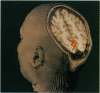
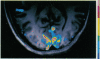
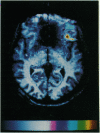
Abstract
The techniques of in vivo magnetic resonance (MR) imaging and spectroscopy have been established over the past two decades. Recent applications of these methods to study human brain function have become a rapidly growing area of research. The development of methods using standard MR contrast agents within the cerebral vasculature has allowed measurements of regional cerebral blood volume (rCBV), which are activity dependent. Subsequent investigations linked the MR relaxation properties of brain tissue to blood oxygenation levels which are also modulated by consumption and blood flow (rCBF). These methods have allowed mapping of brain activity in human visual and motor cortex as well as in areas of the frontal lobe involved in language. The methods have high enough spatial and temporal sensitivity to be used in individual subjects. MR spectroscopy of proton and carbon-13 nuclei has been used to measure rates of glucose transport and metabolism in the human brain. The steady-state measurements of brain glucose concentrations can be used to monitor the glycolytic flux, whereas subsequent glucose metabolism--i.e., the flux into the cerebral glutamate pool--can be used to measure tricarboxylic acid cycle flux. Under visual stimulation the concentration of lactate in the visual cortex has been shown to increase by MR spectroscopy. This increase is compatible with an increase of anaerobic glycolysis under these conditions as earlier proposed from positron emission tomography studies. It is shown how MR spectroscopy can extend this understanding of brain metabolism.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman J. J., Ewy C. S., Becker N. N., Shalwitz R. A. Deuterium nuclear magnetic resonance measurements of blood flow and tissue perfusion employing 2H2O as a freely diffusible tracer. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4099–4102. doi: 10.1073/pnas.84.12.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini P. A., Wong E. C., Hinks R. S., Tikofsky R. S., Hyde J. S. Time course EPI of human brain function during task activation. Magn Reson Med. 1992 Jun;25(2):390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Behar K. L., den Hollander J. A., Stromski M. E., Ogino T., Shulman R. G., Petroff O. A., Prichard J. W. High-resolution 1H nuclear magnetic resonance study of cerebral hypoxia in vivo. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4945–4948. doi: 10.1073/pnas.80.16.4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliveau J. W., Kennedy D. N., Jr, McKinstry R. C., Buchbinder B. R., Weisskoff R. M., Cohen M. S., Vevea J. M., Brady T. J., Rosen B. R. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991 Nov 1;254(5032):716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- Belliveau J. W., Rosen B. R., Kantor H. L., Rzedzian R. R., Kennedy D. N., McKinstry R. C., Vevea J. M., Cohen M. S., Pykett I. L., Brady T. J. Functional cerebral imaging by susceptibility-contrast NMR. Magn Reson Med. 1990 Jun;14(3):538–546. doi: 10.1002/mrm.1910140311. [DOI] [PubMed] [Google Scholar]
- Berkelbach van der Sprenkel J. W., Luyten P. R., van Rijen P. C., Tulleken C. A., den Hollander J. A. Cerebral lactate detected by regional proton magnetic resonance spectroscopy in a patient with cerebral infarction. Stroke. 1988 Dec;19(12):1556–1560. doi: 10.1161/01.str.19.12.1556. [DOI] [PubMed] [Google Scholar]
- Blamire A. M., Ogawa S., Ugurbil K., Rothman D., McCarthy G., Ellermann J. M., Hyder F., Rattner Z., Shulman R. G. Dynamic mapping of the human visual cortex by high-speed magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11069–11073. doi: 10.1073/pnas.89.22.11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley P. A., Edelstein W. A., Foster T. H., Adams W. A. In vivo solvent-suppressed localized hydrogen nuclear magnetic resonance spectroscopy: a window to metabolism? Proc Natl Acad Sci U S A. 1985 Apr;82(7):2148–2152. doi: 10.1073/pnas.82.7.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn H., Frahm J., Gyngell M. L., Merboldt K. D., Hänicke W., Sauter R. Cerebral metabolism in man after acute stroke: new observations using localized proton NMR spectroscopy. Magn Reson Med. 1989 Jan;9(1):126–131. doi: 10.1002/mrm.1910090115. [DOI] [PubMed] [Google Scholar]
- Damadian R., Goldsmith M., Minkoff L. NMR in cancer: XVI. FONAR image of the live human body. Physiol Chem Phys. 1977;9(1):97-100, 108. [PubMed] [Google Scholar]
- Fitzpatrick S. M., Hetherington H. P., Behar K. L., Shulman R. G. The flux from glucose to glutamate in the rat brain in vivo as determined by 1H-observed, 13C-edited NMR spectroscopy. J Cereb Blood Flow Metab. 1990 Mar;10(2):170–179. doi: 10.1038/jcbfm.1990.32. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E., Mintun M. A., Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988 Jul 22;241(4864):462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E. Stimulus rate determines regional brain blood flow in striate cortex. Ann Neurol. 1985 Mar;17(3):303–305. doi: 10.1002/ana.410170315. [DOI] [PubMed] [Google Scholar]
- Frahm J., Merboldt K. D., Bruhn H., Gyngell M. L., Hänicke W., Chien D. 0.3-second FLASH MRI of the human heart. Magn Reson Med. 1990 Jan;13(1):150–157. doi: 10.1002/mrm.1910130114. [DOI] [PubMed] [Google Scholar]
- Graham G. D., Blamire A. M., Howseman A. M., Rothman D. L., Fayad P. B., Brass L. M., Petroff O. A., Shulman R. G., Prichard J. W. Proton magnetic resonance spectroscopy of cerebral lactate and other metabolites in stroke patients. Stroke. 1992 Mar;23(3):333–340. doi: 10.1161/01.str.23.3.333. [DOI] [PubMed] [Google Scholar]
- Gruetter R., Novotny E. J., Boulware S. D., Rothman D. L., Mason G. F., Shulman G. I., Shulman R. G., Tamborlane W. V. Direct measurement of brain glucose concentrations in humans by 13C NMR spectroscopy. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1109–1112. doi: 10.1073/pnas.89.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter R., Rothman D. L., Novotny E. J., Shulman G. I., Prichard J. W., Shulman R. G. Detection and assignment of the glucose signal in 1H NMR difference spectra of the human brain. Magn Reson Med. 1992 Sep;27(1):183–188. doi: 10.1002/mrm.1910270118. [DOI] [PubMed] [Google Scholar]
- Hanstock C. C., Rothman D. L., Prichard J. W., Jue T., Shulman R. G. Spatially localized 1H NMR spectra of metabolites in the human brain. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1821–1825. doi: 10.1073/pnas.85.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss W. D., Pawlik G., Herholz K., Wagner R., Göldner H., Wienhard K. Regional kinetic constants and cerebral metabolic rate for glucose in normal human volunteers determined by dynamic positron emission tomography of [18F]-2-fluoro-2-deoxy-D-glucose. J Cereb Blood Flow Metab. 1984 Jun;4(2):212–223. doi: 10.1038/jcbfm.1984.30. [DOI] [PubMed] [Google Scholar]
- KETY S. S. The theory and applications of the exchange of inert gas at the lungs and tissues. Pharmacol Rev. 1951 Mar;3(1):1–41. [PubMed] [Google Scholar]
- Kim S. G., Ashe J., Georgopoulos A. P., Merkle H., Ellermann J. M., Menon R. S., Ogawa S., Ugurbil K. Functional imaging of human motor cortex at high magnetic field. J Neurophysiol. 1993 Jan;69(1):297–302. doi: 10.1152/jn.1993.69.1.297. [DOI] [PubMed] [Google Scholar]
- Kwong K. K., Belliveau J. W., Chesler D. A., Goldberg I. E., Weisskoff R. M., Poncelet B. P., Kennedy D. N., Hoppel B. E., Cohen M. S., Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong K. K., Hopkins A. L., Belliveau J. W., Chesler D. A., Porkka L. M., McKinstry R. C., Finelli D. A., Hunter G. J., Moore J. B., Barr R. G. Proton NMR imaging of cerebral blood flow using H2(17)O. Magn Reson Med. 1991 Nov;22(1):154–158. doi: 10.1002/mrm.1910220116. [DOI] [PubMed] [Google Scholar]
- Mason G. F., Rothman D. L., Behar K. L., Shulman R. G. NMR determination of the TCA cycle rate and alpha-ketoglutarate/glutamate exchange rate in rat brain. J Cereb Blood Flow Metab. 1992 May;12(3):434–447. doi: 10.1038/jcbfm.1992.61. [DOI] [PubMed] [Google Scholar]
- Merboldt K. D., Bruhn H., Hänicke W., Michaelis T., Frahm J. Decrease of glucose in the human visual cortex during photic stimulation. Magn Reson Med. 1992 May;25(1):187–194. doi: 10.1002/mrm.1910250119. [DOI] [PubMed] [Google Scholar]
- Michaelis T., Merboldt K. D., Hänicke W., Gyngell M. L., Bruhn H., Frahm J. On the identification of cerebral metabolites in localized 1H NMR spectra of human brain in vivo. NMR Biomed. 1991 Apr;4(2):90–98. doi: 10.1002/nbm.1940040211. [DOI] [PubMed] [Google Scholar]
- Moon R. B., Richards J. H. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973 Oct 25;248(20):7276–7278. [PubMed] [Google Scholar]
- Ogawa S., Lee T. M., Kay A. R., Tank D. W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Lee T. M., Nayak A. S., Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990 Apr;14(1):68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Tank D. W., Menon R., Ellermann J. M., Kim S. G., Merkle H., Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S. E., Fox P. T., Posner M. I., Mintun M., Raichle M. E. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988 Feb 18;331(6157):585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Petersen S. E., Fox P. T., Snyder A. Z., Raichle M. E. Activation of extrastriate and frontal cortical areas by visual words and word-like stimuli. Science. 1990 Aug 31;249(4972):1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- Prichard J. W., Shulman R. G. NMR spectroscopy of brain metabolism in vivo. Annu Rev Neurosci. 1986;9:61–85. doi: 10.1146/annurev.ne.09.030186.000425. [DOI] [PubMed] [Google Scholar]
- Prichard J., Rothman D., Novotny E., Petroff O., Kuwabara T., Avison M., Howseman A., Hanstock C., Shulman R. Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5829–5831. doi: 10.1073/pnas.88.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radda G. K., Bore P. J., Rajagopalan B. Clinical aspects on 31P NMR spectroscopy. Br Med Bull. 1984 Apr;40(2):155–159. doi: 10.1093/oxfordjournals.bmb.a071962. [DOI] [PubMed] [Google Scholar]
- Rosen B. R., Belliveau J. W., Vevea J. M., Brady T. J. Perfusion imaging with NMR contrast agents. Magn Reson Med. 1990 May;14(2):249–265. doi: 10.1002/mrm.1910140211. [DOI] [PubMed] [Google Scholar]
- Rothman D. L., Behar K. L., Hetherington H. P., Shulman R. G. Homonuclear 1H double-resonance difference spectroscopy of the rat brain in vivo. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6330–6334. doi: 10.1073/pnas.81.20.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman D. L., Behar K. L., Hetherington H. P., den Hollander J. A., Bendall M. R., Petroff O. A., Shulman R. G. 1H-Observe/13C-decouple spectroscopic measurements of lactate and glutamate in the rat brain in vivo. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1633–1637. doi: 10.1073/pnas.82.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman D. L., Howseman A. M., Graham G. D., Petroff O. A., Lantos G., Fayad P. B., Brass L. M., Shulman G. I., Shulman R. G., Prichard J. W. Localized proton NMR observation of [3-13C]lactate in stroke after [1-13C]glucose infusion. Magn Reson Med. 1991 Oct;21(2):302–307. doi: 10.1002/mrm.1910210215. [DOI] [PubMed] [Google Scholar]
- Rothman D. L., Novotny E. J., Shulman G. I., Howseman A. M., Petroff O. A., Mason G., Nixon T., Hanstock C. C., Prichard J. W., Shulman R. G. 1H-[13C] NMR measurements of [4-13C]glutamate turnover in human brain. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9603–9606. doi: 10.1073/pnas.89.20.9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappey-Marinier D., Calabrese G., Fein G., Hugg J. W., Biggins C., Weiner M. W. Effect of photic stimulation on human visual cortex lactate and phosphates using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 1992 Jul;12(4):584–592. doi: 10.1038/jcbfm.1992.82. [DOI] [PubMed] [Google Scholar]
- Turner R., Jezzard P., Wen H., Kwong K. K., Le Bihan D., Zeffiro T., Balaban R. S. Functional mapping of the human visual cortex at 4 and 1.5 tesla using deoxygenation contrast EPI. Magn Reson Med. 1993 Feb;29(2):277–279. doi: 10.1002/mrm.1910290221. [DOI] [PubMed] [Google Scholar]
- Turner R., Le Bihan D., Moonen C. T., Despres D., Frank J. Echo-planar time course MRI of cat brain oxygenation changes. Magn Reson Med. 1991 Nov;22(1):159–166. doi: 10.1002/mrm.1910220117. [DOI] [PubMed] [Google Scholar]
- Ueki M., Linn F., Hossmann K. A. Functional activation of cerebral blood flow and metabolism before and after global ischemia of rat brain. J Cereb Blood Flow Metab. 1988 Aug;8(4):486–494. doi: 10.1038/jcbfm.1988.89. [DOI] [PubMed] [Google Scholar]
- Utz J. A., Herfkens R. J., Johnson C. D., Shimakawa A., Pelc N., Glover G., Johnson G. A., Spritzer C. E. Two-second MR images: comparison with spin-echo images in 29 patients. AJR Am J Roentgenol. 1987 Mar;148(3):629–633. doi: 10.2214/ajr.148.3.629. [DOI] [PubMed] [Google Scholar]
- Villringer A., Rosen B. R., Belliveau J. W., Ackerman J. L., Lauffer R. B., Buxton R. B., Chao Y. S., Wedeen V. J., Brady T. J. Dynamic imaging with lanthanide chelates in normal brain: contrast due to magnetic susceptibility effects. Magn Reson Med. 1988 Feb;6(2):164–174. doi: 10.1002/mrm.1910060205. [DOI] [PubMed] [Google Scholar]
- Weinberger D. R., Berman K. F., Zec R. F. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986 Feb;43(2):114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Williams D. S., Detre J. A., Leigh J. S., Koretsky A. P. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]