (Laule et al., 2006, Laule et al., 2008) have demonstrated strong correlations between histological measures of myelin and the myelin water fraction measurement provided by multi-component relaxation imaging. mcDESPOT (Deoni et al., 2008) provides a rapid, high-volumetric coverage approach to multi-component relaxation imaging that makes this technique a viable option for clinical trials.
In this study, the utility of mcDESPOT, which produces quantitative measurements of brain and spinal cord myelin contents in addition to volume, was examined in relation to commonly used clinical measures of disability for monitoring PPMS. This study demonstrated that cervical spinal cord myelin water volume fraction is significantly reduced in PPMS compared to controls, corresponding well with post mortem studies (Gilmore et al., 2005). PPMS whole brain and regional white matter also had decreased VFM that correlated with disability, indicating that diffuse white matter damage is an important pathological feature in PPMS that can be monitored with mcDESPOT.
The cohort was small for this study, thus correlations with clinical indices should be interpreted with caution. However, taken concurrently, the brain and spinal cord atrophy and VFM measurements came neatly together to describe the studied aspects of clinical disability. (1) Cognition: declining cognitive scores were largely represented by brain volume changes. (2) Dexterity: reduced dexterity was reflected by reduced brain volume, but more specifically, by reduced VFM in both corpus callosum and spinal cord. (3) Ambulation: ambulation was related to reduced spinal cord volume, likely demyelination (as indicated by VFM) in particular.
vCSF correlated with both the PASAT and 9HPT scores, while the more specific corpus callosum VFM was only related to the 9HPT. This result may suggest that the PASAT score is more closely associated with decreases in whole brain volume rather than white matter myelin content while the manual dexterity task deficits were more influenced by demyelination in important pathways.
For the ambulatory task, the T25FW was negatively correlated with both spinal cord CCV (implicating atrophy) and VFM (implicating myelin loss). Interestingly, spinal cord VFM also had a trend to correlate with the 9HPT (R = 0.49, p = 0.06) while CCV did not (R = 0.11, p = 0.7).
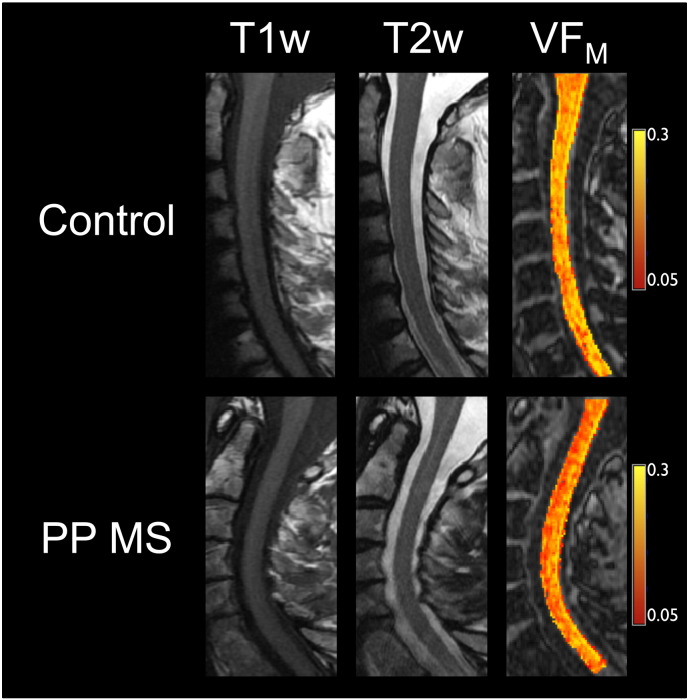
While the ambulation scores correlated with both CCV and spinal cord VFM, they are not redundant measures. Across all participants, there was a significant correlation between CCV and VFM (R = 0.56, p = 0.005), thus reductions in VFM account for roughly 30% of the variance in CCV. This correlation was largely bimodal; i.e. control and PPMS data were well separated in the correlation plots, rather than forming part of a continuum; the correlation was driven by the inter-group differences (in both scores) between the groups, with no significant intra-group differences between CCV and VFM (controls: R = − 0.08, p = 0.8; PPMS: R = 0.21, p = 0.5). A similar effect was seen in brain, with a weak correlation between vCSF and global normal appearing white matter VFM (R = − 0.45, p = 0.02 for all participants, dropping to − 0.34 (p = 0.3) for controls and − 0.27 (p = 0.3) for PPMS). Thus while a substantial reduction in myelin is reflected by a decrease in volume, only 20–30% of the change in volume is a reflection of the change in VFM; volume changes also reflect other tissue or water loss. Thus VFM and volume are complementary measures; by obtaining VFM in addiction to measuring volume we are gleaning more specific information about what is driving atrophy. In addition, spinal cord VFM correlated more strongly with the overall disability measures and was linked to the dexterity task, and therefore may be more clinically relevant than CCV. Measuring both volume and VFM could therefore aid in targeting therapies and monitoring progression more effectively. Fig. 4 illustrates the utility of spinal cord VFM for rapid assessment of myelin changes in the spinal cord; for the age-matched healthy control the VFM values are visibly higher, while the conventional images do not demonstrate the changes apparent in the PPMS VFM image.
Fig. 4.
Illustrative case of spinal cord pathology evident for a subject with PPMS (age 58, EDSS 6.5) compared to a control (age 58). The same colour scale was used for both participants. While 2 lesions were noted within the PPMS cervical spinal cord by a neuroradiologist (NS), markedly lower mcDESPOT VFM values are apparent throughout the PPMS spinal cord (particularly in anterior regions); the T1- and T2-weighted images do not reflect this diffuse pathology.
Volume measurements in the brain (Shiee et al., 2012) and spinal cord (Bieniek et al., 2006, Laule et al., 2010) were in line with literature reports, indicating that the images from the mcDESPOT acquisition are well suited to this analysis. Using a standard protocol across centres for atrophy measurements as part of a mcDESPOT imaging protocol would improve consistency of atrophy results for multi-centre trials.
The traditional approach to multi-component relaxation imaging (multiple spin-echo T2 relaxation) has not previously been applied to study brain in a cohort of people with PPMS. The more recently introduced approach of mcDESPOT has been applied in PPMS brain (Kolind et al., 2012), revealing reduced VFM in PPMS compared to controls, and demonstrating correlations with EDSS functional subscores. The results from the present study were consistent with those past results, but provide further detail with the inclusion of the MSFC and its components.
Traditional multi-component relaxation imaging has been applied to PPMS spinal cord (Laule et al., 2010), however the reduction in myelin water fraction in PPMS compared to controls did not reach significance (12% reduction, p = 0.09, 20 PPMS and 24 healthy controls). Laule et al. did however detect a significant reduction in PPMS spinal cord myelin water fraction over 2 years (− 10.5%, p = 0.01). In the current study, using mcDESPOT, we successfully detected a reduction in VFM between PPMS and controls (19%, p = 0.000004, 15 PPMS and 9 healthy controls). The increased significance in this study is likely due to the smaller inter- and intra-subject coefficient of variation for mcDESPOT measures of VFM compared to traditional myelin water fraction measurements (Kolind and Deoni, 2011). It is therefore very encouraging that mcDESPOT spinal cord VFM measurements will be sensitive to changes over time and able to detect treatment effects with relatively small cohorts.
While atrophy has been proven to be relevant to disease progression and is known to differ between PPMS and controls, it lacks specificity. A marker related to myelin provides further information on what is driving the atrophy and may be more sensitive in a case with opposing pathologies. For instance, upon commencement of therapy, pseudoatrophy (reduction in brain volume due to resolution of inflammation) is common. A finding of atrophy in the absence of a decrease in VFM may elucidate the difference between an effective or detrimental intervention.
Despite the small cohort, the results of this study clearly demonstrate the ability of mcDESPOT to distinguish between PPMS and healthy controls, and the clinical relevance of the calculated measures. This quantitative measurement is equally applicable at other field strengths, across centres, system manufacturers, and over time (Deoni et al., 2009, Kolind et al., 2012, Kolind et al., 2013), providing a robust method for assessing patients consistently. mcDESPOT is also more time efficient than traditional spin-echo based multi-component relaxation techniques, covering the entire brain at 1.7 mm-slice thickness or cervical spinal cord at 1 mm-slice thickness compared to only between 1 and 20 5 mm-slices in similar time with traditional approaches (MacKay et al., 1994, MacMillan et al., 2011, Minty et al., 2009, Prasloski et al., 2012, Wu et al., 2006). Perhaps more important will be the investigation of whether the measured values reflect clinical changes over time, through longitudinal studies.
5. Conclusions
In summary, mcDESPOT acquisition for whole brain and cervical spinal cord differentiated between PPMS and healthy controls, and provided a comprehensive description of clinical scores in this cohort. Brain volume was linked to scores for cognitive processing and manual dexterity, and spinal cord volume to ambulation. VFM in brain correlated with manual dexterity, while in the cervical spinal cord it correlated with all clinical scores except cognitive processing. Volume measurements and VFM were not strongly correlated, and thus are likely sensitive to different processes. Together, these measurements provide complementary information, with increased specificity for myelin provided by VFM. This protocol promises to present a full picture of progression in PPMS and provide effective measures in clinical trials for therapeutic treatment of progression.
Acknowledgements
Sincere thanks to the participants and radiographers. The authors wish to thank Katrina McMullen and Tobias Wood for helpful discussions and editing. This study was supported by the MS Society of Canada, the Milan & Maureen Ilich Foundation, and a Wellcome Trust VIP Award.
References
- Antel J., Antel S., Caramanos Z., Arnold D.L., Kuhlmann T. Primary progressive multiple sclerosis: part of the MS disease spectrum or separate disease entity? Acta Neuropathol. May 2012;123(5):627–638. doi: 10.1007/s00401-012-0953-0. [DOI] [PubMed] [Google Scholar]
- Kantarci O. Treatment of primary progressive multiple sclerosis. Semin. Neurol. Feb 2013;33(1):74–78. doi: 10.1055/s-0033-1343798. [DOI] [PubMed] [Google Scholar]
- Rice C.M., Cottrell D., Wilkins A., Scolding N.J. Primary progressive multiple sclerosis: progress and challenges. J. Neurol. Neurosurg. Psychiatry. Oct 2013;84(10):1100–1106. doi: 10.1136/jnnp-2012-304140. [DOI] [PubMed] [Google Scholar]
- Tiberio M., Chard D.T., Altmann D.R. Gray and white matter volume changes in early RRMS: a 2-year longitudinal study. Neurology. Mar 22 2005;64(6):1001–1007. doi: 10.1212/01.WNL.0000154526.22878.30. [DOI] [PubMed] [Google Scholar]
- De Stefano N., Narayanan S., Francis G.S. Evidence of axonal damage in the early stages of multiple sclerosis and its relevance to disability. Arch. Neurol. 2001;58(1):65–70. doi: 10.1001/archneur.58.1.65. [DOI] [PubMed] [Google Scholar]
- Sastre-Garriga J., Ingle G.T., Chard D.T. Grey and white matter volume changes in early primary progressive multiple sclerosis: a longitudinal study. Brain. Jun 2005;128(Pt 6):1454–1460. doi: 10.1093/brain/awh498. [DOI] [PubMed] [Google Scholar]
- Tedeschi G., Lavorgna L., Russo P. Brain atrophy and lesion load in a large population of patients with multiple sclerosis. Neurology. Jul 26 2005;65(2):280–285. doi: 10.1212/01.wnl.0000168837.87351.1f. [DOI] [PubMed] [Google Scholar]
- Pagani E., Rocca M.A., Gallo A. Regional brain atrophy evolves differently in patients with multiple sclerosis according to clinical phenotype. AJNR Am. J. Neuroradiol. Feb 2005;26(2):341–346. [PMC free article] [PubMed] [Google Scholar]
- Grassiot B., Desgranges B., Eustache F., Defer G. Quantification and clinical relevance of brain atrophy in multiple sclerosis: a review. J. Neurol. Sep 2009;256(9):1397–1412. doi: 10.1007/s00415-009-5108-4. [DOI] [PubMed] [Google Scholar]
- De Stefano N., Giorgio A., Battaglini M. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology. Jun 8 2010;74(23):1868–1876. doi: 10.1212/WNL.0b013e3181e24136. [DOI] [PubMed] [Google Scholar]
- Lukas C., Sombekke M.H., Bellenberg B. Relevance of spinal cord abnormalities to clinical disability in multiple sclerosis: MR imaging findings in a large cohort of patients. Radiology. Nov 2013;269(2):542–552. doi: 10.1148/radiology.13122566. [DOI] [PubMed] [Google Scholar]
- Bieniek M., Altmann D.R., Davies G.R. Cord atrophy separates early primary progressive and relapsing remitting multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2006;77(9):1036–1039. doi: 10.1136/jnnp.2006.094748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard D.T., Griffin C.M., Rashid W. Progressive grey matter atrophy in clinically early relapsing-remitting multiple sclerosis. Mult. Scler. Aug 2004;10(4):387–391. doi: 10.1191/1352458504ms1050oa. [DOI] [PubMed] [Google Scholar]
- Sastre-Garriga J., Ingle G.T., Rovaris M. Long-term clinical outcome of primary progressive MS: predictive value of clinical and MRI data. Neurology. Aug 23 2005;65(4):633–635. doi: 10.1212/01.wnl.0000173061.12776.1f. [DOI] [PubMed] [Google Scholar]
- Laule C., Vavasour I.M., Zhao Y. Two-year study of cervical cord volume and myelin water in primary progressive multiple sclerosis. Mult. Scler. Jun 2010;16(6):670–677. doi: 10.1177/1352458510365586. [DOI] [PubMed] [Google Scholar]
- MacKay A., Whittall K., Adler J., Li D., Paty D., Graeb D. In vivo visualization of myelin water in brain by magnetic resonance. Magn. Reson. Med. 1994;31(6):673–677. doi: 10.1002/mrm.1910310614. [DOI] [PubMed] [Google Scholar]
- Deoni S.C., Rutt B.K., Arun T., Pierpaoli C., Jones D.K. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn. Reson. Med. Dec 2008;60(6):1372–1387. doi: 10.1002/mrm.21704. [DOI] [PubMed] [Google Scholar]
- Deoni S.C. Correction of main and transmit magnetic field (B(0) and B(1)) inhomogeneity effects in multicomponent-driven equilibrium single-pulse observation of T(1) and T(2) Magn. Reson. Med. Apr 2011;65(4):1021–1035. doi: 10.1002/mrm.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolind S., Matthews L., Johansen-Berg H. Myelin water imaging reflects clinical variability in multiple sclerosis. Neuroimage. Mar 2012;60(1):263–270. doi: 10.1016/j.neuroimage.2011.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolind S.H., Deoni S.C. Rapid three-dimensional multicomponent relaxation imaging of the cervical spinal cord. Magn. Reson. Med. Feb 2011;65(2):551–556. doi: 10.1002/mrm.22634. [DOI] [PubMed] [Google Scholar]
- Polman C.H., Reingold S.C., Edan G. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann. Neurol. Dec 2005;58(6):840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Fischer J.S., Rudick R.A., Cutter G.R., Reingold S.C. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult. Scler. Aug 1999;5(4):244–250. doi: 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Zhang Y., Jenkinson M. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. Sep 2002;17(1):479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Tench C.R., Morgan P.S., Constantinescu C.S. Measurement of cervical spinal cord cross-sectional area by MRI using edge detection and partial volume correction. J. Magn. Reson. Imaging. Mar 2005;21(3):197–203. doi: 10.1002/jmri.20253. [DOI] [PubMed] [Google Scholar]
- Deoni S.C., Matthews L., Kolind S.H. One component? Two components? Three? The effect of including a nonexchanging “free” water component in multicomponent driven equilibrium single pulse observation of T1 and T2. Magn. Reson. Med. Jul 2013;70(1):147–154. doi: 10.1002/mrm.24429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontaneda O., Fox R.J., Chataway J. Clinical trials in progressive multiple sclerosis: lessons learned and future perspectives. Lancet Neurol. Feb 2015;14(2):208–223. doi: 10.1016/S1474-4422(14)70264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallantyre E.C., Bo L., Al-Rawashdeh O. Greater loss of axons in primary progressive multiple sclerosis plaques compared to secondary progressive disease. Brain. May 2009;132(Pt 5):1190–1199. doi: 10.1093/brain/awp106. [DOI] [PubMed] [Google Scholar]
- van Waesberghe J.H., Castelijns J.A., Scheltens P. Comparison of four potential MR parameters for severe tissue destruction in multiple sclerosis lesions. Magn. Reson. Imaging. 1997;15:155–162. doi: 10.1016/s0730-725x(96)00340-2. [DOI] [PubMed] [Google Scholar]
- Mottershead J.P., Schmierer K., Clemence M. High field MRI correlates of myelin content and axonal density in multiple sclerosis—a post-mortem study of the spinal cord. J. Neurol. 2003;250(11):1293–1301. doi: 10.1007/s00415-003-0192-3. [DOI] [PubMed] [Google Scholar]
- Bot J.C., Blezer E.L., Kamphorst W. The spinal cord in multiple sclerosis: relationship of high-spatial-resolution quantitative MR imaging findings to histopathologic results. Radiology. 2004;233(2):531–540. doi: 10.1148/radiol.2332031572. (Epub 2004 Sep 22) [DOI] [PubMed] [Google Scholar]
- Schmierer K., Tozer D.J., Scaravilli F. Quantitative magnetization transfer imaging in postmortem multiple sclerosis brain. JMRI. 2007;26:41–51. doi: 10.1002/jmri.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau P.J., Rutt B.K., Karlik S.J., Mitchell J.R. Magnetization transfer and multicomponent T2 relaxation measurements with histopathologic correlation in an experimental model of MS. J. Magn. Reson. Imaging. 2000;11(6):586–595. doi: 10.1002/1522-2586(200006)11:6<586::aid-jmri3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Serres S., Anthony D.C., Jiang Y. Comparison of MRI signatures in pattern I and II multiple sclerosis models. NMR Biomed. 2009;22:1014–1024. doi: 10.1002/nbm.1404. [DOI] [PubMed] [Google Scholar]
- Vavasour I.M., Laule C., Li D.K., Traboulsee A.L., MacKay A.L. Is the magnetization transfer ratio a marker for myelin in multiple sclerosis? J. Magn. Reson. Imaging. Mar 2011;33(3):713–718. doi: 10.1002/jmri.22441. [DOI] [PubMed] [Google Scholar]
- Fox R.J., Fisher E., Tkach J., Lee J.-C., Cohen J.A., Rudick R.A. Brain atrophy and magnetization transfer ratio following methylpredni- solone in multiple sclerosis: short-term changes and long-term implications. Mult. Scler. 2005;11:140145. doi: 10.1191/1352458505ms1142oa. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Harsan L.A., Poulet P., Guignard B. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J. Neurosci. Res. 2006;83(3):392–402. doi: 10.1002/jnr.20742. [DOI] [PubMed] [Google Scholar]
- Kozlowski P., Raj D., Liu J., Lam C., Yung A.C., Tetzlaff W. Characterizing white matter damage in rat spinal cord with quantitative MRI and histology. J. Neurotrauma. Jun 2008;25(6):653–676. doi: 10.1089/neu.2007.0462. [DOI] [PubMed] [Google Scholar]
- Odrobina E.E., Lam T.Y., Pun T., Midha R., Stanisz G.J. MR properties of excised neural tissue following experimentally induced demyelination. NMR Biomed. 2005;18(5):277–284. doi: 10.1002/nbm.951. [DOI] [PubMed] [Google Scholar]
- Webb S., Munro C.A., Midha R., Stanisz G.J. Is multicomponent T2 a good measure of myelin content in peripheral nerve? Magn. Reson. Med. 2003;49(4):638–645. doi: 10.1002/mrm.10411. [DOI] [PubMed] [Google Scholar]
- McCreary C.R., Bjarnason T.A., Skihar V., Mitchell J.R., Yong V.W., Dunn J.F. Multiexponential T2 and magnetization transfer MRI of demyelination and remyelination in murine spinal cord. Neuroimage. 2009;45(4):1173–1182. doi: 10.1016/j.neuroimage.2008.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisz G.J., Webb S., Munro C.A., Pun T., Midha R. MR properties of excised neural tissue following experimentally induced neuroinflammation. Magn. Reson. Med. 2004;51:473–479. doi: 10.1002/mrm.20008. [DOI] [PubMed] [Google Scholar]
- Laule C., Kozlowski P., Leung E., Li D.K., Mackay A.L., Moore G.R. Myelin water imaging of multiple sclerosis at 7 T: correlations with histopathology. Neuroimage. May 1 2008;40(4):1575–1580. doi: 10.1016/j.neuroimage.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Laule C., Leung E., Li D.K. Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Mult. Scler. 2006;12(6):747–753. doi: 10.1177/1352458506070928. [DOI] [PubMed] [Google Scholar]
- Gilmore C.P., DeLuca G.C., Bo L. Spinal cord atrophy in multiple sclerosis caused by white matter volume loss. Arch. Neurol. Dec 2005;62(12):1859–1862. doi: 10.1001/archneur.62.12.1859. [DOI] [PubMed] [Google Scholar]
- Shiee N., Bazin P.L., Zackowski K.M. Revisiting brain atrophy and its relationship to disability in multiple sclerosis. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni S.C., Samson R., Wheeler-Kingshott C.A. Proceedings of the 17th Annual Meeting of the International Society of Magnetic Resonance in Medicine. 2009. Intra and inter-site reproducibility of myelin water volume fraction values derived using mcDESPOT; p. 4530. Honolulu, USA. [Google Scholar]
- Kolind S., Sharma R., Knight S., Johansen-Berg H., Talbot K., Turner M.R. Myelin imaging in amyotrophic and primary lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. Dec 2013;14(7–8):562–573. doi: 10.3109/21678421.2013.794843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty E.P., Bjarnason T.A., Laule C., MacKay A.L. Myelin water measurement in the spinal cord. Magn. Reson. Med. Apr 2009;61(4):883–892. doi: 10.1002/mrm.21936. [DOI] [PubMed] [Google Scholar]
- Wu Y., Alexander A.L., Fleming J.O., Duncan I.D., Field A.S. Myelin water fraction in human cervical spinal cord in vivo. J. Comput. Assist. Tomogr. 2006;30(2):304–306. doi: 10.1097/00004728-200603000-00026. [DOI] [PubMed] [Google Scholar]
- MacMillan E.L., Madler B., Fichtner N. B, Fichtner N, et al. Myelin water and T(2) relaxation measurements in the healthy cervical spinal cord at 3.0 T: repeatability and changes with age. Neuroimage. Jan 15 2011;54(2):1083–1090. doi: 10.1016/j.neuroimage.2010.08.076. [DOI] [PubMed] [Google Scholar]
- Prasloski T., Rauscher A., Mackay A.L. Rapid whole cerebrum myelin water imaging using a 3D GRASE sequence. Neuroimage. Oct 15 2012;63(1):533–539. doi: 10.1016/j.neuroimage.2012.06.064. [DOI] [PubMed] [Google Scholar]