Abstract
Aims
To determine the short-term efficacy of corneal collagen crosslinking (CXL) treatment in patients with progressive Keratoconus (KCN) in comparison with no treatment.
Settings and design
This controlled clinical trial study was carried out at a tertiary eye hospital, Eastern Province, Saudi Arabia.
Methods and material
A prospective controlled clinical study of patients being treated for Keratoconus at a tertiary eye care hospital in the Eastern province of Saudi Arabia. 51 eyes of 43 patients with progressive KCN who received corneal collagen crosslinking (treatment group) and 50 eyes of 34 patients with KCN and no treatment (control group) were included in our study. A one year clinical data were collected preoperatively as well as at 1, 3, 6 and 12 months postoperatively for the treatment group patients. A baseline and 1 year clinical data were collected for the control group patients. The short-term efficacy of the treatment in preventing progression of KCN in comparison with no treatment was analysed at one year.
Results
At one year after crosslinking there was significant flattening of the average keratometry by 0.61 D (p = 0.001) [95% CI: 0.25, 0.97] compared to 0.40 D (p = 0.210) steepening in the control group; difference between treatment and control was 1.01 D (p = 0.006) [95%CI: 0.29, 1.72]. Pachymetry in treatment group thinned by 20.21 μm (p < 0.0001) [95% CI: 12.77, 27.66] compared to 0.32 μm (p = 0.912) in the control group. Visual acuity remained stable at the preoperative level of 20/30 (p = 0.397) in the treatment group and 20/40 (p = 0.553) in the control group at one year.
Conclusions
Corneal CXL is an effective treatment for halting the progression of KCN as shown by reduced keratometry and stability of vision.
Keywords: Collagen crosslinking, Keratoconus, Riboflavin, Ultraviolet A, Ectasia
Introduction
Keratoconus (KCN) is a progressive degenerative disease of the cornea, usually bilateral, however asymmetric.1 It affects mostly young patients, and an early age of onset is a negative prognostic factor for corneal transplantation.2 There are several treatment options available, depending on the stage of KCN. However, none of these options restore vision to a near normal vision. Recently, there has been a major breakthrough in blocking the progression of the disease in the form of corneal collagen crosslinking with Riboflavin and Ultraviolet A (UVA) light.3, 4 This treatment when offered to patients with progressive KCN and, treated early, can dramatically improve the outcome of KCN, especially in younger patients.
KCN is a very common corneal ectasia in Saudi Arabia and accounts for high percentage of corneal transplants in Saudi Arabia.5 No controlled studies have been conducted reporting results of corneal collagen crosslinking with Riboflavin and UVA in patients with KCN in Saudi Arabia. The aim of this prospective controlled study was to report our results of collagen crosslinking treatment in comparison with no treatment over a period of one year in Saudi population with KCN.
Subjects and methods
The study was conducted at a tertiary care ophthalmic hospital in Eastern Province of Saudi Arabia. It was approved by institutional review board of the hospital. The inclusion criteria for the treatment group was diagnosis of KCN, with either subjective worsening of vision or at least one diopter steepening of the cornea on keratometry over a period of one year and, the thinnest corneal pachymetry of more than 400 μm. The exclusion criteria were patients with previous intracorneal segments placement, severe dry-eye, and corneal pachymetry less than 400 μm, central corneal scar or no subjective or objective progression of KCN. The matched control group patients were randomly selected from our medical records, patients who had been diagnosed with KCN but did not undergo any intervention for treatment of KCN during their follow-up in the clinic. These patients were historic controls and did not undergo any treatment as CXL was not readily available as treatment modality in the past.
A full ocular assessment, including the measurement of pre-operative Visual acuity, Refraction and, Specular Microscopy (Konan Medical, Inc., Hyogo, Japan), was carried out for all patients undergoing corneal collagen crosslinking. Best Corrected Visual Acuity (BCVA), refraction and Pentacam measurements were repeated at every subsequent visit.
Collagen crosslinking technique
An informed consent was obtained from all patients undergoing the procedure. The eye to be treated was prepped with Iodine and topical anesthesia. A lid speculum was used. An 8 mm zone was treated with an 8 mm sponge soaked in 20% alcohol for 20 s followed by rigorous irrigation to wash out any residual alcohol. The epithelium was removed with cellulose sponges. Pachymetry was performed after epithelium removal to ascertain that it was more than 400 μm. A 3.0 ml single use isotonic solution of Riboflavin (>0.1%) with Dextran500 (20%) (LightMed Corporation, New Taipei City, Taiwan), was instilled in the eye every 4 min and Balanced Salt Solution (BSS) every two minutes for a total of 30 min. Pachymetry was obtained at the end of 30 min to make sure the corneal thickness was still more than 400 microns before the UVA light application. UVA light from VEGA CBM-X-Linker (Costruzione Strumenti Oftalmici, or CSO, Italy) with cropped light beam of 9 mm diameter was applied for 30 min with 3 mW/cm2 energy. Riboflavin drops and BSS were continued in the same manner during UVA irradiation. At the end of procedure, a bandage contact lens was applied. The patient was instructed to use antibiotic Vigamox (Alcon, Dallas Fort Worth, USA) and steroid eye drops, Vexol (Allergan, Irvine California, USA) four times a day for a week following the procedure. The steroid drops were gradually tapered over 1 month and antibiotics were discontinued after one week. Patients were followed in clinic at post-op day one, 1 week, 1 month, 3 months, 6 months and then one year after the procedure.
Statistical analysis
Independent t-test for parametric data, and one way repeated-measure ANOVA were performed with SPSS statistical software (version 17.0.1, IBM Corp., Somers, NY, USA) to compare groups and trends. p < 0.05 was considered statistically significant. Multivariate and multiple regression models were used to investigate influencing factors such as age and gender.
Results
Fifty eyes of 43 (34 males and 9 females) patients with a mean age of 24.81 ± 6.71 years and age range 12–42 years with Keratoconus were included in the treatment group. Fifty eyes of 33 patients (20 males and 13 females) with mean age 28.18 ± 6.00 years and age range 16–41 years with Keratoconus were included in the control group.
In repeated measurement/paired/matched (pre-post) design, the same eye acted as control (preoperative/untreated) case and treatment (postoperative/treated) case. It constituted the perfect match in every aspect. Noting this, our study compared changes and not the baseline measurements or the end measurements.
Keratometry
The average Keratometry of treatment group steepened by 0.74 D in immediate postoperative at 1 month (Table 1, Fig. 1A). However, at 12 months, flattening by 0.61 D was noted (p-value 0.001) (Table 1); the control group, on the other hand, steepened by 0.40 D (p-value 0.21) over one year (Table 2). At one year, treatment group compared to control group, flattened by 1.00 D (p-value 0.006) (Fig. 1B). Incidentally, a difference between responses of males and females to treatment was also noted (Fig. 1C): males (malesnt − malest) flattened more by 0.81 D (p-value 0.005) compared to females (femalesnt − femalest).
Table 1.
Topographic and refractive changes over 1, 3, 6, and 12 months from baseline in treatment group.
| Parameter | Pre-treatment Mean ± SD | Post-treatment |
|||
|---|---|---|---|---|---|
| Post treatment interval | Difference value from baseline | 95% confidence interval | p-value | ||
| Average keratometry Kav | 47.35 ± 3.81 | 1 month | 0.74 | 0.23, 1.26 | 0.006 |
| 3 months | −0.53 | −1.06, 0.01 | 0.054 | ||
| 6 months | −0.73 | −1.17, −0.29 | 0.002 | ||
| 12 months | −0.61 | −0.97, −0.25 | 0.001 | ||
| Maximum keratometry Kmax | 55.03 ± 7.39 | 1 month | 1.11 | −0.18, 2.40 | 0.091 |
| 3 months | −0.61 | −1.94, 0.72 | 0.362 | ||
| 6 months | −1.39 | −2.29, −0.49 | 0.003 | ||
| 12 months | −0.54 | −1.73, 0.66 | 0.371 | ||
| Asphericity Q value | −0.82 ± 0.53 | 1 month | −0.157 | −0.25, −0.06 | 0.001 |
| 3 months | −0.018 | −0.10, 0.06 | 0.654 | ||
| 6 months | 0.07 | −0.01, 0.15 | 0.080 | ||
| 12 months | 0.093 | 0.004, 0.18 | 0.042 | ||
| Pachymetry thinnest (μm) | 460.53 ± 27.80 | 1 month | −38.69 | −49.25, −28.13 | <0.001 |
| 3 months | −32.03 | −39.32, −24.74 | <0.001 | ||
| 6 months | −21.12 | −28.68, −13.55 | <0.001 | ||
| 12 months | −20.21 | −27.66, −12.77 | <0.001 | ||
| Spherical equivalent (D) | −3.37 ± 3.32 | 1 month | 0.49 | −3.26, 4.24 | 0.794 |
| 3 months | 0.30 | −0.63, 1.23 | 0.516 | ||
| 6 months | −0.14 | −1.11, 0.83 | 0.769 | ||
| 12 months | 0.02 | −0.55, 0.60 | 0.935 | ||
| Best Corrected Visual Acuity (Decimal) | 0.63 ± 0.26 | 1 month | 0.08 | −0.27, 0.42 | 0.663 |
| 3 months | 0.142 | −0.02, 0.27 | 0.028 | ||
| 6 months | 0.010 | −0.07, 0.09 | 0.790 | ||
| 12 months | 0.029 | −0.04, 0.10 | 0.397 | ||
Figure 1.
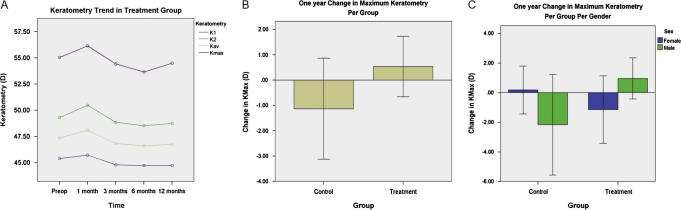
(A) Keratometry trends over one year in the treatment group. (B) Comparison of maximum keratometry between treatment and control groups at one year. (C) Maximum keratometry difference between control and treatment groups based on gender.
Table 2.
Topographic changes at 12 months from baseline in treatment and control groups.
| Parameter | Control group |
Treatment group |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline mean ± SD | Change at 1 year | Confidence interval | p-value | Baseline mean ± SD | Change at 1 year | Confidence interval | p-value | |
| Average keratometry Kav (D) | 50.77 ± 7.77 | 0.40 | −0.23,1.03 | 0.210 | 47.35 ± 3.81 | −0.61 | −0.97, −0.25 | 0.001 |
| Maximum keratometry (D) | 61.02 ± 13.99 | 1.14 | −0.86,3.13 | 0.259 | 55.03 ± 7.39 | −0.54 | −1.73, 0.66 | 0.371 |
| Asphericity | −0.98 ± 0.77 | −0.09 | −0.24,0.06 | 0.231 | −0.82 ± 0.5 | 0.093 | 0.004, 0.18 | 0.042 |
| Pachymetry thinnest (μm) | 441.90 ± 65.64 | −0.32 | −6.14,5.50 | 0.912 | 460.53 ± 27.80 | −20.21 | −27.66, −12.77 | <0.001 |
| Spherical equivalent (D) | −4.53 ± 4.98 | −0.29 | −0.97,0.39 | 0.398 | −3.37 ± 3.32 | 0.02 | −0.55, 0.60 | 0.935 |
| Best Corrected Visual Acuity (Decimal) | 0.58 ± 0.33 | 0.02 | −0.04,0.08 | 0.553 | 0.63 ± 0.26 | 0.029 | −0.04, 0.1 | 0.397 |
The maximum Keratometry of treatment group steepened by 1.11 D (p-value 0.091) in the immediate postoperative period at 1 month, and by 12 months flattening was noted with an average of 0.54 D (p-value 0.371) (Table 1) whereas those of control group steepened by 1.14 D (p-value 0.259) over one year (Table 2). Compared to control group, treatment group flattened by 1.67 D (p-value 0.152) (Fig. 1B); males (malesnt − malest) flattened by 3.12 D (p-value 0.029) compared to females (femalesnt − femalest) (Fig. 1C).
Pachymetry
At one month postoperatively, pachymetry at the thinnest location showed thinning by 38.69 μm (p-value < 0.000). At 12 months, thinning became less at 20.21 μm (p-value < 0.0001) (Table 1 and Fig. 2A). On the contrary, control corneas thinned by 0.32 with a p-value of 0.912 by 12 months (Table 2); treatment corneas thinned by 19.89 μm (p-value < 0.0001) compared to control corneas (Fig. 2B); males (malesnt − malest) thinned more than females (femalesnt − femalest) by 22.90 μm (p-value < 0.0001) (Fig. 2C).
Figure 2.
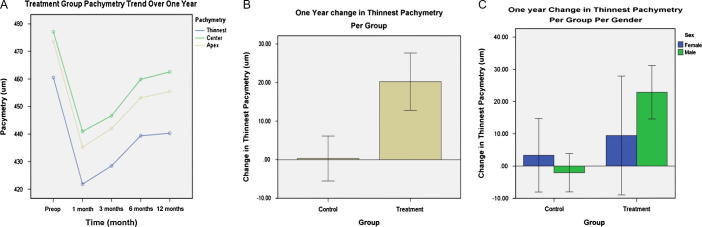
Pachymetry changes over one year in treatment and control groups. (A) Treatment group pachymetry trend over one year. (B) Change in the thinnest pachymetry over one year in the treatment and control groups. (C) Change in the pachymetry over one year per gender per group.
Corneal shape
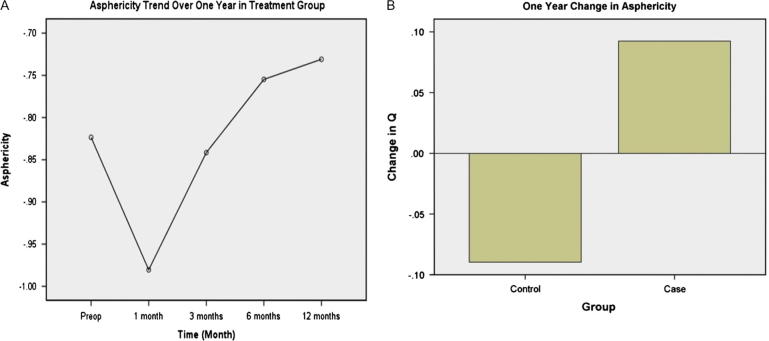
Corneas took a more prolate shape in the treatment group, as asphericity increased by 0.152 (p-value 0.001) one month postoperatively. This decreased by 0.093 (less prolate) (p-value 0.042) at 12 months (Table 1 and Fig. 3A). On the other hand, control corneas became more prolate by 0.090 (p-value 0.231) at 12 months (Table 2). The treatment corneas differed by 0.182 (p-value 0.037) (less prolate) from control corneas (Fig. 3B); also males (malesnt − malest) differed by 0.291(less prolate) (p-value 0.006) from females (femalesnt − femalest).
Figure 3.
(A) Trend in the asphericity over one year in the treatment group. (B) Asphericity difference between control and treatment groups at one year.
Refraction
Spherical equivalent of treatment group had a hyperopic shift by 0.49 D (p-value 0.794) in immediate postoperative period at 1 month; by 12 months, hyperopic shift was 0.024 D (p-value 0.935) (Table 1) whereas those of control group had myopic shift by 0.29 D (p-value 0.398) over one year (Table 2); compared to control group, treatment group had a hyperopic shift by 0.31 D (p-value 0.483).
Visual acuity
The mean baseline Best Corrected Visual Acuity of treatment group was 20/30 preoperatively; after treatment, it quickly lost one line (0.142) to 20/40 (p-value 0.028). However, it regained the loss and improved to pre-op baseline level at 20/30 (p-value 0.397) at the end of the year (Table 1). The control group, on the other hand, maintained visual acuity at 20/40 (p-value 0.553) (Table 2). The difference between control and treatment groups was −0.011 (p-value 0.799).
Discussion
There is high prevalence of KCN in Saudi Arabia.6 The onset of disease is usually very early with rapid deterioration of vision which often times requires a corneal transplant.5, 6, 7 The available treatment in the form corneal collagen crosslinking is projected to dramatically change the visual prognosis in the long run.
Collagen crosslinking as a treatment option is commonly offered to progressive KCN patients in all tertiary level ophthalmic care hospitals in Saudi Arabia. However, very little has been published about the results of this procedure in this part of the world, where a very early onset and severe presentation of the disease is not uncommon.5 We studied our KCN patient population that was treated with collagen crosslinking treatment. We also randomly selected clinical controls with the comparable demographics to investigate their progression and justify crosslinking for all KCN patients.
Our findings in the study are not vastly different from the published data on crosslinking throughout the world.8, 9, 10, 11, 12, 14 Although, there was deterioration of BCVA at 1 and 3 months, all our patients in the intervention group were able to either maintain or improve their Best Corrected Visual Acuity at the end of 12 month period. In this respect, our study shows results comparable to published results showing stability,8, 13 or improved visual outcome after crosslinking.10, 11, 14, 15, 16 The keratometry K1, K2, Kmax, Kav of the intervention group showed a marked steepening in the 1 and 3 months post op period. However, it returned back to baseline at 1-year (Fig. 1A). On an average, there was a 1-diopter flattening at 1-year in the treatment group which is similar to the data in literature.12, 17, 18 On the other hand, the control group had a trend towards steeping keratometry at one year. Keratoconus is a slowly progressive disease and one year may not be enough time to show clinically significant progression in the control group.19, 20
The corneal pachymetry in treatment group decreased dramatically in the initial 3 months post treatment, which slowly crept towards the baseline, however, not to the preoperative level in any case (Fig. 2A). In our study-population this detriment of corneal thickness post crosslinking is relatively more than other study populations.21, 22, 23, 24 The reason of thinner pachymetry post crosslinking is not fully understood but may be explained by more compact structure due to increased interfibrillar and intrafibrillar chemical bonds,25, 26, 27 keratocyte apoptosis,20, 28, 29 epithelial remodelling, and changes in glycosaminoglycans.27, 30 Many studies have shown that these bonds in the corneal stroma after crosslinking increase the tensile strength of the cornea despite the fact the cornea is thinner postprocedure.31, 32, 33, 34 Our control group also had mild progressive thinning of the cornea over a period of time, which is explained by the natural progression of the disease rather than any structural modification. Again one year may not be enough to show marked thinning in the control group.
The asphericity of the cornea decreased over time, which is consistent with the flattening of the cornea. A study by Hersh et al. had showed significant decrease in higher order aberrations after crosslinking.35 The refractive error (spherical equivalent) went through the same change of worsening initially and then returning back to baseline. The control group remained to have stable refractive error over one-year. Vinciguerra et al. concluded that refractive outcomes were achieved by both flattening of the cone apex and a steepening of the part of the cornea symmetrically opposite to the cone.14 In conclusion, in our population, after crosslinking there is rapid deterioration of BCVA, average keratometry, pachymetry and, astigmatism mostly at one month which gradually returns back to baseline at one year with the exception of pachymetry.
Interestingly we found a difference in corneal response to crosslinking between males and females. Our male patients responded more effectively to the treatment as compared to the female patients as to average and maximum keratometry, pachymetry, and asphericity. This difference has not been noted yet in the published data according to our knowledge. It needs to be explored more with further studies.
No patients who underwent corneal collagen crosslinking had any sight threatening complications. The epithelial defect created iatrogenically at time of surgery healed within a week time for all patients. There was a mild corneal haze after crosslinking in some patients; however, it was visually insignificant.
In summary, we conclude from our study that crosslinking is an effective treatment in eyes with progressive Keratoconus by significantly reducing average mean K readings and decreasing asphericity, after one year follow-up. Further clinical studies with longer follow-up are needed to determine the efficacy of the CXL in time, despite the fact that the results of this study are promising for a long-term efficacy.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
Contributor Information
Ashbala Khattak, Email: ashbalakhattak@gmail.com.
Fouad R. Nakhli, Email: fouadraja@hotmail.com.
Haider R. Cheema, Email: drrac@hotmail.com.
References
- 1.Pouliquen Y. Keratoconus. The Doyne Lecture. Eye. 1987;1:1–14. doi: 10.1038/eye.1987.2. [DOI] [PubMed] [Google Scholar]
- 2.Jiménez J.L.O., Jurado J.C.G., Rodriguez F.J.B., Laborda D.S. Keratoconus. Age of onset and natural history. Optometry Vision Sci. 1997;74:147–151. doi: 10.1097/00006324-199703000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Curr Opin Ophthalmol. 2006;17:356–360. doi: 10.1097/01.icu.0000233954.86723.25. [DOI] [PubMed] [Google Scholar]
- 4.Wollensak G., Spoerl E., Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of Keratoconus. Am J Ophthalmol. 2003 May;135(5):620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 5.Al-Towerki A.E., Gonnah el-S., Al-Rajhi A., Wagoner M.D. Changing indications for corneal transplantation at the King Khaled Eye Specialist Hospital (1983–2002) Cornea. 2004;23(6):584–588. doi: 10.1097/01.ico.0000121708.58571.5b. [DOI] [PubMed] [Google Scholar]
- 6.Wagoner M.D., Gonnah el-S., Al-Towerki A.E. King Khaled Eye Specialist Hospital Cornea Transplant Study Group. Outcome of primary adult optical penetrating keratoplasty with imported donor corneas. Int Ophthalmol. 2010;30(2):127–136. doi: 10.1007/s10792-009-9295-x. [DOI] [PubMed] [Google Scholar]
- 7.Al-Mohaimeed M.M. Penetrating keratoplasty for keratoconus: visual and graft survival outcomes. Int J Health Sci (Qassim) 2013 Jan;7(1):67–74. doi: 10.12816/0006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grewal D.S., Brar G.S., Jain R., Sood V., Singla M., Grewal S.P.S. Corneal collagen crosslinking using riboflavin and ultraviolet-A light for Keratoconus: one-year analysis using Scheimpflug imaging. J Cataract Refract Surg. 2009 Mar;35(3):425–432. doi: 10.1016/j.jcrs.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 9.Caporossi A., Mazzotta C., Baiocchi S., Caporossi T. Long-term results of riboflavin ultraviolet A corneal collagen cross-linking for keratoconus in Italy: the Siena Eye Cross Study. Am J Ophthalmol. 2010;149:585–593. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Hersh P.S., Greenstein S.A., Fry K.L. Corneal collagen crosslinking for keratoconus and corneal ectasia: one year results. J Cataract Refract Surg. 2011;37:149–160. doi: 10.1016/j.jcrs.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 11.Asri D., Touboul D., Fournie P., Malet F., Garra C., Gallois A. Corneal collagen crosslinking in progressive keratoconus: Multicenter results from the French National Reference Center for Keratoconus. J Cataract Refract Surg. 2011;37(12):2137–2143. doi: 10.1016/j.jcrs.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Wittig-Silva C., Whiting M., Lamoureux E., Lindsay R.G., Sullivan L.J., Snibson G.R. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: preliminary results. J Refract Surg. 2008;24(7):S720–S725. doi: 10.3928/1081597X-20080901-15. [DOI] [PubMed] [Google Scholar]
- 13.Raiskup-Wolf F., Hoyer A., Spoerl E., Pillunat L.E. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 14.Vinciguerra P., Albè E., Trazza S., Rosetta P., Vinciguerra R., Seiler T. Refractive, topographic, tomographic, and aberrometric analysis of keratoconic eyes undergoing corneal cross-linking. Ophthalmology. 2009;116:369–378. doi: 10.1016/j.ophtha.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 15.Rechichi M., Daya S., Scorcia V., Meduri A., Scorcia G. Epithelial-disruption collagen crosslinking for keratoconus: one-year results. J Cataract Refract Surg. 2013;(June 21) doi: 10.1016/j.jcrs.2013.05.022. pii: S0886-3350(13)00646-9. [Epub ahead of print] PMID: 23796620. [DOI] [PubMed] [Google Scholar]
- 16.Brooks N.O., Greenstein S., Fry K., Hersh P.S. Patient subjective visual function after corneal collagen crosslinking for keratoconus and corneal ectasia. J Cataract Refract Surg. 2012;38(4):615–619. doi: 10.1016/j.jcrs.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 17.Koller T., Pajic B., Vinciguerra P., Seiler T. Flattening of the cornea after collagen crosslinking for keratoconus. J Cataract Refract Surg. 2011;37(8):1488–1492. doi: 10.1016/j.jcrs.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 18.Coskunseven E., Jankov M.R., 2nd, Hafezi F. Contralateral eye study of corneal collagen cross-linking with riboflavin and UVA irradiation in patients with keratoconus. J Refract Surg. 2009;25(4):371–376. doi: 10.3928/1081597X-20090401-02. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki M., Amano S., Honda N. Longitudinal changes in corneal irregular astigmatism and visual acuity in eyes with keratoconus. Jpn J Ophthalmol. 2007;51:265–269. doi: 10.1007/s10384-007-0453-2. [DOI] [PubMed] [Google Scholar]
- 20.Barr J.T., Wilson B.S., Gordon M.O. Estimation of the incidence and factors predictive of corneal scarring in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) study; the CLEK study group. Cornea. 2005;25:16–25. doi: 10.1097/01.ico.0000164831.87593.08. [DOI] [PubMed] [Google Scholar]
- 21.Greenstein S.A., Shah V.P., Fry K.L., Hersh P.S. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37(4):691–700. doi: 10.1016/j.jcrs.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 22.Hassan Z., Szalai E., Módis L., Jr., Berta A., Németh G. Assessment of corneal topography indices after collagen crosslinking for keratoconus. Eur J Ophthalmol. 2013;(March 7) doi: 10.5301/ejo.5000249. [Epub ahead of print] PMID: 23483510. [DOI] [PubMed] [Google Scholar]
- 23.Viswanathan D, Males. Prospective longitudinal study of corneal collagen crosslinking in progressive keratoconus. J.Clin Experiment Ophthalmol2012 Nov 13.doi: 10.1111/ceo.12035. [Epub ahead of print]PMID:23145528. [DOI] [PubMed]
- 24.Guber I., Guber J., Kaufmann C., Bachmann L.M., Thiel M.A. Graefes Arch Visual recovery after corneal crosslinking for keratoconus: a 1-year follow-up study. Clin Exp Ophthalmol. 2013;251(3):803–807. doi: 10.1007/s00417-012-2133-2. [DOI] [PubMed] [Google Scholar]
- 25.Bottos K.M., Dreyfuss J.L., Regatieri C.V.S., Lima-Filho A.A.S., Schor P., Nader H.B. Immunofluorescence confocal microscopy of porcine corneas following collagen cross-linking treatment with riboflavin and ultraviolet A. J Refract Surg. 2008;24:S715–S719. doi: 10.3928/1081597X-20080901-14. [DOI] [PubMed] [Google Scholar]
- 26.Seiler T., Hafezi F. Corneal cross-linking-induced stromal demarcation line. Cornea. 2006;25:1057–1059. doi: 10.1097/01.ico.0000225720.38748.58. [DOI] [PubMed] [Google Scholar]
- 27.Wollensak G., Spörl E., Mazzotta C., Kalinski T., Sel S. Interlamellar cohesion after corneal crosslinking using riboflavin and ultraviolet A light. Br J Ophthalmol. 2011 Jun;95(6):876–880. doi: 10.1136/bjo.2010.190843. [DOI] [PubMed] [Google Scholar]
- 28.Mazzotta C., Traversi C., Baiocchi S., Caporossi O., Bovone C., Sparano M.C. Corneal healing after riboflavin ultraviolet-A collagen cross-linking determined by confocal laser scanning microscopy in vivo: early and late modifications. Am J Ophthalmol. 2008;146:527–533. doi: 10.1016/j.ajo.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 29.Wollensak G., Spoerl E., Wilsch M., Seiler T. Keratocyte apoptosis after corneal collagen cross-linking using riboflavin/UVA treatment. Cornea. 2004;23:43–49. doi: 10.1097/00003226-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Michelacci Y.M. Collagens and proteoglycans of the corneal extracellular matrix. Braz J Med Biol Res. 2003;36:1037–1046. doi: 10.1590/s0100-879x2003000800009. < http://www.scielo.br/pdf/bjmbr/v36n8/4920.pdf> (accessed 27.11.10) [DOI] [PubMed] [Google Scholar]
- 31.Wollensak G., Spoerl E., Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29:1780–1785. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 32.Ahearne M., Yang Y., Then K.Y., Liu K.-K. Non-destructive mechanical characterisation of UVA/riboflavin crosslinked collagen hydrogels. Br J Ophthalmol. 2008;92:268–271. doi: 10.1136/bjo.2007.130104. [DOI] [PubMed] [Google Scholar]
- 33.Terai N., Raiskup F., Haustein M., Pillunat L.E., Spoerl E. Identification of biomechanical properties of the cornea: the ocular response analyzer. Curr Eye Res. 2012 Jul;37(7):553–562. doi: 10.3109/02713683.2012.669007. [DOI] [PubMed] [Google Scholar]
- 34.Wollensak G., Wilsch M., Spoerl E., Seiler T. Collagen fiber diameter in the rabbit cornea after collagen crosslinking by riboflavin/UVA. Cornea. 2004;23(5):503–507. doi: 10.1097/01.ico.0000105827.85025.7f. [DOI] [PubMed] [Google Scholar]
- 35.Greenstein S.A., Fry K.L., Hersh M.J., Hersh P.S. Higher-order aberrations after corneal collagen crosslinking for keratoconus and corneal ectasia. J Cataract Refract Surg. 2012;38(2):292–302. doi: 10.1016/j.jcrs.2011.08.041. [DOI] [PubMed] [Google Scholar]