Abstract
The psychosis high-risk state is accompanied by alterations in functional brain activity during working memory processing. We used binary automatic pattern-classification to discriminate between the at-risk mental state (ARMS), first episode psychosis (FEP) and healthy controls (HCs) based on n-back WM-induced brain activity. Linear support vector machines and leave-one-out-cross-validation were applied to fMRI data of matched ARMS, FEP and HC (19 subjects/group).
The HC and ARMS were correctly classified, with an accuracy of 76.2% (sensitivity 89.5%, specificity 63.2%, p = 0.01) using a verbal working memory network mask. Only 50% and 47.4% of individuals were classified correctly for HC vs. FEP (p = 0.46) or ARMS vs. FEP (p = 0.62), respectively. Without mask, accuracy was 65.8% for HC vs. ARMS (p = 0.03) and 65.8% for HC vs. FEP (p = 0.0047), and 57.9% for ARMS vs. FEP (p = 0.18). Regions in the medial frontal, paracingulate, cingulate, inferior frontal and superior frontal gyri, inferior and superior parietal lobules, and precuneus were particularly important for group separation.
These results suggest that FEP and HC or FEP and ARMS cannot be accurately separated in small samples under these conditions. However, ARMS can be identified with very high sensitivity in comparison to HC. This might aid classification and help to predict transition in the ARMS.
Keywords: Working memory, Classification, Machine learning, Magnetic resonance imaging, Schizophrenia, Risk factors
Highlights
-
•
The ARMS was accurately identified based on an individual patient's response within a WM network.
-
•
Regional cortical activations were particularly important for group separation.
-
•
Based on WM alterations, FEP and HC or FEP and ARMS could not be accurately separated in small samples.
1. Introduction
Working memory deficits are considered to be a central manifestation of the pathophysiology of schizophrenia (Forbes et al., 2009) and behavioural deficits in working memory processing (Pflueger et al., 2007) are already evident before the onset of the disorder in individuals with an at-risk mental state (ARMS) (Fusar-Poli et al., 2012d). Alterations in functional brain activity (Smieskova et al., 2012a) during working memory processing have been reported in ARMS subjects. In comparison to healthy controls (HCs), subjects at high risk for psychosis exhibited reduced prefrontal and parietal activation during the n-back task (Fusar-Poli et al., 2010).
There is increasing evidence that vulnerability to psychosis is associated with dysfunctional connectivity (Schmidt et al., 2013b). For example, Crossley et al. (2009) demonstrated a progressive increase in dysfunctional frontotemporal connectivity during a working memory task from HC to ARMS individuals and further to patients with FEP. Beyond connectivity, ARMS is also associated with abnormalities of regional brain structure (Fusar-Poli et al., 2012e; Smieskova et al., 2010), activity (Broome et al., 2010), and neurochemistry (Allen et al., 2012; Fusar-Poli et al., 2011b) that are qualitatively similar to but less severe than those in patients with overt psychosis (Fusar-Poli et al., 2007b). There is also evidence suggesting that within ARMS individuals, prefrontal dysfunction during WM is related to grey matter abnormalities in the same regions (Fusar-Poli et al., 2011a).
However, it is still unclear whether functional brain activity during working memory processing can be used for the individual classification and prognosis of patients at high clinical risk.
At present, individuals considered at high-risk for psychosis are clinically identified according to the PACE (Personal Assessment and Crisis Evaluation Clinic, Melbourne) criteria if they present with “attenuated” psychotic symptoms, full-blown psychotic symptoms that are brief and self-limiting (Riecher-Rossler et al., 2007a; Riecher-Rössler et al., 2009; Yung et al., 2004), or a significant decrease in functioning in the context of a family history of schizophrenia (Fusar-Poli et al., 2012b). This can lead to the correct prediction of a subsequent transition to a first psychotic episode in only 29% (Fusar-Poli et al., 2012a) of ARMS individuals after 2 years and in 35 (Nelson et al., 2013)–49% (Klosterkotter et al., 2001) after 3–10 years. Thus, in practical terms, it is difficult to predict which subjects with an ARMS will later develop psychosis on the basis of their presentation of clinical features and neuropsychological markers (Fusar-Poli et al., 2012c).
Multivariate automatic pattern classification of individuals at high-risk may be a promising approach to predicting the development of psychoses in individuals with ARMS (Lao et al., 2004; Mandl et al., 2013a). These methods categorise individuals by identification of the multivariate statistical properties of the data that discriminate between groups of subjects (Klöppel et al., 2008b; Lao et al., 2004). In this context, support vector machines (SVMs) have emerged as a powerful tool, as these machines can learn to categorise complex, high dimensional training data and to generalise the learned classification rules to new data (Koutsouleris et al., 2012; Noble, 2006). SVMs use information from all available voxels, which are combined to reflect differences between groups (Klöppel et al., 2008a), in order to create classifiers that allow the clinician to make predictions for newly acquired (or unseen) data (Klöppel et al., 2008b; Rizk-Jackson et al., 2011). SVMs have been successfully applied to structural MRI data and can distinguish between ARMS subjects and healthy controls (HCs) with high accuracy (Borgwardt et al., 2013b; Koutsouleris et al., 2012; Koutsouleris et al., 2009a). A limited number of studies have employed MRI data to investigate neurofunctional classifiers in individuals at risk of psychosis, in order to demonstrate that subtle differential functional patterns subserving emotional processing may make a major contribution towards identifying individuals who tend towards psychosis (Modinos et al., 2012; Modinos et al., 2013).
2. Aims of the study
Despite substantial evidence of working memory deficits both, at the time of first episode of the disease (Mesholam-Gately et al., 2009), and predating the onset of psychosis (Fusar-Poli et al., 2012c), so far it has not been assessed if discriminative information regarding vulnerability for psychosis resides in working memory alterations. In this study, we sought to examine whether subjects with an ARMS can be identified on the basis of their individual response within a working memory network of regions activated in a verbal identity-monitoring variant of the n-back task (Owen et al., 2005). We used previously collected contrast images of fMRI data (Smieskova et al., 2012a) and applied pattern classification using linear SVMs and leave-one-out cross-validation (Klöppel et al. 2008b, Klöppel et al. 2009).
Based on previous structural (Borgwardt et al., 2013b; Koutsouleris et al. 2009a, Koutsouleris et al. 2012) and functional (Modinos et al. 2012, Modinos et al. 2013) SVM MRI studies of subjects with an ARMS, we hypothesised that prefrontal activations could make a predominant contribution to the classification of the ARMS. On the assumption that increasing task demand increases the magnitude of neurofunctional abnormalities in ARMS (Fusar-Poli et al., 2007b), we expected robust discrimination of ARMS and HC, with high classification accuracies. On the other hand, we expected that it would be much more difficult to differentiate ARMS and FEP patients on the basis of their working memory activations (Borgwardt et al., 2013a).
3. Methods
3.1. Participants
Subjects with an ARMS and FEP patients were assessed at the time of MRI scan. Inclusion required one or more of the following: (a) “attenuated” psychotic symptoms, (b) brief limited intermittent psychotic symptoms (BLIPS), or (c) a first degree relative with a psychotic disorder plus at least two indicators of a clinical change, such as a marked decline in social or occupational functioning. All ARMS individuals were antipsychotic-naive. Subjects were assessed using the ‘Basel Screening Instrument for Psychosis’ (BSIP) (Riecher-Rossler et al., 2007b), the Brief Psychiatric Rating Scale (BPRS) (Lukoff et al., 1986), the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1989), and the Global Assessment of Functioning (GAF). The BSIP evaluates “prodromal” symptoms occurring in the previous 5 years; nonspecific “prodromal” signs (Riecher-Rossler et al., 2007b) occurring in the previous 2 years; previous or current psychotic symptoms, psychosocial functioning over the last 5 years, substance dependency; and psychotic disorders in first and second degree relatives (Riecher-Rossler et al., 2008). The group of individuals with an ARMS corresponds to the Personal Assessment and Crisis Evaluation (PACE) criteria by Yung et al. (1998). The FEP patients met the operational criteria for FEP according to Breitborde et al. (2009). Current and previous psychotropic medication, alcohol, nicotine, cannabis, and consumption of other illegal drugs were investigated by using a semi-structured interview adapted from the Early Psychosis Prevention and Intervention Centre (EPPIC) Drug and Alcohol Assessment Schedule (http://www.eppic.org.au). The exclusion criteria for these subjects were: history of previous psychotic disorder treated with antipsychotics; psychotic symptomatology secondary to an “organic” disorder; substance abuse according to the ICD-10 research criteria; psychotic symptomatology associated with an affective psychosis or a borderline personality disorder; age under 18 years; insufficient knowledge of the German language; and IQ less than 70 (Lehrl et al., 1995).
In ARMS subjects, clinical follow-up occurred on average 4.8 ± 8.6 months (range = 0–24 months) after their baseline MRI scans. During the follow-up period, 4 ARMS subjects (21%) made a transition to a first episode of psychosis, according to the PACE criteria (Yung et al., 1998). HCs were recruited from the same geographical area as the other groups. All subjects were representative of the local population of individuals presenting with an ARMS or FEP in terms of age, sex, handedness, and alcohol and cannabis consumption. These individuals had no current psychiatric disorder, no history of psychiatric illness, head trauma, neurological illness, serious medical or surgical illness, substance abuse, and no family history of any psychiatric disorder as assessed by an experienced psychiatrist in a detailed clinical semi-structured interview. All participants provided written informed consent, and the study was approved by the Research Ethics Committee.
3.2. MR image acquisition
Functional data were acquired on a 3 T scanner (Siemens Magnetom Verio, Siemens Healthcare, Erlangen, Germany) using an echo planar sequence with a repetition time of 2.5 s, echo time of 28 ms, matrix 76 × 76, 126 volumes and 38 slices with 0.5 mm interslice gap, that gave a resolution of 3 × 3 × 3 mm3, and a field of view of 228 × 228 cm2.
3.3. n-back working memory task
A well-established n-back working memory task (Smieskova et al., 2012a) was administered to patients and controls. With an inter-stimulus interval of 2 s, all subjects were presented with a series of black letters on a white background in a prismatic mirror. Each stimulus was presented for 1 s. The size of the letters was 8 cm projected onto the screen at the end of the scanner. During the baseline (0-back) condition, subjects were required to press the button with the right hand when the letter “X” appeared. During 1-back and 2-back conditions, participants were instructed to press the button if the currently presented letter was the same as that presented 1 (1-back condition) or 2 (2-back condition) trials previously. The three conditions were presented in 10 alternating.
30 s blocks (2 × 1-back, 3 × 2-back, and 5 × 0-back), matched for the number of target letters per block (i.e. 2 or 3), in a pseudo-random order. The reaction times and response accuracy were recorded on-line.
3.4. Image preprocessing
3.4.1. fMRI analysis
We pre-processed and analysed the MR images using the Statistical Parametric Mapping software (SPM8; Wellcome Department of Cognitive Neurology, London, United Kingdom). All MRI volumes were realigned to the first volume, corrected for motion artefacts, mean adjusted by proportional scaling, with affine and non-linear registration, normalised into standard space (MNI template), and smoothed using an 8 mm FWHM Gaussian kernel (Modinos et al., 2013). Maximum likelihood parameter estimates were calculated at the first level at each voxel using the general linear model. Our design matrix included an autoregressive AR(1) model of serial correlations and a high-pass filter with a cutoff of 128 s. We convolved the onset times for each block in seconds with a canonical haemodynamic response function. The first level design matrix included four conditions (with onset times of 0-back, 1-back, 2-back, and errors in seconds) in a block design and a multiple regressor for motion corrections. Each task condition (1-back and 2-back) was then contrasted against the baseline condition (0-back) in each subject. For SVM analysis we used 2-back more than 0-back contrasts known to comprise information about attention-independent modality with a higher load level of working memory.
For the fMRI n-back analyses only the correct responses were used. Clinical and sociodemographic differences were previously analysed and published by Selnes et al. ( 2012) and Smieskova et al. (2012a). Additionally, behavioural performances during the 2-back working memory condition were previously analysed by Schmidt et al. (2013a) using signal detection theory. The sensitivity index was calculated using the formula z(Hits) − z(False Alarms). The equal weighting of hits and false alarms provides an objective measure of sensitivity that is independent of participant response bias. The sensitivity index values were further subjected to a 1-way ANOVA. When the ANOVA null hypothesis of equal means was rejected, we used Bonferroni-corrected post-hoc t tests.
3.5. Pattern classification analysis
3.5.1. SVM prediction scores
The pattern classification of the contrast images obtained under the 2-back > 0-back condition was based on three different 2-class prediction models: HC versus ARMS, HC versus FEP, and ARMS versus FEP, using linear SVM combined with a leave-one-out cross-validation strategy (Klöppel et al., 2008b).
An anatomical region of interest mask was created using WFU PickAtlas (theory of the Talairach brain atlas) toolbox implemented in SPM8 (Maldjian et al., 2003). The mask included the main regional loci of brain activation of verbal identity-monitoring variant of the n-back task identified by a meta-analysis: lateral premotor cortex (BA6), dorsal cingulate/medial premotor (SMA) (BA 32,6), dorsolateral prefrontal (BA46,9), ventrolateral prefrontal (BA44), frontal pole (BA10), medial posterior parietal (BA7), inferior parietal lobule (BA40), medial and lateral cerebellum, and thalamus (Owen et al., 2005).
For comparison, pattern classification without this mask was also performed.
3.6. Support vector machine (SVM)
LIBSVM, a library for SVMs (http://www.csie.ntu.edu.tw/-cjlin/libsvm), running under MatLab 7.1 (MathWorks, USA), was used for pattern classification analysis.
The SVM methodology has been detailed elsewhere (Klöppel et al., 2008b). In brief, SVMs include a training step to learn about systematic differences in the data with respect to the two groups that are to be classified. In the context of machine learning, individual BOLD images are treated as points located in a high dimensional space, where the total number of dimensions is determined by the number of voxels within the mask. SVMs are trained to establish a decision rule that should be sufficiently general to allow discrimination of new (testing) data. Scans enter the training process alongside their label. New scans can then be tested against trained sets and in turn categorised as members of a particular clinical group (Klöppel et al., 2008b).
A linear kernel matrix was created from the images, which can be viewed as a similarity measure among subjects belonging to a characterised group. The images cluster in subspaces containing images that are very similar, so that image normalisation into a standard space is an important pre-requisite.
The use of an SVM for image classification is an example of linear discrimination. As a binary classifier, it divides the space of BOLD images into two classes separated by a hyperplane. The optimal separating hyperplane (Forbes et al., 2009) produced by an SVM is defined by those images that are closest to the separating boundary between them, i.e. the images that are most ambiguous, called ‘support vectors’. After training, an OSH contains learned differences between classes; for example, in the present case, this may be ARMS and HC images. The trained classifier can be used on scans that have not yet been presented to the SVM algorithm. This information is used in the prediction step to assign any new (or previously retained) image to one of the learned classes. Leave-one-out cross-validation iteratively leaves successive images out of the training data set for subsequent class assignation until each one has been used to make a prediction. Thereby, the method permits the estimation of the overall classification accuracy and the potential generalisability of a prediction model (Klöppel et al., 2008b).
Weight vector maps were created to show the brain regions that best discriminate between groups. In order to test how well SVMs can differentiate different stages of psychosis, 6 separate prediction models for different 2-group predictions were created — with or without a mask (Models I–VI; see Table 3 for listing) (results for contrast IV — see Fig. 2).
Table 3.
Results of SVM classification using n-back (2-back > 0-back) BOLD contrast maps for image analysis.
| Model | Sensitivity (%) | Specificity (%) | Correctly classified (%) | ||
|---|---|---|---|---|---|
| Mask | I | HC–ARMS | 89.5 | 63.2 | 76.2 |
| II | HC–FE | 52.6 | 47.4 | 50.0 | |
| III | ARMS–FE | 42.1 | 52.6 | 47.4 | |
| No mask | IV | HC–ARMS | 73.7 | 57.9 | 65.8 |
| V | HC–FE | 73.7 | 57.9 | 65.8 | |
| VI | ARMS–FE | 52.6 | 63.2 | 57.9 | |
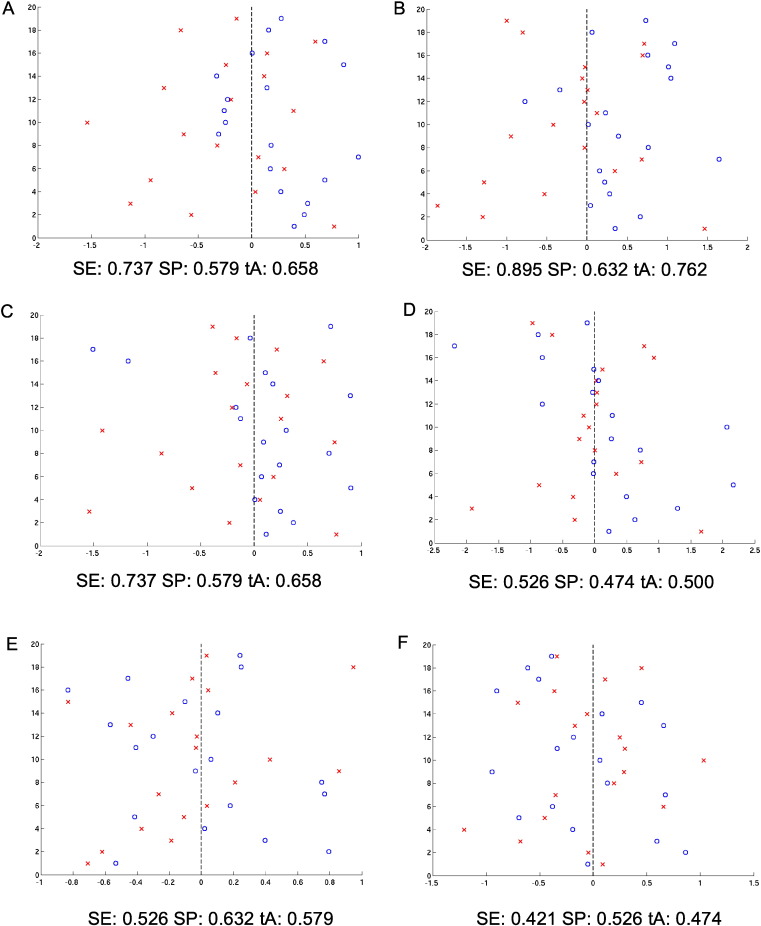
Fig. 2.
Classification score, i.e. projection of subjects onto the weight vector with positive patterns (blue circles) discriminating for group I, and negative patterns (red crosses) for group II.
Statistical inferences were made at a significance level of p < 0.05. p-Values were calculated using random labelling and permutation testing. The statistical significance of classification accuracy was determined by permutation testing (for a total of N = 100 permutations). This involved repeating the classification procedure with training group labels randomly allocated by the computer N times in order to generate a null distribution of accuracies. The fraction of permutations achieving a higher accuracy than the true labels provides an estimate of the significance of the obtained accuracy relative to chance.
The slack parameter C was optimised for each instance during leave-one-out cross-validation by performing a parameter search, where the highest classification accuracy determined the optimal value of C.
3.7. Statistical analysis of demographic data
Population variables are expressed as means and standard deviations (ordinal scales) or as totals and percentages (nominal scales). Group comparisons were performed via analysis of variance (ANOVA) for parametric data and via chi-square tests for non-parametric data. The Tukey-HSD correction was calculated for ANOVA post-hoc analysis. A statistical level of p < 0.05 was considered significant. Statistical analysis was performed with IBM SPSS Version 19.0.
4. Results
4.1. Sample characteristics
Groups were well-matched for age (F(2,54) = 0.549, p = 0.581), sex (χ2(2) = 2.927, p = 0.231), and handedness (χ2(2) = 0.538, p = 0.764). They differed in positive symptoms (sum of suspiciousness, hallucinations, delusions, and conceptual disorganisation scores of BPRS; F(2,40) = 27.587, p < 0.0001), negative symptoms (total score of SANS, F(2,52) = 22.226, p < 0.0001), and global functioning (total score of GAF; F(2,54) = 38.237, p < 0.0001). The FEP and ARMS groups had more positive (p = 0.007 and p < 0.001) and negative symptoms (p = 0.0001), and worse global functioning (p = 0.001) than those of HC, as calculated in ANOVA with Tukey's honest significance difference tests. The FEP and ARMS groups did not differ regarding antidepressant medication (37% in both groups) at the MRI date. Additionally, 32% in the FEP group was receiving antipsychotic medication. Groups did not differ with respect to alcohol, cannabis and cigarette consumption at MRI. Study population characteristics are summarised in Table 1.
Table 1.
Study population characteristics.
| FEP (n = 19) | ARMS (n = 19) | HC (n = 19) | Statistics | Post-hoc | |
|---|---|---|---|---|---|
| BPRS positive symptoms | 11.73 (4.6) |
7.07 (2.3) | 4.00 (0.0) | F(2,40) = 27.587 p < 0.0001 |
FEP > HC, FEP > ARMS, ARMS > HC |
| BPRS total (SD) | 49.50 (16.5) |
37.89 (6.5) | 24.58 (1.2) | F(2,52) = 27.966 p < 0.0001 |
FEP > HC, FEP > ARMS, ARMS > HC |
| SANS total (SD) | 21.17 (13.1) |
17.06 (12.4) | 0.00 (0.0) | F(2,52) = 22.226 p < 0.0001 |
FEP > HC, ARMS > HC |
| GAF total (SD) | 54.95 (17.0) | 60.21 (13.5) | 88.63 (4.5) | F(2,54) = 38.237 p < 0.0001 |
FEP < HC, ARMS < HC |
| Antipsychotic n AN/AF/Med | 9/4/6 | 18/1/0 | 19/0/0 | χ2(4) = 21.157 p < 0.0001 |
|
| Antidepressants n (%) | 7 (37%) | 7 (37%) | 0 | χ2(2) = 24.281 p < 0.0001 |
|
| Alcohol n No/Mod/Uncon |
4/14/1 | 3/12/4 | 0/17/2 | χ2(4) = 6.598 p = 0.159 |
|
| Cannabis currently (%) | 6 (32%) | 7 (37%) | 4 (21%) | χ2(2) = 1.411 p = 0.487 |
|
| Smoking (cig /day) | 10.11 (10.97) | 7.29 (9.9) | 3.00 (6.0) | F(2,54) = 2.853 p = 0.066 |
Abbreviations: Alcohol n, number of subjects consuming alcohol; No, no alcohol; Mod, moderate intake of alcohol; Uncon, uncontrolled drinking; Antipsychotic, antipsychotic medication on the date of MRI; AF, antipsychotic free; AN, antipsychotic naive; Med, antipsychotic medicated; ARMS, at-risk mental state individuals = A under the Post-hoc column; BPRS, Brief Psychiatric Rating Scale; BPRS positive symptoms = BPRS 9 + BPRS 10 + BPRS 11 + BPRS 15, sum of suspiciousness, hallucinations, delusions, and conceptual disorganisation; FEP, first episode psychosis patients; GAF, Global Assessment of Functioning; HC, healthy control = H under the Post-hoc column; MWT, intelligence quotient test (multiple choice-vocabulary-intelligence test) and SANS, Scale for the Assessment of Negative Symptoms.
Tukey-HSD correction (at p = 0.05) was calculated for post-hoc analysis in SPSS 19.0.
4.2. Pattern classification results
Table 2 shows the regions most relevant to discriminating between ARMS and HC (as given by SVM weights).
Table 2.
Most important activation regions (top 5%) discriminating between groups (ARMS and HC).
| Anatomical region | Hemisphere | MNI coordinates |
wi | ||
|---|---|---|---|---|---|
| x | y | z | |||
| ARMS > HC | |||||
| Middle frontal gyrus | L | −28 | 60 | 16 | 18.6 |
| −46 | 48 | 10 | 12.7 | ||
| Paracingulate Paracingulate (BA10) |
L | −8 | 50 | −6 | 13.7 |
| R | 6 | 54 | 0 | 7.75 | |
| Cingulate | R | 10 | 42 | 14 | 8.66 |
| Precentral/inferior frontal gyrus (BA44) | L | −56 | 6 | 20 | 13.6 |
| Precentral gyrus (BA6) |
R | 52 | 0 | 34 | 8.05 |
| Medial frontal gyrus (BA9) | R | 6 | 52 | 18 | 6.39 |
| L | −10 | 32 | 30 | 6.44 | |
| Superior frontal gyrus (BA10) (BA9) |
L | 0 | 14 | 58 | 8.75 |
| −40 | 52 | 20 | 33.1 | ||
| −42 | 44 | 28 | 18.8 | ||
| HC > ARMS | |||||
| Middle frontal gyrus | R | 32 | 4 | 62 | 38 |
| Inferior parietal lobule (BA40) | L | −38 | −52 | 58 | 53 |
| R | 54 | −46 | 48 | 42.1 | |
| Superior parietal lobule (BA7) | L | −20 | −60 | 62 | 41.5 |
| Precuneus | L | 0 | −60 | 58 | 50.8 |
| R | 30 | −68 | 54 | 45.1 | |
If the analysis was constrained to the verbal n-back network (mask), of the three prediction models, the highest accuracy (SE 89.5%, SP 63.2%, tA 76.2%, p = 0.01) was found for ARMS vs. HC (model I), only 2 ARMS individuals of 19 were wrongly classified as HC. Low accuracies were observed in models II (SE 52.6%, SP 47.4%, tA 50.0%, p = 0.46) and III (SE 42.1%, SP 52.6%, tA 47.4%, p = 0.62); here, half of the individuals were wrongly classified. Without analysis mask, classification accuracies were equal for model IV (SE 73.7%, SP 57.9%, tA 65.8%, p = 0.03) and model V (SE 73.7%, SP 57.9%, tA 65.8%, p = 0.0047), and low for model VI (SE 52.6%, SP 63.2%, tA 57.9%, p = 0.18) (Table 3).
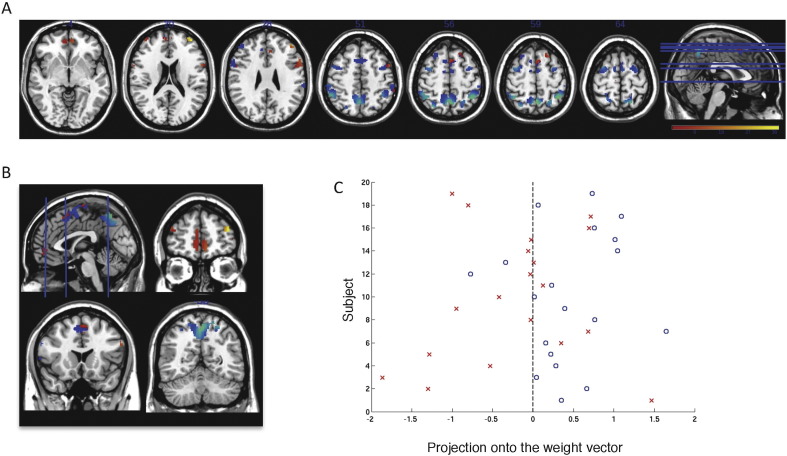
Fig. 1 shows the weight vector map with the most discriminating brain regions between the groups (top 5%). The threshold of classification strength was set to show the top 5% of the weight vector scores, as done in previous studies addressing other aspects of pattern classification and psychosis proneness (Modinos et al., 2013; Modinos et al., 2012).
Fig. 1.
Weight vector maps showing the most discriminating brain regions between HC and ARMS (n = 19 per group) using the verbal n-back mask (top 5%). Regions that contributed more to classifying individuals with ARMS are shown in red/yellow, while regions that contributed more to the classification of controls are shown in blue/green, in axial (A) and coronal (B) views; z = (−4, 20, 28, 51, 56, 59, 64). (C) Classification score, found by the projection of each subject onto the weight vector, with positive patterns (blue circles) discriminating for ARMS, and negative patterns (red crosses) for controls (p = 0.032).
Fig. 2 shows the classification scores, i.e. the projection of subjects onto the weight vector with positive patterns (blue circles) discriminating for group I, and negative patterns (red crosses) for group II for HC versus ARMS ((A) no mask, (B) verbal n-back mask), HC versus FE ((C) no mask, (D) verbal n-back mask), and ARMS versus FE ((E) no mask, (F) verbal n-back).
Models (group I vs. group II): HC–ARMS (A) no mask and (B) verbal n-back mask; HC–FE (C) no mask and (D) verbal n-back mask and ARMS–FE (E) no mask and (F) verbal n-back.
5. Discussion
The present study combines task-induced fMRI data and machine learning to explore whether functional brain activity during n-back working memory processing demonstrates a functional correlation with susceptibility for psychosis. Using SVM we demonstrate that people at high-risk for psychosis can be correctly classified with accuracies of up to 74–90%. The best performance was achieved when the analysis was restricted to brain regions within the verbal working memory network (Owen et al., 2005).
A large number of fMRI studies focusing on the neural basis of verbal working memory show a remarkably consistent network of neural areas active when verbal working memory tasks are carried out (for review see Owen et al., 2005). The main components making up the working memory network within the lateral prefrontal cortex are the dorsal lateral prefrontal cortex (BA9, 10 and 46, mostly bilateral), the ventral lateral prefrontal cortex (BA44; Brodmann's area) and BA45) and the premotor cortex (BA6, mostly bilateral). In the frontal lobe there are also secondary motor areas (BA6), which include parts of the anterior cingulate cortex (ACC) when activated. Bilateral activations in the posterior parietal lobes are found in the dorsal areas of the supra-marginal gyrus (BA40) and in the cerebellum. The exact activation patterns vary, depending on the characteristics of the given verbal working memory task.
Restricting the SVM analysis to these regions, we accurately identified differential activation patterns between ARMS subjects and HC (cf. Table 2). This is in line with our hypothesis that a network of working memory activations, within the lateral prefrontal cortex contributes predominantly to the identification of high-risk subjects.
The low accuracies found by classifying ARMS versus FEP (Table 3, models III and VI) support our second hypothesis that increasing task demand increases the magnitude of neurofunctional abnormalities in ARMS and makes it nearly impossible to distinguish ARMS from FEP using SVM (Borgwardt et al., 2013b).
Lawrie et al. (2002) predicted that individuals with schizophrenia would exhibit decreased connectivity between dorsal lateral prefrontal cortex (Brodmann's areas 9 and 10) and superior temporal gyrus (STG) which is involved in auditory processing. Most of the fMRI studies reviewed in a meta-analysis of the neurofunctional correlates of vulnerability to psychosis (Fusar-Poli et al., 2007b) reported reduced dorsal lateral prefrontal cortex activation in FEP subjects during cognitive tasks, which suggests that hypofrontality in the dorsolateral prefrontal cortex may be a feature of FEP. Three studies of working memory in FEP reported decreasing activation in the dorsolateral prefrontal cortex during the hard level of the task (Mendrek et al., 2004; Rasser et al., 2005; Riehemann et al., 2001), and only one study reported greater prefrontal activation in FEP subjects (Fusar-Poli et al., 2007b). In contrast, a number of genetic high-risk studies reported relatively greater prefrontal activation in high-risk subjects than in controls (Callicott et al., 2003; Seidman et al., 2006; Thermenos et al., 2004), which most probably reflects a compensatory response to maintain adequate performance (Fusar-Poli et al., 2007b).
Notably, in the present study, FEP patients could not be distinguished from HCs. Previous fMRI studies have shown that antipsychotic treatment can modulate brain activity during cognitive tasks (Fusar-Poli et al., 2007a; Snitz et al., 2005) and, more specifically, functional connectivity in circuits that are involved in mediating the cognitive symptoms in schizophrenia (Stephan et al., 2001).
In FEP patients after 6 weeks of treatment with second-generation antipsychotic drugs, frontoparietal–temporal network connectivity during resting state was no longer significantly different from controls (Lui et al., 2010). Antipsychotic treatment also normalises frontoparietal connectivity during a working memory task in FEP patients (Schmidt et al., 2013a), indicating an improved cognitive performance after antipsychotic medication (see also the Limitations section).
This is consistent with studies in early-psychosis patients (Keefe et al., 2007a) and in those with chronic schizophrenia (Keefe et al., 2007b), which reported significant improvements in neurocognition after the treatment with antipsychotics.
Over the past years, the prodromal phase of psychosis has been intensively studied using MRI. Potential MRI-based biomarkers of vulnerability to psychosis have been described in only a few studies (Borgwardt et al., 2013b; Broome et al., 2010; Koutsouleris et al., 2012; Koutsouleris et al., 2009a; Modinos et al., 2012), despite increasing evidence supporting structural and functional brain alterations prior to the onset of schizophrenia (Schmidt et al., 2013b; Smieskova et al., 2012a; Smieskova et al., 2010; Smieskova et al., 2012b). Deficits in neurocognitive tasks indexing working memory in high-risk subjects were shown in a quantitative meta-analysis (Fusar-Poli et al., 2012b). Those subjects at high-risk who later developed psychosis, showed poorer performance in working memory than those who did not develop the illness. Thus, impairments in working memory specifically predicted the later transition to psychosis (Fusar-Poli et al., 2012b).
Our previously published conventional fMRI analysis showed that functional working memory abnormalities may occur prior to the transition to psychosis (Smieskova et al., 2012a). Significant activations were found in the bilateral SPL and MFG during the n-back task in all groups (Smieskova et al., 2012a), consistent with previous n-back studies in ARMS individuals (Broome et al., 2010; Fusar-Poli et al., 2011a). Furthermore, Schmidt et al. (2013b) showed that effective connectivity between frontal and parietal regions modulated by a high-load WM condition was gradually reduced from HCs to individuals with an ARMS for psychosis and further to non-treated FEP patients (Schmidt and Borgwardt, 2013; Schmidt et al., 2014).
The main potential of SVM-based classification is that it might be useful for predicting the clinical transition to psychosis at the individual level. Most recently, automatic pattern classification methods have been considered to promote a potentially accessible and objective way to improve clinical decision making (Mandl et al., 2013b), and may present a measure of the risk of developing psychosis in individuals with an ARMS if sufficiently accurate (Borgwardt et al., 2013b).
Recent efforts have investigated the diagnostic value of neuroimaging biomarkers in the very earliest stages of the illness. For example Sun et al. (2009) used a cortical pattern matching method to compare HCs to patients with recent-onset psychosis. Patients showed lower grey matter density, particularly in the prefrontal, cingulate and lateral temporal brain regions relative to the controls, and the pattern classification analysis using a leave-one-out cross-validation was able to discriminate between the two groups with an accuracy of 86.1%.
Consistent with this finding, structural MRI studies discriminated between HCs and those with an ARMS based on grey matter volume with 82% accuracy (Borgwardt et al., 2013b; Koutsouleris et al., 2012; Koutsouleris et al., 2009b). Furthermore, pattern classification based on brain activation during emotional processing classified subjects with susceptibility to psychosis with accuracies up to 69.4% (Modinos et al., 2012).
Focusing on changes along the psychosis timeline, SVMs based on structural grey matter patterns have discriminated between individuals who did, and did not, transition from the ARMS to full blown psychosis with accuracies of up to 92% (Borgwardt et al., 2013a; Koutsouleris et al., 2012; Koutsouleris et al., 2009a).
Mourao-Miranda et al. (2012) used an SVM whole-brain classification approach to predict future illness course at the individual level from MRI data obtained at the first psychotic episode. The authors were able to classify ‘continuous psychosis’ from ‘episodic psychosis’ and ‘continuous psychosis’ from HCs with accuracies of 70% and 67%, respectively, but they were unable to distinguish ‘episodic psychosis’ from HCs.
Very recently, Kambeitz-Ilankovic et al. (2015) showed that cortical surface-based pattern classification predicted good vs. poor outcome status at follow-up with an accuracy of 82% as determined by nested leave-one-cross-validation. Neuroanatomical prediction involved cortical area reductions in the superior temporal, inferior frontal and inferior parietal areas and was not confounded by functional impairment at baseline, or antipsychotic medication and transition status over the follow-up period.
The classification accuracies based on these structural studies suggest, that longitudinal measures may in the future also further improve classification based on SVM analysis of fMRI data.
5.1. Limitations
There are some limitations to this study that should be taken into account.
Firstly, the rather small sample sizes of the present study may have limited the HC versus psychosis classification, and thus the findings may have been influenced by the heterogeneity of the psychosis subgroup. To account for this, we controlled for the potential effects of covariates, such as age, gender, and education.
Secondly, to ensure that the data used for testing does not overlap with those used for training two completely different sets of data would be best practice. However, this also increases the total amount of data to be acquired. Therefore, in order to obtain a relatively unbiased estimate of generalisability (Hastie et al., 2001), here we used leave one cross-validation, in which the total sample is split into training and testing data several times using different partitions and the resulting accuracies of classification are averaged across repetitions (Lemm et al., 2011).
Thirdly, although we performed pattern classification in matched groups with possibly low antipsychotic rates, we cannot fully exclude the confounding effects of antipsychotic medications (Ettinger et al., 2011; Fusar-Poli et al., 2013). Individuals who performed the task badly had been excluded from the fMRI n-back analysis (Selnes et al., 2012; Smieskova et al., 2012a). Furthermore, instead of evaluating the number of omissions or error rates, Schmidt et al. (2013a) analysed the 2-back working memory performances using signal-detection theory. The sensitivity index provides an objective measure independent of the individual's response bias. When equally weighting hit rates and false alarms, the 2-back working memory performance did significantly distinguish non-treated FEP patients from HCs, whereas the working memory performance in ARMS individuals came out in the middle of both groups. Moreover, no significant difference was found in antipsychotic-treated FEP individuals compared with HCs, indicating an improved cognitive performance after antispychotic medication (Yao et al., 2013).
Fourthly, other factors such as cannabis use and smoking might have played a confounding role. However, the latter seems to be rather unlikely, as we did not find significant differences between groups (Table 1).
Fifthly, it would have been interesting to perform a transition analysis. However, given the low number of n = 4 individuals with psychosis resulting from ARMS after 33.3 months of clinical follow-up, this was not possible in the present study. Transition analysis may in the future further improve classification accuracy.
Generally, it is unclear whether supervised MRI-based pattern recognition can achieve the level of sensitivity and specificity needed in order to be integrated into clinical applications. In the present study, only 63% of controls were correctly classified, which limits the diagnostic applicability of the model to the healthy population. However, the sensitivity of detecting the ARMS was nearly 90%. In the future, feature selection methods and the use of an independent test data set may further increase classification accuracy. However, a reduced number of voxels would also limit the interpretation of the results, in particular if different regions are compared.
Brodersen et al. applied linear SVM to n-back fMRI data (2 > 0 back) in 41 patients with chronic schizophrenia and 42 HCs (Brodersen et al., 2014). Parameter estimates from a dynamic causal model (DCM) of a visual–parietal–prefrontal network were used to define a model-based feature space for the subsequent application of supervised and unsupervised (Gaussian mixture modelling) learning techniques. Accuracy was 55% when local activity was used, but rose to 62% when functional connectivity was used and was even better (78%) with DCM-based effective connectivity estimates. With DCM parameters, an unsupervised approach showed nearly the same accuracy (71%).
Automatic pattern classification based on functional working memory data might contribute to monitor disease development in people at clinical high-risk for psychosis and improve clinical decisions in clinically diagnosed ARMS. Furthermore, the shift from single predictive models to ensembles of classifiers may produce more generalisable diagnostic biomarkers by averaging the diagnostic decisions of numerous predictive models (Koutsouleris et al., 2010).
Because it is difficult to predict which high-risk individuals will go on to develop psychosis (Fusar-Poli et al., 2012a) there is a clear clinical need for other markers that could be used to help clinicians identify the subgroup of subjects that will benefit most from preventive interventions (Fusar-Poli et al., 2013). Our results reveal that SVM analysis of working memory-related fMRI data can identify ARMS subjects with high accuracy when the analysis is restricted to a distributed and subtle set of brain regions within the verbal working memory network.
This might have implications for clinical decision making concerning the early detection of individuals at high-risk of developing psychosis. Thus, in addition to the abnormalities in neuroanatomical patterns (Koutsouleris et al., 2009a), including measures of neuro-functional activity may improve the classification accuracy for people at high-risk for psychosis.
References
- Allen P., Chaddock C.A., Howes O.D., Egerton A., Seal M.L., Fusar-Poli P., Valli I., Day F., McGuire P.K. Abnormal relationship between medial temporal lobe and subcortical dopamine function in people with an ultra high risk for psychosis. Schizophr. Bull. 2012;38(5):1040–1049. doi: 10.1093/schbul/sbr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N.C. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br. J. Psychiatry Suppl. 1989;7:49–58. [PubMed] [Google Scholar]
- Borgwardt S., Koutsouleris N., Aston J., Studerus E., Smieskova R., Riecher-Rossler A., Meisenzahl E.M. Distinguishing prodromal from first-episode psychosis using neuroanatomical single-subject pattern recognition. Schizophr. Bull. 2013;39(5):1105–1114. doi: 10.1093/schbul/sbs095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgwardt S., Koutsouleris N., Aston J., Studerus E., Smieskova R., Riecher-Rössler A., Meisenzahl E.M. Distinguishing prodromal from first-episode psychosis using neuroanatomical single-subject pattern recognition. Schizophr. Bull. 2013;39(5):1105–1114. doi: 10.1093/schbul/sbs095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitborde N.J.K., Srihari V.H., Woods S.W. Review of the operational definition for first-episode psychosis. Early Interv. Psychiatry. 2009;3(4):259–265. doi: 10.1111/j.1751-7893.2009.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen K.H., Deserno L., Schlagenhauf F., Lin Z., Penny W.D., Buhmann J.M., Stephan K.E. Dissecting psychiatric spectrum disorders by generative embedding. Neuroimage Clin. 2014;4:98–111. doi: 10.1016/j.nicl.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome M.R., Fusar-Poli P., Matthiasson P., Woolley J.B., Valmaggia L., Johns L.C., Tabraham P., Bramon E., Williams S.C.R., Brammer M.J., Chitnis X., Zelaya F., McGuire P.K. Neural correlates of visuospatial working memory in the ‘at-risk mental state’. Psychol. Med. 2010;40(12):1987–1999. doi: 10.1017/S0033291710000280. [DOI] [PubMed] [Google Scholar]
- Callicott J.H., Egan M.F., Mattay V.S., Bertolino A., Bone A.D., Verchinksi B., Weinberger D.R. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am. J. Psychiatry. 2003;160(4):709–719. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- Crossley N.A., Mechelli A., Fusar-Poli P., Broome M.R., Matthiasson P., Johns L.C., Bramon E., Valmaggia L., Williams S.C.R., McGuire P.K. Superior temporal lobe dysfunction and frontotemporal dysconnectivity in subjects at risk of psychosis and in first-episode psychosis. Hum. Brain Mapp. 2009;30(12):4129–4137. doi: 10.1002/hbm.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger U., Williams S.C.R., Fannon D., Premkumar P., Kuipers E., Möller H.J., Kumari V. Functional magnetic resonance imaging of a parametric working memory task in schizophrenia: relationship with performance and effects of antipsychotic treatment. Psychopharmacology. 2011;216(1):17–27. doi: 10.1007/s00213-011-2214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes N.F., Carrick L.A., McIntosh A.M., Lawrie S.M. Working memory in schizophrenia: a meta-analysis. Psychol. Med. 2009;39(06):889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P. Predicting psychosis. Arch. Gen. Psychiatry. 2012;69(3) doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Borgwardt S., Bechdolf A. The psychosis high-risk state: a comprehensive state-of-the-art review. J.A.M.A. Psychiatry. 2013;70(1):107–120. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Broome M.R., Matthiasson P., Williams S.C.R., Brammer M., McGuire P.K. Effects of acute antipsychotic treatment on brain activation in first episode psychosis: an fMRI study. Eur. Neuropsychopharmacol. 2007;17(6):492–500. doi: 10.1016/j.euroneuro.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Broome M.R., Woolley J.B., Johns L.C., Tabraham P., Bramon E., Valmaggia L., Williams S.C., McGuire P. Altered brain function directly related to structural abnormalities in people at ultra high risk of psychosis: longitudinal VBM-fMRI study. J. Psychiatr. Res. 2011;45(2):190–198. doi: 10.1016/j.jpsychires.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Deste G., Smieskova R., Barlati S., Yung A.R., Howes O., Stieglitz R.D., Vita A., McGuire P., Borgwardt S. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch. Gen. Psychiatry. 2012;69(6):562–571. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Deste G., Smieskova R., Barlati S., Yung A.R., Howes O., Stieglitz R.D., Vita A., McGuire P., Borgwardt S. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch. Gen. Psychiatry. 2012;69(6):562–571. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Howes O.D., Allen P., Broome M., Valli I., Asselin M.C., Grasby P.M., McGuire P.K. Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Arch. Gen. Psychiatry. 2010;67(7):683–691. doi: 10.1001/archgenpsychiatry.2010.77. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Howes O.D., Allen P., Broome M., Valli I., Asselin M.C., Montgomery A.J., Grasby P.M., McGuire P. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol. Psychiatry. 2011;16(1):67–75. doi: 10.1038/mp.2009.108. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., McGuire P., Borgwardt S. Mapping prodromal psychosis: a critical review of neuroimaging studies. European Psychiatry. 2012;27(3):181–191. doi: 10.1016/j.eurpsy.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Perez J., Broome M., Borgwardt S., Placentino A., Caverzasi E., Cortesi M., Veggiotti P., Politi P., Barale F., McGuire P. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2007;31(4):465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Radua J., McGuire P., Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr. Bull. 2012;38(6):1297–1307. doi: 10.1093/schbul/sbr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie T., Tibshirani R., Friedman J. Springer-Verlag; New York, NY: 2001. The Elements of Statistical Learning: Data Mining, Inference and Prediction. [Google Scholar]
- Kambeitz-Ilankovic L., Meisenzahl E.M., Cabral C., von Saldern S., Kambeitz J., Falkai P., Möller H.-Jr, Reiser M., Koutsouleris N. Prediction of outcome in the psychosis prodrome using neuroanatomical pattern classification. Schizophr. Res. 2015 doi: 10.1016/j.schres.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Keefe R.S.E., Bilder R.M., Davis S.M. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch. Gen. Psychiatry. 2007;64(6):633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Keefe R.S.E., Sweeney J.A., Gu H., Hamer R.M., Perkins D.O., McEvoy J.P., Lieberman J.A. Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. Am. J. Psychiatry. 2007;164(7):1061–1071. doi: 10.1176/ajp.2007.164.7.1061. [DOI] [PubMed] [Google Scholar]
- Klöppel S., Chu C., Tan G.C., Draganski B., Johnson H., Paulsen J.S., Kienzle W., Tabrizi S.J., Ashburner J., Frackowiak R.S.J. Automatic detection of preclinical neurodegeneration. Neurology. 2009;72(5):426–431. doi: 10.1212/01.wnl.0000341768.28646.b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöppel S., Stonnington C.M., Barnes J., Chen F., Chu C., Good C.D., Mader I., Mitchell L.A., Patel A.C., Roberts C.C., Fox N.C., Jack C.R., Ashburner J., Frackowiak R.S.J. Accuracy of dementia diagnosis — a direct comparison between radiologists and a computerized method. Brain. 2008;131(11):2969–2974. doi: 10.1093/brain/awn239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöppel S., Stonnington C.M., Chu C., Draganski B., Scahill R.I., Rohrer J.D., Fox N.C., Jack C.R., Ashburner J., Frackowiak R.S.J. Automatic classification of MR scans in Alzheimer's disease. Brain. 2008;131(3):681–689. doi: 10.1093/brain/awm319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosterkötter J., Hellmich M., Steinmeyer E.M., Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch. Gen. Psychiatry. 2001;58(2):158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N., Borgwardt S., Meisenzahl E.M., Bottlender R., Moller H.-J., Riecher-Rössler A. Disease prediction in the at-risk mental state for psychosis using neuroanatomical biomarkers: results from the FePsy Study. Schizophr. Bull. 2012;38(6):1234–1246. doi: 10.1093/schbul/sbr145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N., Meisenzahl E.M., Davatzikos C. Use of neuroanatomical pattern classification to identify subjects in at-risk mental states of psychosis and predict disease transition. Arch. Gen. Psychiatry. 2009;66(7):700–712. doi: 10.1001/archgenpsychiatry.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N., Meisenzahl E.M., Davatzikos C., Bottlender R., Frodl T., Scheuerecker J., Schmitt G., Zetzsche T., Decker P., Reiser M., Möller H., Gaser C. Use of neuroanatomical pattern classification to identify subjects in at-risk mental states of psychosis and predict disease transition. Arch. Gen. Psychiatry. 2009;66(7):700–712. doi: 10.1001/archgenpsychiatry.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N., Patschurek-Kliche K., Scheuerecker J., Decker P., Bottlender R., Schmitt G., Rujescu D., Giegling I., Gaser C., Reiser M., Möller H., Meisenzahl E.M. Neuroanatomical correlates of executive dysfunction in the at-risk mental state for psychosis. Schizophr. Res. 2010;123(2–3):160–174. doi: 10.1016/j.schres.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Lao Z., Shen D., Xue Z., Karacali B., Resnick S.M., Davatzikos C. Morphological classification of brains via high-dimensional shape transformations and machine learning methods. NeuroImage. 2004;21(1):46–57. doi: 10.1016/j.neuroimage.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Lawrie S.M., Buechel C., Whalley H.C., Frith C.D., Friston K.J., Johnstone E.C. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol. Psychiatry. 2002;51(12):1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- Lehrl S., Triebig G., Fischer B. Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol. Scand. 1995;91(5):335–345. doi: 10.1111/j.1600-0404.1995.tb07018.x. [DOI] [PubMed] [Google Scholar]
- Lemm S., Blankertz B., Dickhaus T., Müller K. Introduction to machine learning for brain imaging. NeuroImage. 2011;56(2):387–399. doi: 10.1016/j.neuroimage.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Lui S., Li T., Deng W. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch. Gen. Psychiatry. 2010;67(8):783–792. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- Lukoff D., Liberman R.P., Nuechterlein K.H. Symptom monitoring in the rehabilitation of schizophrenic patients. Schizophr. Bull. 1986;12(4):578–603. doi: 10.1093/schbul/12.4.578. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mandl R.C.W., Brouwer R.M., Cahn W., Kahn R.S., Hulshoff Pol H.E. Family-wise automatic classification in schizophrenia. Schizophr. Res. 2013;149(1–3):108–111. doi: 10.1016/j.schres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Mandl R.C.W., Brouwer R.M., Cahn W., Kahn R.S., Hulshoff Pol H.E. Family-wise automatic classification in schizophrenia. Schizophr. Res. 2013;149(1–3):108–111. doi: 10.1016/j.schres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Mendrek A. Changes in distributed neural circuitry function in patients with first-episode schizophrenia. Br. J. Psychiatry. 2004;185(3):205–214. doi: 10.1192/bjp.185.3.205. [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately R.I., Giuliano A.J., Goff K.P., Faraone S.V., Seidman L.J. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Modinos G., Mechelli A., Pettersson-Yeo W., Allen P., McGuire P., Aleman A. Pattern classification of brain activation during emotional processing in subclinical depression: psychosis proneness as potential confounding factor. PeerJ. 2013;1:e42. doi: 10.7717/peerj.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G., Pettersson-Yeo W., Allen P., McGuire P.K., Aleman A., Mechelli A. Multivariate pattern classification reveals differential brain activation during emotional processing in individuals with psychosis proneness. NeuroImage. 2012;59(3):3033–3041. doi: 10.1016/j.neuroimage.2011.10.048. [DOI] [PubMed] [Google Scholar]
- Mourao-Miranda J., Reinders A.A.T.S., Rocha-Rego V., Lappin J., Rondina J., Morgan C., Morgan K.D., Fearon P., Jones P.B., Doody G.A., Murray R.M., Kapur S., Dazzan P. Individualized prediction of illness course at the first psychotic episode: a support vector machine MRI study. Psychol. Med. 2012;42(05):1037–1047. doi: 10.1017/S0033291711002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B., Yuen H.P., Wood S.J., Lin A., Spiliotacopoulos D., Bruxner A., Broussard C., Simmons M., Foley D.L., Brewer W.J., Francey S.M., Amminger G.P., Thompson A., McGorry P.D., Yung A.R. Long-term follow-up of a group at ultra high risk (“prodromal”) for psychosis: the PACE 400 Study. J.A.M.A. Psychiatry. 2013;70(8):793–802. doi: 10.1001/jamapsychiatry.2013.1270. [DOI] [PubMed] [Google Scholar]
- Noble W.S. What is a support vector machine? Nat. Biotechnol. 2006;24(12):1565–1567. doi: 10.1038/nbt1206-1565. [DOI] [PubMed] [Google Scholar]
- Owen A.M., McMillan K.M., Laird A.R., Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflueger M.O., Gschwandtner U., Stieglitz R.-D., Riecher-Rössler A. Neuropsychological deficits in individuals with an at risk mental state for psychosis — working memory as a potential trait marker. Schizophr. Res. 2007;97(13):14–24. doi: 10.1016/j.schres.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Rasser P.E., Johnston P., Lagopoulos J., Ward P.B., Schall U., Thienel R., Bender S., Toga A.W., Thompson P.M. Functional MRI BOLD response to tower of London performance of first-episode schizophrenia patients using cortical pattern matching. NeuroImage. 2005;26(3):941–951. doi: 10.1016/j.neuroimage.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Riecher-Rössler A., Aston J., Ventura J., Merlo M., Borgwardt S., Gschwandtner U., Stieglitz R.-D. The Basel Screening Instrument for Psychosis (BSIP): development, structure, reliability and validity. Fortschr. Neurol. Psychiatr. 2008;76(4):207–216. doi: 10.1055/s-2008-1038155. [DOI] [PubMed] [Google Scholar]
- Riecher-Rössler A., Gschwandtner U., Aston J., Borgwardt S., Drewe M., Fuhr P., Pflüger M., Radü W., Schindler C., Stieglitz R.-D. The Basel early-detection-of-psychosis (FEPSY)-study — design and preliminary results. Acta Psychiatr. Scand. 2007;115(2):114–125. doi: 10.1111/j.1600-0447.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- Riecher-Rössler A., Gschwandtner U., Aston J., Borgwardt S., Drewe M., Fuhr P., Pflüger M., Radü W., Schindler C., Stieglitz R.-D. The Basel early-detection-of-psychosis (FEPSY)-study — design and preliminary results. Acta Psychiatr. Scand. 2007;115(2):114–125. doi: 10.1111/j.1600-0447.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- Riecher-Rössler A., Pflueger M.O., Aston J., Borgwardt S.J., Brewer W.J., Gschwandtner U., Stieglitz R.-D. Efficacy of using cognitive status in predicting psychosis: a 7-year follow-up. Biol. Psychiatry. 2009;66(11):1023–1030. doi: 10.1016/j.biopsych.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Riehemann S., Volz H.P., Stützer P., Smesny S., Gaser C., Sauer H. Hypofrontality in neuroleptic-naive schizophrenic patients during the Wisconsin card sorting test — a fMRI study. Eur. Arch. Psychiatry Clin. Neurosci. 2001;251(2):66–71. doi: 10.1007/s004060170055. [DOI] [PubMed] [Google Scholar]
- Rizk-Jackson A., Stoffers D., Sheldon S., Kuperman J., Dale A., Goldstein J., Corey-Bloom J., Poldrack R.A., Aron A.R. Evaluating imaging biomarkers for neurodegeneration in pre-symptomatic Huntington's disease using machine learning techniques. Neuroimage. 2011;56(2):788–796. doi: 10.1016/j.neuroimage.2010.04.273. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Borgwardt S. Abnormal effective connectivity in the psychosis high-risk state. NeuroImage. 2013;81:119–120. doi: 10.1016/j.neuroimage.2013.05.035. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Smieskova R., Aston J. Brain connectivity abnormalities predating the onset of psychosis: correlation with the effect of medication. J.A.M.A. Psychiatry. 2013;70(9):903–912. doi: 10.1001/jamapsychiatry.2013.117. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Smieskova R., Aston J., Simon A., Allen P., Fusar-Poli P., McGuire P.K., Riecher-Rössler A., Stephan K.E., Borgwardt S. Brain connectivity abnormalities predating the onset of psychosis: correlation with the effect of medication. J.A.M.A. Psychiatry. 2013;70(9):903–912. doi: 10.1001/jamapsychiatry.2013.117. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Smieskova R., Simon A., Allen P., Fusar-Poli P., McGuire P., Bendfeldt K., Aston J., Lang U., Walter M., Radue E., Riecher-Rössler A., Borgwardt S. Abnormal effective connectivity and psychopathological symptoms in the psychosis high-risk state. J Psychiatry Neurosci. 2014;39(4):239–248. doi: 10.1503/jpn.130102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman L.J., Thermenos H.W., Poldrack R.A., Peace N.K., Koch J.K., Faraone S.V., Tsuang M.T. Altered brain activation in dorsolateral prefrontal cortex in adolescents and young adults at genetic risk for schizophrenia: an fMRI study of working memory. Schizophr. Res. 2006;85(1–3):58–72. doi: 10.1016/j.schres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Selnes P., Fjell A.M., Gjerstad L., Bjørnerud A., Wallin A., Due-Tønnessen P., Grambaite R., Stenset V., Fladby T. White matter imaging changes in subjective and mild cognitive impairment. Alzheimer's & Dementia. 2012;8(5 Suppl.):S112–S121. doi: 10.1016/j.jalz.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Smieskova R., Allen P., Simon A., Aston J., Bendfeldt K., Drewe J., Gruber K., Gschwandtner U., Klarhoefer M., Lenz C., Scheffler K., Stieglitz R., Radue E., McGuire P., Riecher-Rössler A., Borgwardt S.J. Different duration of at-risk mental state associated with neurofunctional abnormalities. A multimodal imaging study. Hum. Brain Mapp. 2012;33(10):2281–2294. doi: 10.1002/hbm.21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smieskova R., Fusar-Poli P., Allen P., Bendfeldt K., Stieglitz R.D., Drewe J., Radue E.W., McGuire P.K., Riecher-Rössler A., Borgwardt S.J. Neuroimaging predictors of transition to psychosis — a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2010;34(8):1207–1222. doi: 10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Smieskova R., Fusar-Poli P., Aston J., Simon A., Bendfeldt K., Lenz C., Stieglitz R.-D., McGuire P., Riecher-Rössler A., Borgwardt S.J. Insular volume abnormalities associated with different transition probabilities to psychosis. Psychol. Med. 2012;42(08):1613–1625. doi: 10.1017/S0033291711002716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz B.E., MacDonald A., Cohen J.D., Cho R.Y., Becker T., Carter C.S. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. Am. J. Psychiatry. 2005;162(12):2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- Stephan K.E., Magnotta V.A., White T., Arndt S., Flaum M., O’Leary D.S., Andreasen N.C. Effects of olanzapine on cerebellar functional connectivity in schizophrenia measured by fMRI during a simple motor task. Psychol. Med. 2001;31(06):1065–1078. doi: 10.1017/s0033291701004330. [DOI] [PubMed] [Google Scholar]
- Sun D., van Erp T.G.M., Thompson P.M., Bearden C.E., Daley M., Kushan L., Hardt M.E., Nuechterlein K.H., Toga A.W., Cannon T.D. Elucidating a magnetic resonance imaging-based neuroanatomic biomarker for psychosis: classification analysis using probabilistic brain atlas and machine learning algorithms. Biol. Psychiatry. 2009;66(11):1055–1060. doi: 10.1016/j.biopsych.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermenos H.W., Seidman L.J., Breiter H., Goldstein J.M., Goodman J.M., Poldrack R., Faraone S.V., Tsuang M.T. Functional magnetic resonance imaging during auditory verbal working memory in nonpsychotic relatives of persons with schizophrenia: a pilot study. Biol. Psychiatry. 2004;55(5):490–500. doi: 10.1016/j.biopsych.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Yao L., Yi X., Wei Y. Gray matter alteration in isolated congenital anosmia patient: a voxel-based morphometry study. Eur. Arch. Otorhino-Laryngology. 2013;270(9):2569–2573. doi: 10.1007/s00405-013-2595-9. [DOI] [PubMed] [Google Scholar]
- Yung A.R., Phillips L.J., McGorry P.D., McFarlane C.A., Francey S., Harrigan S., Patton G.C., Jackson H.J. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br. J. Psychiatry Suppl. 1998;172(33):14–20. [PubMed] [Google Scholar]
- Yung A.R., Phillips L.J., Yuen H.P., McGorry P.D. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr. Res. 2004;67(2–3):131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]