The most important risk factor for stroke and neurodegeneration is aging. In fact, survival after stroke diminishes largely with aging. In fact, recovery after brain artery occlusion is dramatically worsened by aging, even normal aging is associated with neuron damage and cognitive decline. Mechanisms involved in aging-related, cognitive decline and susceptibility to neuron damage in stroke and neurodegeneration are largely unknown. One of the most important mechanisms contributing to neural dysfunction and death is excitotoxicity. This process is based on the fact that the excessive glutamate receptor stimulation may lead to neuronal damage. This overstimulation may be due to increased concentration of glutamate, or the prolonged activation of receptors.
Protecting the aging brain against damage remains a big challenge for neurologists and neuroscientists. Interestingly, a large number of basic and clinical studies have provided strong evidence indicating that the prolonged use of non-steroidal anti-inflammatory drugs (NSAIDs) may reduce the incidence of Alzheimer's disease (AD) (Wang et al., 2015), the most common form of dementia. NSAIDs also decreased glutamate excitotoxicity both in vitro, in rat primary neuronal cultures and hippocampal slices (Grilli et al., 1996), and in vivo, protecting rats against rotenone-induced parkinsonism (Madathil et al., 2013). Recent evidence suggests also that NSAIDs may even protect against the cognitive decline associated to healthy aging in humans (Kern et al., 2012).
NSAIDs present antipyretic, anti-inflammatory and analgesic effects. Therefore, they are mainly used to relief pain, fever and inflammation. Their best characterized action is inhibition of cyclooxygenase (COX), and thus the synthesis of prostaglandins, which participate in the inflammatory response. NSAIDs can be non-selective COX inhibitors such as aspirin, ibuprofen, indomethacin or sulindac; or selective COX-2 inhibitors, such as rofecoxib and celecoxif. The action mechanism of neuroprotection by NSAIDs is unknown, but reports suggest that it is not related to the classic anti-inflammatory activity of these drugs. It is widely accepted that neuronal excitotoxicity induced by glutamate is mainly caused by one kind of ionotropic glutamate receptor, the N-methyl-D-aspartate receptor (NMDAR), probably because of its high permeability to Ca2+. The combination of different subunits constitutes NMDARs: The NR1 subunit, is ubiquitous and essential whereas the NR2 subunit, is a regulatory subunit (NR2A - NR2D). There is also a third subunit named NR3 (NR3A - NR3B). A functional NMDA receptor requires the binding of two NR1 subunits with two other NR2 subunits or with the combination of a NR2 and NR3 subunits. In normal synaptic transmission, the NMDAR, blocked by the Mg2+ located in the channel, is activated for short periods of time.
However, in pathological conditions, like the prolonged depolarization that takes place in ischemic events, during which Mg2+ is fully removed from its binding site at the NMDARs, an overly activation of the receptor causes excessive Ca2+ entry through the channel. This Ca2+ entry, together with the Ca2+ released from the intracellular stores, increases the cytosolic free Ca2+ concentration to levels that exceed the capacity of the intracellular Ca2+ clearing mechanisms and pumps leading to mitochondrial Ca2+ overload. This may cause impaired metabolism and certain processes that trigger cell death such as the one in the neurodegenerative disorders (Pivovarova et al., 2004).
We have reported that oligomers, but not fibrils, of the amyloid β peptide 1–42 (Aβ1–42), the most likely toxin in AD, induce also a sustained entry of Ca2+ followed by mitochondrial Ca2+ overload leading to cell death in cultures of rat cerebellar granule cells (Sanz-Blasco et al., 2008). The pathway for Ca2+ entry remains unknown, but several reports suggest it could be mediated, at least partially, by NMDA receptors. Interestingly, we showed that a series of NSAIDs, including salicylate (the major metabolite of aspirin), ibuprofen, sulindac sulfide, indomethacin and the structural analogue lacking anti-inflammatory activity R-flurbiprofen, are able to depolarize partially mitochondria preventing mitochondrial Ca2+ overload without affecting Ca2+ influx induced by oligomers of Aβ1–42. All these effects were achieved at fairly low concentrations of NSAIDs, in the μM range, far from those required for preventing inflammation or reducing the Aβ burden. Mitochondrial depolarization could be easily explained by the chemical structure of carboxylic NSAIDs, resembling mild mitochondrial uncouplers. These class of compounds are able to decrease partially mitochondrial potential, the huge driving force for mitochondrial Ca2+ uptake, in a similar manner to low concentrations of established mitochondrial uncouplers as carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP).
Understanding the mechanisms of aging-related susceptibility to neuron damage and neuroprotection by NSAIDs is critical since they may provide feasible ways of preventing brain damage in the elderly. However, this study has been hampered by the lack of suitable models of brain aging. Recently, several authors have provided evidence indicating that long-term cultures of hippocampal neurons show many of the typical hallmarks of aged neurons including accumulation of reactive oxygen species (ROS), lipofuscin granules, heterochromatic foci, activation of the Jun N-terminal protein kinase (pJNK) and p53/p21 pathways, gradual loss of cholesterol, and changes in Ca2+ channel density and NMDA receptor expression (Sodero et al., 2011). In addition, studies show the increased vulnerability of hippocampal neurons with age in culture (Brewer et al., 2007). We have recently used long-term cultures of neonatal rat hippocampal neurons to investigate age-related susceptibility to excitotoxicity and neuroprotection by NSAIDS. We found that NMDA promoted cell death only in aged neurons cultured for several weeks in vitro but not in young cultures (Calvo et al., 2015), despite both expressed NMDA receptors and showed NMDA-induced rises in cytosolic [Ca2+]. However, the increases in cytosolic [Ca2+] induced by NMDA were much larger in older cultures than in younger neurons. These changes correlated with changes in the composition and density of NMDA receptor subunits consistently with those observed in vivo (Cui et al., 2013). Most importantly, NMDA induced mitochondrial Ca2+ uptake only in aged neurons. Thus, mitochondria from young neurons are seemingly not sensitive to the changes in cytosolic [Ca2+].
Several mechanisms may contribute to differential behavior of mitochondria from young and old neurons, but the most likely one is the increased rises in cytosolic [Ca2+] and enhanced resting cytosolic [Ca2+] observed in older neurons (Calvo et al., 2015). Whereas the mechanism for enhanced resting cytosolic [Ca2+] remains unknown, the basis for increased responses to NMDA can be explained by the changes in NMDA receptor density and composition (Calvo et al., 2015). In summary, mitochondrial Ca2+ overload is critical for enhanced susceptibility to cell death in aged neurons (Figure 1). This view is supported by the fact that NMDA promotes permeability transition and release of cytochrome c in older neurons and these effects are prevented by mitochondrial depolarization with FCCP. The role of mitochondria is further supported by the fact that several NSAIDs, including salicylate, indomethacin, sulindac sulphide and the structural analogue R-flurbiprofen depolarize mitochondria and prevent also mitochondrial Ca2+ uptake and neuron cell death without affecting the rise in cytosolic [Ca2+] induced by NMDA. Therefore, these results strongly suggest that enhanced mitochondrial Ca2+ uptake contributes largely to aging-related susceptibility to neuron cell damage and cognitive decline. Most importantly, they also support the view that these processes could be prevented to some extent by the use of low concentrations of NSAIDs (Figure 2). Of course, clinical research is required to fully support this view.
Figure 1.
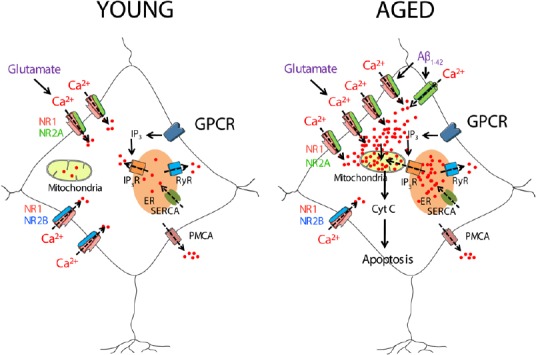
A model of neurotoxicity induced by excessive Ca2+ entry induced by glutamate or by Aβ1–42 oligomers in aged neurons.
Young hippocampal neurons show a similar expression of NR1/NR2A and NR1/NR2B receptors. However, aged neurons show an increased expression of NR1/NR2A receptors. NMDA receptor activation induces a greater Ca2+ entry in aged neurons than in the young ones, which together with the Ca2+ released from the intracellular stores can cause mitochondrial Ca2+ overload, cytochrome c release and apoptosis. Furthermore, aged neurons are more susceptible to the Aβ1–42 oligomers induced Ca2+ entry. Aβ1–42: Amyloid β peptide 1–42; Cyt C: cytochrome C; ER: endoplasmic reticulum; GPCR: G protein-coupled receptor; IP3: inositol trisphosphate; IP3R: inositol trisphosphate receptor; NR1, NR2A, NR2B: subunits 1, 2A and 2B of the N-methyl-D-aspartate receptor; PMCA: plasma membrane Ca2+-ATPase; RyR: ryanodine receptor; SERCA: sarcoplasmic and endoplasmic reticulum Ca2+-ATPase.
Figure 2.
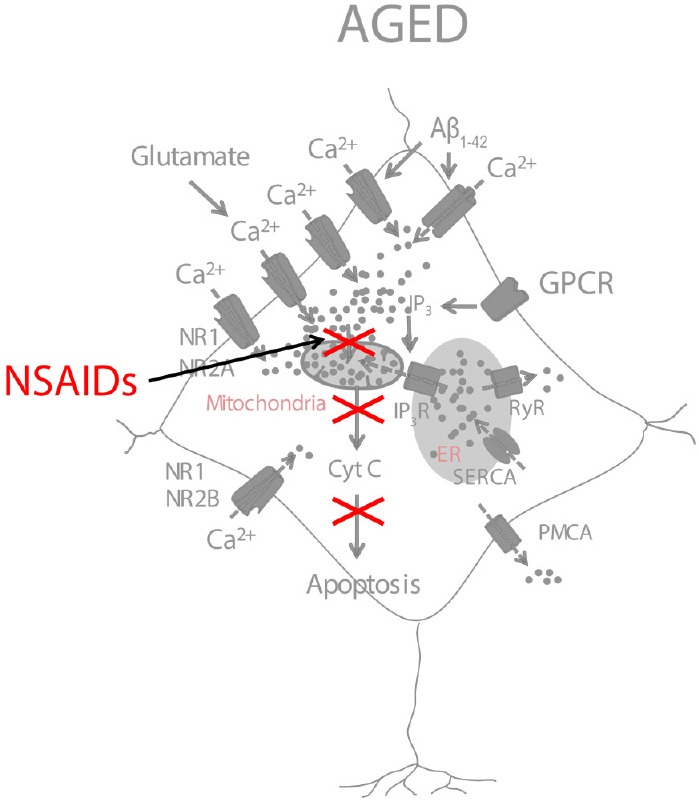
A model of NSAID neuroprotection based on the inhibition of mitochondrial Ca2+ overload.
NSAIDs, used at low concentrations, partially depolarize mitochondria and inhibit mitochondrial Ca2+ overload, thus preventing the release of cytochrome c and NMDA- or Aβ1–42-induced apoptosis. Aβ1–42: Amyloid β peptide 1–42; NSAIDs: non steroidal anti-inflammatory drugs.
Interestingly, some NSAIDs have been already proposed or even tested to prevent neuron cell death in brain pathologies. For example, R-flurbiprofen (Flurizan™) was tested for preventing AD in a large-scale clinical trial that failed in its phase three. Notably, this compound was used at large concentrations aimed at reducing the Aβ burden. The reasons for the failure of R-flurbiprofen in this trial are not clear at present but it has been proposed that damage in AD patients might be too severe to be reversed by even the best drugs. In support of this view, it has been shown recently that R-flurbiprofen, a drug that lacks anti-inflammatory activity, prevents and attenuates primary progressive experimental multiple sclerosis in mice, even if the treatment commenced on or after the first signs of the disease (Schmitz et al., 2014). Further research is required to test the use of selected NSAIDs and structural analogues without anti-inflammatory activity in neuron damage associated to aging.
This work was supported by grants VA145U13, BIO/VA33/13, BIO103/VA45/11 from Junta de Castilla y León, Spain and BFU2012-37146 from Ministerio de Economía y Competitividad, Spain. MCR was supported by a pre-doctoral fellowship from Junta de Castilla y León, Spain and The European Social Fund.
References
- Brewer LD, Thibault O, Staton J, Thibault V, Rogers JT, Garcia-Ramos G, Kraner S, Landfield PW, Porter NM. Increased vulnerability of hippocampal neurons with age in culture: temporal association with increases in NMDA receptor current, NR2A subunit expression and recruitment of L-type calcium channels. Brain Res. 2007;1151:20–31. doi: 10.1016/j.brainres.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Calvo M, Sanz-Blasco S, Caballero E, Villalobos C, Nunez L. Susceptibility to excitotoxicity in aged hippocampal cultures and neuroprotection by non-steroidal anti-inflammatory drugs: role of mitochondrial calcium. J Neurochem. 2015;132:403–417. doi: 10.1111/jnc.13004. [DOI] [PubMed] [Google Scholar]
- Cui Z, Feng R, Jacobs S, Duan Y, Wang H, Cao X, Tsien JZ. Increased NR2A:NR2B ratio compresses long-term depression range and constrains long-term memory. Sci Rep. 2013;3:1036. doi: 10.1038/srep01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M, Pizzi M, Memo M, Spano P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-kappaB activation. Science. 1996;274:1383–1385. doi: 10.1126/science.274.5291.1383. [DOI] [PubMed] [Google Scholar]
- Kern S, Skoog I, Ostling S, Kern J, Borjesson-Hanson A. Does low-dose acetylsalicylic acid prevent cognitive decline in women with high cardiovascular risk. A 5-year follow-up of a non-demented population-based cohort of Swedish elderly women. BMJ Open. 2012:2. doi: 10.1136/bmjopen-2012-001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madathil SK, Karuppagounder SS, Mohanakumar KP. Sodium salicylate protects against rotenone-induced parkinsonism in rats. Synapse. 2013;67:502–514. doi: 10.1002/syn.21658. [DOI] [PubMed] [Google Scholar]
- Pivovarova NB, Nguyen HV, Winters CA, Brantner CA, Smith CL, Andrews SB. Excitotoxic calcium overload in a subpopulation of mitochondria triggers delayed death in hippocampal neurons. J Neurosci. 2004;24:5611–5622. doi: 10.1523/JNEUROSCI.0531-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Blasco S, Valero RA, Rodriguez-Crespo I, Villalobos C, Nunez L. Mitochondrial Ca2+ overload underlies Abeta oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS One. 2008;3:e2718. doi: 10.1371/journal.pone.0002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz K, de Bruin N, Bishay P, Mannich J, Haussler A, Altmann C, Ferreiros N, Lotsch J, Ultsch A, Parnham MJ, Geisslinger G, Tegeder I. R-flurbiprofen attenuates experimental autoimmune encephalomyelitis in mice. EMBO Mol Med. 2014;6:1398–1422. doi: 10.15252/emmm.201404168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodero AO, Weissmann C, Ledesma MD, Dotti CG. Cellular stress from excitatory neurotransmission contributes to cholesterol loss in hippocampal neurons aging in vitro. Neurobiol Aging. 2011;32:1043–1053. doi: 10.1016/j.neurobiolaging.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Wang J, Tan L, Wang HF, Tan CC, Meng XF, Wang C, Tang SW, Yu JT. Anti-inflammatory drugs and risk of Alzheimer's disease: an updated systematic review and meta-analysis. J Alzheimers Dis. 2015;44:385–96. doi: 10.3233/JAD-141506. [DOI] [PubMed] [Google Scholar]


