Abstract
The present study aimed to investigate the effect of ZnO nanoparticles on alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) enzyme expressions in C2C12 cells. ZnO nanoparticles are widely used in the several cosmetic lotions and other biomedical products. Several studies report on ZnO nanoparticle mediated cytotoxicity. However, there are no reports on the effect of ZnO nanoparticles on ALT, AST, ALP and LDH enzyme expressions in C2C12 cells. A cytotoxicity assay was carried out to determine the effect of ZnO nanoparticles (1–5 mg/ml) on C2C12 cell viability at 48 and 72 h. ZnO nanoparticles increased ALT, AST, ALP and LDH enzyme mRNA expression and their activities in C2C12 cells. In conclusion, the present study showed that ZnO nanoparticles increased these enzyme activities and its mRNA expression in C2C12 cells in a dose-dependent manner.
Keywords: C2C12 cells, Antioxidant, ZnO nanoparticles, Cytotoxicity, Enzymes, mRNA
1. Introduction
Nanoparticles are key scientific tools that have been used in several biotechnological and pharmacological fields. Nanostructural ZnO has been used in the various biomedical applications in the modern world. Metal oxide nanoparticles are believed to be safe for applications because they are more stable and with salient properties (Zhao and Castranova, 2011). Titanium dioxide (TiO2) and ZnO are widely used as UV blockers in cosmetic lotions (Becheri et al., 2008). ZnO nanoparticle increases antibacterial activity (Nagarajan and Rajagopalan, 2008), and it has been used in cotton fabric, rubber and food packaging industries (Qun et al., 2007). Oral and intra-peritoneal administration of ZnO nanoparticles showed its distribution in the mice liver, spleen, kidneys and adipose tissue (Li et al., 2012). It is systemically absorbed, which elevates the zinc level in the liver, adipose tissue and pancreas (Umrani and Paknikar, 2014). However, the cytotoxicity of nanoparticles, and its interaction with the biological system is still unclear (Snyder-Talkington et al., 2012).
C2C12 cells are mouse myoblast cells obtained from C3H mice. C2C12 cells are useful to study the expression of various proteins, and to explore mechanistic pathways. In addition, they are useful to study the differentiation of myoblast and osteoblast (Yaffe and Saxel, 1977). Our previous studies reported the cytotoxicity of ZnO nanoparticles on antioxidant enzyme activities and mRNA expression in the co-cultured C2C12 and 3T3-L1 cells (Muthuraman et al., 2015), and the dose-dependent effect of ZnO nanoparticles on oxidative stress, and antioxidant enzyme activity in adipocytes (Muthuraman et al., 2014). However, there are no reports on the effect of ZnO nanoparticles on the ALT, AST, ALP and LDH expressions in C2C12 cells. Therefore, the study is unique, and helps to understand the cytotoxicity of ZnO nanoparticle in C2C12 cells.
2. Materials and methods
ZnO nanoparticles (35 nm particle size) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Laboratory wares were purchased from Falcon Lab ware (Becton-Dickinson, Franklin Lakes, NJ, USA). C2C12 cells (mouse, muscle) were purchased from ATCC.
2.1. Cell culture
C2C12 cells were incubated at a density of 9000 cells/cm2 and grown in DMEM containing 10% FBS and 1% penicillin/streptomycin at 37 °C in 5% CO2. Confluent C2C12 cells (3rd passage) were induced to differentiate with a standard differentiation medium consisting of DMEM supplemented with 2% horse serum and 1% antibiotics. Cultures were re-fed every 2 days to allow 90% cells to reach complete differentiation. Cells with differentiation medium were considered as the control. Differentiated cells with ZnO nanoparticles were considered as treatment.
2.2. Sulforhodamine B (SRB) assay
SRB assay is widely used for the cell viability and proliferation The cytotoxic effect of ZnO nanoparticles on C2C12 cells was measured by the SRB assay (Vichai and Kirtikara, 2006). C2C12 cells were seeded at a density of 2.5 × 104 cells/well into 96-well plates, and allowed to adhere for 24 h at 37 °C. Cells were treated with ZnO nanoparticles at different concentrations (1–5 mg/ml) for 48 and 72 h. At the end of the treatment, cells were fixed with acetone, and air dried. After being fully dried, each well was added with 100% of SRB solution (0.4% w/v), and incubated for 3 h at room temperature. Microplate was washed with 1% of acetic acid, and then dried under a drying oven. Stained C2C12 cells were observed for morphology using inverted light microscope. Finally, 10 mM of Tris-base was added and kept incubation for overnight. Following complete dissolution of SRB in Tris-base, the absorbance was measured at 540 nm.
2.3. Enzyme activities
ALT and AST are transaminase enzymes. It is measured by kit (Biovision, Life Science) method and expressed as units/liter (Waller-Evans et al., 2013). These enzymes catalyze reversible transamination and products were measured at 570 nm. ALP enzyme assay kit (Abcam, ab83369) uses p-nitrophenyl phosphate (pNPP) as a substrate which turns yellow measured at 405 nm (Grog and Pearse, 1952). LDH enzyme activity is determined by measuring the absorbance at 340 nm resulting from the oxidation of NADH. One unit represents the oxidation of one micromole of NADH/minute at 25 °C and pH 7.3 (Hess et al., 1958).
2.4. qRT-PCR
Total RNA was isolated from all the samples (Chomczynski and Mackey, 1995). The first-strand c-DNA was synthesized from 1 μg of the total RNA using the M-MLV reverse transcriptase with the anchored oligo d(T)12–18 primer. qPCR was performed using a cDNA equivalent of 10 ng of total RNA from each sample with primers specific for ALT (forward:5′-TTCAAGCAGAGAGACAGGAG-3′, reverse: 5′-TGAGGGAAGGAATACATGG-3′), AST (forward:5′-TGTTCAGCTTCACTGGGTTG-3′, reverse: 5′-CCCAGTCCTGGTAAATGTGG-3′), ALP (forward: 5′-CCAACTCTTTTGTGCCAGAGA-3′, reverse: 5′-GGCTACATTGGTGTTGAGCTTTT-3′), LDH (forward:5′-ATCCAGACTCCTGTTGCCCATTCA-3′, reverse: 5′-TTCGCCCTTGAGTTTGTCCTCCAT-3′) and a housekeeping gene GAPDH (forward: 5′-GGTCACCAGGGCTGCTTTT-3′, reverse: 5′-ATCTCGCTCCTGGAAGATGGT-3′). The reaction was carried out in 10 μl using SYBR Green Master Mix (Bioneer) according to the manufacturers’ instructions. Relative ratios were calculated based on the 2−△△CT method (Pfaffl, 2001). PCR was monitored with the use of Mini Opticon Real Time PCR System (Bio-Rad).
2.5. Statistical analysis
All values were expressed as mean ± SEM. Statistical analysis was done using SPSS 17. The statistical significance of differences between means was assessed by an ANOVA and Student’s t-test. Differences between control and treated were calculated. p < 0.05 was found to be to be significant.
3. Results
3.1. Cytotoxicity
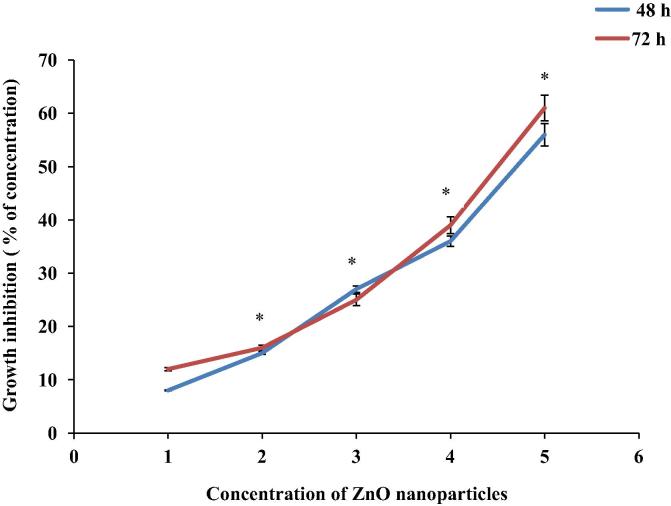
C2C12 cells incubated with different concentrations (1–5 mg/ml) of ZnO nanoparticles for 48 and 72 h, showed a dose-dependent effect that was evident from the SRB assay (Fig. 1). ZnO nanoparticles would be toxic to normal cells at 5 mg/ml. Therefore, 2, 3 and 4 mg/ml of ZnO nanoparticles were used for further investigations.
Figure 1.
Effects of various concentrations of ZnO nanoparticles on cell viability. C2C12 cells were seeded at seeding densities of 2.5 × 104 cells/ml into 96 well plates and treated with 1–5 mg/ml of ZnO nanoparticles at 48 and 72 h. Values are expressed as mean ± SEM, n = 6. Viability is expressed as percentage of cell survival relative to aspartame untreated control (cell viability = 100%). ∗p < 0.05.
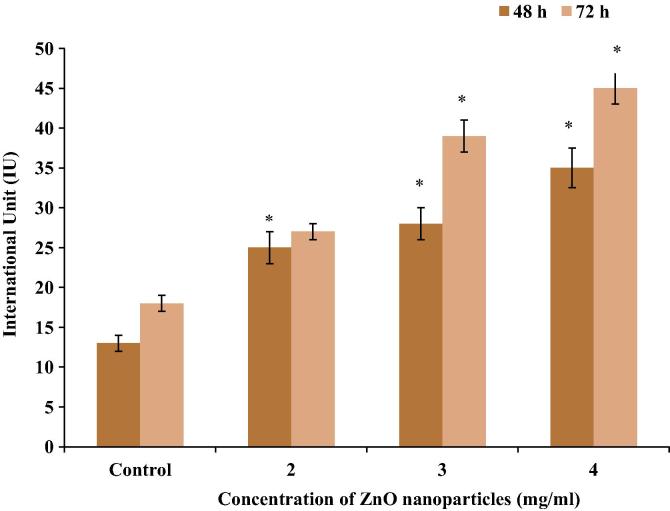
3.2. ALT
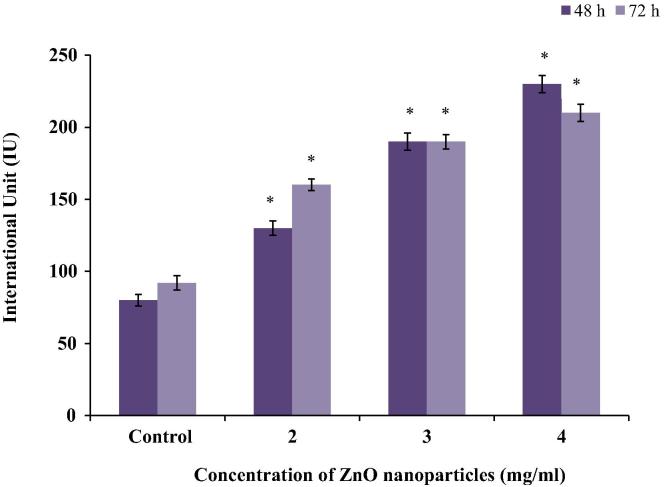
C2C12 cells exposed to different concentrations (2, 3 and 4 mg/ml) of ZnO nanoparticles, increased ALT enzyme activity by 92.3%, 115.4% and 169.2% at 48 h, whereas by 50%, 116.7%, 150% at 72 h, respectively (Fig. 2).
Figure 2.
Measurement of ALT enzyme activity in the C2C12 cells exposed to different concentrations of ZnO nanoparticles. Data represent mean ± SEM, ∗p < 0.05.
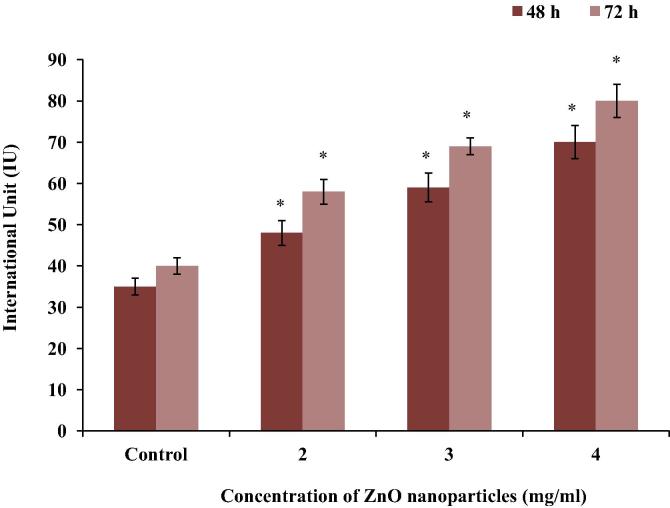
3.3. AST
C2C12 cells exposed to different concentrations (2, 3 and 4 mg/ml) of ZnO nanoparticles, increased AST enzyme activity by 45%, 70% and 125% at 48 h, and by 38.5%, 73%, 107.7% at 72 h, respectively (Fig. 3).
Figure 3.
Measurement of AST enzyme activity in the C2C12 cells exposed to different concentrations of ZnO nanoparticles. Data represent mean ± SEM, ∗p < 0.05.
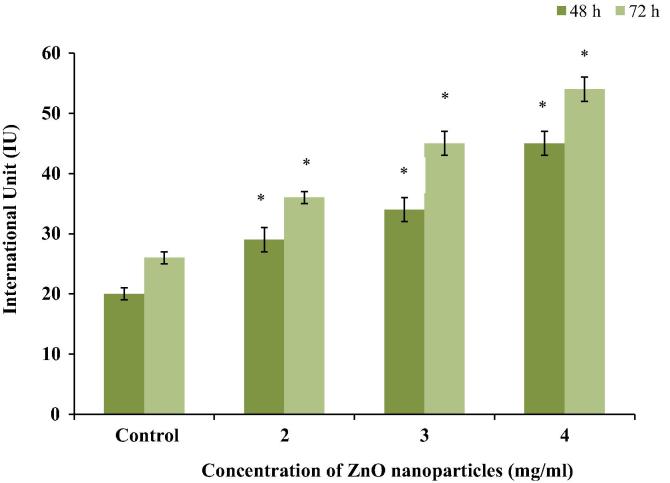
3.4. ALP
C2C12 cells exposed to different concentrations (2, 3 and 4 mg/ml) of ZnO nanoparticles, increased ALP enzyme activity by 37.1%, 68.6% and 100% at 48 h, whereas by 45%, 72.5%, 91% at 72 h, respectively (Fig. 4).
Figure 4.
Measurement of ALP enzyme activity in the C2C12 cells exposed to different concentrations of ZnO nanoparticles. Data represent mean ± SEM, ∗p < 0.05.
3.5. LDH
C2C12 cells exposed to different concentrations (2, 3 and 4 mg/ml) of ZnO nanoparticles, increased LDH enzyme activity by 62.5%, 137% and 187.2% at 48 h, and by 73.9%, 106.3%, 128.3% at 72 h, respectively (Fig. 5).
Figure 5.
Measurement of LDH enzyme activity in the C2C12 cells exposed to different concentrations of ZnO nanoparticles. Data represent mean ± SEM, ∗p < 0.05.
3.6. mRNA expression
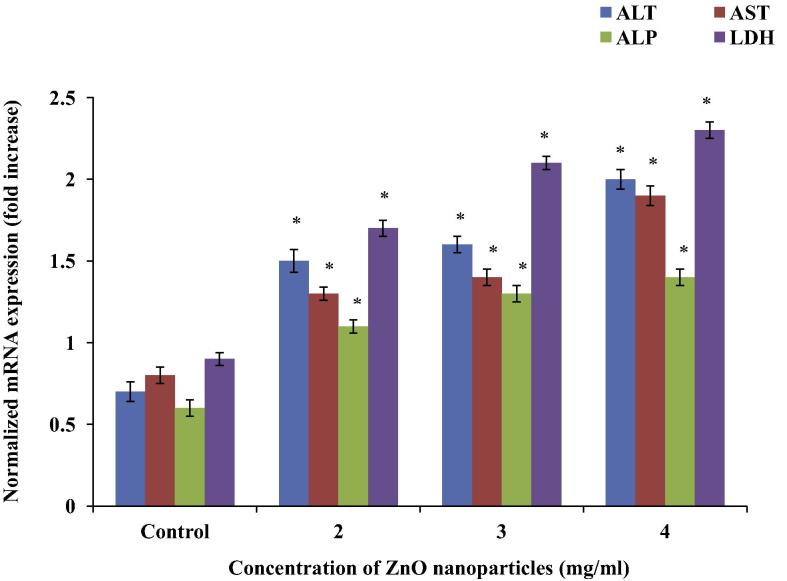
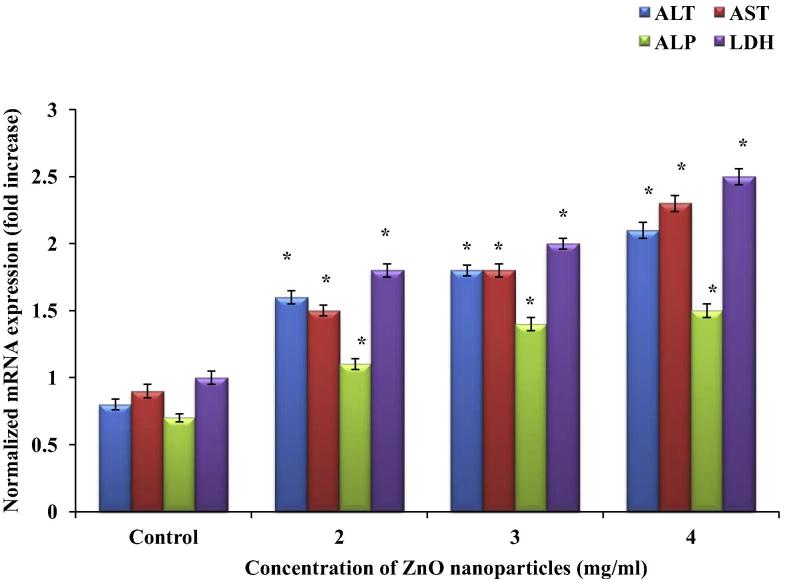
C2C12 cells exposed to 2, 3 and 4 mg/ml of ZnO nanoparticles for 48 & 72 h showed significant changes in ALT, AST, ALP and LDH mRNA expressions in a dose-dependent manner. ALT mRNA expression was increased 1.14, 1.28 and 1.85-fold at 48 h, and 1, 1.25 and 1.62 at 72 h, respectively. AST mRNA expression was increased by 0.62, 0.75 and 1.37-fold at 48 h, and 0.4, 1 and 1.55-fold at 72 h, respectively. ALP mRNA expression was increased by 0.45, 1.17 and 1.33-fold at 48 h, and by 0.57, 1 and 1.14-fold at 72 h respectively. LDH mRNA expression was increased by 0.89, 1.33 and 1.55-fold at 48 h, and by 0.8, 1 and 1.5-fold at 72 h, respectively (Figure 6, Figure 7).
Figure 6.
Up-regulation of ALT, AST, ALP and LDH mRNA expression by ZnO nanoparticles in C2C12 cells at 48 h. Expressions of ALT, AST, ALP and LDH mRNA were related to GAPDH, and presented as fold. Relative expression values were normalized as mean ± SEM, n = 6. ∗p < 0.05.
Figure 7.
Up-regulation of ALT, AST, ALP and LDH mRNA expression by ZnO nanoparticles in C2C12 cells at 72 h. Expressions of ALT, AST, ALP and LDH mRNA were related to GAPDH, and presented as fold. Relative expression values were normalized as mean ± SEM, n = 6. ∗p < 0.05.
4. Discussion
ZnO nanoparticles have been widely used in food products and in biomedical applications (Van Tassel and Goldman, 2011). Copper oxide nanoparticles showed dose-dependent cytotoxicity and oxidative stress in the airway epithelial cells. TiO2 nanoparticles produced oxidative stress and apoptosis in animal cells (Shukla et al., 2013). Reduced zinc concentration are associated with several metabolic and antioxidant enzymes (Kumar et al., 2011). High level of zinc is essential for cells and zinc is a component of many enzymes and transcription factors (Boreiko, 2010). Alteration of cellular zinc homeostasis associated with loss of viability, oxidative stress and dysfunction of mitochondria (Kao et al., 2012). Deng et al. (2009) reported that the ZnO nanoparticles caused neural stem cell toxicity in the culture medium. pH-triggered intracellular release of ionic Zn2+ is responsible for the cytotoxicity of ZnO nanowires (Müller et al., 2010). The adipose tissue expansion and adipocyte enlargement are associated with oxidative stress (Gregor and Hotamisligil, 2007). ZnO nanoparticles administration in mice showed its tissue distribution in the liver, spleen, kidneys and adipose tissue (Li et al., 2012). Elevated zinc level in the liver, adipose tissue and pancreas following absorption (Umrani and Paknikar, 2014), induction of oxidative stress, DNA damage and cell death was also reported (Kumar et al., 2011).
Cytotoxicity of ZnO nanoparticles is due to their increased solubility. High concentration of metal oxide nanoparticles in the environment and food chain may affect human health (De Berardis et al., 2010). Increase of inflammation in the lymph nodes, the cells involved in the inflammatory reaction is due to nanoparticles (Qian, 2011, Su et al., 2009). Enzymatic peroxidation of fatty acids leads to the generation of the reactive oxygen species (Cohen et al., 2011). ROS is derived from mitochondria and endoplasmic reticulum (Wang and Joseph, 1999). The cells are affected when exposed to a higher concentration of reactive oxygen species (Dawei et al., 2010). ZnO nanoparticles are not toxic at low concentration, but at higher concentration increase ROS through increased MDA content (Syama et al., 2013). In our previous study, MDA content was altered significantly even at low concentrations of ZnO nanoparticles. LDH was present in adipose tissues of rat, and their distribution was significantly altered by metabolic stress (Moore and Yontz, 1969), increased LDH activity was reported in H1355 cells (Kao et al., 2012). Our results showed that the ZnO nanoparticles increase LDH activity in cells.
Cytotoxicity of ZnO nanoparticles is due to dissolution outside the cell. Our study demonstrates that the induction of oxidative stress is the vital part of the cytotoxicity of ZnO nanoparticles. In summary, ALT, AST, ALP and LDH enzyme mRNA expressions and their activities were significantly increased in a dose-dependent manner. The present study showed that the ZnO nanoparticles significantly produced cytotoxicity in C2C12 cells.
Acknowledgements
This paper was supported by the KU Research Professor Program of the Konkuk University.
Footnotes
Peer review under responsibility of King Saud University.
References
- Becheri A., Durr M., Nostro P.L., Baglioni P. Synthesis and characterization of zinc oxide nanoparticles: application to textiles as UV-absorbers. J. Nanopart. Res. 2008;10:679–689. [Google Scholar]
- Boreiko C.J. Overview of health risk assessments for zinc. J. Toxicol. Environ. Health A. 2010;73:166–174. doi: 10.1080/15287390903340427. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Mackey K. Short technical report. Modification of the TRIZOL reagent procedure for isolation of RNA from Polysaccharide- and proteoglycan-rich sources. Biotechniques. 1995;19:942–945. [PubMed] [Google Scholar]
- Cohen G., Riahi Y., Sasson S. Lipid peroxidation of poly-unsaturated fatty acids in normal and obese adipose tissues. Arch. Physiol. Biochem. 2011;117:131–139. doi: 10.3109/13813455.2011.557387. [DOI] [PubMed] [Google Scholar]
- Dawei A.I., Zhisheng W., Anguo Z. Protective effects of nano-ZnO on the primary culture mice intestinal epithelial cells in in vitro against oxidative injury. World J. Agric. Sci. 2010;6:149–153. [Google Scholar]
- De Berardis B., Civitelli G., Condello M., Lista P., Pozzi R., Arancia G., Meschini S. Exposure to ZnO nanoparticles induces oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol. Appl. Pharmacol. 2010;246:116–127. doi: 10.1016/j.taap.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Deng X., Luan Q., Chen W., Wang Y., Wu M., Zhang H., Jiao Z. Nanosized zinc oxide particles induce neural stem cell apoptosis. Nanotechnology. 2009;20:115101. doi: 10.1088/0957-4484/20/11/115101. [DOI] [PubMed] [Google Scholar]
- Gregor M.F., Hotamisligil G.S. Thematic review series: adipocyte biology adipocyte stress: the endoplasmic reticulum and metabolic disease. J. Lipid Res. 2007;48:1905–1914. doi: 10.1194/jlr.R700007-JLR200. [DOI] [PubMed] [Google Scholar]
- Grog E., Pearse A.G.E. Coupling azo dye methods for histochemical demonstration of alkaline phosphatase. Nature. 1952;170:578–579. doi: 10.1038/170578b0. [DOI] [PubMed] [Google Scholar]
- Hess R., Scarpeli D., Pearse A. The cytochemical localization of oxidative enzymes. II. Pyridine nucleotide linked dehydrogenase. J. Biophys. Biochem. Cytol. 1958;4:753–760. doi: 10.1083/jcb.4.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao Y.Y., Chen Y.C., Cheng T.J., Chiung Y.M., Liu P.S. Zinc oxide nanoparticles interfere with zinc ion homeostasis to cause cytotoxicity. Toxicol. Sci. 2012;125:462–472. doi: 10.1093/toxsci/kfr319. [DOI] [PubMed] [Google Scholar]
- Kumar A., Pandey A.K., Singh S.S., Shanker R., Dhawan A. Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic. Biol. Med. 2011;51:1872–1881. doi: 10.1016/j.freeradbiomed.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Li C.H., Shen C.C., Cheng Y.W., Huang S.H., Wu C.C., Kao C.C., Liao Y.W., Kang J.J. Organ biodistribution, clearance, and genotoxicity of orally administered zinc oxide nanoparticles in mice. Nanotoxicology. 2012;6:746–756. doi: 10.3109/17435390.2011.620717. [DOI] [PubMed] [Google Scholar]
- Moore R.O., Yontz F.D. Effect of thiamine deficiency in rats on adipose tissue lactate dehydrogenase isozyme distribution. J. Nutr. 1969;98:325–329. doi: 10.1093/jn/98.3.325. [DOI] [PubMed] [Google Scholar]
- Müller K.H., Kulkarni J., Motskin M., Goode A., Winship P., Skepper J.N., Ryan M.P., Porter A.E. PH-dependent toxicity of high aspect ratio ZnO nanowires in macrophages due to intracellular dissolution. ACS Nano. 2010;4:6767–6779. doi: 10.1021/nn101192z. [DOI] [PubMed] [Google Scholar]
- Muthuraman P., Ramkumar K., Kim D.H. Analysis of dose-dependent effect of zinc oxide nanoparticles on the oxidative stress and antioxidant enzyme activity in adipocytes. Appl. Biochem. Biotechnol. 2014;174:2851–2863. doi: 10.1007/s12010-014-1231-5. [DOI] [PubMed] [Google Scholar]
- Muthuraman P., Muthuviveganandavel V., Kim D.H. Cytotoxicity of zinc oxide nanoparticles on antioxidant enzyme activities and mRNA expression in the cocultured C2C12 and 3T3-L1 cells. Appl. Biochem. Biotechnol. 2015;175:1270–1280. doi: 10.1007/s12010-014-1351-y. [DOI] [PubMed] [Google Scholar]
- Nagarajan P., Rajagopalan V. Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Sci. Technol. Adv. Mater. 2008;9:035004. doi: 10.1088/1468-6996/9/3/035004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J.L. The surface properties and photocatalytic activities of ZnO ultrafine particles. Appl. Surf. Sci. 2011;180:308–314. [Google Scholar]
- Qun L., Shui-Lin C., Wan-Chao J. Durability of nano ZnO antibacterial cotton fabric to sweat. J. Appl. Polymer Sci. 2007;103:412–416. [Google Scholar]
- Shukla R.K., Kumar A., Gurbani D., Pandey A.K., Singh S., Dhawan A. TiO2 nanoparticles induce oxidative DNA damage and apoptosis in human liver cells. Nanotoxicology. 2013;7:48–60. doi: 10.3109/17435390.2011.629747. [DOI] [PubMed] [Google Scholar]
- Snyder-Talkington B.N., Qian Y., Castranova V., Guo N.L. New perspectives for in vitro risk assessment of multiwalled carbon nanotubes: application of co-culture and bioinformatics. J. Toxicol. Environ. Health B. 2012;15:468–492. doi: 10.1080/10937404.2012.736856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y.K., Peng S.M., Ji L.W., Wu C.Z., Cheng W.B., Liu C.H. Ultraviolet ZnO nanorod photosensors. Langmuir. 2009;26:603–606. doi: 10.1021/la902171j. [DOI] [PubMed] [Google Scholar]
- Syama S., Reshma S.C., Sreekanth P.J., Varma H.K., Mohanan P.V. Effect of zinc oxide nanoparticles on cellular oxidative stress and antioxidant defense mechanisms in mouse liver. Toxicol. Environ. Chem. 2013;95:495–503. [Google Scholar]
- Umrani D.R., Paknikar K.M. Zinc oxide nanoparticles show antidiabetic activity in streptozotocin-induced Types-1 and 2 diabetic rats. Nanomedicine. 2014;9:89–104. doi: 10.2217/nnm.12.205. [DOI] [PubMed] [Google Scholar]
- Van Tassel K.A., Goldman R. The growing consumer exposure to nanotechnology in everyday product: regulating innovative technologies in light of lessons from the past. Connect. Law Rev. 2011;44:481. [Google Scholar]
- Vichai V., Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nature Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- Waller-Evans H., Hue C., Fearnside J., Rothwell A.R., Lockstone H.E., Caldérari S., Wilder S.P., Cazier J.B., Scott J., Gauguier D. Nutrigenomics of high fat diet induced obesity in mice suggests relationships between susceptibility to fatty liver disease and the proteasome. PLoS One. 2013;8:e82825. doi: 10.1371/journal.pone.0082825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Joseph J.A. Structure-activity relationships of Quercetin in antagonizing hydrogen peroxide-induced calcium dysregulation in PC12 cells. Free Radic. Biol. Med. 1999;27:683–694. doi: 10.1016/s0891-5849(99)00119-7. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Zhao J., Castranova V. Toxicology of nanomaterials used in nanomedicine. J. Toxicol. Environ. Health B. 2011;14:593–632. doi: 10.1080/10937404.2011.615113. [DOI] [PubMed] [Google Scholar]