Abstract
Abiotic stresses cause changes in the balance of phytohormones in plants and result in inhibited root growth and an increase in the susceptibility of plants to root rot disease. The aim of this work was to ascertain whether microbial indole-3-acetic acid (IAA) plays a role in the regulation of root growth and microbially mediated control of root rot of cotton caused by Fusarium solani. Seed germination and seedling growth were improved by both NaCl and Mg2SO4 (100 mM) solutions when treated with root-associated bacterial strains Pseudomonas putida R4 and Pseudomonas chlororaphis R5, which are able to produce IAA. These bacterial strains were also able to reduce the infection rate of cotton root rot (from 70 to 39%) caused by F. solani under gnotobiotic conditions. The application of a low concentration of IAA (0.01 and 0.001 μg/ml) stimulated plant growth and reduced disease incidence caused by F. solani (from 70 to 41–56%, respectively). Shoot and root growth and dry matter increased significantly and disease incidence was reduced by bacterial inoculants in natural saline soil. These results suggest that bacterial IAA plays a major role in salt stress tolerance and may be involved in induced resistance against root rot disease of cotton.
Keywords: Salinity, Cotton, Indole-3-acetic acid, Fusarium solani, Plant growth promoting rhizobacteria
1. Introduction
Numerous studies have shown that salt stress affects physiological processes of plants, causing a nutrient imbalance, altering the levels of growth regulators, inhibiting photosynthesis and protein synthesis, all of which lead to reduced plant growth (Ahmad, 2013, Dolatabadian et al., 2011, Egamberdieva et al., 2013a, Egamberdieva et al., 2013b, Hashem et al., 2014a, Hashem et al., 2015, Hameed et al., 2014). An increase in salinity led to a decrease in the radical length of Cicer arietinum (Egamberdieva et al., 2014a), Arachis hypogaea (Mensah et al., 2006), Raphanus sativus (Egamberdieva, 2008), Vicia faba (Hashem et al., 2014a), Ochradenus baccatus (Hashem et al., 2014b) and Solanum lycopersicum (Hashem et al., 2015). Previous reports also indicated that salt stress increases susceptibility of plants toward various phytopathogens (Triky-Dotan et al., 2005; Egamberdieva et al., 2011). Goudarzi et al. (2011) observed increased root colonization of sorghum (Sorghum bicolor) by Macrophomina phaseolina under 1400 mg of NaCl kg−1 soil condition. In other studies, the severity of crown and root rot disease of tomato caused by Fusarium oxysporum f. sp. radicis-lycopersici significantly increased when plants were irrigated with saline water (EC = 4.6 dS m) (Triky-Dotan et al., 2005).
A decrease in root growth under salt stress is related to a decline of endogenous levels of phytohormones such as auxins, gibberellins, jasmonic acid and salicylic acid caused by NaCl toxicity (Debez et al., 2001, Egamberdieva, 2009, Egamberdieva, 2013, Alqarawi et al., 2014a). Indole-3-acetic acid (IAA) is the most abundant naturally occurring auxin with a well-documented ability to regulate many aspects of plant development, including the differentiation of vascular tissues, elongation, growth, stimulation of seed and tuber germination, lateral root initiation, biosynthesis of various metabolites and resistance to stressful conditions (Woodward and Bartel, 2005, Javid et al., 2011, Ramalingam and In-Jung, 2013). In addition, IAA is able to reduce the infection of tomato caused by F. oxysporum f. sp. radicis-lycopersici by inducing resistance in host tissue (Sharaf and Farrag, 2004).
Several studies have suggested that the exogenous application of phytohormones such as auxins (Afzal et al., 2005), cytokinins (Gul et al., 2000), and gibberellins (Khan et al., 2011) can stimulate plant cell proliferation, which in turn results in an enlarged root system and enhances the utilization of important nutrients from the soil (Egamberdieva, 2009). The root-associated microorganisms of various plants possess the ability to synthesize and release gibberellins, auxins, and cytokinins as secondary metabolites, which are subsequently re-taken up by plants for growth and development (Spaepen et al., 2007, Egamberdiyeva and Höflich, 2002, Egamberdiyeva and Hoflich, 2003, Egamberdieva and Lugtenberg, 2014, Egamberdieva et al., 2014b, Turan et al., 2014). The ability of a plant species to adapt to stress conditions often appears to depend on their association with certain microbes, which produce phytohormones such as indole-3-acetic acid, cytokinins, and gibberellic acid under stress conditions (Berg et al., 2013). Stimulation of plant growth and development of various plants by inoculation with PGPR having IAA producing ability has been repeatedly documented (Bano et al., 2013, Egamberdieva, 2013). The involvement of phytohormone IAA in the Pseudomonas fluorescens-mediated control of Fusarium head blight disease of barley was reported by Petti et al. (2012). However, to the best of our knowledge, no information is available on the role of IAA in biological control of pathogens under salt stress condition. The aim of this work was to ascertain whether microbial IAA plays a role in the regulation of root growth, and microbially mediated control of root rot of cotton caused by Fusarium solani.
2. Materials and methods
2.1. Plant seeds
The seeds of cotton (Gossypium hirsutum L.) variety ‘C-6524’ were obtained from the Department of Plant Production, Tashkent State University of Agriculture, Uzbekistan and used in this study. Seeds were sorted to eliminate broken, small and infected seeds. Seeds of cotton were surface-sterilized for 5 min with concentrated sulfuric acid followed by 70% ethanol for 3 min and rinsed 5 times with sterile, distilled water.
2.2. Microorganisms
Bacterial strains) Pseudomonas putida R4 and Pseudomonas chlororaphis R5(and plant pathogenic mold F. solani (Mart.) Sacc. (teleomorph: Nectria haematococca Berk. et Br.) were kindly provided by the culture collection of the Department of Microbiology and Biotechnology, National University of Uzbekistan. The isolation and pathogenicity test of plant pathogen mold were carried out previously from the rhizosphere of cucumber grown in saline soil, as described by Egamberdieva et al. (2011). Typical symptoms of root rot disease were observed in a previous study on both tomato (S. lycopersicum L.) and cotton (G. hirsutum L.) grown under saline soil conditions (Egamberdieva et al., 2011).
In order to determine the optimum salt concentration for growth, bacterial strains were cultured in King’s B (KB) medium supplemented with different concentrations of NaCl (3%, 4%, 5%, and 6%, w/v). The growth rate of bacterial strains was determined spectrophotometrically (Beckman DU 800 Spectrophotometer) at an optical density of 600 nm at regular time intervals for 24, 48 and 72 h.
2.3. IAA production
The production of indole 3-acetic acid (IAA) by bacterial strains was determined using a previously described colorimetric method (Egamberdieva et al., 2008). Briefly, the tested strains were inoculated in KB supplemented with 1.5% NaCl and Mg2SO4 and incubated at 28 °C at 150 rpm/min. After cultivation for 3 days, aliquots of bacterial cultures were centrifuged at 13,000 rpm for 10 min. 2 ml of supernatant was added to a tube with 100 μl 10 mM orthophosphoric acid and 4 ml of Salkowski reagent. The mixture was incubated at room temperature for 25 min and the absorbance of the developed pink color was read at 530 nm. The IAA (Sigma–Aldrich, St. Louis, MO, USA) concentration in culture was determined by using a calibration curve of pure IAA as a standard.
2.4. Seed germination
Seeds were germinated in 85 mm × 15 mm tight fitting plastic Petri dishes with 5 ml of test solution consisting of either 0.0 (control), 100 mM NaCl or 100 mM Mg2SO4. Thirty healthy and uniform seeds were sown in each Petri dish as three replications. To determine the effects of auxin on seed germination and seedling growth, IAA was used at 0.1, 0.01 and 0.001 μM under non-saline (control) and saline (100 mM NaCl or 100 mM Mg2SO4) conditions. The sterility of seeds was tested on Nutrient agar by incubating plates in the dark for three days at 28 °C. To study the effect of IAA-producing bacterial strains P. putida R4 and P. chlororaphis R5 on the germination of cotton seed, bacterial strains were grown in Nutrient broth (NB) medium for 24 h. The bacterial culture (1.0 ml) was sedimented by centrifugation (13,000×g) and the supernatant was discarded. Cells were washed with 1 ml of phosphate buffered saline (PBS) and re-suspended in PBS. Cell suspensions were adjusted to OD620 = 0.1, which corresponds to a cell density of about 1 × 108 cells/ml. The seeds were coated with bacteria by soaking them in the bacterial suspension, plated in Petri dishes and germinated in a plant growth chamber at 28 °C. The percentage germination was recorded after 5 days. The seed was considered to have germinated when the radicle had emerged >0.5 cm.
2.5. Plant growth in gnotobiotic sand system
The effect of the two IAA-producing bacterial strains on root and shoot growth and plant weight of cotton seedlings exposed to salt stress (100 mM NaCl and 100 mM Mg2SO4) was studied in gnotobiotic sand tubes (25 mm in diameter, 200 mm in length), as described by Simons et al. (1996). The tubes contained 60 g of sterilized sand soaked with 6 ml of plant nutrient solution (Kuiper et al., 2001). The growth and preparation of bacterial inoculants as well as the inoculation of seeds were as described by Egamberdieva and Kucharova (2009). Sterile germinated seeds were treated with bacterial suspension and planted into sterile glass tubes, one seed per tube with 10 replicates. Plants were grown for three weeks in climate-controlled chambers with 16 h of light at 24 °C and an 8-h dark period at 20 °C. After 3 weeks, the length of shoots and roots, and the fresh weight of whole plants were measured.
2.6. Biological control of fungal pathogen
A small piece of agar disk (0.5 cm in diameter) with F. solani culture was homogenized, placed on Chapek-Dox agar plates and cultivated for 3 days at 28 °C. The fungal material was poured over sterile glass wool to remove the mycelium while the filtrate, containing the spores, was adjusted to a concentration of 5 × 106 spores/ml. For sand infestation, spores were mixed thoroughly with sterilized sand to 6.0 × 106 spores/kg sand. The germinated cotton seeds were coated with bacteria by soaking them in a suspension of 1 × 108 colony-forming units (CFU)/ml bacteria in sterile PBS buffer, whereas control seeds were soaked in sterile PBS buffer. One seed was sown per tube with sand as described above. The treatments were as follows: (a) uninoculated control plants, (b) seeds inoculated with bacterial strains and (c) seeds treated with 0.1, 0.01, and 0.001 μM IAA grown in infested soil with F. solani spores. Each treatment contained four groups with 12 plants each. Plants were grown in gnotobiotic sand supplemented with NaCl at final concentrations of 100 mM in climate-controlled chambers with 16 h of daylight at 24 °C and an 8-h dark period at 18 °C. After 21 days, plants were removed from the sand, roots washed with water, and were examined for typical foot and root rot symptoms as indicated by browning and lesions. Roots without any disease symptoms were classified as healthy.
2.7. Enumeration of Fusarium colonies from the root
After 21 days of growth in gnotobiotic sand, the plants were carefully removed from the sand and were gently shaken to remove all but the tightly adhering sand. A section of 1 cm of root tip with attached sand particles was cut from the plantlets and transferred into a tube containing 1 ml of PBS. The root tip was vortexed in PBS and the homogenates were serially diluted. The appropriate dilutions, 10−3 and 10−4, were spread on Potato dextrose agar (PDA) plates and colonies were counted after 5 days incubation at 28 °C. Three replicates per dilution level were used to enumerate fungal colonies. The number of fungi colonizing the root was calculated as CFU/cm root.
2.8. Plant growth experiments in pots
The effect of IAA-producing bacterial strains on plant growth was studied in pot experiments using saline soil sampled from the Syrdarya province of Uzbekistan. The main chemical soil properties are: organic matter 0.79%; Ct 2.39%; Nt 0.07%; CO32−–C 1.59%, Ca2+ 54.3 g/kg; Mg2+ 26.1 g/kg; K+ 6.7 g/kg; P 1.2 g/kg; Cl− 0.1 g/kg; Na+ 0.8 g/kg; pH 7.8. The electrical conductivity (EC) value of saline soil was 6.8 dS/m. This soil contained 27 ± 9 g sand kg−1, 693 ± 12 g silt kg−1, and 280 ± 13 g clay kg−1 with a total cation exchange capacity (CEC) of 24.7 ± 1 (cmol+) kg−1 (Egamberdieva and Kucharova, 2009). The preparation of a bacterial suspension for seed inoculation was described previously (Egamberdieva and Kucharova, 2009). Briefly, bacteria were grown in KB medium for 24 h and bacterial suspensions were adjusted to an OD620 = 0.1 corresponding to a cell density of about 1 × 108 cells/ml. Cotton seeds were coated with bacteria by dipping them in bacterial suspensions for 15 min. Inoculated seedlings were sown in plastic pots (12 cm diameter; 15 cm deep) containing 350 g of soil. The inoculation treatments were set-up in a randomized design with six replications. The pot experiment had two treatments: uninoculated seeds and seeds inoculated with bacteria. Plants were grown under open field conditions, where temperature ranges from 20 to 24 °C during the day and from 15 to 17 °C at night and after six weeks the shoot and root length and dry matter of cotton plants were measured.
2.9. Statistical analyses
Data were tested for statistical significance using the analysis of variance package included in Microsoft Excel 2007, and comparisons were performed with a Student’s t-test. Mean comparisons were conducted using a least significant difference (LSD) test (P = 0.05).
3. Results
The two studied bacterial strains were able to grow in the presence of NaCl concentrations up to 5% and produce IAA in media containing 1.5% NaCl or Mg2SO4. The production of IAA by P. putida R4 was 8.9 and 4.1 μg/ml in the presence of NaCl and Mg2SO4, respectively, whereas P. chlororaphis R5 produced 6.4 and 5.1 μg/ml, respectively.
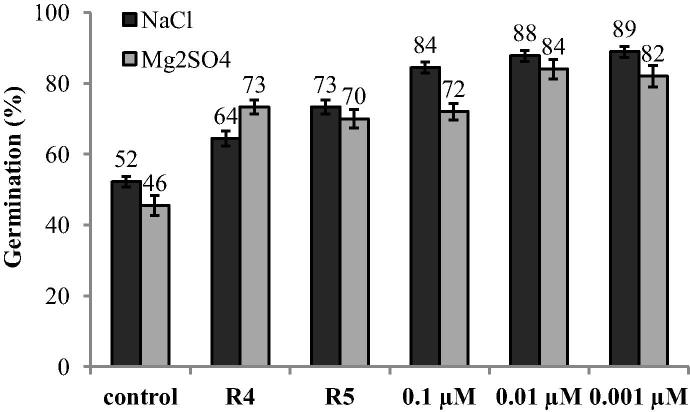
A negative effect of salinity on germination, and shoot and root length of cotton seedlings was observed (Figure 1, Figure 2, Figure 3). Under non-saline conditions, 98% of cotton seeds germinated but a much lower germination percentage was observed at 100 mM NaCl (52%) and Mg2SO4 (46%). Exposure to Mg2SO4 suppressed germination more than NaCl. Seed germination was improved by various concentrations of IAA (0.1, 0.01 and 0.001 μM) and by the presence of IAA-producing bacterial strains. The lowest concentration of IAA (0.001 μM) effectively alleviated salt stress caused by both salt NaCl and Mg2SO4 and improved seed germination (from 46 to 89%) (Fig. 1). Seed germination improved up to 64 and 73% in response to NaCl stress and up to 73 and 70% in response to Mg2SO4 stress respectively when seeds were inoculated with IAA-producing bacterial strains P. putida R4 and P. chlororaphis R5.
Figure 1.
The effect of IAA-producing Pseudomonas putida R4 and P. chlororaphis R5 and IAA concentrations (0.1, 0.01, 0.001 μM) on the seed germination of cotton under the influence of salt stress (100 mM NaCl or Mg2SO4). The percentage of germination was recorded after 5 days. Columns represent means for three petri dishes (each has thirty healthy and uniform seeds) (N = 3) with error bars showing standard error.
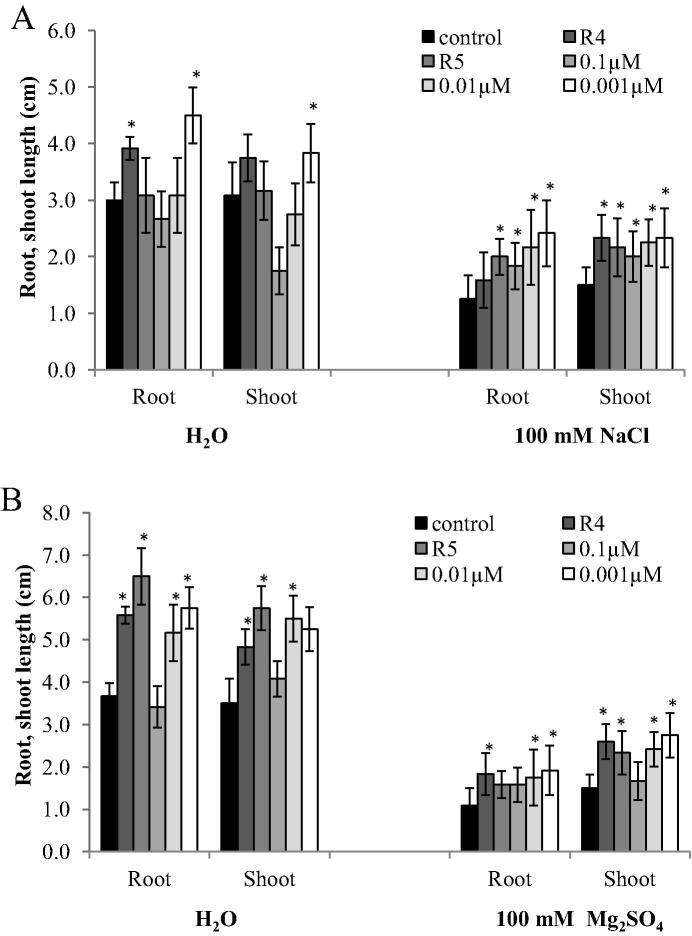
Figure 2.
Effect of inoculation of salt-stressed cotton seedlings with IAA-producing Pseudomonas putida R4 and P. chlororaphis R5 strains and IAA concentrations (0.1, 0.01, 0.001 μM) on root and shoot length of seedlings. Seedlings were grown for three weeks in gnotobiotic sand system containing 0 and 100 mM NaCl (A), or 100 mM Mg2SO4 (B). Columns represent means for six seedlings (N = 6) with error bars showing standard error. Columns marked with an asterisk differed significantly from untreated plants at P < 0.05.
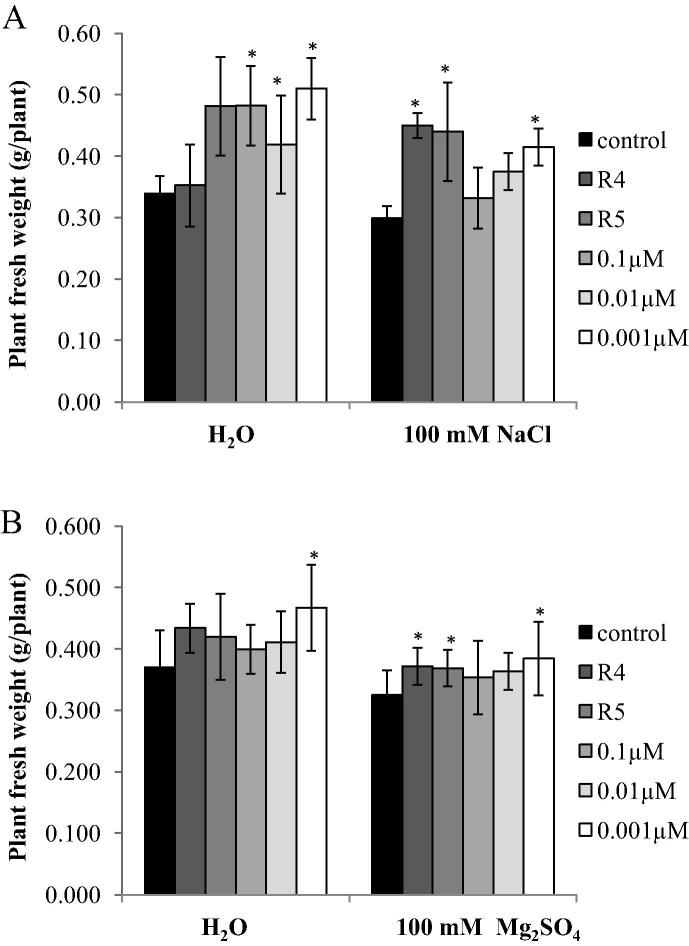
Figure 3.
Effect of inoculation of salt-stressed cotton seedlings with IAA-producing Pseudomonas putida R4 and P. chlororaphis R5 strains and IAA concentrations (0.1, 0.01, 0.001 μM) on seedling fresh weight. Seedlings were grown for 3 weeks in gnotobiotic sand containing 0 and 100 mM NaCl (A) or 100 mM Mg2SO4 (B). Columns represent means for six seedlings (N = 6) with error bars showing standard error. Columns marked with an asterisk differed significantly from untreated plants at P < 0.05.
At 100 mM NaCl or Mg2SO4, the fresh weight of whole plants as well as the length of roots and shoots was lower than those of non-stressed seedlings. However, the decrease in root and shoot length was more pronounced under Mg2SO4 than under NaCl stress. NaCl and Mg2SO4 stress reduced root length by 58% and 70%, shoot length by 51% and 57%, and fresh weight by 12%, respectively (Fig. 2a and b; Fig. 3a and b). The three concentrations of IAA as well as the use of the two IAA-producing bacterial strains improved seedling growth in non-stressed conditions and also ameliorated the negative effects caused by salt stress on cotton seedlings. P. putida R4 and P. chlororaphis R5 significantly increased root length by 26-60%, shoot length of cotton seedling by 55–44% at 100 mM NaCl respectively compared to the uninoculated seedlings (Fig. 2a). At 100 mM Mg2SO4, the root and shoot length of cotton seedlings inoculated with bacterial strains R4 and R5 increased by 69–73% and 46–55%, respectively (Fig. 2b). Under NaCl and Mg2SO4 stress conditions, a lower concentration of IAA (0.001 μM) had a greater stimulatory effect on root length (98–76%), shoot length (55–83%) and fresh weight (38–18%) of cotton seedlings.
In this study, various concentrations of IAA and two IAA-producing bacteria were also used to control cotton root rot caused by F. solani in gnotobiotic conditions. The strain R4 could reduce the level of infection of cotton root rot by 39% (Fig. 4). A lower concentration of IAA (0.01 and 0.001 μM) reduced the incidence of disease by 41–56% respectively.
Figure 4.
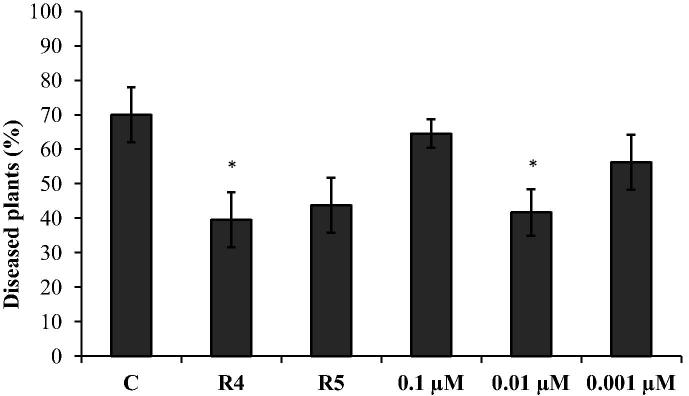
Control of cotton root rot caused by Fusarium solani in gnotobiotic conditions by IAA-producing Pseudomonas putida R4 and P. chlororaphis R5 and IAA. Plants were grown for 21 days in gnotobiotic sand infested with F. solani. Treatments were: C – control plants, grown in soil infested with F. solani but not treated with bacteria and IAA; seeds treated with P. putida R4 and P. chlororaphis R5; seeds treated with 0.1, 0.01 or 0.001 μM IAA. Columns marked with an asterisk differed significantly from untreated plants at P < 0.05.
The root surface of cotton grown in sand infested with the fungal pathogen (6.0 × 106 spores/kg sand) appeared to be heavily colonized by F. solani (7.6 × 102 CFU/1 cm root). The plants inoculated with P. putida R4, and P. chlororaphis R5 had lower fungal colonization (4.0 and 3.6 × 102 CFU/cm root respectively). F. solani root colonization was similar in the plant roots treated with IAA: 6.0 for 0.1 μM IAA, 6.3 for 0.01 μM IAA and 5.6 (CFU/cm root tip) for 0.001 μM IAA.
The plant growth-promoting properties of strains and their ability to serve as biological control agents of cotton root rot were also tested in naturally salinated soil. P. putida R4 and P. chlororaphis R5 significantly stimulated shoot and root growth and dry matter of cotton in saline soil in comparison with the uninoculated control plants (Table 1). The disease incidence in control plants grown in naturally saline soil was 21% and seed coating with bacterial strains reduced this level to 12% (Table 1).
Table 1.
The effect of IAA producing Pseudomonas strains on shoot and root growth, and plant dry matter and the control of root rot of cotton grown in saline soil.
| Bacterial strains | Shoot length (cm) | Root length (cm) | Dry matter (g/plant) | Disease incidence (%) |
|---|---|---|---|---|
| Control | 9.0 | 10.2 | 0.092 | 21 |
| P. putida R4 | 10.8 | 11.0 | 0.119⁎ | 15 |
| P. chlororaphis R5 | 11.0⁎ | 12.2 | 0.118⁎ | 12⁎ |
Significantly different from the control at P < 0.05; plants were grown under open natural conditions in pots containing saline soil not infested with F. solani for 6 weeks.
4. Discussion
Numerous studies have shown that salt stress affects physiological processes of plants, which may cause nutrient imbalance, altered levels of growth regulators, inhibited photosynthesis and protein synthesis, which collectively lead to reduced plant growth (Dolatabadian et al., 2011, Hashem et al., 2014a, Hashem et al., 2014b, Alqarawi et al., 2014b). In this study, salt stress caused by NaCl and Mg2SO4 (100 mM) inhibited cotton seed germination and also the growth of seedling roots and shoots. A reduction in germination and seedling growth of plants by increasing salinity levels has been described in safflower (Carthamus tinctorius L.) (Ghazizade et al., 2012), wheat (Triticum aestivum), (Egamberdieva, 2009), groundnut (A. hypogaea) (Mensah and Ihenyen, 2009), and chickpea (C. arietinum) (Egamberdieva et al., 2014a, Egamberdieva et al., 2014b) and other medicinal plants such as taily weed O. baccatus, Ephedra alata (Hashem et al., 2014b, Alqarawi et al., 2014a, Alqarawi et al., 2014b). Salinity inhibits the synthesis of phytohormones, such as cytokinins and auxins, in plants (Figueiredo et al., 2008). According to Iqbal and Ashraf (2010) and Alqarawi et al. (2014b), salinity perturbs the hormonal balance of plants; therefore, hormonal homeostasis under salt stress might be one possible mechanism of phytohormone induced plant salt tolerance. The exogenous application of phytohormones such as gibberellins (Afzal et al., 2005), auxins (Egamberdieva, 2009), and cytokinins (Gul et al., 2000) mitigate salt stress and stimulate plant root and shoot growth under stress. The content of phytohormones in plants may also be affected by root-associated microorganisms (Turan et al., 2014). In earlier studies, Fulchieri et al. (1993) and Lucangeli and Bottini (1997) reported that maize (Zea mays) seedlings inoculated with PGPR strains Azospirillum lipoferum exhibited relatively higher amounts of IAA, and GA3, relative to non-inoculated controls. The inoculation of chickpea with Bradyrhizobium japonicum caused an increase in IAA content of leaves, and stimulated root growth (Bano et al., 2010). Phytohormone-producing root-associated bacteria that efficiently colonize plant roots might supply additional IAA into the rhizosphere and stimulate the growth of lateral roots and root hairs (Egamberdiyeva and Hoflich, 2003, Egamberdieva, 2012, Bano et al., 2013). From those studies, it is assumed that the presence of phytohormone-producing PGPR strains may affect the metabolism of endogenous phytohormones in the plant, thus increasing the root surface, which facilitates higher absorption of essential nutrients. In this study, IAA production by root-associated PGPR strains P. putida R4 and P. chlororaphis R5 was not affected by salt stress (1.5% NaCl and Mg2SO4) and bacterial strains alleviated salt stress in cotton seedlings under gnotobiotic sand and saline soil conditions. Bianco and Defez (2009) reported that IAA enhanced different cellular defence systems, thus protecting plants from salt stress conditions. Wu et al. (2014) observed an increase in cotton growth and tolerance to salt stress by Klebsiella oxytoca Rs-5. The germination rates of cotton seeds treated with bacteria Rs-5 increased by 15.40% compared with the control treatment. In addition, bacterial inoculation also improved plant physiological parameters such as chlorophyll a, soluble sugar, malondialdehyde, and proline content. A salt-tolerant strain K. oxytoca Rs-5 was also able to produce IAA (Yue et al., 2007).
We also investigated in this study whether IAA is a mechanism of biological control when used by PGPR. By using various IAA concentrations and IAA-producing bacteria, it was possible to control cotton root rot caused by F. solani under gnotobiotic conditions. Our results show that P. putida R4 and P. chlororaphis R5, which produce IAA, were able to reduce the infection rate of cotton root rot caused by F. solani. A lower concentration of IAA (0.01 and 0.001 μg/ml) also reduced the incidence of disease in plants grown under salt stress. Similar results obtained by Fernández-Falcón et al. (2003) in which the exogenous application of IAA to banana plants (Musa paradisiaca) induced resistance to Fusarium wilt caused by F. oxysporum f. sp. cubense, and that resistance was more effective when performed using low doses.
Beneficial PGPR efficiently colonize the rhizosphere of plants, protecting them from various soil-borne pathogens (Kamilova et al., 2008; Egamberdieva et al., 2011). The inoculation of IAA-producing bacterial strains R4 and R5 reduced the CFU of F. solani in comparison with uninoculated plants grown in sand infested with the pathogen, and reduced the disease incidence. Disease incidence was also reduced in plants treated with lower IAA concentrations, although the colonization of F. solani was almost similar to uninoculated control plants. This might be explained by the fact that the application of IAA primes the defence response such that plants are activated to respond rapidly to the pathogen and thus increase plant disease resistance (Petti et al., 2012). We observed that IAA producing bacterial strains R4 and R5 were able to reduce disease incidence in saline soil conditions.
5. Conclusion
The inoculation of plants by PGPR which colonize plant roots and produce IAA may be a good means of alleviating salt stress, stimulating plant growth and protecting plants from root rot disease caused by F. solani under saline soil conditions. These results suggest that bacterial IAA plays a major role in salt stress tolerance and is involved in induced resistance against root rot disease of cotton.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project no. RGP-VPP-271.
Footnotes
Peer review under responsibility of King Saud University.
References
- Afzal I., Basra Sh., Iqbal A. The effect of seed soaking with plant growth regulators on seedling vigor of wheat under salinity stress. J. Stress Physiol. Biochem. 2005;1(1):6–14. [Google Scholar]
- Ahmad P. Academic Press, Elsevier; USA: 2013. Oxidative Damage to Plants, Antioxidant Networks and Signaling. [Google Scholar]
- Alqarawi A.A., Abd Allah E.F., Hashem A., Al Huqail Asma A., Abdulaziz A., Al Sahli A.A. Impact of abiotic salt stress on some metabolic activities of Ephedra alata Decne. J. Food Agric. Environ. 2014;12(2):620–625. [Google Scholar]
- Alqarawi A.A., Hashem A., Abd Allah E.F., Alshahrani T.S., Al-Huail Asma A. Effect of salinity on moisture content, pigment system, and lipid composition in Ephedra alata Decne. Acta Biol. Hung. 2014;65(1):61–71. doi: 10.1556/ABiol.65.2014.1.6. [DOI] [PubMed] [Google Scholar]
- Bano A., Batool R., Dazzo F. Adaptation of chickpea to desiccation stress is enhanced by symbiotic rhizobia. Symbiosis. 2010;50:129–133. [Google Scholar]
- Bano Q., Ilyas N., Bano A., Zafar N., Akram A., Hassan F. Effect of Azospirillum inoculation on maize (Zea mays L.) under drought stress. Pak. J. Bot. 2013;45(S1):13–20. [Google Scholar]
- Berg G., Alavi M., Schmidt C.S., Zachow C., Egamberdieva D., Kamilova F., Lugtenberg B. Biocontrol and osmoprotection for plants under saline conditions. In: de Bruijn Frans J., editor. Molecular Microbial Ecology of the Rhizosphere. Wiley-Blackwell; USA: 2013. [Google Scholar]
- Bianco C., Defez R. Medicago truncatula improves salt tolerance when nodulated by anindole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J. Exp. Bot. 2009;60:3097–3107. doi: 10.1093/jxb/erp140. [DOI] [PubMed] [Google Scholar]
- Debez A., Chaibi W., Bouzid S. Effect du NaCl et de regulatoeurs de croissance sur la germination d’ Atriplex halimus L. Cahiers Agric. 2001;10:135–138. [Google Scholar]
- Dolatabadian A., Modarres Sanavy S.A.M., Ghanati F. Effect of salinity on growth, xylem structure and anatomical characteristics of soybean. Not. Sci. Biol. 2011;3:41–45. [Google Scholar]
- Egamberdieva D., Shurigin V., Gopalakrishnan S., Sharma R. Growth and symbiotic performance of chickpea (Cicer arietinum) cultivars under saline soil conditions. J. Biol. Chem. Res. 2014;31(1):333–341. [Google Scholar]
- Egamberdieva D., Lugtenberg B. PGPR to alleviate salinity stress on plant growth. In: Miransari M., editor. Use of microbes for the alleviation of soil stresses. Springer; New York: 2014. pp. 73–96. [Google Scholar]
- Egamberdieva D., Botir H., Hashem A., Abd-Allah E.F. Characterization of salt tolerant Enterobacter hormaechei strain associated with tomato root grown in arid saline soil. J. Pure Appl. Microbiol. 2014;8(5):4231–4239. [Google Scholar]
- Egamberdieva D. The role of phytohormone producing bacteria in alleviating salt stress in crop plants. In: Miransari M., editor. Biotechnological techniques of stress tolerance in plants. Stadium Press; USA: 2013. pp. 21–39. [Google Scholar]
- Egamberdieva D., Jabborova D., Wirth S. Alleviation of salt stress in legumes by co-inoculation with Pseudomonas and Rhizobium. In: Arora N.K., editor. Plant Microbe Symbiosis – Fundamentals and Advances. Springer; India: 2013. pp. 291–303. [Google Scholar]
- Egamberdieva D., Jabborova D., Mamadalieva N. Salt tolerant Pseudomonas extremorientalis able to stimulate growth of Silybum marianum under salt stress condition. Med. Aromat. Plant Sci. Biotechnol. 2013;7(1):7–10. [Google Scholar]
- Egamberdieva D. Indole-acetic acid production by root associated bacteria and its involvement in plant growth and development. In: Keller A.H., Fallon M.D., editors. Auxins: Structure, Biosynthesis and Functions. Nova Science Publishers Inc.; USA: 2012. pp. 103–122. [Google Scholar]
- Egamberdieva D. Alleviation of salinity stress in radishes with phytohormone producing rhizobacteria. J. Biotechnol. 2008;136S:262. [Google Scholar]
- Egamberdieva D. Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol. Plant. 2009;31:861–864. [Google Scholar]
- Egamberdieva D., Kucharova Z. Selection for root colonising bacteria stimulating wheat growth in saline soils. Biol. Fertil. Soils. 2009;45:561–573. [Google Scholar]
- Egamberdiyeva D., Hoflich G. The effect of associative bacteria from different climates on plant growth of pea at different soils and temperatures. Arch. Agric. Soil Sci. 2003;49(2):203–213. [Google Scholar]
- Egamberdiyeva D., Höflich G. Root colonization and growth promotion of winter wheat and pea by Cellulomonas spp. at different temperatures. J. Plant Growth Regul. 2002;38:219–224. [Google Scholar]
- Egamberdieva D., Kamilova F., Validov S., Gafurova L., Kucharova Z., Lugtenberg B. High incidence of plant growth-stimulating bacteria associated with the rhizosphere of wheat grown in salinated soil in Uzbekistan. Envir. Microb. 2008;19:1–9. doi: 10.1111/j.1462-2920.2007.01424.x. [DOI] [PubMed] [Google Scholar]
- Egamberdieva D., Kucharova Z., Davranov K., Berg G., Makarova N., Azarova T., Chebotar V., Tikhonovich I., Kamilova F., Validov S., Lugtenberg B. Bacteria able to control foot and root rot and to promote growth of cucumber in salinatedsoils. Biol. Fert. Soils. 2011;47:197–205. [Google Scholar]
- Fernández-Falcón M., Borges A.A., Borges-Pérez B. Induced resistance to Fusarium wilt of banana by exogenous applications of indoleacetic acid. Phytoprotection. 2003;84:149–153. [Google Scholar]
- Figueiredo M.V.B., Burity H.A., Martınez C.R., Chanway C.P. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 2008;4:182–188. [Google Scholar]
- Fulchieri M., Lucangeli C., Bottini R. Inoculation with Azospirillum lipoferum affects growth and gibberellin status of corn seedling roots. Plant Cell Physiol. 1993;34:1305–1309. [Google Scholar]
- Ghazizade M., Golkar P., Salehinejad F. Effect of salinity stress on germination and seedling characters in safflower (Carthamus tinctorius L.) genotypes. Ann. Biol. Res. 2012;3(1):114–118. [Google Scholar]
- Goudarzi S., Banihashemi Z., Maftoun M. Effect of salt and water stress on root infection by Macrophomina phaseolina and ion composition in shoot in sorghum. Iran. J. Plant Pathol. 2011;47(3):69–83. [Google Scholar]
- Gul B., Khan M.A., Weber D.J. Alleviation salinity and dark-enforced dormancy in Allenrolfea occidentalis seeds under various thermoperiods. Aust. J. Bot. 2000;48:745–752. [Google Scholar]
- Hameed A., Egamberdieva D., Abd-Allah E.F., Hashem A., Kumar A., Ahmad P. Salinity stress and arbuscular mycorrhizal symbiosis in plants. In: Miransari M., editor. Use of microbes for the alleviation of soil stresses. Springer; New York: 2014. pp. 139–159. [Google Scholar]
- Hashem A., Abd Allah E.F., Alqarawi A.A., Alwhibi Mona S., Alenazi M.M., Egamberdieva D., Ahmad P. Arbuscular mycorrhizal fungi mitigates NaCl induced adverse effects on Solanum lycopersicum L. Pak. J. Bot. 2015;47(1):327–340. [Google Scholar]
- Hashem A., Abd Allah E.F., Alqarawi A.A., El-Didamony G., Alwhibi Mona S., Egamberdieva D., Ahmad P. Alleviation of adverse impact of salinity on faba bean (Vicia Faba L.) by arbuscular mycorrhizal fungi. Pak. J. Bot. 2014;46(6):2003–2013. [Google Scholar]
- Hashem A., Abd Allah E.F., Alqarawi A.A., Al Huqail Asma A., Egamberdieva D. Alleviation of abiotic salt stress in Ochradenus baccatus (Del.) by Trichoderma hamatum (Bonord.) Bainier. J. Plant Interact. 2014;9(1):857–868. [Google Scholar]
- Iqbal M., Ashraf M. Gibberellic acid mediated induction of salt tolerance in wheat plants: growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environ. Exp. Bot. 2010;86:76–85. [Google Scholar]
- Javid M.G., Sorooshzadeh A., Moradi F., Sanavy S.A.M.M., Allahdadi I. The role of phytohormones in alleviating salt stress in crop plants. Aust. J. Crop Sci. 2011;5(6):726–734. [Google Scholar]
- Kamilova F., Lamers G., Lugtenberg B. Biocontrol strain Pseudomonas fluorescens WCS365 inhibits germination of Fusarium oxysporum spores in tomato root exudate as well as subsequent formation of new spores. Environ. Microbiol. 2008;10(6):2455–2461. doi: 10.1111/j.1462-2920.2008.01638.x. [DOI] [PubMed] [Google Scholar]
- Khan A.L., Hamayun M., Yoon-Ha Kim., Kang S.M., Lee J.H., Lee I.N. Gibberellins producing endophytic Aspergillus fumigatus sp. LH02 influenced endogenous phytohormonal levels, isoflavonoids production and plant growth in salinity stress. Process Biochem. 2011;46:440–447. [Google Scholar]
- Kuiper I., Bloemberg G.V., Lugtenberg B.J. Selection of a plant-bacterium pair as a novel tool for rhizostimulation of polycyclic aromatic hydrocarbon-(PAH)-degrading bacteria. Mol. Plant-Microbe Interact. 2001;14:1197–1205. doi: 10.1094/MPMI.2001.14.10.1197. [DOI] [PubMed] [Google Scholar]
- Lucangeli C., Bottini R. Effects of Azospirillum spp. on endogenous gibberellin content and growth maize (Zea mays L.) treated with uniconazole. Symbiosis. 1997;23:63–71. [Google Scholar]
- Mensah J.K., Ihenyen J. Effects of salinity on germination, seedling establishment and yield of three genotypes of mung bean (Vigna mungo L. Hepper) in Edo State, Nigeria. Nigerian Ann. Nat. Sci. 2009;8(2):17–24. [Google Scholar]
- Mensah J.K., Akomeah P.A., Ikhajiagbe B., Ekpekurede E.O. Effects of salinity on germination, growth and yield of five groundnut genotypes. Afr. J. Biotechnol. 2006;5(20):1973–1979. [Google Scholar]
- Petti S., Reiber K., Ali S.S., Berney M., Doohan F.M. Auxin as a player in the biocontrol of Fusarium head blight disease of barley and its potential as a disease control agent. BMC Plant Biol. 2012;12:224. doi: 10.1186/1471-2229-12-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam R., In-Jung L. Ameliorative effects of spermine against osmotic stress through antioxidants and abscisic acid changes in soybean pods and seeds. Acta Physiol. Plant. 2013;35:263–269. [Google Scholar]
- Spaepen S., Vanderleyden J., Remans R. Indole-3-acetic acid in microbial and microorganism-plant signalling. FEMS Microbiol. Rev. 2007;31:425–448. doi: 10.1111/j.1574-6976.2007.00072.x. [DOI] [PubMed] [Google Scholar]
- Sharaf E.F., Farrag A.A. Induced resistance in tomato plants by IAA against Fusarium oxysporum lycopersici. Pol. J. Microbiol. 2004;53(2):111–116. [PubMed] [Google Scholar]
- Simons M., van der Bij A.J., Brand I., de Weger L.A., Wijffelman C.A., Lugtenberg B.J. Gnotobiotic system for studying rhizosphere colonization by plant growth-promoting Pseudomonas bacteria. Mol. Plant-Microbe Interact. 1996;9:600–607. doi: 10.1094/mpmi-9-0600. [DOI] [PubMed] [Google Scholar]
- Triky-Dotan S., Yermiyahu U., Katan J., Gamliel A. Development of crown and root rot disease of tomato under irrigation with saline water. Phytopathology. 2005;95:1438–1444. doi: 10.1094/PHYTO-95-1438. [DOI] [PubMed] [Google Scholar]
- Turan M., Ekinci M., Yildirim E., Gneş A., Karagz K., Kotan R., Dursun A. Plant growth-promoting rhizobacteria improved growth, nutrient, and hormone content of cabbage (Brassica oleracea) seedlings. Turk. J. Agric. For. 2014;38:327–333. [Google Scholar]
- Woodward A.W., Bartel B. Auxin: regulation, action and interaction. Ann. Bot. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Peng Y., Guo L., Li C. Root colonization of encapsulated Klebsiella oxytoca Rs-5 on cotton plants and its promoting growth performance under salinity stress. Eur. J. Soil Biol. 2014;60:81–87. [Google Scholar]
- Yue H., Mo W., Li C., Zheng Y., Li H. The salt stress relief and growth promotion effect of Rs5 on cotton. Plant Soil. 2007;297(1):139–145. [Google Scholar]