Abstract
Virtually all drug interventions that have been successful pre-clinically in experimental stroke have failed to prove their efficacy in a clinical setting. This could be partly explained by the complexity and heterogeneity of human diseases as well as the associated co-morbidities which may render neuroprotective drugs less efficacious in clinical practice. One aspect of crucial importance in the physiopathology of stroke which is not completely understood is neuroinflammation. At the present time, it is becoming evident that subtle, but continuous neuroinflammation can provide the ground for disorders such as cerebral small vessel disease. Moreover, advanced aging and a number of highly prevalent risk factors such as obesity, hypertension, diabetes and atherosclerosis could act as “silent contributors” promoting a chronic proinflammatory state. This could aggravate the outcome of various pathological entities and can contribute to a number of subsequent post-stroke complications such as dementia, depression and neurodegeneration creating a pathological vicious cycle. Moreover, recent data suggests that the inflammatory process might be closely linked with multiple neurodegenerative pathways related to depression. In addition, pro-inflammatory cytokines could play a central role in the pathophysiology of both depression and dementia.
Keywords: aging, stroke, neuroinflammation, comorbidities, depression, dementia
Introduction
Cerebrovascular diseases are one of the most prevalent health care problems in Europe. Total European cost of brain disorders in 2010 was 798 billion € of which 64.1 billion € was related to stroke alone. In many cases, the result of cerebrovascular disorders is a loss of independent living and secondary health problems affecting not only patients but also their families. The number of elderly people is increasing with a number of co-morbidities increasing the risk of cerebrovascular diseases. Thus, strategies in guiding patient selection and patient selection and novel preventive and neuroprotective therapies are urgently needed. Emerging evidence suggests that several diseases show overlapping mechanisms with neuroinflammation as one possible common pathway the leading to an increased risk of cerebrovascular neurological diseases.
Although neuropathological conditions differ in their aetielogy and in the way in which the inflammatory response is mounted, cellular and molecular mechanisms of neuroinflammation are probably similar in aging, hypertension, depression and cognitive impairment or after cerebral insults such as stroke (Allison and Ditor, 2014). Moreover, aging and a number of highly prevalent risk factors such as hypertension, diabetes and atherosclerosis are considered to act as “silent contributors” to neuroinflammation – not only establishing the condition as a central pathophysiological mechanism, but also constantly fuelling it (Figure 1). In this review, we describe the relationship between aging, comorbidities and neuroinflammation as the final link which aggravates the outcome of cerebrovascular diseases and precipitates the development of post-event subsequent complications including depression and neurodegenerative disorders.
Figure 1.
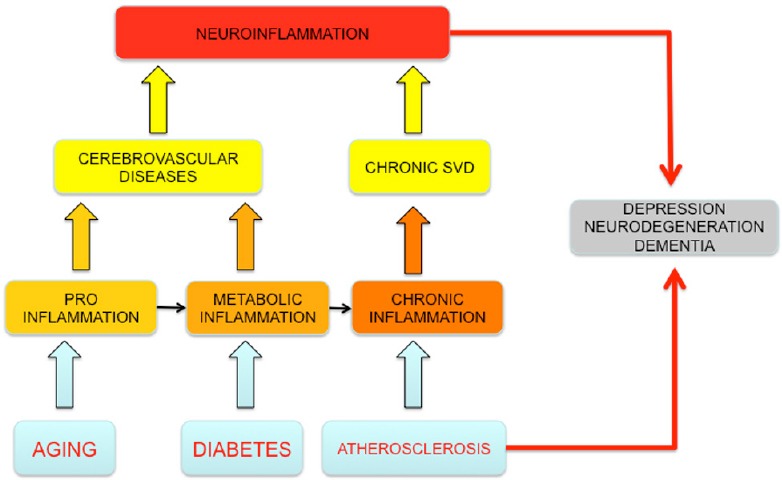
Neuroinflammation and comorbidities in central nervous system (CNS) pathologies.
Aging, comorbidities and neuroinflammation aggravates the outcome of cerebrovascular diseases and precipitates the development of post-event subsequent complications including depression and neurodegen-erative disorders. SVD: Small vessel disease.
Cerebral Small Vessel Disease (“Vascular Dementia”) and Neuroinflammation
In older individuals, inflammatory mechanisms have been linked to the pathogenesis of both dementia and functional impairment. Increasing evidence suggests that systemic and local neuroinflammation significantly contributes to cerebral small vessel disease (cSVD)–vascular dementia. For example, adhesion molecule serum levels are increased in patients with white matter lesions (de Leeuw et al., 2002). A relationship between inflammatory processes and cSVD may also be assumed since chronic inflammation plays an important role in hypertension which is the primary risk factor for cSVD (Schiffrin, 2014). One hypothesis is that these microvascular changes result in a state of chronic hypoperfusion leading to continuous oligodendrocyte death and consecutive degeneration of myelinated fibers. This may not only cause progressive white matter damage on a macroscopic scale, but also may foster the onset of inflammatory processes. Further, increased low-grade inflammation amplifies the risk of stroke (Shimizu et al., 2011). However, in a cross-sectional study investigating the possible association between biomarkers of systemic inflammation and functional status in older patients with late onset Alzheimer's disease and elderly patients with vascular dementia it was found that interleukin 6 (IL-6) plasma levels negatively correlated with vascular dementia (Zuliani et al., 2007).
Atherosclerosis and Chronic Inflammation
Atherosclerosis, a major risk factor for stroke and central nervous system (CNS) tissue destruction, is a disease of arteries characterized by vascular inflammation caused primarily by infiltrated monocytes into the injured vascular wall.
Several studies have suggested that inflammation may be important for accelerated progression of atherosclerosis. In a study investigating the association between inflammatory biomarkers and progression of intracranial large artery stenosis after ischemic stroke, it was found that in addition to traditional risk factors, circulating levels of IL-6 after stroke were associated with future intracranial large artery stenosis progression (Tousoulis et al., 2011). Likewise, it is widely accepted that in addition to other established cardiovascular risk factors, markers of inflammation such as C-reactive protein (CRP) is a strong predictor of subclinical and clinical atherosclerosis (Rizzo et al., 2009) and progression of hemorrhagic stroke (Di Napoli et al., 2012, 2014). Thus, in patients with hypertension, elevated CRP levels may predict clinical events. These patients also showed a significant relationship between clinical events and quintiles of CRP levels (Rizzo et al., 2009). Other studies have reported on pathological vicious cycles related to C-reactive protein and atherosclerosis. For example, elevated circulating levels of C-reactive protein independently predict the development of new plaques in older persons with carotid arteries free from atherosclerotic lesions (Molino-Lova et al., 2011; Shimizu et al., 2013).
Virtually all drug interventions that have been successful pre-clinically in experimental stroke have failed to demonstrate positive results in stroke patients. Our research as well as other group's studies indicate that ignoring the molecular characteristics of ageing and the associated co-factors present in clinical stroke results in disappointing results in clinical trials (Petcu et al., 2010; Murray et al., 2012; Buga et al., 2013; Popa-Wagner et al., 2014).
Studies conducted on aged rats have demonstrated that neurological impairment is more severe and functional recovery less successful than in young rats (Lindner et al., 2003; Buchhold et al., 2007; Popa-Wagner et al., 2011). Indeed, elderly individuals recover less well from stroke than young individuals (Manwani et al., 2011).
Stroke, Obesity and Neuroinflammation
Age represents the most important risk factor for stroke. Virtually all drug interventions that have been successful pre-clinically in experimental stroke have failed to demonstrate positive results in stroke patients.
Our research as well as other group's studies indicate that ignoring the molecular characteristics of ageing and the associated co-factors present in clinical stroke results in disappointing results in clinical trials (Petcu et al., 2010; Buga et al., 2013; Murray et al., 2013; Popa-Wagner et al., 2014).
Epidemiological studies have revealed an age-dependent increase of stroke susceptibility in men and women, with half of all strokes occurring in people over 75 years, and one third of cases in people over 85 years (Roger et al., 2012; Willey et al., 2012). Studies conducted on aged rats have demonstrated that neurological impairment is more severe and functional recovery less successful than in young rats (Lindner et al., 2003; Buchhold et al., 2007; Popa-Wagner et al., 2011). In addition, elderly individuals recover less well from stroke than young individuals (Manwani et al., 2011).
Stroke patients are at highest risk of death in the first weeks after the event, and between 20% to 50% die within the first month depending on type, severity, age, co- morbidity and effectiveness of treatment of complications. Patients who survive may be left with no disability or with mild, moderate or severe disability. Considerable spontaneous recovery occurs up to about six months (Bonita et al., 1988). However, patients with a history of stroke are at risk of a subsequent event of around 10% in the first year and 5% per year thereafter (Burn et al., 2014).
In obese mice, the adipose tissue is characterised by a lower interstitial oxygen partial pressure (PO2) (Ye et al., 2007; Rausch et al., 2008). During surgery, the obese patients present with a lower PO2 in the subcutaneous adipose tissue of the lateral upper arm compared with non-obese patients (Kabon et al., 2004). Furthermore, abdominal subcutaneous adipose tissue PO2 is slightly lower in overweight/obese compared with lean subjects (Pasarica et al., 2009). Thus adipose tissue dysfunction in obesity contributes to chronic, low-grade inflammation that predisposes to type 2 diabetes melitus and cardiovascular disease (Goossens et al., 2011).
The obesity paradox has been reported in many articles as an inverse relationship between the body mass index (BMI) and mortality in stroke patients. However, the relationship between BMI and functional recovery in post stroke patients has not been well described (Olsen et al., 2008; Towfighi et al., 2009; Ovbiagele et al., 2011; Ryu et al., 2011; Vemmos et al., 2011; Andersen et al., 2013).
A cohort study from the China National Stroke Registry analyzed the relationship between the body mass index (BMI), mortality and post stroke functional recovery at 3 months after disease onset. This study enrolled and analyzed 10,905 eligible patients with acute ischemic stroke. Favourable functional recovery was seen in 52.4 % of underweight (BMI 18.5 kg/m2), 55.0% of normal weight (BMI 18.5–22.9 kg/m2), 61% of overweight (BMI 23–27.4 kg/m2), 59.2% of obese (27.5–32.4 kg/m2) and 60.3% of severe obese (BMI > 32.5 kg/m2) stroke survivors. The overweight acute ischemic stroke survivors had a better 3-month functional recovery. Remarkably, patients with obesity (BMI less than 32.5 kg/m2) showed a positive outcome. However, severe obesity was associated with higher mortality while an overweight status was not a protective factor of survival at 3 months after stroke (Zhao et al., 2014).
A study evaluating the effect of BMI on stroke rehabilitation conducted in 819 patients revealed that overweighted patients had better functional progression than patients included in other weight categories (Burke et al, 2014). In a large retrospective cohort study from the Danish Stroke Register, 53,812 patients were evaluated for BMI, age, sex, civil status, stroke severity, stroke subtype, a predefined cardiovascular profile, and the socioeconomic status. There was no evidence of an obesity paradox in patients with reported stroke. However, stroke occurred at a significantly younger age in patients with higher BMI (Burke et al., 2014).
In conclusion, obesity could determine a worse outcome in stroke patients, yet it is not known the exact molecular pathways (Howcroft et al., 2013). However, since obesity represents a state of chronic inflammation it is likely that this factor plays a crucial role in the general evolution of these patients.
Diabetes Mellitus and Metabolic Inflammation
Diabetes mellitus (DM) is a great challenge for the healthcare system accounting for ~6% of global mortality in industrialized countries. Half of DM-associated deaths are attributed to cardiovascular (macro- and micro-vascular) complications.
Neuropathic complications are also frequent, occurring in about 60% of people with DM, and often overlap with, and worsen the consequences of vascular disease. Sensory neuropathy is a typical form of peripheral neuropathy characterized by an altered perception of noxious stimuli or ischemic pain. This promotes the foot ulcers caused by pressure or traumas and abrogates warning symptoms during a heart attack.
It is becoming well established that lifestyles, especially dietary habits, greatly affect metabolic health. Bad nutritional habits can lead to metabolic disorders, triggered by a system-wide chronic inflammation, also called metaflammation, metabolic inflammation (Olefsky and Glass, 2010). A metaflammation state can lead to a series of disorders and diseases, including hypertension, metabolic syndrome, CVD, stroke, insulin resistance and type 2 diabetes mellitus (T2DM). It is postulated that lipid hormones including sphingolipids and eicosanoids in concert with cytokines and adipokines play an important role in this process by inducing adverse regulatory responses in target cells such as macrophages. The role of genetics in driving metabolic disease development is strongly indicated by the higher concordance rate of T2DM in monozygotic than in dizygotic twins. It has been estimated that 30% to 70% of T2DM risk can be attributed to genetics (Poulsen et al., 1999). The investigation of gene-environment interactions through large collaborative efforts holds promise in furthering our understanding of the interplay between genetic and environmental factors (Cornelis and Hu, 2012).
Since the completion of the HapMap project and the availability of whole genome SNP assays, genome-wide analysis of correlations between genetic variants (SNPs) and phenotypes has become an important approach to find disease-causative genes. Genome wide SNP typing is often performed in very large groups of human individuals (cohorts), and a large number of loci underlying disease have now been catalogued (http://www.genome.gov/gwastudies/), including variants that increase susceptibility to T2DM. However, these loci confer effects of only modest size and do not add to the clinical prediction of diabetes beyond that of traditional risk factors, such as obesity, physical inactivity, family history of diabetes, and certain clinical parameter. Furthermore, recent studies led to the identification of new genetic loci linking adipocyte and insulin biology to body fat distribution (Locke et al., 2015; Shungin et al., 2015). The combination of GWAS with metabolomics is now breaking new grounds (Bictash et al., 2010), as it allows making associations between SNPs and so-called intermediate phenotypes that can be obtained through exact measurements.
Metabolomics facilitates the exact quantitative measurement of large sets of lipid molecules and other metabolites, and GWAS has allowed the mapping of numerous metabolic phenotypes on the genome, as demonstrated by the discovery of substantial numbers of loci with relative strong effects (Illig et al., 2010; Teslovich et al., 2010; Suhre et al., 2011; Kettunen et al., 2012). Therefore, we could speculate diabetes mellitus is characterised by a state of increased general inflammation including at the CNS level which might impair recovery and outcome in a wide range of neurological conditions.
Depression and Neuroinflammation
Major depressive disorder (MDD) is a severe psychiatric illness that is associated with significant morbidity and mortality. In addition to mortality associated with suicide (Kessler et al., 2005), depressed patients are more likely to develop dementia, coronary artery disease and type 2 diabetes (Knol et al., 2006). Depression also complicates the prognosis of other chronic diseases (Evans et al., 2005; Gildengers et al., 2008). However, biological mechanisms underlying depression remains poorly understood.
Despite advances in the treatment of major depression, one-third of depressed patients fail to respond to conventional antidepressant medication (Rush et al., 2006). One pathophysiologic mechanism hypothesized to contribute to treatment resistance in depression is inflammation. Inflammation has been linked to depression and dementia by a number of putative mechanisms involving neuroinflammation, oxidative stress, endothelial nitric oxide synthase uncoupling, and hyperglutamatergia, as well as their relationships to indirect evidence of neurovascular dysfunction in MDD (Najjar et al., 2013; Zunszain et al., 2013).
Recent evidence has shown that MDD is associated with increased levels of inflammatory markers in the periphery. A number of inflammatory biomarkers (including inflammatory cytokines, acute phase proteins, chemokines, and adhesion molecules) in the periphery have been found to be reliably elevated in one third of all depressed patients with a decreased likelihood of response to conventional antidepressants (Lanquillon et al., 2000; Miller et al., 2009; Papakostas et al., 2011). Conversely, patients treated with cytokines for various illnesses are at increased risk of developing major depressive illness (Krishnadas and Cavanagh, 2012). A recent study reported that treatment-resistant depression (TRD) who has highly increased inflammation (i.e., elevated baseline hs-CRP concentration) responded preferentially to infliximab while infliximab-treated participants with a low level of inflammation appeared to do worse than placebo-treated participants (Raison et al., 2013). Of note, increased inflammatory markers in depressed patients have also been associated with remitted stages of depression in response to treatment with conventional antidepressant medications (Sluzewska et al., 1997; Lanquillon et al., 2000; Nemeroff et al., 2003; Baune et al., 2012).
On a background of systemic inflammation, proinflammatory cytokines can access the central nervous system and interfere with serotonin metabolism, and reduce both synaptic plasticity and hippocampal neurogenesis (Maes, 2009; Caraci et al., 2010). Behavioral consequences of these effects of the immune system on the brain include depression (Capuron and Miller, 2011; Raison et al., 2012).
Cross-sectional (Penninx et al., 2003; Tiemeier et al., 2003; Bremmer et al., 2008) and prospective (van den Biggelaar et al., 2007; Milaneschi et al., 2009) epidemiological studies have focused on peripheral inflammatory markers, such as cytokines and acute phase reactans, on the assumption that peripheral inflammatory markers are etiological factors in the development of depressive symptoms (Dantzer et al., 2008a, b; Baune et al., 2012) as well as induce neurotransmitter changes in the brain as seen in depression (Anisman et al., 2008). The most consistent finding has been the association of elevated cytokines IL-6 and IL-8 with depressive symptoms (Baune et al., 2012).
Successful antidepressant treatment may reduce proinflammatory markers by improving perfusion or restore endothelial function (Ghiadoni et al., 2000; Miller et al., 2009; Nagata et al., 2010). Etanercept, a soluble tumor necrosis factor-α receptor, and celecoxib, a cyclo-oxygenase-2 inhibitor, may reduce depressive symptoms in patients with inflammatory diseases (Tyring et al., 2006; Kekow et al., 2010) and infliximab may improve depression in patients with greater pre-treatment inflammation (Raison et al., 2012).
Depression, Aging and Neuroinflammation
Normal aging is characterized by a chronic low-grade, pro-inflammatory state (Bruunsgaard et al., 2001), with an over-expression of systemic inflammatory factors, including pro-inflammatory cytokines (Fagiolo et al., 1992, 1993). Age-associated inflammation in the brain manifests primarily the chronic activation of perivascular and parenchymal macrophages/microglia expressing proinflammatory cytokines and an increased number of astrocytes (Ye and Johnson, 1999).
Given the potential role of inflammation in psychopathology, it is possible that chronically activated inflammatory signals in aging may contribute to increased vulnerability to neuropsychiatric disorders (Capuron et al., 2008). Inflammation in obese women is associated with increased concentrations of inflammatory markers (IL-6, CRP and adipokines) that correlated with increased symptoms of depression and anxiety (Capuron et al., 2010). Conversely, removal of fat tissue surgically was associated with reduced inflammation (Cancello et al., 2005).
The prevalence of depression and cognitive dysfunction is particularly elevated in the elderly and obese subjects. Patients with major depression have an increased onset risk of aging-related diseases affecting the cardiovascular, cerebrovascular, neuroendocrine, metabolic, and immune systems (McIntyre et al., 2007; Bauer, 2008; Wolkowitz et al., 2010). Depression can thus significantly compromise successful aging defined subjectively as freedom from chronic disease and disability, along with high physical and cognitive functioning and social engagement (Jeste et al., 2013).
Post-stroke Depression and Neuroinflammation
Emerging evidence suggests that stroke and traumatic brain injury confer vulnerability to a late-onset of neuropsychaitric and neurocognitive symptoms (Alexopoulos, 2006; Fenn et al., 2013).
Brain injury initiates an exaggerated neuroinflammation by activation of an immune-reactive microglial population as a possible triggering mechanism for the development of depressive-like behavior after injury that may last for weeks and months after the event (Fenn et al., 2013). Importantly, a recent meta-analysis found that the frequency of depressive symptoms even tends to increase in the long-term phase of recovery (Hackett et al., 2005). Depression persists after 20 months in 34% of elderly patients with acute stroke and has been linked to both worse cognitive and physical outcome.
Despite the fact that a high proportion of stroke patients develop mood disorders, the mechanisms underlying PSD have so far received little attention from the field of neurobiology. One major factor involving the development of post-stroke depression could be represented by an age-related microglia activation in response to stroke. Persistent neuronal death causes a prolonged neuroinflammatory response in the infarcted area and may contribute substantially to post-stroke depression. After stroke and traumatic brain injury microglia move toward the site of damage and engulf and clear damaged cellular debris (Nimmerjahn et al., 2005; Hanisch and Kettenmann, 2007; Wakselman et al., 2008). Previously we have shown that aged rats showed a fulminant microglia reaction during the acute phase of stroke that persists for weeks thereafter (Badan et al., 2003; Buga et al., 2012; Fenn et al., 2013). Since microglia has been involved in scavenging synapses, these findings suggest that neuroinflammation represents a significant etiopathogenic molecular pathway.
Conclusions
Although neuropathological conditions differ in aetiology and in the way in which the inflammatory response is mounted, cellular and molecular mechanisms of neuroinflammation are probably similar in aging, depression and cognitive impairment or after cerebral insult such as stroke (Goossens, 2008; Allison and Ditor, 2014). Moreover, a number of highly prevalent risk factors such as obesity hypertension, diabetes and atherosclerosis are increasingly understood to act as “silent contributors” to neuroinflammation – not only establishing the condition as a central pathophysiological mechanism, but also constantly fuelling it. Subtle, but continuous neuroinflammation can provide the ground for disorders such as cSVD and subsequent dementia. Acute neuroinflammation, often in the context of traumatic or ischemic CNS lesions, aggravates the damage and can lead to a number of pathologies such as depression, post-stroke dementia and potentially neurodegeneration. All of those sequelae impair recovery and most of them provide the ground for further cerebrovascular events and a vicious cycle develops.
Therefore, understanding the mechanisms associated with vascular dementia, stroke and related complications is of paramount importance in improving current preventive and therapeutic interventions. However, all these pathological entities are associated with neuroinflammation. A thorough understanding of molecular factors and pathways associated with neuroinflammation will eventually enable the discovery and implementation of new diagnostic and therapeutic strategies for a wide range of neurologial conditions.
Footnotes
Funding: Dr. Raluca Elena Sandu was supported by a POSDRU grant no. 159/1.5/S/136893 grant: “Strategic partnership for the increase of the scientific research quality in medical universities through the award of doctoral and postdoctoral fellowships–DocMed.Net_2.0”.
Conflicts of interest: We have no conflict of interest to declare.
References
- Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60:1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Allison DJ, Ditor DS. The common inflammatory etiology of depression and cognitive impairment: a therapeutic target. J Neuroinflammation. 2014;11:151. doi: 10.1186/s12974-014-0151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen KK, Olsen TS. Body mass index and stroke: overweight and obesity less often associated with stroke recurrence. J Stroke Cerebrovasc Dis. 2013;2:26. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.031. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z, Hayley S. Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegenerative disorders. Prog Neurobiol. 2008;85:1–74. doi: 10.1016/j.pneurobio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Badan I, Buchhold B, Hamm A, Gratz M, Walker LC, Plattparallel D, Kessler Ch, Popa-Wagner A. Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery. J Cereb Blood Flow Metab. 2003;23:845–854. doi: 10.1097/01.WCB.0000071883.63724.A7. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Psychiatric and neurophysiological predictors of obesity in HIV/AIDS. Psychophysiology. 2008;45:1055–1063. doi: 10.1111/j.1469-8986.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune BT, Smith E, Reppermund S, Air T, Samaras K, Lux O, Brodaty H, Sachdev P, Trollor JN. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: the prospective Sydney Memory and Aging Study. Psychoneuroendocrinology. 2012;37:1521–1530. doi: 10.1016/j.psyneuen.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Bictash M, Ebbels TM, Chan Q, Loo RL, Yap IK, Brown IJ, de Iorio M, Daviglus ML, Holmes E, Stamler J, Nicholson JK, Elliott P. Opening up the “Black Box”: metabolic phenotyping and metabolome-wide association studies in epidemiology. J Clin Epidemiol. 2010;63:970–979. doi: 10.1016/j.jclinepi.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke. 1988;19:1497–1500. doi: 10.1161/01.str.19.12.1497. [DOI] [PubMed] [Google Scholar]
- Bremmer MA, Beekman A, Deeg DJ, Penninx BW, Dik MG, Hack CE, Hoogendijk WJ. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 2008;106:249–255. doi: 10.1016/j.jad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Buchhold B, Mogoanta L, Suofu Y, Hamm A, Walker L, Kessler Ch, Popa-Wagner A. Environmental enrichment improves functional and neuropathological indices following stroke in young and aged rats. Restor Neurol Neurosci. 2007;25:467–484. [PubMed] [Google Scholar]
- Buga AM, Di Napoli M, Popa-Wagner A. Preclinical models of stroke in aged animals with or without comorbidities: role of neuroinflammation. Biogerontology. 2013;14:651–662. doi: 10.1007/s10522-013-9465-0. [DOI] [PubMed] [Google Scholar]
- Buga AM, Scholz CJ, Kumar S, Herndon J, Alexandru D, Cojocaru GR, Dandekar T, Popa-Wagner A. Identification of new therapeutic targets by genome-wide analysis of gene expression in the ipsilateral cortex of aged rats after stroke. PLoS One. 2012;7:e50985. doi: 10.1371/journal.pone.0050985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DT, Al-Adawi S. Effect of body mass index on stroke rehabilitation. J Stroke Cerebrovasc Dis. 2014;95:1055–1059. doi: 10.1016/j.apmr.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire Community Stroke Project. Stroke. 1994;25:333–337. doi: 10.1161/01.str.25.2.333. [DOI] [PubMed] [Google Scholar]
- Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumie A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clement K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Poitou C, Machaux-Tholliez D, Frochot V, Bouillot JL, Basdevant A, Laye S, Clement K. Relationship between adiposity, emotional status and eating behaviour in obese women: role of inflammation. Psychol Med. 2010;41:1517–1528. doi: 10.1017/S0033291710001984. [DOI] [PubMed] [Google Scholar]
- Capuron L, Su S, Miller AH, Bremner JD, Goldberg J, Vogt GJ, MAisano C, Jones L, Murrah NV, Vaccarino V. Depressive symptoms and metabolic syndrome: is inflammation the underlying link. Biol Psychiatry. 2008;64:896–900. doi: 10.1016/j.biopsych.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraci F, Copani A, Nicoletti F, Drago F. Depression and Alzheimer's disease: neurobiological links and common pharmacological targets. Eur J Pharmacol. 2010;626:64–71. doi: 10.1016/j.ejphar.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Cornelis MC, Hu FB. Gene-environment interactions in the development of type 2 diabetes: recent progress and continuing challenges. Annu Rev Nutr. 2012;32:245–259. doi: 10.1146/annurev-nutr-071811-150648. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, Breteler MM. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125:765–772. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- Di Napoli M, Godoy D DA, Campi V, Masotti L L, Smith CJ, Parry-Jones AR, Hopkins SJ, Slevin M, Papa F, Mogoanta L, Pirici D, Popa-Wagner A. C-reactive protein in intracerebral hemorrhage: time course, tissue localization, and prognosis. Neurology. 2012;79:690–699. doi: 10.1212/WNL.0b013e318264e3be. [DOI] [PubMed] [Google Scholar]
- Di Napoli M, Parry-Jones AR, Smith CJ, Hopkins SJ, Slevin M, Masotti L, Campi V, Singh P, Papa F, Popa-Wagner A, Tudorica V, Godoy DA. C-reactive protein predicts hematoma growth in intracerebral hemorrhage. Stroke. 2014;45:59–65. doi: 10.1161/STROKEAHA.113.001721. [DOI] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, et al. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Fagiolo U, Cossarizza A, Santacaterina S, Ortolani C, Monti D, Paganelli R, Franceschi C. Increased cytokine production by peripheral blood mononuclear cells from healthy elderly people. Ann N Y Acad Sci. 1992;663:490–493. doi: 10.1111/j.1749-6632.1992.tb38712.x. [DOI] [PubMed] [Google Scholar]
- Fagiolo U, Cossarizza A, Scala E, Fanales-Belasio E, Ortolani C, Cozzi E Monti D, Franceschi C, Paganelli R. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23:2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Gensel JC, Huang Y, Popovich PG, Lifshitz J, Godbout JP. Immune activation promotes depression 1 month after diffuse brain injury: a role for primed microglia. Biol Psychiatry. 2013;76:575–584. doi: 10.1016/j.biopsych.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiadoni L, Virdis A, Magagna A, Taddei S, Salvetti A. Effect of the angiotensin II type 1 receptor blocker candesartan on endothelial function in patients with essential hypertension. Hypertension. 2000;35:501–506. doi: 10.1161/01.hyp.35.1.501. [DOI] [PubMed] [Google Scholar]
- Gildengers AG, Whyte EM, Drayer RA, Soreca I, Fagiolini A, Kilbourne AM, Houck PR, Reynolds CF, Frank E, Kupfer DJ, Mulsant BH. Medical burden in late-life bipolar and major depressive disorders. Am J Geriatr Psychiatry. 2008;16:194–200. doi: 10.1097/JGP.0b013e318157c5b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94:206–218. doi: 10.1016/j.physbeh.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JW, Cajlakovic M, Ribitsch V, Clément K, Blaak EE. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- Hackett ML, Anderson CS. Predictors of depression after stroke: a systematic review of observational studies. Stroke. 2005;36:2296–2301. doi: 10.1161/01.STR.0000183622.75135.a4. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Howcroft TK, Campisi J, Louis GB, Smith MT, Wise B, Wyss-Coray T, Augustine AD, McElhaney JE, Kohanski R, Sierra F. Aging. 2013;5:84–93. doi: 10.18632/aging.100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illig T, Gieger C, Zhai G, Römisch-Margl WA, Wang-Sattler R, Prehn C, Altmaier E, Kastenmüller G, Kato BS, Mewes HW, Meitinger T, de Angelis MH, Kronenberg F, Soranzo N, Wichmann HE, Spector TD, Adamski J, Suhre K. A genome-wide perspective of genetic variation in human metabolism. Nat Genet. 2010;42:137–141. doi: 10.1038/ng.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Palmer BW. A call for a new positive psychiatry of ageing. Br J Psychiatry. 2013;202:81–83. doi: 10.1192/bjp.bp.112.110643. [DOI] [PubMed] [Google Scholar]
- Kabon B, Nagele A, Reddy D, Eagon C, Fleshman JW, Sessler DI, Kurz A. Obesity decreases perioperative tissue oxygenation. Anesthesiology. 2004;100:274–280. doi: 10.1097/00000542-200402000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekow J, Moots RJ, Emery P, Durez P, Koenig A, Singh A, Pedersen R, Robertson D, Freundlich B, Sato R. Patient-reported outcomes improve with etanercept plus methotrexate in active early rheumatoid arthritis and the improvement is strongly associated with remission: the COMET trial. Ann Rheum Dis. 2010;69:222–225. doi: 10.1136/ard.2008.102509. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Life time prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kettunen J, Tukiainen T, Sarin AP, Ortega-Alonso A, Tikkanen E, Lyytikäinen LP, Kangas AJ, Soininen P, Würtz P, Silander K, Dick DM, Rose RJ, Savolainen MJ, Viikari J, Kähönen M, Lehtimäki T, Pietiläinen KH, Inouye M, McCarthy MI, Jula A, et al. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat Genet. 2012;44:269–276. doi: 10.1038/ng.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus: A meta-analysis. Diabetologia. 2006;49:837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83:495–502. doi: 10.1136/jnnp-2011-301779. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Gribkoff VK, Donlan NA, Jones TA. Long-lasting functional disabilities in middle-aged rats with small cerebral infarcts. J Neurosci. 2003;23:10913–10922. doi: 10.1523/JNEUROSCI.23-34-10913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Mägi R, Randall JC, Winkler TW, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:664–675. doi: 10.1016/j.pnpbp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Manwani B, Liu F, Xu Y, Persky R, Li J, McCullough LD. Functional recovery in aging mice after experimental stroke. Brain Behav Immun. 2011;25:1689–1700. doi: 10.1016/j.bbi.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Soczynska JK, Konarski JZ, Woldeyohannes HO, Law CW, Miranda A, Fulgosi D, Kennedy SH. Should depressive syndromes be reclassified as “metabolic syndrome type II”? Ann Clin Psychiatry. 2007;19:257–264. doi: 10.1080/10401230701653377. [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Corsi AM, Penninx BW, Bandinelli S, Guralnik JM, Ferrucci L. Interleukin-1 receptor antagonist and incident depressive symptoms over 6 years in older persons: the InCHIANTI study. Biol Psychiatry. 2009;65:973–978. doi: 10.1016/j.biopsych.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molino-Lova R, Macchi C, Gori AM, Marcucci R, Polcaro P, Cecchi F, Lauretani F, Bandinelli S, Abbate R, Beghi E, Guralnik JM, Ferrucci L. High sensitivity C-reactive protein predicts the development of new carotid artery plaques in older persons. Nutr Metab Cardiovasc Dis. 2011;21:776–782. doi: 10.1016/j.numecd.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KN, Buggey HF, Denes A, Allan SM. Systemic immune activation shapes stroke outcome. Mol Cell Neurosci. 2013;53:14–25. doi: 10.1016/j.mcn.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Nagata R, Kawabe K, Ikeda K. Olmesartan an angiotensin II receptor blocker, restores cerebral hypoperfusion in elderly patients with hypertension. J Stroke Cerebrovasc Dis. 2010;19:236–240. doi: 10.1016/j.jstrokecerebrovasdis.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Najjar S, Pearlman DM, Devinsky O, Najjar A, Zagzag D. Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: a review of clinical and experimental evidence. J Neuroinflammation. 2013;10:142. doi: 10.1186/1742-2094-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB. The role of GABA in the pathophysiology and treatment of anxiety disorders. Psychopharmacol Bull. 2003;37:133–146. [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Olsen TS, Dehlendorff C, Petersen HG, Andersen KK. Body mass index and poststroke mortality. Neuroepidemiology. 2008;30:93–100. doi: 10.1159/000118945. [DOI] [PubMed] [Google Scholar]
- Ovbiagele B, Bath PM, Cotton D, Vinisko R, Diener HC. Obesity and recurrent vascular risk after a recent ischemic stroke. Stroke. 2011;42:3397–3402. doi: 10.1161/STROKEAHA.111.624957. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Shelton RC, Kinrys G, Henry ME, Bakow BR, Lipkin SH, Pi B, Thurmond L, Bilello JA. Assessment of a multi-assay, serum-based biological diagnostic test for major depressive disorder: a Pilot and Replication Study. Mol Psychiatry. 2013;18:332–339. doi: 10.1038/mp.2011.166. [DOI] [PubMed] [Google Scholar]
- Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Rubin S, Ferrucci L, Harris T, Pahor M. Inflammatory markers and depressed mood in older persons: results from the health, aging and body composition study. Biol Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- Petcu EB, Smith RA, Miroiu RI, Opris MM. Angiogenesis in old-aged subjects after ischemic stroke: a cautionary note for investigators. J Angiogenes Res. 2010;2:26. doi: 10.1186/2040-2384-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa-Wagner A, Buga AM, Kokaia Z. Perturbed cellular response to brain injury during aging. Ageing Res Rev. 2011;10:71–79. doi: 10.1016/j.arr.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Buga AM, Tica AA, Albu CV. Perfusion deficits, inflammation and aging precipitate depressive behaviour. Biogerontology. 2014;15:439–448. doi: 10.1007/s10522-014-9516-1. [DOI] [PubMed] [Google Scholar]
- Poulsen Olefsky JM, Glass CK. Macrophages inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- Poulsen P, Kyvik KO, Vaag A, Beck-Nielsen H. Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance-a population-based twin study. Diabetologia. 1999;42:132–145. doi: 10.1007/s001250051131. [DOI] [PubMed] [Google Scholar]
- Raison CL, Rutherford RD, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. Arch Gen Psychiatry. 2012;3:1–11. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Corrado E, Coppola G, Muratori I, Mezzani A Novo G, Novo S. The predictive role of C-reactive protein in patients with hypertension and subclinical atherosclerosis. Coron Artery Dis. 2009;20:15–20. doi: 10.1111/j.1445-5994.2009.01955.x. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Corrado E, Coppola G, Muratori I, Novo G G, Novo S. Markers of inflammation are strong predictors of subclinical and clinical atherosclerosis in women with hypertension. Intern Med J. 2009;39:539–545. doi: 10.1097/MCA.0b013e3283109065. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, et al. Executive summary: heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Ryu WS, Lee SH, Kim CK, Yoon BW. Body mass index, initial neurological severity and long-term mortality in ischemic stroke. Cerebrovasc Dis. 2011;32:170–176. doi: 10.1159/000328250. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL. Inflammation, immunity and development of essential hypertension. J Hypertens. 2014;32:228–229. doi: 10.1097/HJH.0000000000000042. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Ishikawa J, Yano Y, Hoshide S, Shimada K, Kario K. The relationship between the morning blood pressure surge and low-grade inflammation on silent cerebral infarct and clinical stroke events. Atherosclerosis. 2011;219:316–321. doi: 10.1016/j.atherosclerosis.2011.06.030. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Shimomura K, Tokuyama Y, Sakurai K, Isahaya K, Takaishi S, Kato B, Usuki N, Shimizu T, Yamada K, Hasegawa Y. Association between inflammatory biomarkers and progression of intracranial large artery stenosis after ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22:211–217. doi: 10.1016/j.jstrokecerebrovasdis.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, Strawbridge RJ, Pers TH, Fischer K, Justice AE, Workalemahu T, Wu JM, Buchkovich ML, Heard-Costa NL, Roman TS, Drong AW, Song C, Gustafsson S, Day FR, Esko T, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518:187–196. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluzewska A, Sobieska M, Rybakowski JK. Changes in acute-phase proteins during lithium potentiation of antidepressants in refractory depression. Neuropsychobiology. 1997;35:123–127. doi: 10.1159/000119332. [DOI] [PubMed] [Google Scholar]
- Suhre K, Shin SY, Petersen AK, Mohney RP, Meredith D, Wägele B, Altmaier E, Deloukas P, Erdmann J, Grundberg E, Hammond CJ, de Angelis MH, Kastenmüller G, Köttgen A, Kronenberg F, Mangino M, Meisinger C, Meitinger T, Mewes HW, et al. CARDIoGRAM. Human metabolic individuality in biomedical and pharmaceutical research. Nature. 2011;477:54–60. doi: 10.1038/nature10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeier H, Breteler MM, van Popele NM, Hofman A, Witteman JC. Late-life depression is associated with arterial stiffness: a population-based study. J Am Geriatr Soc. 2003;51:1105–1110. doi: 10.1046/j.1532-5415.2003.51359.x. [DOI] [PubMed] [Google Scholar]
- Tousoulis D, Kampoli AM, Papageorgiou N, Androulakis E, Antoniades C, Toutouzas K, Stefanadis C. Pathophysiology of atherosclerosis: the role of inflammation. Curr Pharm Des. 2011;17:4089–4110. doi: 10.2174/138161211798764843. [DOI] [PubMed] [Google Scholar]
- Towfighi A, Ovbiagele B. The impact of body mass index on mortality after stroke. Stroke. 2009;40:2704–2708. doi: 10.1161/STROKEAHA.109.550228. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebocontrolled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- van den Biggelaar AH, Gussekloo J, de Craen AJ, Frölich M, Stek ML, van der Mast RC, Westendorp RG. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp Gerontol. 2007;42:693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Vemmos K, Ntaios G, Spengos K, Savvari P, Vemmou A, Pappa T, Manios E, Georgiopoulos G, Alevizaki M. Association between obesity and mortality after acute first-ever stroke: the obesity-stroke paradox. Stroke. 2011;42:30–36. doi: 10.1161/STROKEAHA.110.593434. [DOI] [PubMed] [Google Scholar]
- Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci. 2008;28:8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey JZ, Ortega-Gutierrez S, Petersen N, Khatri P, Ford AL, Rost NS, Ali LK, Gonzales NR, Merino JG, Meyer BC, Marshall RS. Impact of acute ischemic stroke treatment in patients >80 years of age: the specialized program of translational research in acute stroke (SPOTRIAS) consortium experience. Stroke. 2012;43:2369–2375. doi: 10.1161/STROKEAHA.112.660993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depress Anxiety Depression gets old fast: do stress and depression accelerate cell aging. Depress Anxiety. 2010;27:327–338. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Zhao L, Du W, Zhao X, Liu L, Wang C, Wang Y, Xu Y. Favorable functional recovery in overweight ischemic stroke survivors: findings from the China National Stroke Registry. J Stroke Cerebrovasc Dis. 2014;23:e201–206. doi: 10.1016/j.jstrokecerebrovasdis.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Zuliani G, Guerra G, Ranzini M, Rossi L, Munari MR, Zurlo A, Blè A, Volpato S, Atti AR, Fellin R. High interleukin-6 plasma levels are associated with functional impairment in older patients with vascular dementia. Int J Geriatr Psychiatry. 2007;22:305–311. doi: 10.1002/gps.1674. [DOI] [PubMed] [Google Scholar]
- Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Topics Behav Neurosci. 2013;14:135–151. doi: 10.1007/7854_2012_211. [DOI] [PubMed] [Google Scholar]


