Abstract
Chronic stress is implicated as a risk factor for Alzheimer's disease (AD) and other neurodegenerative disorders. While the specific mechanisms linking stress exposure and AD vulnerability have yet to be fully elucidated, our lab and others have shown that acute and repeated restraint stress in rodents leads to an increase in hippocampal tau phosphorylation (tau-P) and tau insolubility, a critical component of tau pathology in AD. Tau phosphorylation induced by a psychological stressor is reversible and is thought to be dependent on intact signaling through the type 1 corticotropin-releasing factor receptor, but how sex steroids or other modulators may also modulate this effect are unknown. A naturally occurring attenuation of stress response is observed in female rats at the end of pregnancy and throughout lactation. To test the hypothesis that decreased sensitivity to stress during lactation modulates stress-induced tau-P, cohorts of virgin, lactating, and weaned female rats were subjected to 30 minutes of restraint stress or no stress (control), and were sacrificed at 20 minutes or 24 hours after the episode. Exposure to restraint stress induced a significant decrease in tau-P in the hippocampus of lactating rats sacrificed 20 minutes after stress compared to lactating controls and virgins subjected to stress treatment. Lactating rats sacrificed 24 hours after exposure to restraint stress showed a significant increase in tau-P compared to the restraint-stressed lactating rats sacrificed only 20 minutes after stress exposure, expressing phosphorylation levels similar to control animals. Further, GSK3-α levels were significantly decreased in stressed lactating animals at both timepoints. This suggests a steep, yet transient stress-induced dephosphorylation of tau, influenced by GSK3, in the hippocampus of lactating rats.
Keywords: Alzheimer's disease (AD), stress, lactation, corticotropin-releasing, tau, hippocampus, GSK3, steroid, prolaction
Introduction
Stress is implicated as a risk factor for the development of Alzheimer's disease (AD), a neurodegenerative disorder defined pathologically by the accumulation of extracellular beta-amyloid (Aβ) plaques and intracellular neurofibrillary tangles comprised of hyperphosphorylated tau (tau-P) aggregates. The role of maternal and ovarian hormones in the development or prevention of neurodegenerative diseases like AD has become a subject of intense study as certain hormones seem to demonstrate neuroprotective and neurogenic qualities. For example, during lactation, remarkable adaptations occur in the female brain, including attenuation of the hypothalamic-pituitary-adrenal (HPA) axis to stress and changes in hippocampal plasticity (1-3). The observed increase in plasticity can be influenced by hormonal fluctuations that occur during lactation in conjunction with suckling stimulation from the litter (4).
Studies have found that over-activation of the HPA axis results in decreased hippocampal neurogenesis, increased neurodegeneration, and increased cognitive impairment (5-7). Stress-inducing environmental factors can play a role in AD development and, more specifically, can induce tau-P (7-14). Studies show increased tau-P in rodents subjected to cold water stress, and cognitive deficits as a result of excess glucocorticoid (stress hormone) exposure (9,11). Moreover, a single exposure to restraint, an emotional stressor, leads to a significant increase in tau-P in the rodent hippocampus, with repeated exposures to restraint stress or CRF overexpression resulting in cumulative increases in an insoluble, potentially pathogenic form of tau-P (13-15). These studies implicate the corticotropin-releasing-factor pathway (CRF) is mechanistically involved in stress-induced tau-P as this phenomenon was not observed in mice with pharmacologic blockade or genetic knockout of CRF receptor 1 (CRFR1) (7,13,14).
The physiological changes that occur during pregnancy and lactation may confer neuroprotection against excitotoxins, such as kainic acid, and lead to a decreased sensitivity to stress (16,17). The morphological and functional changes in the maternal brain occur not only in areas that support lactation, but also in areas of learning and memory such as the CA1 region of the hippocampus (2,3) and areas related to neurogenesis such as the subventricular zone and the dentate gyrus (18,19). Reproduction also facilitates learning and memory and decreases the prevalence of neuronal markers of aging (20). Potentially due to fluctuations of maternal hormones (i.e. prolactin, progesterone, and estrogen) during pregnancy and lactation, and increasing evidence of their effect on the hippocampus, learning, and memory, the number of studies aiming to determine the relationship of these hormones to neurodegeneration and AD pathology is rapidly growing. For example, in rodents, prolactin has been shown to decrease anxiety, prevent stress-induced decreases in neurogenesis (21, 22), and diminish excitotoxic cell damage in the hippocampus (23). In the hippocampus of AD mice, progesterone significantly reduces tau-P and estrogen prevents Aβ accumulation (24). The mechanisms by which these hormones regulate tau and Aβ are not fully understood; however, these studies suggest that maternal hormones work to attenuate the stress response and could potentially play a role in preventing AD pathology.
Lactation is a reproductive condition in which the circadian fluctuation of corticosterone is abolished but basal levels are chronically elevated (1). Thus, a more in-depth understanding of the mechanisms by which lactation reduced sensitivity to stress may further elucidate the specific circuitry and cellular mechanisms which influence AD vulnerability. Here we address the impact of the reproductive condition on stress-induced tau-P and major tau kinases (GSK, ERK, and JNK) in female rats.
Materials and methods
Animals
Adult (250-300g), virgin or pregnant female Wistar rats were housed individually under controlled temperature and lighting conditions (12-h:12-h light:dark cycle, lights on at 06:00 h), with food and water available ad libitum. One day after parturition, litter sizes were culled to 8-10 pups. Mothers and their litters were undisturbed until postpartum days 14-19 (lactating rats) or one month after weaning at day 22 (weaned rats). Vaginal smears of virgin and weaned female rats were followed for at least three estrous cycles, and they were stressed or handled in the diestrous phase of the cycle (virgin rats). The Institutional Animal Care and Use Committee of the Institute for Neurobiology at the UNAM approved all experimental protocols. Animals were handled in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Acute Restraint Stress
Rats were placed in acrylic restrainers with ventilation for 30 minutes, and were sacrificed either 20 minutes or 24 hours after the restraint episode. Control rats were handled similarly, but were not subjected to any stress episodes. N=5 rats were included in each group.
Western blot analysis
One set of rats was euthanized by decapitation, and brains were immediately removed and hippocampus was collected, immediately frozen on dry ice and stored at −70°C until assay. Tissues were homogenized in radio-immunoprecipitation assay (RIPA) buffer as previously described (14,25). 15ug of protein was separated on 10% SDS-PAGE and transferred to nitrocellulose membrane at 100V for fifty minutes. Nonspecific binding was blocked by incubating membranes in 5% milk-PBS-T for thirty minutes. Membranes were incubated with primary antibodies diluted in 5% milk-PBS-T overnight at 4°C. The primary antibodies were detected by anti-mouse or anti-rabbit HRP-linked secondary antibodies for one hour (1:2500; EMD Biosciences, La Jolla, CA) and developed using an enhanced chemiluminescence Western blot detection kit (Supersignal West Pico; Pierce Biotechnology). Quantitative band intensity readings were obtained using the NIH ImageJ software. All readings were normalized to internal standard band intensity readings.
Corticosterone and prolactin determination
Trunk blood was collected to determine serum corticosterone and prolactin levels in groups of rats either from control or stress treatment at 20 min after 30 min-restraint (N=5). Serum was separated by centrifugation (10 min at 10,000 rpm) and stored at −20° C until assay. Corticosterone was determined using immunoassay kits (Catalog No. ADI-900-097 Enzo Life Sciences, Farmingdale, NY) that employed a competitive immunoassay for quantitative determinations. The optical density of the samples and controls from the standard curve were read immediately in a microplate reader at 450 nm (Bio Rad, Hercules, CA, USA). For prolactin determination, microtiter plates were coated overnight with 10 ng of rat PRL (NHPP-NIH). Serial dilutions of standard rat PRL (0.06–64 ng/ml) in TPBS were incubated for 16 h with 100 μl primary anti-rPRL polyclonal antiserum (1:40,000; NHPP-NIH) in TPBS containing 1% (w/v) non-fat dry milk (Bio-Rad). Samples and standards (100 μl) were then added to the coated wells and incubated for 2 h at room temperature. Secondary goat anti-rabbit IgG peroxidase conjugate (Bio-Rad) was added (1:3000 in TPBS with 1% non-fat dry milk) and incubated for 2 h at room temperature. Bound secondary antibodies were detected by reaction with 2,20 azino-di-(3-ethylbenzothiazoline sulfonate) substrate (Roche, Mannheim, Germany). Plates were read in a microplate reader at 450 nm.
Antibodies
Two well-characterized antibodies were used to probe for specific phosphorylated residues on rat hippocampal tau: S202/T205 (AT8, 1:500; Pierce Biotechnology) and S396/404 (PHF-1, 1:1000; gift from Dr. P. Davies, Albert Einstein College of Medicine, Bronx, NY). Kinase expression and activity was assessed using antibodies specific to total glycogen synthase kinase-3 (GSK-3β, 1:1000; BD Biosciences, San Diego, CA), inactive GSK-3 (pS9, 1:1000; Cell Signaling Technology, Danvers, MA), active GSK-3 (pY216, 1:1000; BD Biosciences), mitogen-activated protein kinases [extracellular signal-regulated kinases 1 and 2 (ERK1/2)], 1:500; Cell Signaling Technology] and phosphorylated c-Jun-N-terminal kinase (pJNK, 1:1000; Cell Signaling Technology). Actin (1:2000; Sigma-Aldrich) was used as a protein-loading control.
Immunohistochemistry
Another set of rats were anesthetized with a lethal dose of urethane (1.5-2 g/kg bw, ip), perfused with 4% paraformaldehyde and processed for immunohistochemistry as previously described (13). Frozen sections were cut into 30 μm thick slices on a sliding microtome and stored at −20 °C in antifreeze until use. PHF-1 (1:5000) was used to probe for tau-P on free-floating sections of rat hippocampus. 0.3% hydrogen peroxide was used to quench endogenous peroxidases and 0.1% sodium borohydride was used to reduce reactive free aldehydes. The staining reaction was carried out using a nickel-enhanced DAB Peroxidase Substrate kit (Vector Laboratories).
Statistical analyses
Integrated intensity readings from Western blots were analyzed using either a one- or two-way ANOVA using R and GraphPad Prism software; pair comparison was estimated by t-test. Resultant data were plotted on bar graphs, with data expressed as mean ± SEM percentage of control values.
Results
Basal levels of tau-P and tau kinase expression in the hippocampus of female rats at various reproductive stages
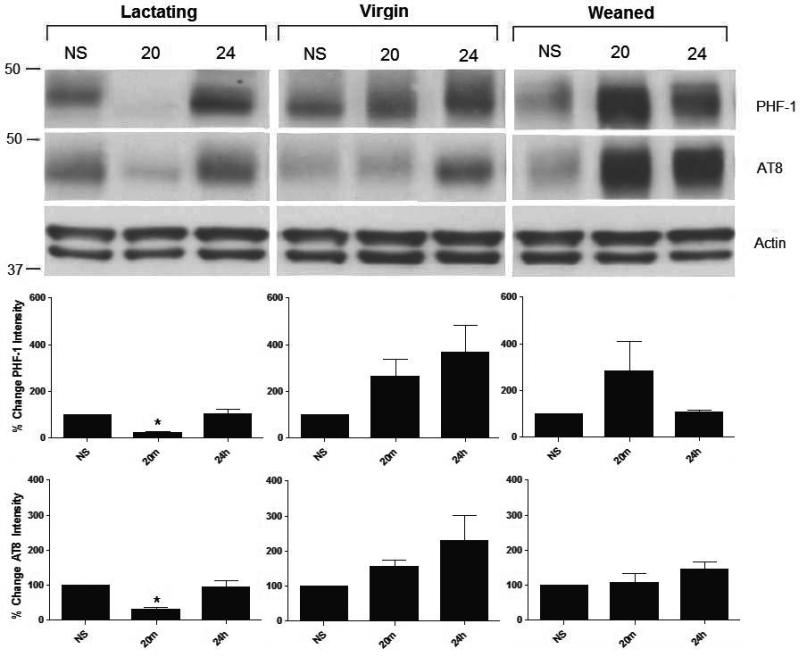
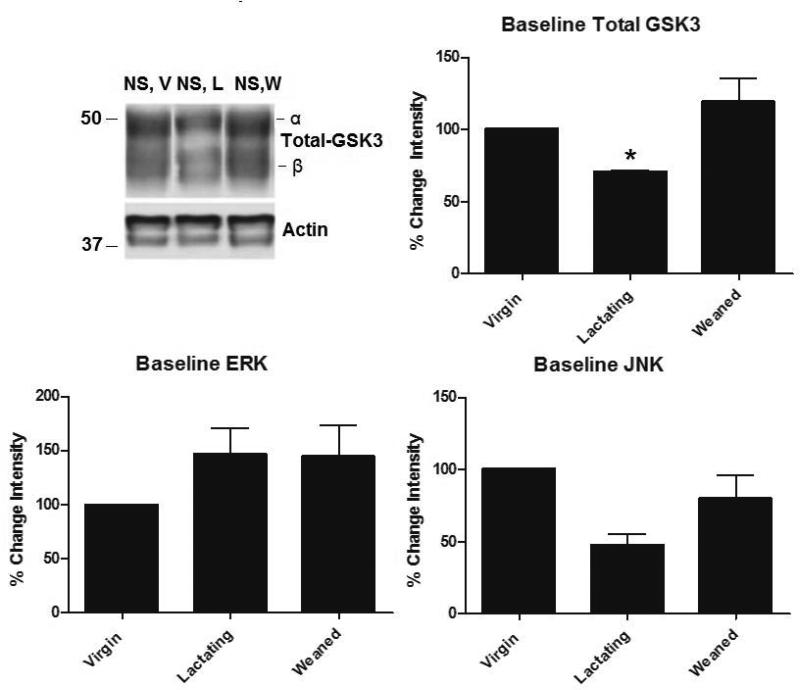
Western blot analysis was used to determine the effects of stress on hippocampal tau-P and tau kinases in female rats at two well-characterized phospho-epitopes, AT8 and PHF-1.Control animals showed no significant baseline differences in tau-P at the AT8 and PHF-1 phosphorylation sites throughout all reproductive stages studied (Figure 1). However, as shown in Figure 2, non-stressed lactating control animals express significantly lower levels of total GSK3-α (F (7,28)= 42.4, p<0.001) compared to weaned (p= 0.016) and virgin (p = 0.008) non-stressed controls. There were no significant differences in ERK or JNK levels across all controls (F (7,28)= 32.1, p>0.05) (Figure 2).
Figure 1. Acute stress-induced hippocampal tau dephosphorylation in lactating rats.
Female rats that were virgin, lactating (14-19 postpartum), or weaned (21 days postpartum)(N=5, each) were sacrificed after either no stress (NS), 20 minutes (20) or 24 hours (24) following a single restraint episode. Lactating rats had significantly reduced levels of stress-induced tau-P at both PHF-1 and AT8 epitopes compared to virgin (p=0.008) and weaned rats (p=0.008); however, tau-P returned to control/basal levels after 24 hours. Data are presented as percent change ± SEM.
Figure 2. Western blot analysis of baseline kinase levels in unstressed female rats.
We assessed differences in major tau kinases in female rat hippocampus under basal conditions (not stressed) in virgin (V), lactating (L), or weaned (W) (N=5, each), Western blot analysis was performed using antibodies specific to GSK3, ERK, and JNK. We found that NS lactating rats have significantly less hippocampal GSK3-α compared to both non-stressed virgin and weaned rats (p = 0.016, p = 0.016). There were no significant differences in ERK or JNK. Data are presented as percent change from the V condition ± SEM.
Effects of stress on tau-P in the hippocampus of lactating rats
Western blot analysis was used to determine the effects of stress on hippocampal tau-using AT8 and PHF-1. As shown in Figure 1, 20-minutes following restraint stress exposure, lactating rats showed significantly lower levels of tau-P at both the AT8 (F (7,28)= 42.4, p=0.0008) (p = 0.032) and PHF-1 (F (7,28)= 2.5, p=0.042) (p = 0.03) epitopes compared to lactating controls. This significant change was also observed when compared to stressed virgin (p = 0.008) and weaned (p = 0.008) rats as shown in Figure 1. 24 hours following stress, tau-P levels in lactating rats at the PHF-1 site significantly increased (p = 0.008) compared to the levels observed at the 20-minute, returning to near-control levels. No significant changes in tau-P levels were observed in virgin or weaned rats in response to stress.
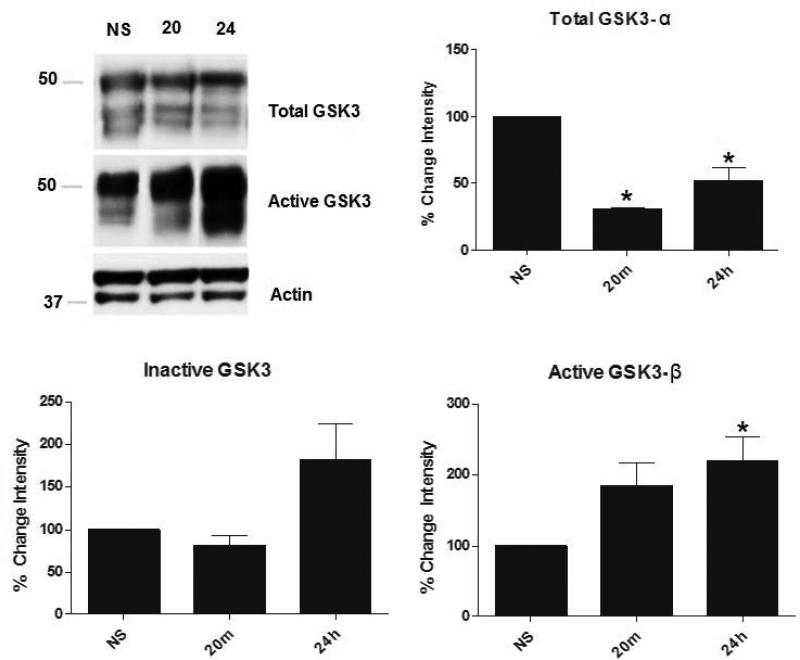
To elucidate possible mechanisms of this stress-induced dephosphorylation of tau, changes in several known tau kinases, such as GSK3, were investigated using Western blot analysis. As shown in Figure 3, overall levels of total GSK3-α significantly decreased in lactating rats compared to lactating controls (F (7,28)= 42.4, p<0.001) (p = 0.008) twenty minutes following stress, and these levels remained low twenty-four hours after stress exposure (p = 0.008). Interestingly, active GSK3-β significantly increased (F (7,28)= 17.61, p<0.0001) in lactating rats twenty-four hours after stress exposure compared to lactating controls (p = 0.016), while there were no significant changes in active GSK3-α or inactive GSK3(α or β) in stress-exposed lactating animals compared to lactating controls. This significant drop in GSK3 twenty minutes following stress could play a key role in the stress-induced dephosphorylation of hippocampal tau observed in restraint-stressed lactating rats.
Figure 3. GSK3 levels in control and stressed lactating rats.
Lactating rats were sacrificed after no stress (NS), or 20 minutes (20) or 24 hours (24) (N=5, each) following acute restraint stress. Twenty minutes after stress, lactating rats had significantly lower levels of total hippocampal GSK3-α compared to their controls (p=0.008) and these low levels were sustained 24 hours later (p=0.008). However, 24 hours following stress, active GSK3-β levels increased compared to control (p=0.016), although active GSK3-α levels stayed relatively the same. There were no significant changes in inactive GSK3 (both α and β) levels. Data are presented as percent change from NS condition ± SEM.
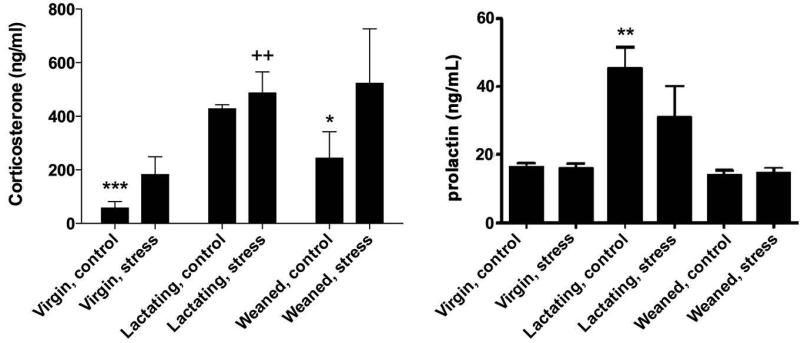
Analysis of corticosterone levels in response to restraint by ELISA showed that basal level of corticosterone was higher (F (2,22)= 5.43, p=0.01) in lactating than in the virgin (p<0.0001; Fig. 4) and the weaned rats (p=0.0377). Because of the variation of data, increases in the concentration of corticosterone caused by stress were not detected in virgin and weaned rats (3-fold and 2-fold of corresponding control, respectively). However, a tendency to increase after stress was detected in virgin rats (p=0.0787 vs control). Corticosterone concentration in lactating rats was equally high independently of the treatment, control or stress. For prolactin, higher concentration was detected in lactating animals, and no change was induced by stress (F (1,24)= 1.57, p=0.22) (Figure 4).
Figure 4. Corticosterone and prolactin plasma concentration in control and stressed rats.
Basal corticosterone and prolactin levels were increased in lactating rats compared to virgin and weaned cohorts (N=5, each), and were unchanged in response to restraint stress. Restraint-virgin rats showed a slight increase in corticosterone but it was not significantly different from control. Values represent mean ± SEM; for corticosterone graph: ***p< 0.0001, *p=0.0377 vs lactating control; ++p=0.0092 vs virgin stress; for prolactin graph: **p< 0.001 vs virgin and weaned controls. Data are presented as percent change ± SEM.
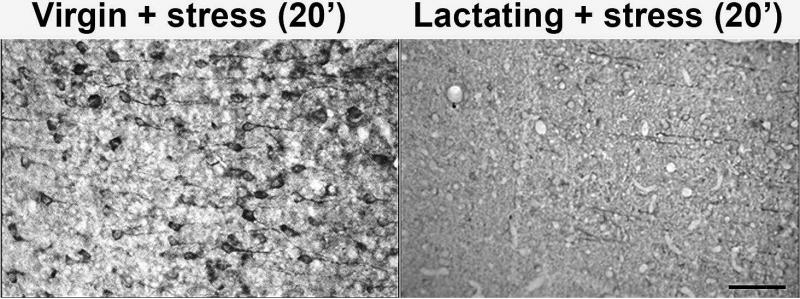
Distribution of tau-P in the female rat hippocampus
Immunohistochemical staining of the hippocampi of virgin and lactating rats was used to assess qualitative differences in tau-P (PHF-1 epitope) intensity and localization in non-stressed controls (not shown) and in animals twenty minutes following stress. Figure 5 shows representative micrographs of the CA3 region of the hippocampus which demonstrate a decrease in somatic tau-P (possibly a shift from soma to dendrites/axons) in lactating rats exposed to restraint stress in comparison to virgin cohorts. The overall decrease in tau-P in stressed lactating rats supports our biochemical data.
Figure 5. Localization of tau-P in the CA3 region of stressed virgin or lactating rat hippocampus.
30um thick sections of the hippocampus were stained with PHF-1 to visualize the localization of tau-P in stressed virgin and lactating rats twenty minutes following stress (N=5, each). This 20x magnification of CA3 pyramidal neurons demonstrates a lack of increase in somatic staining in stressed lactating animals (right) compared to stressed virgin animals (left). Unstressed controls (not shown) had no appreciable tau-P signal. Stressed lactating animals did not significantly differ in tau-P levels compared to unstressed controls (p>0.05). Bar = 100μm.
Discussion
Stress-induced dephosphorylation of tau in the hippocampus of lactating rats
While previous studies demonstrate significant increases in hippocampal tau-P following both acute and chronic restraint stress in male mice (13,14), the current study reveals a stress-induced dephosphorylation of tau in the hippocampus of lactating rats, a change that is not observed in virgin or one-month weaned rats. A number of studies have indicated that females respond differently to stress compared to males, that the hormones involved in lactation help to confer a higher tolerance to stress regulated by the HPA axis, and that these hormones grant neuroprotection that extends to many regions of the brain including the hippocampus (4,17). Our current tau kinase data suggest that a significant decrease in both the α and β isoforms of total GSK3 may play a role in the stress-induced decrease in tau phosphorylation observed in lactating rats exposed to restraint stress. Since maternal hormones are known to play a role in stress response during pregnancy and lactation, it may be possible that one or more of these hormones are responsible for the alteration in total GSK3 levels in response to stress.
Maternal hormones involved in lactation and stress
During lactation, the maternal brain shows a significantly reduced response to stress, a phenomenon that is possibly due to chronically elevated corticosterone levels (1). Basal plasma levels of corticosterone were higher in lactating than in virgin and weaned rats. No significant rise was detected in response to stress, but virgin rats exhibited a trend toward increase. This lack of statistical change may be related to the timing of plasma sampling, which was performed at sacrifice (50 min post-stress). A number of other hormones, such as prolactin, oxytocin and ovarian hormones, show increased expression during either pregnancy or lactation, and may play crucial roles in the attenuated stress response that is observed.
Prolactin is involved in milk production and is primarily produced in the anterior pituitary during lactation, but prolactin receptors have been previously documented in the brain (26). Prolactin is involved in maternal behavior, the sleep-wake cycle, grooming, sexual behavior, and stress coping (26). Animals treated with prolactin demonstrate an attenuated HPA-axis response to stress, but the mechanism by which prolactin is involved in the overall anxiolytic effect is not fully understood (27). In the present study, we found a stress-induced dephosphorylation of tau in conjunction with a decrease in both the α and β isoforms of total GSK3 activity. Furthermore, previous studies have documented relationships between a hyperprolactinemic stage and this tau kinase. One study suggests that GSK3 can work as a prolactin receptor kinase that tags the receptor for degradation, while another describes a pathway that involves prolactin acting to increase Akt activity, which in turn inhibits GSK3 activity (28, 29). It may be that increased prolactin levels during lactation result in decreased GSK3 activity (via an Akt pathway) in order to preserve prolactin receptors during lactation (a critical time period) and could perhaps be one explanation for the decreased baseline levels of GSK3 in lactating rats. However, additional studies are required to determine how stress-induced prolactin activity could cause a large enough decrease in GSK3 activity to cause a significant dephosphorylation of hippocampal tau.
Oxytocin, another hormone present at elevated levels during lactation, facilitates social behavior, maternal bonding and milk ejection, and is known to decrease the corticosterone stress response, decrease glucocorticoid receptor expression, stimulate hippocampal neurogenesis, and modulate behavioral responses to stress (30). In the hippocampus, oxytocin has been shown to influence a number of signaling molecules, to modulate synaptic transmission, and to increase adult hippocampal neurogenesis, even under stressful conditions (31-33). Since oxytocin has such a large influence in the hippocampus during stress and lactation, further work is needed to determine if it plays a role in the stress-induced decrease in hippocampal GSK3 and tau-P seen in lactating rats.
The ovarian hormone progesterone regulates reproduction, the release of neurotransmitters, and neuronal development. Progesterone is present at constant plasma concentrations throughout pregnancy and lactation, until levels drop on postpartum day 18, and has been shown to have a neuroprotective effect in the nervous system (34). Progesterone administration has been shown to decrease tau-P and increase the dephosphorylated form of tau in the hippocampus of ovarectomized rats, but does not affect GSK3 phosphorylation (35). Another study found that chronic progesterone treatment increased total hippocampal tau in ovariectomized rats, but this study did not address tau phosphorylation (36). Interestingly, progesterone increases tau phosphorylation at the PHF-1 epitope in rat cerebellum associated with an increase in GSK3 activity, and decreases PP2A activity in the hippocampus of ovariectomized rats (34,37). These findings suggest a role for progesterone in regulating tau-P and associated kinases; however, these seemingly conflicting studies demonstrate the need for further research on the specific mechanisms by which progesterone regulates these proteins.
The lactation period in the mother has been well established as neuroprotective (16), and previous studies have shown that GSK3 inhibition increases neuroprotection by inhibiting β-catenin and tau-P, modulating apoptotic pathways, promoting neurogenesis, and improving cognitive deficits in models of neurodegenerative diseases like AD and Parkinson's disease (38,39). Our understanding of the role of GSK3 in the cell is continually expanding and broadening, and it has been established that it not only regulates glycogen synthase activity but also plays a role in a large number of other processes in the cell. It is possible that one of the mechanisms by which lactation confers neuroprotection to the mother is by inhibiting GSK3 activity (via regulation by some maternal/ovarian hormone), thereby inhibiting stress-induced tau phosphorylation and apoptosis, and promoting other neuroprotective mechanisms like neurogenesis.
Hormones play a complex role in the female brain throughout adolescence, motherhood, and senescence, including menopause. While hormones like prolactin and oxytocin are important for lactation, their actions in the brain and throughout the body have a much larger impact than just milk production and ejection. Maternal and ovarian hormones are known to have significant anxiolytic effects in response to stress and, in some cases, to increase adult neurogenesis. Furthermore, lactation is considered an enriched environment due to new tasks exteroceptive stimuli from the pups, such as olfactory, auditory, tactile, and visual signals in addition to the neurogenic stimulation provided by suckling. The neuroplasticity of the female brain also changes as the female goes through different reproductive stages, and tau is thought to play a role in the changes in brain circuitry that occur as part of this plasticity. The dephosphorylation of tau seen in stressed lactating rats may be a neuroprotective effect driven by elevated hormone levels and pup's signaling. Functional adaptations in the female brain during lactation include many forms of stress-attenuation both behaviorally and molecularly, but the extent to which these changes affect the future development of neurological diseases like AD remains unclear. Some studies suggest that pregnancy is a risk factor for AD due to decreased levels of estrogen compared to nulliparous women (40, 41). It may be that the neuroprotection conferred by lactation only lasts as long as the lactation period itself, as suggested by the data from our weaned animals. However, further research including studies focused on chronic stress, multiple maternities, and how these variables affect behavior and neuropathological markers, such as neurofibrillary tangles and amyloid-beta plaque formation, are needed to better our understanding of the female brain, stress response, and how these specific circuitries play a role in the development of neurodegenerative diseases like AD.
Acknowledgements
This work was supported by a grant from UC MEXUS – CONTACYT to R.A.R and T.M. and NIH AG032755, AG047484, AG010483 and the Alzheimer's Association (R.A.R.) and UNAMDGAPA-PAPIIT IN202315 (T.M.). We thank Dr. Michael C. Donohue for statistical assistance, Louise Monte for assistance with manuscript preparation, MSc. Martha Carranza for assistance with ELISA, and Dr. Martín García and Dr. Alejandra Castilla for animal care assistance. Importantly, the authors thank Dr. Peter Davies (Albert Einstein) for donating antibody PHF-1.
References
- 1.Lightman SL, Windle RJ, Wood SA, Kershaw YM, Shanks N, Ingram CD. Peripartum plasticity within the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2001;133:111–129. doi: 10.1016/s0079-6123(01)33009-1. [DOI] [PubMed] [Google Scholar]
- 2.Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Marcus LK, Hearon C, Meyer EA, Hester N, Morgan M, Kozub FJ, Lambert KG. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav. 2006;49(2):131–142. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Pawluski JL, Galea LA. Hippocampal morphology is differentially affected by reproductive experience in the mother. J Neurobiol. 2006;66(1):71–81. doi: 10.1002/neu.20194. [DOI] [PubMed] [Google Scholar]
- 4.Cabrera V, Ramos E, González-Arenas A, Cerbón M, Camacho-Arroyo I, Morales T. Lactation reduces glial activation induced by excitotoxicity in the rat hippocampus. J Neuroendocrinol. 2013;25:519–527. doi: 10.1111/jne.12028. [DOI] [PubMed] [Google Scholar]
- 5.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurogeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 6.McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 7.Carroll JC, Iba M, Bangasser DA, Valentino RJ, James MJ, Brunden KR, Lee VM, Trojanowski JQ. Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. J. Neuro. 2011;31(40):14436–14449. doi: 10.1523/JNEUROSCI.3836-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korneyev AY. Stress-induced tau phosphorylation in mouse strains with different brain ERK 1 + 2 immunoreactivity. Neurochem Res. 1998;23:1539–1543. doi: 10.1023/a:1020980004539. [DOI] [PubMed] [Google Scholar]
- 9.Feng Q, Cheng B, Yang R, Sun FY, Zhu CQ. Dynamic changes of phosphorylated tau in mouse hippocampus after cold water stress. Neurosci Lett. 2005;388:13–16. doi: 10.1016/j.neulet.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic psychological distress and risk of Alzheimer's Disease in old age. Neuroepidemiology. 2006;27:143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- 11.Sotiropoulos I, Catania C, Pinto LG, Silva R, Pollerberg GE, Takashima A, Sousa N, Almeida OFX. Stress acts cumulatively to precipitate Alzheimer's Disease-like tau pathology and cognitive deficits. J. Neurosci. 2011;31(21):7840–7847. doi: 10.1523/JNEUROSCI.0730-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson L, Guo X, Hällström T, Norton MC, Waern M, Ostling S, Bengtsson C, Skoog I. Common psychosocial stressors in middle aged women related to longstanding distress and increased risk of Alzheimer's disease: a 38-year longitudinal population study. BMJ Open 2013. 2013;3(9):e003142. doi: 10.1136/bmjopen-2013-003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rissman RA, Lee K, Vale W, Sawchenko PE. Corticotropin-releasing factor receptors differentially regulate stress-induced tau phosphorylation. J. Neurosci. 2007;27(24):6552–6562. doi: 10.1523/JNEUROSCI.5173-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rissman RA, Staup MA, Lee AR, Justice NJ, Rice KC, Vale W, Sawchenko PE. Corticotropin-releasing factor receptor-dependent effects of repeated stress on tau phosphorylation, solubility, and aggregation. Proc Natl Acad Sci USA. 2012;109(16):6277–6282. doi: 10.1073/pnas.1203140109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell SN, Zhang C, Monte L, Roe AD, Rice KC, Tache Y, Masliah E, Rissman R. Increased tau phosphorylation and aggregation in the hippocampus of mice overexpressing conrticotropin-releasing factor. J Alzheimers Dis. 2015;43(3):967–976. doi: 10.3233/JAD-141281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morales T. Recent findings on neuroprotection against excitotoxicity in the hippocampus of female rats. J Neuroendocrinol. 2011;23(11):994–1001. doi: 10.1111/j.1365-2826.2011.02141.x. [DOI] [PubMed] [Google Scholar]
- 17.Slattery D, Neumann I. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J Physiol. 2008;586.2:377–385. doi: 10.1113/jphysiol.2007.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuta M, Bridges RS. Gestation-induced cell proliferation in the rat brain. Brain Res Dev Brain Res. 2005;156(1):61–66. doi: 10.1016/j.devbrainres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Leuner B, Mirescu C, Noiman L, Gould E. Maternal experience inhibits the production of immature neurons in the hippocampus during the postpartum period through elevations in adrenal steroids. Hippocampus. 2007;17(6):434–442. doi: 10.1002/hipo.20278. [DOI] [PubMed] [Google Scholar]
- 20.Gatewood JD, Morgan MD, Eaton M, McNamara IM, Stevens LF, Macbeth AH, Meyer EA, Lomas LM, Kozub FJ, Lambert KG, Kinsley CH. Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Res Bull. 2005;66(2):91–98. doi: 10.1016/j.brainresbull.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Torner L, Toschi N, Pohlinger A, Landgraf R, Neumann ID. Anxiolytic and anti-stress effects of brain prolactin: improved efficacy of anti-sense targeting of the prolactin receptor by molecular modeling. J Neurosci. 2001;21(9):3207–3214. doi: 10.1523/JNEUROSCI.21-09-03207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blume A, Torner L, Liu Y, Subburaju S, Aguilera G, Neumann ID. Prolactin activates mitogen-activated protein kinase signaling and corticotropin releasing hormone transcription in rat hypothalamic neurons. Endocrinology. 2008;150(4):1841–1849. doi: 10.1210/en.2008-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morales T, Lorenson M, Walker AM, Ramos E. Both prolactin (PRL) and a molecular mimic of phosphorylated PRL, S179D-PRL, protect the hippocampus of female rats against excitotoxicity. Neuroscience. 2014;258:211–217. doi: 10.1016/j.neuroscience.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in Female 3xTg-AD mice. J Neurosci. 2007;27(48):13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roe AD, Staup MA, Serrats J, Sawchenko PE, Rissman RA. Lipopolysaccharide-induced tau phosphorylation and kinase activity--modulation, but not mediation, by corticotropin-releasing factor receptors. Eur J Neurosci. 2011;34(3):448–456. doi: 10.1111/j.1460-9568.2011.07764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr Rev. 1996;17:639–669. doi: 10.1210/edrv-17-6-639. [DOI] [PubMed] [Google Scholar]
- 27.Torner L, Neumann ID. The Brain Prolactin System: Involvement in stress response adaptations in lactation. Stress. 2002;5(4):249–257. doi: 10.1080/1025389021000048638. [DOI] [PubMed] [Google Scholar]
- 28.Dominguez-Caceres MA, Garcia-Martinez JM, Calcabrini A, Gonzalez L, Porque PG, Leon J, Martin-Perez J. Prolactin induces c-Myc expression and cell survival through activation of Src/Akt pathway in lymphoid cells. Oncogene. 2004;23:7378–7390. doi: 10.1038/sj.onc.1208002. [DOI] [PubMed] [Google Scholar]
- 29.Plotnikov A, Li Y, Tran TH, Tang W, Palazzo JP, Rui H, Fuchs SY. Oncogene-mediated inhibition of glycogen synthase kinase 3-B impairs degradation of prolactin receptor. Cancer Res. 2008;68:1354–1361. doi: 10.1158/0008-5472.CAN-07-6094. [DOI] [PubMed] [Google Scholar]
- 30.Cohen H, Kaplan Z, Kozlovsky N, Gidron Y, Matar MA, Zohar J. Hippocampal Microinfusion of oxytocin attenuates the behavioral response to stress by means of dynamic interplay with the glucocorticoid-catecholamine responses. J Neuroendo. 2010;22:889–904. doi: 10.1111/j.1365-2826.2010.02003.x. [DOI] [PubMed] [Google Scholar]
- 31.Petersson M, Uvnas-Moberg K. Systemic oxytocin treatment modulate glucocorticoid and mineralicorticoid receptor mRNA in the rat hippocampus. Neurosci Lett. 2003;343(2):97–100. doi: 10.1016/s0304-3940(03)00334-3. [DOI] [PubMed] [Google Scholar]
- 32.Zaninetti M, Raggenbass M. Oxytocin receptor agonists enhance inhibitory synaptic transmission in the rat hippocampus by activating interneurons in stratum pyramidale. Eur J Neurosci. 2002;12(11):3975–3984. doi: 10.1046/j.1460-9568.2000.00290.x. [DOI] [PubMed] [Google Scholar]
- 33.Leuner B, Caponiti JM, Gould E. Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus. 2012;22(4):861–868. doi: 10.1002/hipo.20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amorim MA, Guerra-Araiza C, Pernia O, da Cruz e Silva EF. Garcia-Segura ML. Progesterone regulates the phosphorylation of protein phosphotases in the brain. J Neuro Res. 2010;88:2826–2832. doi: 10.1002/jnr.22442. [DOI] [PubMed] [Google Scholar]
- 35.Pinto-Almazan R, Calzada-Mendoza CC, Campos-Lara MG, Guerra-Araiza C. Effect of chronic administration of estradiol, progesterone, and tibolone on the expression and phosphorylation of glycogen synthase kinase-3B and the microtubule-associated protein tau in the hippocampus and cerebellum of the female rat. J Neuro Res. 2012;90:878–886. doi: 10.1002/jnr.22808. [DOI] [PubMed] [Google Scholar]
- 36.Camacho-Arroyo I, Gonzalez-Arena A, Espinosa-Raya J, Pina-Medina AG, Picazo O. Short- and long-term treatment with estradiol or progesterone modifies the expression of GFAP, MAP2 and Tau in prefrontal cortex and hippocampus. Life Sci. 2011;89:123–128. doi: 10.1016/j.lfs.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Guerra-Araiza C, Amorim MA, Camacho-Arroyo I, Garcia-Segura LM. Effects of progesterone and its reduced metabolites, dihydroprogesterone and tetrahydroprogesterone, on the expression and phosphorylation of glycogen synthase kinase-3 and the microtubule-associated protein tau in the rat cerebellum. Dev Neurobiol. 2007;67(4):510–520. doi: 10.1002/dneu.20383. [DOI] [PubMed] [Google Scholar]
- 38.Culbert AA, Brown MJ, Frame S, Hagen T, Cross DAE, Bax B, Reith AD. GSK-3 inhibition by adenoviral FRAT1 overexpression is neuroprotective and induces tau dephosphorylation and B-catenin stabilisation without elevation of glycogen synthase activity. Febs Letters. 2001;507(3):288–294. doi: 10.1016/s0014-5793(01)02990-8. [DOI] [PubMed] [Google Scholar]
- 39.King MK, Pardo M, Cheng Y, Downey K, Jope RS, Beurel E. Glycogen Synthase Kinase-3 Inhibitors: Rescuers of Cognitive Impairments. Pharmacol Ther. 2014;141(1):1–12. doi: 10.1016/j.pharmthera.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colucci M, Cammarata S, Assini A, Croce R, Clerici F, Novello C, Mazzella L, Dagnino N, Mariani C, Tanganelli P. The number of pregnancies is a risk factor for Alzheimer's disease. Eur J Neurol. 2006;13(12):1374–1377. doi: 10.1111/j.1468-1331.2006.01520.x. [DOI] [PubMed] [Google Scholar]
- 41.Ptok U, Barkow K, Heun R. Fertility and number of children in patients with Alzheimer's disease. Arch Womens Ment Health. 2002;5(2):83–86. doi: 10.1007/s00737-002-0142-6. [DOI] [PubMed] [Google Scholar]