Multiple sclerosis is a chronic inflammatory disease that is accompanied by demyelination and axonal damage resulting in neurological deficits. Remyelination is the natural endogenous repair mechanism of demyelinated axons and it is supposed to protect axons/neurons from degeneration and thus the patient from progressive disability (Franklin and Ffrench-Constant, 2008). Current therapeutics for patients with multiple sclerosis are to some extent very effective in inhibiting neuroinflammation and demyelination. However, to date there are no substances available that can enhance remyelination. Remyelination is the result of recruitment/proliferation of new oligodendrocyte precursor cells (OPC) and differentiation into mature myelin producing oligodendrocytes (Franklin and Ffrench-Constant, 2008). These processes are supported by many factors and signals and failure at any stage might lead to repair failure. Strategies to enhance myelin repair are either the promotion of endogenous repair mechanisms via modulation of OPC proliferation and oligodendrocyte differentiation or the transplantion of myelinating cells into lesions. Due to the multiloculated process in multiple sclerosis and the ethical problems with the cell source, the latter is less favoured. The endogenous promotion of remyelination could be achieved by several approaches such as: (1) treatment with factors that directly influence OPC proliferation, migration and/or differentiation (e.g., growth factors, cytokines), (2) creation of a repair promoting environment in the central nervous system (CNS) via modulation of resident glial cells or peripheral immune cells, (3) modulation of inhibitory factors of OPC proliferation, migration and/or differentiation (e.g., LINGO-1, myelin), or (4) protection of oligodendroglial cells. Alternatively, transplantation of exogenous cells such as mesenchymal stem cells is in the focus of current research on repair mechanisms. To date several approaches have been successfully tested in animal models of remyelination, however, only few clinical trials were performed in patients with multiple sclerosis. Inhibiting LINGO-1 presents a promising option to enhance myelin repair in patients with multiple sclerosis. Treatment with LINGO-1 antagonists showed beneficial effects on remyelination by induction of OPC differentiation in animal models (Mi et al., 2009) and a first clinical trial (phase II) in patients with first optic neuritis has just been completed. Preliminary results indicate that the (primary) endpoint, recovery of the optic nerve latency measured by visual evoked potentials, has been reached, however, there was no effect on other secondary outcome parameters like visual acuity or change in retinal nerve fiber layer (RNFL) thickness measured by optic coherence tomography (OCT) (Biogen, press release 08.01.2015). Thus, there is still further clinical need for the development of remyelinating/regenerating treatments for patients with multiple sclerosis.
Recently, we identified a new mechanism to enhance myelin repair via the choline pathway (Skripuletz et al., 2015). We have tested the substance cytidine 5′-diphosphocholine (citicoline, CDP-choline) for possible neuroprotective and regenerative properties in two animal models of multiple sclerosis. First, we found that CDP-choline ameliorated clinical symptoms of murine myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis (MOG-EAE) when it was applied from the first day of disease induction or from the onset of disease. Histopathological analyses at later time points revealed beneficial effects on oligodendrocytes, myelination, and axons. In our experiments, CDP-choline failed to show beneficial effects on the disease course when it was applied after the peak of disease. We hypothesised that the effects of CDP-choline might be driven by modulation of OPC proliferation which occurs before the peak of disease in this animal model. We have thus utilized an additional model of CNS remyelination, the toxic cuprizone model. The cuprizone model provides the advantage to investigate remyelination processes without the influence of peripheral immune cells which do not play a key role in this model. Furthermore, the cuprizone model offers consistent, anatomically reproducible and well detectable processes of OPC regeneration and remyelination (Skripuletz et al., 2011). We did not find any beneficial effects of CDP-choline on the course of cuprizone induced demyelination and oligodendrocyte loss. However, during remyelination CDP-choline enhanced myelin repair and reversed motor coordination deficits as shown by the beam walking test. The improved remyelination was accompanied by higher numbers of proliferating OPC which resulted in higher numbers of mature oligodendrocytes. Additional in vitro experiments confirmed that CDP-choline increases the proliferation rate of OPC. Thus modulation of OPC proliferation seems to be an important mechanism of action of CDP-choline. Nevertheless, this mechanism may not be exclusively operated.
Additional external confirmation and corroboration of our results comes from not fully published studies in EAE where CDP-choline was shown to be beneficial in EAE in Lewis rats (Grieb, 2015). However, these studies did not look into the mechanisms how this effect was achieved.
CDP-choline has been shown to exert neuroprotective effects in in vitro experiments as well as in animal models of stroke, brain injury, and neurodegenerative diseases (Grieb, 2014). One hypothesis of action is the stimulation of brain phospholipid synthesis. In the CNS, endogenous CDP-choline is an intermediate compound in the biosynthesis of the cell membrane phospholipid phosphatidylcholine (lecithin) and acetylcholine (Adibhatla and Hatcher, 2002). During pathological conditions, the amount of endogenous CDP-choline might be limited in the synthesis of phosphatidylcholine and acetylcholine and thus the substance might be limited during repair processes. Exogenously applied CDP-choline might be a possible tool to promote regeneration. CDP-choline given parenterally or orally quickly degrades into its products cytidine and choline which can cross the blood-brain barrier separately (Grieb, 2014). In the CNS both products can be resynthesized to CDP-choline and might thus increase the biosynthesis of phosphatidylcholine. In the synthesis pathway of phosphatidylcholine, CDP-choline is converted directly with diacyglyceride (DAG) into phosphatidylcholine and cytidine-mono-phosphate (CMP) in a reaction catalysed by the enzyme 1,2-diacylglycerol cholinephosphotransferase (CPT) (Hjelmstad and Bell, 1991). However, the exact mechanisms of action of CDP-choline in animal disease models are not well understood and other pathophysiological mechanisms are possible. Indeed, in our work we found regenerative functions of exogenous CDP-choline that were promoted via increasing the proliferation rate of OPC (Figure 1).
Figure 1.
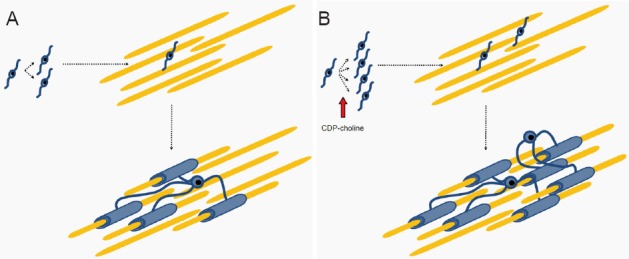
Proposed action of cytidine 5′-diphosphocholine (citicoline, CDP-choline) during remyelination.
(A) During remyelination, oligodendrocyte precursor cells (OPC) proliferate, migrate into the demyelinated area, and finally differentiate into myelinating oligodendrocytes. (B) In the presence of CDP-choline, proliferation of OPC is enhanced and thus more OPC can migrate into the demyelinated area and more axons are remyelinated.
Furthermore, it should be noted that the neuroprective effects of CDP-choline which were found in animal disease models of stroke and brain injury could not be reproduced in humans. In the COBRIT (Citicoline Brain Injury Treatment) trial a 90-day treatment of daily citicoline (2,000 mg) or placebo was performed in 1,213 patients (Zafonte et al., 2012). In patients with traumatic brain injury, CDP-choline failed to improve the functional and cognitive status as compared to placebo controls. In the International Citicoline Trial on Acute Stroke (ICTUS), 2,298 patients with moderate to severe acute ischaemic stroke either received CDP-choline (2,000 mg per day intravenously during the first 3 days, thereafter 1,000 mg per days orally for a total of 6 weeks) or placebo (Davalos et al., 2012). In this trial, CDP-choline did dot show beneficial effects as compared to placebo as well. These results were surprising and might be partly explained by different methodological evaluations of the disease. Furthermore, there is a big difference in the doses used in animals and humans. In animal experiments, usually doses of 500 mg/kg are applied, which would be equivalent to 35,000 mg CDP-choline per day in a 70 kg person. However, in clinical trials doses of 2,000 mg per day were used.
In our opinion, the disappointing results in clinical trials in stroke and brain injury do not neccessarily imply negative results in demyelinating disorders such as multiple sclerosis. In these diseases, there is a completely different mechanism of lesion induction. In contrast to stroke, multiple sclerosis lesions are accompanied by demyelination while axons/neurons are not completely damaged during the onset of disease. Subsequently during repair processes, new OPC proliferate and differentiate into mature oligodendrocytes and build new myelin sheaths.
To date, CDP-choline has been studied in several thousand humans and showed a favourable safety profile (Grieb, 2014; Adibhatla and Hatcher, 2002). Adverse effects were rare and consisted of stomach pain, diarrhea, and headaches. As shown in rodent experiments, there is a lack of toxicity of CDP-choline. The median lethal dose (LD50) is very high with 8,000 mg/kg orally applied CDP-choline in mice and rats (Grieb, 2014). This dose would correspond to 560,000 mg CDP-choline in a 70 kg person, which is almost not possible to ingest.
In conclusion, CDP-choline showed repair promoting functions in two animal models of multiple sclerosis with a new mechanism of action. Due to the known beneficial safety profile, CDP-choline may be a promising substance for patients with multiple sclerosis, which is worth further investigations, e.g., as add-on therapy to established immunomodulators.
References
- Adibhatla RM, Hatcher JF. Citicoline mechanisms and clinical efficacy in cerebral ischemia. J Neurosci Res. 2002;70:133–139. doi: 10.1002/jnr.10403. [DOI] [PubMed] [Google Scholar]
- Davalos A, Alvarez-Sabin J, Castillo J, Diez-Tejedor E, Ferro J, Martinez-Vila E, Serena J, Segura T, Cruz VT, Masjuan J, Cobo E, Secades JJ. Citicoline in the treatment of acute ischaemic stroke: an international, randomised multicentre, placebo-controlled study (ICTUS trial) Lancet. 2012;380:349–357. doi: 10.1016/S0140-6736(12)60813-7. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Grieb P. Neuroprotective properties of citicoline: facts, doubts and unresolved issues. CNS Drugs. 2014;28:185–193. doi: 10.1007/s40263-014-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieb P. Beneficial effects of exogenous CDP-cholinê (citicoline) in EAE. Brain. 2015 doi: 10.1093/brain/awv140. doi: 10.1093/brain/awv140. [DOI] [PubMed] [Google Scholar]
- Hjelmstad RH, Bell RM. Molecular insights into enzymes of membrane bilayer assembly. Biochemistry. 1991;30:1731–1740. doi: 10.1021/bi00221a001. [DOI] [PubMed] [Google Scholar]
- Mi S, Miller RH, Tang W, Lee X, Hu B, Wu W, Zhang Y, Shields CB, Zhang Y, Miklasz S, Shea D, Mason J, Franklin RJ, Ji B, Shao Z, Chédotal A, Bernard F, Roulois A, Xu J, Jung V, Pepinsky B. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann Neurol. 2009;65:304–315. doi: 10.1002/ana.21581. [DOI] [PubMed] [Google Scholar]
- Skripuletz T, Gudi V, Hackstette D, Stangel M. De- and remyelination in the CNS white and grey matter induced by cuprizone: the old, the new, and the unexpected. Histol Histopathol. 2011;26:1585–1597. doi: 10.14670/HH-26.1585. [DOI] [PubMed] [Google Scholar]
- Skripuletz T, Manzel A, Gropengiesser K, Schafer N, Gudi V, Singh V, Salinas TL, Jorg S, Hammer A, Voss E, Vulinovic F, Degen D, Wolf R, Lee DH, Pul R, Moharregh-Khiabani D, Baumgartner W, Gold R, Linker RA, Stangel M. Pivotal role of choline metabolites in remyelination. Brain. 2015;138:398–413. doi: 10.1093/brain/awu358. [DOI] [PubMed] [Google Scholar]
- Zafonte RD, Bagiella E, Ansel BM, Novack TA, Friedewald WT, Hesdorffer DC, Timmons SD, Jallo J, Eisenberg H, Hart T, Ricker JH, Diaz-Arrastia R, Merchant RE, Temkin NR, Melton S, Dikmen SS. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT) JAMA. 2012;308:1993–2000. doi: 10.1001/jama.2012.13256. [DOI] [PubMed] [Google Scholar]


