Abstract
OBJECTIVE
To investigate neuropsychological performance (NP) during acute HIV infection (AHI) before and after combination antiretroviral therapy (cART).
DESIGN
Prospective study of Thai AHI participants examined at 3 and 6 months following initiation of cART.
METHODS
36 AHI participants were evaluated pre-cART at median 19 days since HIV exposure and 3 and 6 months after cART with the Grooved Pegboard test (GP), Color Trails 1 & 2 (CT1, CT2), and Trail Making Test A (TM). Raw scores were standardized to 251 age-and-education-matched HIV-uninfected Thais. To account for learning effects, change in NP performance was compared to that of controls at 6 months. Analyses included multivariable regression, non-parametric repeated measures ANOVA, and Mann-Whitney U test.
RESULTS
Baseline NP scores for the AHI group were within normal range (Z scores range: −0.26 to −0.13). NP performance improved on CT1, CT2, and TM in the initial 3 months (ps <0.01) with no significant change during the last 3 months. Only improvement in CT1 was greater than that seen in controls at 6 months (p=0.018). Participants that performed >1 standard deviation below normative means on >2 tests (n=8) exhibited higher baseline cerebrospinal fluid (CSF) HIV RNA (p=0.047) and had no improvement after cART.
CONCLUSIONS
Most AHI individuals had normal NP performance and early cART slightly improved their psychomotor function. However, approximately 25% had impaired NP performance which correlated with higher CSF HIV RNA, and these abnormalities were not reversed by early cART possibly indicating limited reversibility of cognitive impairment in a subset of AHI individuals.
Keywords: HIV infection, HIV-Associated Neurocognitive Disorder, Neuropsychological Tests, Mild Cognitive Impairment, Antiretroviral Therapy
INTRODUCTION
Despite access to combination antiretroviral therapy (cART), a large percentage of chronically infected HIV-positive individuals exhibit mild forms of cognitive impairment [1, 2], known as HIV-associated neurocognitive disorders (HAND). Prior to widespread use of cART, 15–20% of HIV patients developed HIV-associated dementia (HAD) [3] but that incidence is now greatly reduced. In contrast, the prevalence estimates of milder forms of cognitive impairment have not decreased, with up to 50% of chronically infected HIV-positive individuals still manifesting some degree of cognitive dysfunction [1, 2, 4, 5]. One possible explanation for persistent cognitive impairment in the cART era is that injury to the nervous system may be accrued during the very early stages of infection, prior to initiation of antiretroviral treatment. Limited information is available regarding the neuropsychological status among individuals during acute HIV infection (AHI, prior to antibody seroconversion) and whether cognitive function may be altered by very early initiation of cART.
We previously demonstrated detectable CSF HIV RNA as early as eight days post estimated date of transmission [6], with concurrent evidence of central nervous system (CNS) inflammation, measured by magnetic resonance spectroscopy imaging and CSF markers of immune activation [6, 7]. There are limited data characterizing cognitive function in primary HIV infection, defined as the first year after HIV transmission and these studies have not included individuals captured during the first month [8, 9]. Results from these studies have been mixed, with one study reporting impairments in processing speed, executive function and learning among cART-naïve patients with primary infection [10], while others have not demonstrated statistically significant differences in neuropsychological (NP) performance between primary infection participants and HIV-uninfected controls [11, 12]. No studies have examined neurocognitive performance and HIV disease biomarkers during AHI or the impact of immediate antiretroviral therapy on cognitive performance during this acute stage.
The efficacy of cART in improving cognitive performance in individuals with advanced HIV disease is well understood [13–15]. However, studies demonstrate that a considerable proportion of chronically infected persons continue to have neurocognitive deficits despite cART [16]. Importantly, studies demonstrating chronic neurocognitive impairments despite treatment were focused on individuals who started cART at variable durations since exposure. As such, it is possible that neurological damage sustained during the early stages of HIV infection may have led to NP impairments that were refractory to later treatment. One study longitudinally examined the effect of cART on cognitive performance during early infection [10], identifying mild deficits in NP performance pre-cART and stabilization of cognitive function following cART. However, this observational study did not control for practice effects and assessed participants about four months after estimated HIV transmission, well after the acute phase of infection. Further, in previous studies neurocognitive assessments were conducted at variable times after infection [10].
The current study evaluated Thai participants who were identified within days of estimated HIV exposure and before antibody seroconversion. We examined neuropsychological performance at two time points (3 months and 6 months) following cART initiation, and compared change in NP performance in the AHI cohort with that of Thai normative controls to account for practice effects. We hypothesized that neurocognitive impairment would be detected in AHI and performance on the NP tests would improve significantly above and beyond practice effects following cART initiation.
METHODS
Study design and participants
Acute HIV study participants were recruited from the Thai Red Cross Anonymous Clinic in Bangkok, Thailand. AHI participants were identified through nucleic acid testing, and were characterized according to Fiebig stage defined by the sequential appearance of viral RNA, antigen, and antibodies, as previously described [17, 18]. The inclusion criteria for the AHI group were: confirmed acute HIV-1 infection, age >18 years, ART-naïve, informed consent, and assent to initiating protocol-defined cART. Because the key defining feature of the unique study population was laboratory-defined AHI, we did not exclude any individuals eligible by the above criteria. However, mental health, substance use and educational histories were obtained in all individuals, and no participants included in the analysis had major psychiatric diagnoses.
Participants completed baseline clinical, neurological, CSF sampling (n=21/36), and NP testing prior to starting cART. Due to the nature of the parent study, individuals were randomized either to standard cART (efavirenz, tenofovir, and emtricitabine or lamivudine) or standard cART plus raltegravir and maraviroc (MVC). There were no significant differences in baseline characteristics (age, gender, days post HIV transmission, CD4 count, CD8 count, plasma HIV RNA, and CSF HIV RNA) between the two treatment arms using Mann-Whitney U test. Thus for the purpose of this study, all participants were aggregated into one treatment group for analyses and the effect of MVC was evaluated in multivariable models. All were followed longitudinally with NP testing at 3 and 6 months after initial assessment.
Clinical Characterization
Psychiatric assessment: Participants completed the Thai version of the Hospital Anxiety and Depression Scale (HADS), a 14-item scale with anxiety and depression subscales (7 items per subscale). Each item is scored from 0–3, with a total score range of 0–21 per subscale. HADS scores were correlated with baseline NP scores using Spearman correlation. Scores greater than 11 were considered positive cases. Illicit Drug Use Quantification: Identification, duration, and frequency of drug use was elicited from participants through structured interviews. Time of HIV transmission: AHI was confirmed by serial laboratory testing at 2, 4, 8, 12, 24 weeks after initial detection of positive HIV nucleic acid and negative HIV antibody. HIV transmission dates were estimated from the dates of HIV exposure within the past 30 days reported by participants. When multiple possible dates were given, the mean time point was selected.
Neuropsychological Testing
The 4-test NP battery evaluated fine motor function/manual dexterity [Grooved Pegboard test (GP), non-dominant hand], psychomotor speed [Color Trails 1 (CT1), Trail Making A (TM)], and executive function/set shifting [Color Trails 2 (CT2)].
We utilized an existing normative NP testing database of HIV-uninfected Thai control participants (n= 449) [19]. For the purpose of this study, we utilized only the 251 HIV-uninfected controls in the similar age range as our AHI participants. The normative sample had a median (IQR) age of 34 (27–42) years; 46% (n=115) were male and 30% (n=76) had a bachelor’s degree or higher. These control participants completed NP assessments at baseline and at 6 months follow up. For baseline NP performance analysis, data of all 251 HIV-uninfected controls were used. In longitudinal NP performance analyses, only the 45 controls that had complete NP data for the six-month study period were utilized.
The raw NP scores of AHI participants were standardized using data from the HIV-uninfected control participants from equivalent age and education stratum to calculate z-scores. A composite score (NPZ-4), the arithmetic mean of individual z-scores, was calculated to provide an overall measure on NP performance.
Laboratory Measures
CSF protein and cell count were measured via lumbar puncture. CSF and plasma HIV RNA quantification (viral load) was completed using the Roche Amplicor HIV-1 Monitor Test V1.5 in most participants, but by Roche COBAS TaqMan HIV-1 Test V2.0 for 3 participants due to testing platform change during the parent study. The lower limit of detection in CSF was 100 copies/ml due to dilution correction. CD4 and CD8 cell counts were determined by flow cytometry.
Statistical Analysis
Relationships between baseline demographic and clinical data and baseline NP performance were examined by Spearman correlation. A multivariable regression model was used to identify associations between clinical and laboratory parameters and NP performance at baseline (dependent variable = NPZ-4, independent variables = CD4, CSF HIV RNA, days post transmission). Data were assessed for normalcy prior to analysis. Data that were found to not be normally distributed were analyzed using the appropriate non-parametric tests. The non-parametric repeated measures ANOVA, the Friedman test, was employed to compare baseline to follow up NP values at the 3 and 6 month time point after treatment. The Mann-Whiney U test was utilized to compare the change in longitudinal NP performance of the AHI group to that of the matched controls at the 6-month time point.
RESULTS
Baseline Characteristics of AHI Participants
We enrolled 36 AHI participants with a median (IQR) age of 28 (24–33) years (Table 1). Most were young, educated men (89% male, 58% with bachelor’s degree or higher). The median estimated days since history of HIV exposure was 19 days (interquartile range 15–24 days). Over half (64%) were classified as Fiebig stage I (HIV RNA+, p24 antigen-, HIV IgM−) and II (HIV RNA+, p24 antigen+, HIV IgM−). Most (86%) were infected with circulating recombinant form (CRF) 01_AE, the predominant subtype in Thailand, with the remaining being recombinant CRF01_AE and clade B. HIV-1 tropism data were available for 34 participants; all were R5-tropic. The median (interquartile range) CD4 and CD8 counts were 411 (338–568) cell/mm3 and 578 (399–1013) cells/mm3, respectively. Plasma and CSF HIV RNA was 5.52 (4.56–5.87) log10 copies/ml and 3.37 (2.19–4.35) log10 copies/ml, respectively. Almost three-quarters (72%) denied lifetime drug use or drug use in the four months prior to enrollment.
Table 1.
Baseline Characteristics of AHI Participants, N=36.
| Median (IQR) or Total (%) | |
|---|---|
| Age | 28(24 – 33) |
| Male | 32 (89%) |
| Bachelor’s degree or higher | 21 (58%) |
| Days post HIV transmission | 19 (15–24) |
| Fiebig I/II* | 23 (64%) |
| CRF01_AE | 31 (86%) |
| CD4 Count (cells/mm3) | 411 (338–568) |
| CDS Count (cells/mm3) | 578 (399–1013) |
| Plasma HIV RNA (Iog10 copies/ml) | 5.52 (4.56–5.87) |
| CSF HIV RNA (Iog10 copies/ml) | 3.37 (2.19–4.35) |
| No drug use** | 26 (72%) |
Fiebig I: HIV RNA+; Fiebig II: HIV RNA+, p24 antigen +.
Denies any lifetime drug use or any drug use in 4 months prior to enrollment
Baseline NP Performance and Correlates
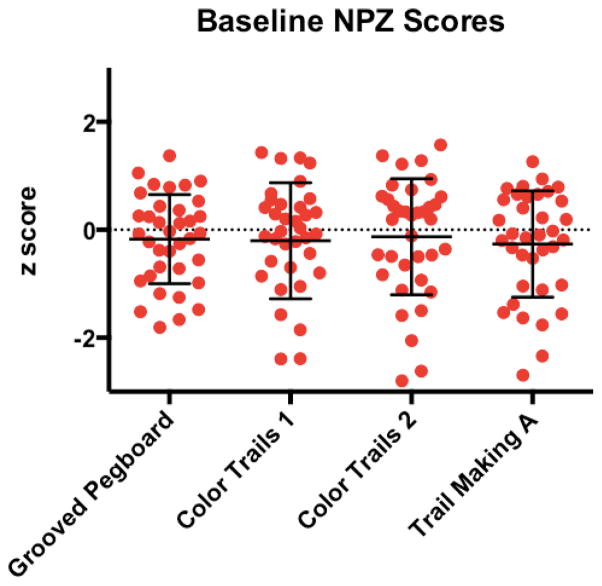
The mean z-scores were close to zero for each NP test supportive of normal performance compared to controls (Figure 1). Specifically, the mean z-scores on GP, CT1, CT2, TM were −0.17, −0.20, −0.13, and −0.26, respectively. Mean NPZ-4 was −0.19. However, eight participants performed greater than one standard deviation below the mean performance of matched participants on at least two NP tests. A subset met threshold criteria for anxiety (n=16, 44%), or depression (n=8, 22%) on the HADS scale. The composite NP score, NPZ-4, did not correlate to depression (p=0.336) or anxiety scores (p=0.861).
Figure 1. Baseline Neuropsychological (NP) Performance of AHI Participants.
Z-scores on Grooved Pegboard = −0.17; Color Trails 1 = −0.20; Color Trails 2 = −0.13; and Trail Making A = −0.26. Eight participants performed >1 SD below norm means on ≥2 NP tests.
The baseline NP performance inversely correlated with CSF HIV RNA (r=−0.493, p=0.023) and estimated days post transmission (r=−0.389, p=0.019) (Figure 2). There was no significant correlation seen with NP performance and plasma HIV RNA (p=0.203), CD4 count (p=0.440), CD8 count (p=0.468), CSF WBC (p=0.073), or CSF protein (p=0.995). However, in multivariable modeling CSF HIV RNA, CD4 count and estimated duration of infection were found to explain 31% of the variance in NP performance at baseline (adjusted R-square = 0.310, p= 0.025) with individual effects significant for CSF HIV RNA (β= −0.725, p=0.013) and CD4 (β= −0.882, p =0.004). Given the unexpected inverse relationship between CD4 count and NP performance, we included a CSF HIV RNA—CD4 count interaction term, which was significant (β=0.663, p<0.001) and resulted in an increase in the explained variance of the full model to 72%. The 21 participants with LP were separated into 3 equal groups of low, moderate, and high CD4 count with ranges of 132–338 cells/mm3, 339–555 cells/mm3, and 565–970 cells/mm3, respectively. As CD4 count increased, the strength of the correlation between CSF HIV RNA and NPZ-4 decreased (low CD4 group, R2 = 0.37; moderate CD4 group, R2 = 0.16; high CD4 group, R2 = 0.02).
Figure 2. Baseline Correlations.
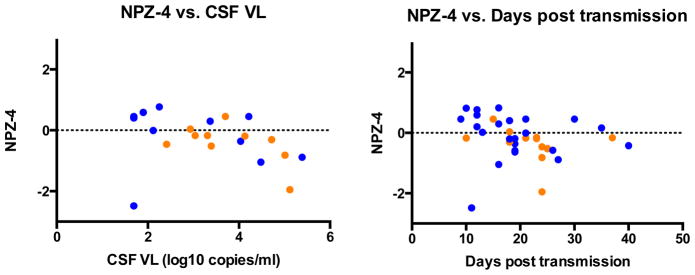
Blue dot = Fiebig I/II (HIV RNA+, HIV IgM−), Orange dot = Fiebig III/IV (HIV IgM+, HIV IgG−). NP performance was negatively correlated with cerebrospinal fluid (CSF) viral load (VL) (r=−0.493, p=0.023) and days post-transmission (r=−0.389, p=0.019). NPZ-4 scores did not correlate with depression (p=0.336) or anxiety measures (p=0.861).
Analysis of demographic differences between the subgroup of AHI participants (n=8) that displayed NP testing impairment at baseline and the rest of the AHI participants (n=28) revealed that the impaired subgroup had significantly higher levels of CSF HIV RNA (U = 16, p = 0.047) than the non-impaired AHI participants (available CSF HIV RNA in impaired group (n=5/8) vs. rest of AHI group (n=16/28)).
Longitudinal NP Performance
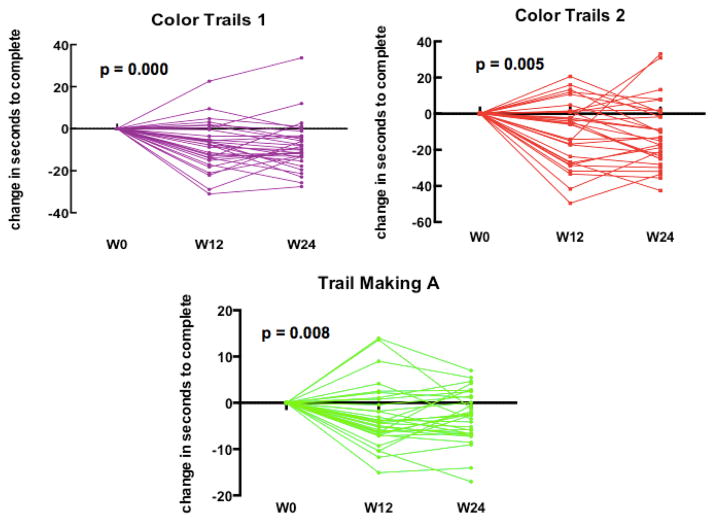
Thirty-one of the thirty six participants completed NP testing at both the three month and six month follow-up period. There were no baseline demographic differences between participants retained and those lost to follow-up using Mann-Whitney U test. We noted no change in motor performance over 6 months, n=31 (χ2(2) = 1.613, p=0.446). However, there was a significant improvement noted in CT1, χ2(2) = 20.387, p=0.000 TM, χ2(2) = 8.581, p=0.014) and CT2, χ2(2) = 9.484, p=0.009) over the 6 month period. Post-hoc analysis with Wilcoxon signed-ranks tests identified significant improvement only in the initial 3 months following initial assessment (ps <0.01) with no significant change in performance during the last 3 months, Figure 3. In the 8 participants who initially performed greater than one standard deviation below the mean of their matched controls on two or more NP tests, NP performance did not significantly change at either the 3 or 6 month time point in any domain ((CT1, χ2(2) = 5.250, p=0.072; CT2, χ2(2) = 4.750, p=0.093; TM, χ2(2) = 1.750, p=0.417; GP, χ2(2) = 1.750, p=0.417)).
Figure 3. Longitudinal Neuropsychological Performance after ART Initiation.
Significant improvement was seen in week 0 to week 12 in processing speed (Color Trials 1, p = 0.000; Trail Making A, p = 0.008) and executive functioning (Color Trails 2, p=0.005) in the total cohort. No change in motor performance.
In order to determine how much of the improvement in the AHI group could be due to practice effects, we compared the change in longitudinal performance of the AHI group (n=31) to that of the matched controls. Of the initial 251 HIV-uninfected controls used for baseline NP performance analysis, 45 control participants had complete NP data for the six-month study duration and were used for the longitudinal NP performance analysis. This 45-participant subgroup had a median (IQR) age of 36 (25–45) years; 51% (n=23) were male and 13% (n=6) had a bachelor’s degree or higher. In comparison, the AHI group followed over time (n=31) was younger (median (IQR) age of 29 (23–33) years), predominately male (90%, n=28), and more educated (52% (n=16) with bachelor’s degree or higher).
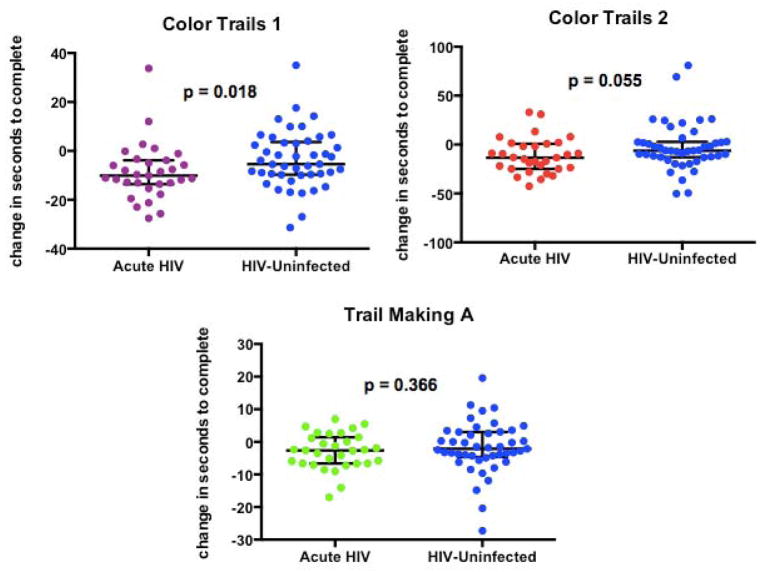
We found that the degree of NP improvement in the AHI group was greater than that seen in matched controls in one processing speed test, CT1 (U = 473, p = 0.018), Figure 4. The observed improvement in the AHI group was similar to that of controls on the other 3 NP tests (CT2, U = 516, p = 0.055; TM, U = 612, p = 0.366; GP, U = 654, p = 0.646).
Figure 4. Comparison of Neuropsychological Performance Improvement in AHI participants and HIV-Uninfected Controls.
When compared to HIV-uninfected controls in NP performance at 6 months, AHI participants only had a greater improvement in one processing test (Color Trails 1, p = 0.018).
In multivariable modeling (dependent variable = change in performance in each NP test, independent variables = MVC, CD4, CSF HIV RNA, days post transmission), MVC was not found to be a significant predictor of longitudinal NP performance in any NP test (CT1: β= −0.129, p=0.652; CT2: β= −0.042, p=0.876; GP: β=0.129, p=0.642; TM: β= −0.418, p=0.069).
DISCUSSION
This prospective longitudinal study characterized NP performance in Thai participants during AHI, beginning a median estimated 19 days since history of HIV exposure and up to six months after cART. The baseline performance of the AHI group as a whole did not significantly differ from that of an age-and-education-matched group of HIV-uninfected controls in the domains of psychomotor speed, executive function, and fine motor performance. However, a subgroup of individuals exhibited cognitive impairment at baseline that correlated with higher CSF HIV RNA.
There are several possible explanations for the relatively normal performance of most AHI participants at baseline. First, given the very early timing following estimated exposure, participants may have been at too early of a stage to have incurred processes such as neuronal injury that would underlie NP impairment. Neuronal injury can be measured by CSF neurofilament light chain (NFL); and we have shown that cART-naïve HIV participants have normal CSF NFL levels and no evidence of axonal injury during very acute HIV infection [20]. Taking into consideration the finding of a notable subset of participants (~25%) that demonstrated baseline neurocognitive impairment, it is most likely that there is a high level of heterogeneity in baseline NP performance in AHI with some performing above average and others exhibiting significant impairment. Secondly, small sample size could have limited our ability to identify very small differences in NP performance. And lastly, it is possible that our neuropsychological battery was too brief to have sufficient sensitivity in identifying HIV-related neurocognitive impairment in acute HIV. Though some studies have successfully identified neurocognitive impairment with a short battery [13], these brief batteries may be more useful in chronic HIV rather than acute HIV.
Factors such as drug use, anxiety, and depression can impact NP performance [21–23]. It is difficult to ascertain how much of an effect prior drug use and psychiatric symptoms has on neurocognitive performance, but the relatively low incidence of reported illicit drug use (28%) and the lack of correlation between anxiety/depressive symptoms and NP scores in this cohort reduces concern regarding the confounding nature of these premorbid factors.
We investigated clinical and biological markers that might render AHI participants at a higher risk for cognitive impairment. NP performance inversely correlated to CSF HIV RNA level and estimated days post HIV transmission, supporting the concept that NP impairment might be, in part, related to the level of viral burden in the CNS during this period. This was further supported by comparison analysis between the small subgroup of AHI participants that displayed cognitive impairment at baseline and the rest of the AHI participants, which demonstrated that those with baseline impairment had significantly higher levels of CSF HIV RNA than their fellow AHI counterparts. These correlations between NP performance and the selected clinical markers are preliminary evidence of associations and would require replication.
Interestingly, there was no significant association between CD4 count and NP performance in univariate modeling in this cohort. Previous work has shown that low CD4 count, especially a low CD4 nadir, is associated with neurocognitive impairment, and that higher CD4 counts confer a lower risk of impairment [24, 25]. Our findings might be explained by differential effects of the CD4 count in acute versus chronic HIV infection. Unlike in chronic infection, low CD4 counts during AHI are less of a marker of sustained immunosuppression and related neurocognitive damage, but instead reflect acute and potentially variable immunologic responses with unclear significance for the CNS.
Although we did not identify an association between CD4 count and NP performance in univariate analyses, a significant relationship was found in multivariable modeling between these two factors. In addition to CD4 count, CSF HIV RNA and days post-transmission were also significant predictors of NP performance in a multivariable regression model. As expected, CSF HIV RNA and days post transmission had an inverse relationship with cognitive performance. However, there was an unexpected inverse relationship between CD4 count and NP performance evident from regression analyses. Because CD4 count drops and plasma viral load increases during the initial weeks of HIV infection [26], it was hypothesized that higher CD4 would be associated with better NP performance. Our finding is most likely related to the fact that CSF HIV RNA and CD4 are closely related, in fact, our correlational analysis showed a significant negative correlation of r = −0.63 between these two factors thus suggesting multicollinearity and distortion of the relationship between CD4 and NP performance.
We found that CD4 level moderated the negative relationship between CSF HIV RNA and NP performance, suggesting that CD4 count may play a protective role in maintaining neurocognitive function despite high levels of CSF HIV RNA. This finding is important because it suggests that utilization of CD4 count alone as a clinical guidepost to facilitate treatment decisions to address neurocognitive performance may not be ideal. Further, preventing advanced immune suppression and initiating cART at higher CD4 counts may result in better overall cognitive outcomes among HIV-infected individuals.
Following immediate cART initiation, we observed improvement in most tests during the first 3 months, but change in only one test (CT1) at 6 months exceeded that of change observed in controls, supportive of practice effects. This contrasts with some reports that have demonstrated deleterious effect of cART on cognition [27, 28]. The few participants (n=8) who demonstrated NP impairment at baseline did not improve at 6 months. They had higher levels of CSF HIV RNA at baseline, which could support early unresolved cognitive deficits over 6 months. Premorbid factors may influence this outcome, but it is noteworthy that only two of the eight individuals reported recent illicit drug use. As such, premorbid substance abuse is not a key driver of poor neuropsychological outcome after cART among individuals with impaired performance at baseline.
The most commonly described cognitive deficits in chronic HIV occur in the domains of motor and psychomotor function, executive function, processing speed, and attention [29–31]. Psychomotor performance and processing speed are regarded as more reliable markers of cognitive impairment longitudinally, given that they are less influenced by practice effects [10]. In our cohort, there were no deficits detected on these tests at baseline. Given that our cohort was in the very early stages of HIV infection, the lack of deficit in motor performance is not surprising. It has been documented that impairment in this domain usually becomes evident in more advanced disease [31]. The use of culturally matched longitudinal control data is a strength of our study; but, since our study group was Thai and mostly infected with clade AE virus, broad generalizability may be limited.
In sum, we identify limited abnormalities on neuropsychological tests among individuals with AHI at baseline with improvement following treatment. We also identify about one-quarter of participants having performance in an impaired range during AHI with limited improvement after 6 months of cART. This study and future research should compare the long-term NP performance trajectory from AHI to chronic disease compared to those who initiate treatment later in disease. Such comparison may provide conclusive evidence to support the need for earlier initiation of cART in order to prevent risk of developing HIV-associated neurocognitive impairment.
Acknowledgments
We thank our study participants and collaborators at TRCARC, UCSF, Yale, and SEARCH, Thailand.
The RV254/SEARCH 010 Study Group includes from SEARCH/HIV-NAT/Thai Red Cross AIDS Research Center: Praphan Phanuphak, Nittaya Phanuphak, Nipat Teeratakulpisarn, Eugene Kroon, Mark de Souza, Nitiya Chomchey, Somprartthana Rattanamanee, Sasiwimol Ubolyam, Suteeraporn Pinyakorn; from AFRIMS: Rapee Trichavaroj, Vatcharain Assawadarachai, Nantana Tantibul, Bessara Nuntapinit, Siriwat Akapirat, Kultida Poltavee, Nampueng; from UCSF: Akash Desai, Stephanie Chiao, Edgar Busovaca, and Lauren Wendelken; from the US Military HIV Research Program: Sodsai Tovanabutra, Merlin Robb, Nelson Michael; from Monogram Biosciences: Laura Napolitano, Molly Martel.
Conflicts of Interest and Source of Funding: Dr. Spudich has received travel support and an honorarium for presentation at a scientific meeting from Abbvie, Inc. For the remaining authors none were declared.
These studies were funded by the National Institutes of Health (R01MH095613, R21MH086341, R01MH09561302-S1, R01NS061696) and the US Military HIV Research Program, Walter Reed Army Institute of Research, Rockville, Maryland, under a cooperative agreement (W81XWH-07-2-0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense. Idil Kore was directly supported by R01MH09561302-S1 and the Yale School of Medicine Office of Student Research. Antiretrovirals were supported by the Thai Government Pharmaceutical Organization, Gilead, Merck and ViiV Healthcare. Monogram Biosciences supported the Trofile® test.
Footnotes
DISCLAIMER
The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army or the Department of Defense or the US National Institutes of Health Some data from this manuscript have been presented at the Conference on Retroviruses and Opportunistic Infections (CROI) in March 2013, Atlanta, GA.
The content of this presentation is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
References
- 1.Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 3.Rosca EC, Rosca O, Simu M, Chirileanu RD. HIV-associated neurocognitive disorders: a historical review. Neurologist. 2012;18:64–67. doi: 10.1097/NRL.0b013e318247bc7a. [DOI] [PubMed] [Google Scholar]
- 4.Chan P, Brew BJ. HIV associated neurocognitive disorders in the modern antiviral treatment era: prevalence, characteristics, biomarkers, and effects of treatment. Curr HIV/AIDS Rep. 2014;11:317–324. doi: 10.1007/s11904-014-0221-0. [DOI] [PubMed] [Google Scholar]
- 5.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 6.Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206:275–282. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spudich S, Gisslen M, Hagberg L, Lee E, Liegler T, Brew B, et al. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis. 2011;204:753–760. doi: 10.1093/infdis/jir387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkinson JH, Higgins JA, Vigil O, Dubrow R, Remien RH, Steward WT, et al. Psychiatric context of acute/early HIV infection. The NIMH Multisite Acute HIV Infection Study: IV. AIDS Behav. 2009;13:1061–1067. doi: 10.1007/s10461-009-9585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson JLE, Hecht F, Price RW, Robertson K, Spudich S. Neurocognitive Performance during Primary HIV-1 Infection [Abstract]. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, California. 2010. [Google Scholar]
- 10.Peterson JLE, Hecht FM, Pilcher C, Price R, Yiannoutsos C, et al. Changes in Neurocognitive Performance from Early HIV-1 Infection to Initiation of Antiretroviral Therapy [Abstract]. 19th Conference on Retroviruses and Opportunistic Infections; Seattle, Washington. 2012. [Google Scholar]
- 11.Crum-Cianflone NF, Moore DJ, Letendre S, Poehlman Roediger M, Eberly L, Weintrob A, et al. Low prevalence of neurocognitive impairment in early diagnosed and managed HIV-infected persons. Neurology. 2013;80:371–379. doi: 10.1212/WNL.0b013e31827f0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore DJ, Letendre SL, Morris S, Umlauf A, Deutsch R, Smith DM, et al. Neurocognitive functioning in acute or early HIV infection. J Neurovirol. 2011;17:50–57. doi: 10.1007/s13365-010-0009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson K, Jiang H, Kumwenda J, Supparatpinyo K, Evans S, Campbell TB, et al. Improved neuropsychological and neurological functioning across three antiretroviral regimens in diverse resource-limited settings: AIDS Clinical Trials Group study a5199, the International Neurological Study. Clin Infect Dis. 2012;55:868–876. doi: 10.1093/cid/cis507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson KR, Robertson WT, Ford S, Watson D, Fiscus S, Harp AG, et al. Highly active antiretroviral therapy improves neurocognitive functioning. J Acquir Immune Defic Syndr. 2004;36:562–566. doi: 10.1097/00126334-200405010-00003. [DOI] [PubMed] [Google Scholar]
- 15.Sacktor N, Nakasujja N, Skolasky R, Robertson K, Wong M, Musisi S, et al. Antiretroviral therapy improves cognitive impairment in HIV+ individuals in sub-Saharan Africa. Neurology. 2006;67:311–314. doi: 10.1212/01.wnl.0000225183.74521.72. [DOI] [PubMed] [Google Scholar]
- 16.Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 17.Ananworanich J, Phanuphak N, de Souza M, Paris R, Arroyo M, Trichavaroj R, et al. Incidence and characterization of acute HIV-1 infection in a high-risk Thai population. J Acquir Immune Defic Syndr. 2008;49:151–155. doi: 10.1097/QAI.0b013e318183a96d. [DOI] [PubMed] [Google Scholar]
- 18.Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 19.Heaps J, Valcour V, Chalermchai T, Paul R, Rattanamanee S, Siangphoe U, et al. Development of normative neuropsychological performance in Thailand for the assessment of HIV-associated neurocognitive disorders. J Clin Exp Neuropsychol. 2013;35:1–8. doi: 10.1080/13803395.2012.733682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peluso M, Valcour V, Ananworanich J, Sithinamsuwan P, Chalermchai T, Chomchey N, et al. Immediate Antiretroviral Therapy Mitigates the Development of Neuronal Injury in Acute HIV [Abstract]. 21st Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts. 2014. [Google Scholar]
- 21.Devlin KN, Gongvatana A, Clark US, Chasman JD, Westbrook ML, Tashima KT, et al. Neurocognitive effects of HIV, hepatitis C, and substance use history. J Int Neuropsychol Soc. 2012;18:68–78. doi: 10.1017/S1355617711001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu SM, Chow DC, Valcour V, Masaki K, Nakamoto B, Kallianpur KJ, et al. The Impact of Depressive Symptoms on Neuropsychological Performance Tests in HIV-Infected Individuals: A Study of the Hawaii Aging with HIV Cohort. World J AIDS. 2011;1:139–145. doi: 10.4236/wja.2011.14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25:1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munoz-Moreno JA, Fumaz CR, Ferrer MJ, Prats A, Negredo E, Garolera M, et al. Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res Hum Retroviruses. 2008;24:1301–1307. doi: 10.1089/aid.2007.0310. [DOI] [PubMed] [Google Scholar]
- 26.Picker LJ. Immunopathogenesis of acute AIDS virus infection. Curr Opin Immunol. 2006;18:399–405. doi: 10.1016/j.coi.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23:1359–1366. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson KR, Su Z, Margolis DM, Krambrink A, Havlir DV, Evans S, et al. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology. 2010;74:1260–1266. doi: 10.1212/WNL.0b013e3181d9ed09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldewicz TT, Leserman J, Silva SG, Petitto JM, Golden RN, Perkins DO, et al. Changes in neuropsychological functioning with progression of HIV-1 infection: results of an 8-year longitudinal investigation. AIDS Behav. 2004;8:345–355. doi: 10.1023/B:AIBE.0000044081.42034.54. [DOI] [PubMed] [Google Scholar]
- 30.Martin EM, Sorensen DJ, Edelstein HE, Robertson LC. Decision-making speed in HIV-1 infection: a preliminary report. AIDS. 1992;6:109–113. doi: 10.1097/00002030-199201000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc. 2002;8:410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]