Each year approximately 360,000 people in the United States suffer a peripheral nerve injury (PNI), which is a leading source of lifelong disability (Kelsey et al., 1997; Noble et al., 1998). The most frequent cause of PNIs is motor vehicle accidents, while gunshot wounds, stabbings, and birth trauma are also common factors. Patients suffering from disabilities as a result of their PNIs are also burdensome to the healthcare system, with average hospital stays of 28 days each year (Kelsey et al., 1997; Noble et al., 1998).
The technique of autologous nerve grafting is considered the gold standard and the most reliable choice in repair of major defects in peripheral nerves (Chiu and Ishii, 1986; Huang et al., 2004; Lee and Wolfe, 2000; Lundborg, 2000). The introduction of an autologous axon segment provides a physical and biological scaffolding over which axonal outgrowth may occur. Complications arise, however, because of the limited supply of donor nerves and the risks associated with the harvesting surgery. Donor sites are vulnerable to infections, the formation of painful neuromas, and loss of function associated with the harvested nerve.
In recent years there has been considerable interest in developing alternative strategies to repair damaged peripheral nerves through transplanted materials of biologic or synthetic origin if donor nerves are scarce (Midha et al., 1993; Hudson et al., 2000; Scherman et al., 2001; Cheng and Chen, 2002).
Despite extensive research into nonautologous alternatives, the autograft has remained the gold standard for the repair of peripheral nerve injuries. Many studies have identified elements that encourage neural regeneration – from mechanisms of axon development, to experimental treatments – but individual breakthroughs remain as separate components, and even if paired, fail to completely address the complexities of real injuries.
A recent article in Nature Medicine by Stuart Forbes and Nadia Rosenthal accurately captures this short-coming, and recommends that “seed-and-soil” concepts be applied to the development of future cell-based regenerative therapies. The seed-and-soil perspective posits that in failing to prepare the embedding environment (the ‘soil’) with the necessary cellular and signaling conditions, it is unreasonable to expect that stem cells (the ‘seeds’) will successfully engraft. In injury, where cellular and signaling responses diverge from the norm, addressing these numerous components is an obstacle to the development of effective treatments (Forbes and Rosenthal, 2014).
At first, it is tempting to extend this notion of seed-and-soil beyond stem cells. In the case of peripheral nerve repair, where novel grafting techniques are regularly heralded, the same principles of preparing the embedding environment might apply. But unlike other organs, peripheral nerves have limited regenerative potential, and require solutions involving autologous donations, tissue-engineered nerve grafts, and other biomaterials. These sizable grafts do not fit the ‘seed’ depiction.
Regardless of whether nerves are grafted with donor nerves or synthetic conduits, the axons and many supportive cells of the disconnected portion of the nerve degenerate, resulting in the loss of the labeled pathway necessary to guide axon outgrowth. This factor coupled with the relatively slow growth of sprouting axons (approximately 1 mm/day) commonly results in poor functional recovery of extremities that are far away from nerve damage. For example, as is commonly found with brachial plexus injury, while elbow flexion may ultimately be regained, hand function is not, resulting in significant impairment of the activities of daily living.
While a primary strategy to repair major peripheral nerve injury (PNI) is to bridge the damage with axons, producing axons of sufficient length and number has posed a significant challenge. The gold standard in peripheral nerve repair, the autologous nerve graft, is limited by the availability of donor axons and complications arising from the harvesting surgery. In addition, most alternative bridges currently used for nerve damage (e.g., synthetic tubes) are limited in the length that they can span to promote repair and are typically used for gaps of less than 2–3 cm. During the past decade, our research teams have been able to utilize a novel tissue engineering technique to create transplantable nervous tissue constructs for major peripheral nerve repair. The key to this procedure is to use a specially designed motorized micro-stepper to produce continuous mechanical tension on axons spanning two initially apposed populations of cultured neurons. Using dorsal root ganglia (DRG) neurons, this technique has rapidly produced nerve tracts consisting of 106 axons grown at rates of 10 mm/day, reaching a remarkable 10 cm in length. To form transplantable nervous tissue constructs, the elongated cultures are embedded in collagen and placed into tubes composed of polyglycolic acid (PGA). In our preliminary studies, stretch-grown cultures used to repair a 1.3 cm rat sciatic nerve deficit were found to survive long-term (4 months), demonstrating robust incorporation of grafted axons within a regenerating network of host axons, including outgrowth of graft axons into host nerve (Huang et al., 2009), and improved restoration of hindlimb motor function.
Ideally, nervous tissue engineered to recapitulate the geometry and orientation of the nervous system could act as a bridge across regions of damage and promote functional recovery. Towards this goal, we have been using axon stretch-growth to create long axon tracts spanning two populations of neurons to serve as living nervous tissue constructs for transplantation and repair of even extensive PNIs (Figure 1). Limiting factors of inducing axon growth for nerve repair include the length the axons can be grown, the rate of growth, the total number of axons in each preparation, and the viability of the axons. To greatly increase this growth rate to produce much longer nervous tissue constructs in a shorter period of time, the stretch growth potential of PNS axons from dorsal root ganglia (DRG) was investigated. It has been well established that PNS axon outgrowth is more rapid and robust in culture than that of CNS axons. In addition, nervous tissue constructs of DRG axons could conceivably be used to repair lesions in both the CNS and PNS. More importantly, we have found that DRGs are an ideal source of neurons that can be used clinically, harvested either from the patient themselves to create autologous grafts, or recovered from organ donors to make allografts. In preliminary studies, we have successfully cultured DRG neurons from 16 patients undergoing therapeutic ganglionectomies and from 4 organ donors. Furthermore, we have been able to elongate cultured human axon tracts from 6 patients (Huang et al., 2008).
Figure 1.
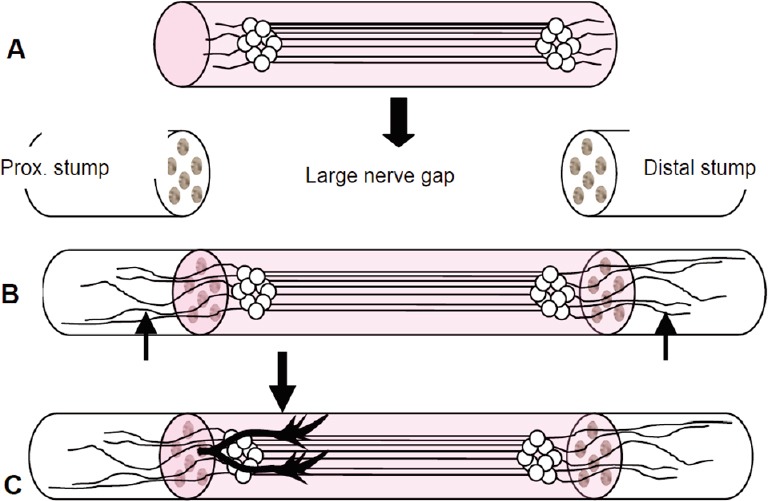
The engineered nervous tissue construct consists of living pure stretch-grown axon tracts spanning two neuron populations (without glial elements), embedded in collagen and inserted into a polyglycolic acid tube.
(A) The construct is transplanted to bridge extensive regions of nerve damage (large arrow). (B) After transplantation, axons from the construct grow out both proximally and distally penetrating into the host nerve (arrows). (C) The construct serves as a living labeled pathway to guide sprouting axons from the host proximal (prox.) nerve stump across extensive nerve damage (arrow) to reinnervate tissue distal to the lesion.
The function recovery of peripheral nerves hinges upon a number of considerations beyond ‘soil’ conditions. These include gap length, nerve diameter and type, availability of donor grafts, fascicular matching and orientation and tissue handling, not to mention immune compatibility (Chiu and Ishii, 1986). But the embedding environment presents other challenges that directly weigh into these consideration.
Time is an important variable that may dictate repair technique. Following injury, a cascade of events continue to remodel the injury site over months and years. Certain repairs may be better suited for an environment when the time between injury and treatment is in days, whereas others may have advantages when the duration is in weeks.
In peripheral nerves, the prospect for regeneration is dependent upon the initial degenerative processes. These include immune and inflammatory responses that prepare the environment for new growth. Axonal degeneration initiates these responses at approximately a week following injury. At the point of detachment, the cytoskeleton quickly disintegrates, and the blood-nerve barrier becomes more permeable as the lengths of axon adjacent to the stump become compromised. This breakdown serves to prepare the site for the subsequent events that occur within a month after injury, as Schwann cells proliferate and recruit macrophages via secreted cytokines. As inhibitory debris is cleared, Schwann cells release trophic factors to stimulate axon regrowth. But if denervation is prolonged, support for regeneration is lost within months as Schwann cells begin to die (Gaudet et al., 2011).
There are a variety of cell types present within peripheral nerves in addition to neurons (axons) and Schwann cells, including perineurial cells, pericytes, endothelials and endoneurial fibroblast-like cells. Cells like fibroflasts have been considered a relatively inert population of cells. In contrary to “traditional” fibrobrasts, endoneurial fibroblast-like cells have a mesenchymal origin and appear to derive from the neural crest. These cells could become interesting actors to increase the efficacy of our transplanted elongated nerve constructs, which only contain neurons.
Scar formation is another concern. As connective tissue infiltrates the nerve gap and fills available, the likelihood that regenerative axons reinnervate their target is reduced. Scarring across the embedding site also impedes a graft's access to vascularization. In the case where a nerve would face prolonged ischemia and poor nutrient availability due to scarring, repair would require a vascularized graft (Grinsell and Keating, 2014).
Electrical stimulation is yet another factor. In the absence of stimulation, muscles and sensory organs will experience deterioration and loss of function. To prevent this, reinnervation must be quickly achieved (Grinsell and Keating, 2014). However, reinnervation appears to benefit from electrical stimulation as well, both in cell culture, and in vivo, possibly providing axon outgrowths directionality (Haan and Song, 2013).
With novel tissue engineering techniques, we hope that transplantation of these nervous tissue constructs will promote regeneration of transected nerves by providing a living pathway to guide host axons from the proximal nerve stump across nerve lesions with significant gaps. Thus, we hope to use our new technique to provide a significant advancement for major nerve reconstruction.
References
- Cheng B, Chen Z. Fabricating autologous tissue to engineer artificial nerve. Microsurgery. 2002;22:133–137. doi: 10.1002/micr.21740. [DOI] [PubMed] [Google Scholar]
- Chiu DT, Ishii C. Management of peripheral nerve injuries. Orthop Clin North Am. 1986;17:365–373. [PubMed] [Google Scholar]
- Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med. 2014;20:857–869. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int 2014. 2014 doi: 10.1155/2014/698256. 698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan N, Song B. Therapeutic application of electric fields in the injured nervous system. Adv Wound Care. 2013;3:156–165. doi: 10.1089/wound.2013.0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JH, Zaghloul K, Zager EL. Surgical management of brachial plexus region tumors. Surg Neurol. 2004;61:372–378. doi: 10.1016/j.surneu.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Huang JH, Zager EL, Groff RG, Zhang J, Pfister BJ, Chen X, Iwata A, Maloney-Wilensky E, Grady MS, Smith DH. Harvested Human Neurons as Live Nervous Tissue Constructs: Implications for Transplantatioin. J Neurosurg. 2008;108:343–347. doi: 10.3171/JNS/2008/108/2/0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JH, Cullen DK, Browne KD, Groff R, Zhang J, Pfister BJ, Zager EL, Smith DH. Long-term survival and integration of transplanted engineered nervous tissue constructs promotes peripheral nerve regeneration. Tissue Eng Part A. 2009;15:1677–1685. doi: 10.1089/ten.tea.2008.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson TW, Evans GR, Schmidt CE. Engineering strategies for peripheral nerve repair. Orthop Clin North Am. 2000;31:485–498. doi: 10.1016/s0030-5898(05)70166-8. [DOI] [PubMed] [Google Scholar]
- Kelsey JK, Nelson L, Felberg A, Rice D. Philadelphia: W.B. Saunders Company; 1997. Upper extremity disorders: frequency, impact, and cost. [Google Scholar]
- Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2002;8:243–252. doi: 10.5435/00124635-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Lundborg G. Brain plasticity and hand surgery: an overview. J Hand Surg. 2000;25:242–252. doi: 10.1054/jhsb.1999.0339. [DOI] [PubMed] [Google Scholar]
- Midha R, Mackinnon SE, Evans PJ, Best TJ, Hare GM, Hunter DA, Falk-Wade JA. Comparison of regeneration across nerve allografts with temporary or continuous cyclosporin A immunosuppression. J Neurosurg. 1993;78:90–100. doi: 10.3171/jns.1993.78.1.0090. [DOI] [PubMed] [Google Scholar]
- Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45:116–122. doi: 10.1097/00005373-199807000-00025. [DOI] [PubMed] [Google Scholar]
- Scherman P, Lundborg G, Kanje M, Dahlin LB. Neural regeneration along longitudinal polyglactin sutures across short and extended defects in the rat sciatic nerve. J Neurosurg. 2001;95:316–323. doi: 10.3171/jns.2001.95.2.0316. [DOI] [PubMed] [Google Scholar]


