Midline destructive lesion (MDL), also known as “lethal midline granuloma”, represents a fibroinflammatory condition characterized by relentless erosion of the nose, paranasal sinuses, and palate [1–2]. Disease attribution remains elusive for many cases, which continue to be labelled “idiopathic” [2].
Four patients, two males and two females, were referred because of progressive erosion of midline facial structures that had occurred over 2–3 years. Their median age was 40 years (range: 27–54). Presenting symptoms included chronic sore throat, difficulty swallowing (nasal regurgitation), and progressive nasalization of speech. Tissue destruction developed insidiously in all four patients, initially as small ulcerative lesions of the soft palate and nasal septum that enlarged gradually into perforations of palatal and nasal structures. Spontaneous loss of the uvula occurred in three patients. Three had palatal prostheses assembled in order to prevent the passage of food into the nasopharynx (Figure 1A-B). All four developed saddlenose deformities.
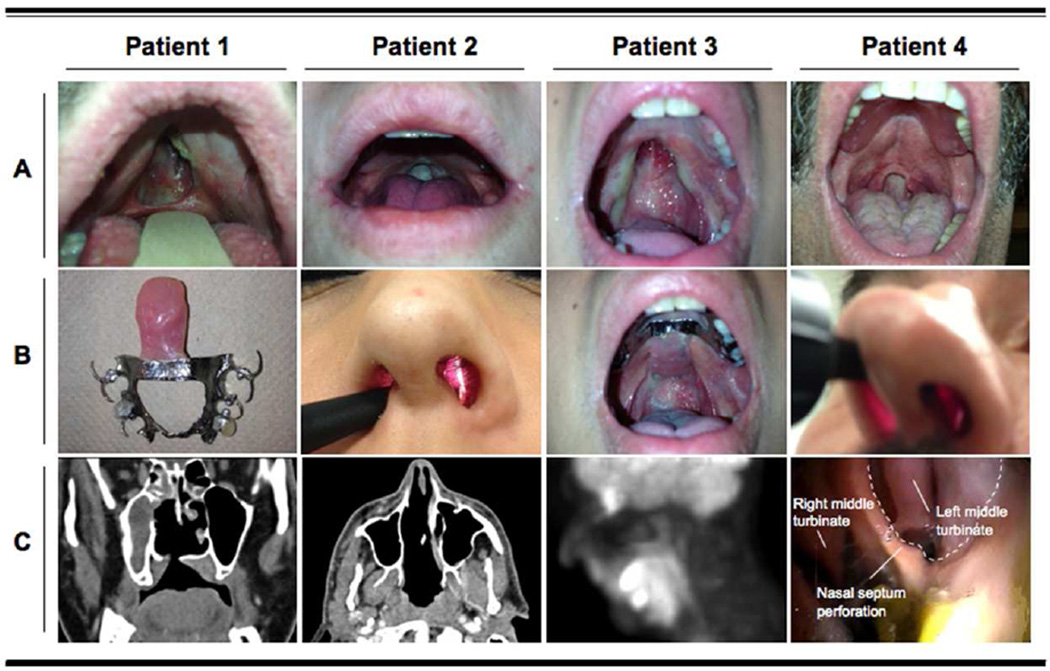
Figure 1. Clinical and radiological features of IgG4-related midline destructive lesion in four patients.
A. Soft palate erosion with uvula re-absorption.
B. Nasal septum perforations (Patients 2 and 4) and the palatal prostheses used by Patients 1 and 3.
C. Computed tomography scan of the head and neck, showing destruction of the hard palate with communication between the oral and nasal cavities (Patient 1), and erosion of the nasal septal cartilage, ethmoid bone, and vomer (Patient 2). A positron emission tomography study revealed 18fluoro-deoxyglucose uptake in the soft palate (Patient 3). Naso-laryngoscopy showing nasal septal perforation (Patient 4).
Two patients had histories of allergic rhinitis, chronic sinusitis, and recurrent ear infections since childhood. One of those also reported bilateral symmetric submandibular enlargement and the other had a history of cholangitis and multiple biliary duct strictures. All patients were active, heavy tobacco smokers. One reported cocaine inhalation, but had not used cocaine for six years before the onset of her symptoms. Physical examination was normal except for palatal and nasal septal defects, absent uvulae, and saddlenose deformities. Nasolaryngoscopic evaluation revealed erosions of the nasal septum and soft palate, confirmed by computed tomography (Figure 1C).
The differential diagnosis in these patients initially focused on malignancies (extranodal NK/T cell lymphoma, sarcomas, and carcinomas) and chronic infections (mycobacterial, fungal, spirochetal). We also considered granulomatosis with polyangiitis, relapsing polychondritis, sarcoidosis, and cocaine-induced MDL [2–3]. These alternative diagnoses were excluded by negative assays for antineutrophil cytoplasmic antibodies and other autoantibodies, serological tests for syphilis, toxicology screens, and cultures for fungi and acid-fast bacilli. Serum IgG4 concentrations were normal in all cases.
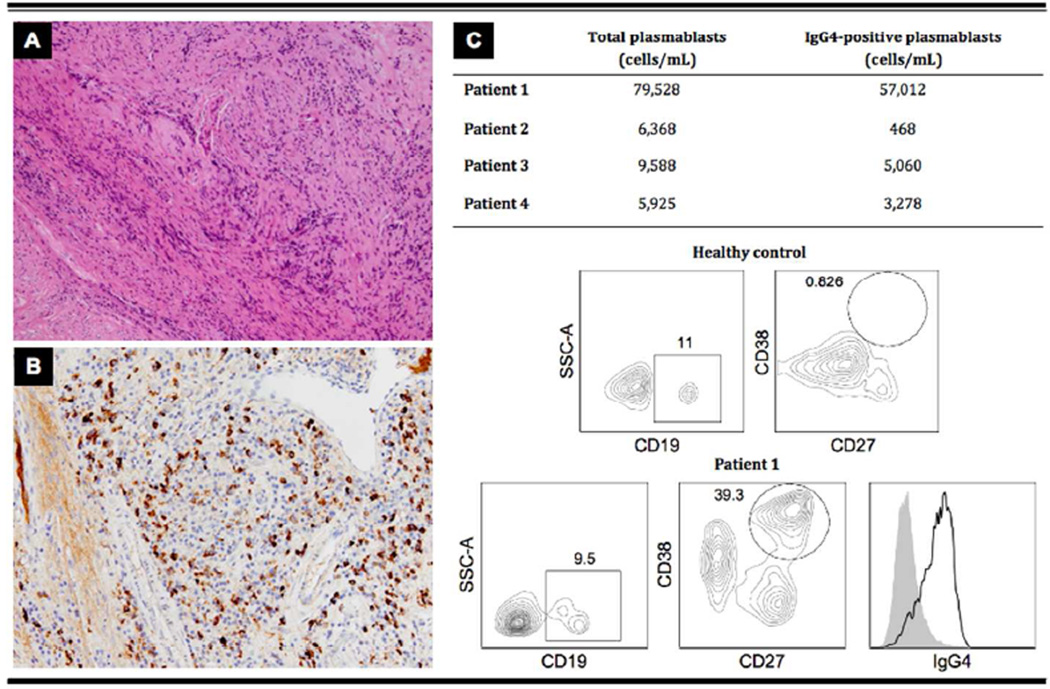
All four patients had histopathological evidence of IgG4-related disease (IgG4-RD). Biopsies from the edges of the palatal perforations revealed lymphoplasmacytic infiltrates and storiform fibrosis (Figure 2A). Immunohistochemistry showed abundant IgG4-positive plasma cells (>50/HPF; IgG4/IgG range: 30–60%)(Figure 2B). No cellular atypia, microabscesses, leukocytoclastic vasculitis, fibrinoid necrosis, granulomatous inflammation, or multinucleated giant cells were observed [2–3]. In situ hybridization for the Epstein-Barr encoding region, immunohistochemistry for cytoplasmic CD3, kappa and lambda chains, and PCR for T cell receptor, all performed in two of the patients, revealed no clonal lymphoprolipherative disorders. All four patients had dramatic oligoclonal expansions of circulating CD19+CD27+CD38+IgG4+ plasmablasts, a cell population recently described as an IgG4-RD biomarker (Figure 2C) [4,5].
Figure 2. Histological and immunopathological features of IgG4-related midline destructive lesion (MDL).
Biopsy from the edges of the palatal perforation of Patient 1.
A. A lymphoplasmacytic infiltrate surrounded by storiform fibrosis (magnification ×200).
B. The number of IgG4-positive plasma cells/high power field was 100; the ratio of IgG4 to total IgG in the tissue was > 0.4 (magnification ×400).
C. Plasmablasts were identified by flow cytometry as cells positive for CD19, CD27, and CD38. A majority of these plasmablasts also expressed surface IgG4. Data from a healthy control and Patient 1 are representative of the 14 healthy donors and the 4 patients with IgG4-related MDL, respectively. Data are expressed in percentages.
One patient was treated with multiple regimens of antibiotic and antifungal therapies together with mycophenolate mofetil (1500 mg daily) for one year with no improvement. Rituximab (1g at day 0 and 15) stabilized the disease for several months. A similar evolution was noted in a second patient who received a two month trial of prednisone (60 mg/day), gradually tapered over two months. One year later, the patient lost the superior teeth necessary for anchoring the palate prosthesis.
IgG4-RD is a fibroinflammatory disorder characterized by tumefactive lesions. Hallmark pathology features are lymphoplasmacytic infiltrates with abundant IgG4-positive plasma cells, storiform fibrosis, obliterative phlebitis, and tissue eosinophilia [6]. Over the past decade, IgG4-RD has been recognized as a cause of lesions in nearly every organ system and identified as a unifying diagnosis for multiple conditions previously regarded as separate entities [7,9]. These cases of IgG4-related MDL presented with tissue destructive lesions, which have been described in other patients with IgG4-RD involving the aorta, middle ear, sinuses, and other organs. The management for such patients remains unclear but likely involves a combination of immunosuppression to control inflammation and surgery to close mucosal lesions and improve oronasopharyngeal function. The optimal time for surgery ideally corresponds to the absence of active inflammation and, thus, peripheral plasmablast measurements may be useful in following disease activity.
In summary, we demonstrate that a proportion of cases of idiopathic MDL are manifestations of IgG4-RD. Distinguishing this entity from the other conditions known to cause MDL is essential because of the important differences in the management approaches.
Acknowledgments
Funding info: This study was funded by grants AI 064930 and AI 076505 from the NIH and a pilot grant from the Harvard Institute of Translational Immunology supported by the Helmsley Foundation
Footnotes
Licence for Publication: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in ARD and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in our licence (http://group.bmj.com/products/journals/instructions-for-authors/licence-forms).
Competing Interests: None declared. The authors have no conflicts of interest and no conflicts of publication to declare.
Contributorship: EDT and JHS conceived of and conducted the clinical studies. HM and VSM conducted laboratory studies with guidance from SP. DK and PS helped with clinical diagnosis and management of patients. VD helped with pathologic diagnosis of patients. EDT and JHS drafted the manuscript. All authors read and approved the final version.
Ethical approval information: this study involves human subjects and was approved by the Ethics Committee/Institutional Review Board of the Massachusetts General Hospital (Partners).
Data sharing statement: there are no additional unpublished data from this study available.
REFERENCES
- 1.Borges A, Fink J, Villablanca P, et al. Midline destructive lesions of the sinonasal tract: simplified terminology based on histopathologic criteria. Am J Neuroradiol. 2000;21:331–336. [PMC free article] [PubMed] [Google Scholar]
- 2.Parker NP, Pearlman AN, Conley DB, et al. The dilemma of midline destructive lesions: a case series and diagnostic review. Am J Otolaryngol. 2010;31:104–109. doi: 10.1016/j.amjoto.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Trimarchi M, Gregorini G, Facchetti F, et al. Cocaine-induced midline destructive lesions: clinical, radiographic, histopathologic, and serologic features and their differentiation from Wegener granulomatosis. Medicine (Baltimore) 2001;80:391–404. doi: 10.1097/00005792-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Mattoo H, Mahajan VS, Della-Torre E, et al. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol. 2014 Sep;134(3):679–687. doi: 10.1016/j.jaci.2014.03.034. PMID: 24815737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015 Jan;74(1):190–195. doi: 10.1136/annrheumdis-2014-205233. PMID: 24817416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181–1192. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 7.Stone JH, Khosroshahi A, Deshpande V, et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012;64:3061–3067. doi: 10.1002/art.34593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamisawa T, Zen Y, Pillai S, et al. IgG4-related disease. Lancet. doi: 10.1016/S0140-6736(14)60720-0. (invited review) [DOI] [PubMed] [Google Scholar]
- 9.Della Torre E, Bozzolo EP, Passerini G, et al. IgG4-related pachymeningitis: evidence of intrathecal IgG4 on cerebrospinal fluid analysis. Ann Intern Med. 2012;156:401–403. doi: 10.7326/0003-4819-156-5-201203060-00025. [DOI] [PubMed] [Google Scholar]