Conventional vs. polyethylene glycol (PEG)-fusion technologies to repair severed spinal axons: Most spinal cord injuries (SCIs) involve cut- or crush-severance of spinal tract axons in the central nervous system (CNS). Clinical outcomes after CNS axonal severance is very poor because proximal segments of CNS axons lack a suitable environment for outgrowth (Kakulas, 1999; Fitch and Silver, 2008; Rowland et al., 2008; Kwon et al., 2010) and therefore do not naturally regenerate (Ramon y Cajal, 1928). Current strategies to try to increase behavioral recovery after SCI are focused on enhancing the environment for axonal outgrowth. These strategies have had limited success to enhance return of function in animal model systems (Kwon et al., 2010). Encouraging outgrowths from surviving damaged axons may also provide significant benefits when spinal severance is not complete (Bittner and Fishman, 2000). However, these approaches, even if beneficial, do not prevent Wallerian degeneration of severed distal axonal segments, although PEG (Luo et al., 2002; Kwon et al., 2009, 2010), methylene blue (MB) (Rojas et al., 2009), and melatonin (MEL) (Stavisky et al., 2005; Raza et al., 2008) administered in low systemic concentrations may have some neuroprotective effects following SCI.
For several decades, we have been developing and improving a novel technology using a well-specified sequence of bioengineered solutions of varying tonicity and concentration. These bioengineered solutions of PEG, MB, and calcium (Ca2+) rapidly (within minutes) fuse/connect cut- or crush-severed ends of closely apposed nerve axons in completely severed nerves to restore axolemmal and axoplasmic continuity and action potential conduction across the lesion site (Bittner et al., 1986; Bittner, 2000; Riley et al., 2015). This technology first included PEG and varying osmotic and Ca2+ concentrations to repair invertebrate giant axons ex vivo (Bittner et al., 1986; Krause and Bittner, 1990), then in vivo (Lore et al, 1999), and then mammalian peripheral nerve axons (PNAs) and CNS axons ex vivo (Lore et al., 1999; Bittner et al., 2000; Marzullo et al., 2002). More recently, the use of MB and micro-sutures were added to this evolving PEG-fusion technology to repair completely crush- or cut-severed sciatic nerves in rats in vivo to restore 40–80% of lost behaviors within 4–6 weeks and prevent or retard much Wallerian degeneration (Britt et al., 2010; Bittner et al., 2012). Even more recently, micro-sutured and PEG-fused autografts (Sexton et al., 2012) and (especially) allografts (Riley et al., 2015) have been used to restore lost behavioral functions within 6 weeks postoperatively, in some cases almost complete recovery within 1–2 weeks after ablating a 0.5–1.0 cm segment from a completely cut-severed sciatic nerve.
This PEG-fusion technology uses hypotonic Ca2+-free saline to open and expand and closely appose the partially collapsed vesicle-filled ends of severed axons that are connected by glial sheaths if crushed, or brought into close apposition (touching) by micro-sutures if cut. Next, the lesion site is bathed in hypotonic Ca2+-free saline with the anti-oxidant MB to prevent vesicle formation. A hypotonic solution of PEG is then applied to fuse the open, vesicle-free axonal ends. Finally, the lesion site is bathed in Ca2+-containing isotonic saline that induces vesicles to form and accumulate and seal any remaining axolemmal holes (Lore et al., 1999; Riley et al., 2015).
We hypothesized that this PEG-fusion technology that successfully rejoins cut- and crush-severed PNAs in vivo could also rejoin crush-severed CNS axons (Lore et al., 1999). We also hypothesized that the lipophilic properties of an amphoteric substance like PEG would allow it to readily penetrate the spinal cord white matter. Our data show that our PEG-fusion technology can indeed significantly enhance restoration of lost behaviors following SCI crush-severed injuries produced by a MASCIS device. We speculate that this PEG-fusion technology could produce a paradigm-shift in the treatment of traumatic injuries to CNS axons.
Materials and methods to induce PEG-fusion repair of crush-severed spinal axons: Animal studies were approved by the Institutional Animal and Care Use Committee (IACUC) of Wayne State University, USA. Male adult Lewis rats (260–285 g, n = 12) were maintained on a 12-hour dark/light cycle, given food and water ad libitum and allowed to acclimate for several days before surgery. At 30 minutes before surgery, rats were given Buprenex (0.05 mg/kg body weight) and were then anesthetized with 5% isoflurane via an inhalation chamber. The rats were maintained at 1.5–1.8% isoflurane using a nose cone. Paralube ointment was applied to eyes and rats were placed on water circulating heating pad. Rats were shaved and skin was cleaned with betadine scrub, then with 70% alcohol. A laminectomy was performed at thoracic vertebrae 9–10 and the dura exposed. A MASCIS device (NY University, NYC, NY, USA; 10 g, 12.5 cm drop) delivered a moderate contusive injury (Gruner, 1992; Constantini and Young, 1994) that crush-severs many spinal tract axons. The dura over the injury site was immediately opened and the site flooded with the following series of four solutions to PEG-fuse crushed CNS axons:
(1) For 3 minutes, hypotonic calcium (Ca2+)-free Krebs saline solution containing (in mM) 0.5 EGTA, 99 NaCl, 5 KCl, 1.2 KH2PO4, 1.3 MgSO4, 26 NaHCO3, 10 Na ascorbate, 10 dextrose, pH 7.35, 274 mOsm, in double distilled H2 O (ddH2 O) to open the cut ends of the axons, expel vesicles, and expand the axoplasm and axolemma so that open axonal membranes at severed ends come in close apposition. See Figure 1 of Britt et al. (2010).
Figure 1.
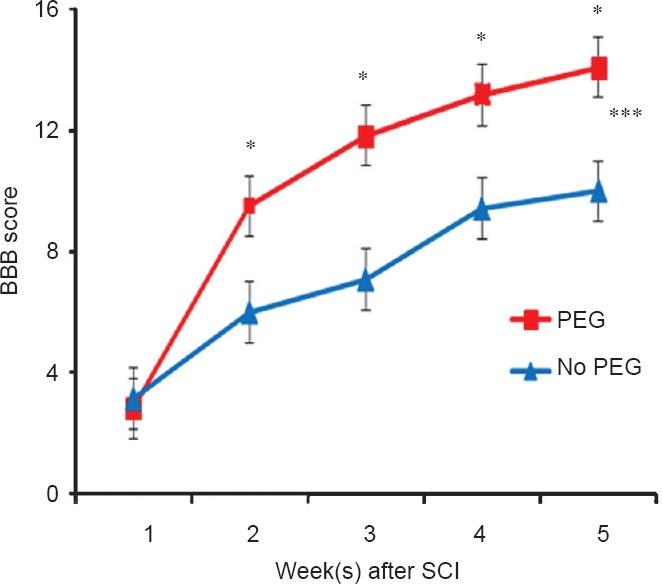
Effect of polyethylene glycol (PEG) treatment on hindlimb locomotor function following spinal cord injury (SCI).
A MASCIS rod-drop device (set at 10 g, 12.25 cm) was used to produce a mild contusive SCI at T9–10 in 12 male Lewis rats randomly assigned to receive a series of experimental solutions that included methylene blue and PEG or the same series of solutions without PEG. *The Basso, Beattie and Bresnahan (BBB) locomotor test scores at the same postoperative time differ by P < 0.05 (two-tailed Student's t-test). ***Curves differ by P < 0.001 according to a two-way analysis of variance (ANOVA) with Bonferroni post-hoc test.
(2) For 2 minutes, hypotonic Ca2+-free saline as described above containing 100 μM methylene blue (Faulding, Aguadilla, PR, USA) to prevent vesicle formation and interactions.
(3) For 2 minutes, a hypotonic solution of 500 mM 3.25 kDa PEG (Sigma Aldrich, St. Louis, MO, USA) in ddH2 O to fuse the membranes of closely apposed open axonal ends.
(4) For 2–4 minutes, wash at least 3× with an isotonic Ca2+-containing physiological saline consisting of (in mM) 124 NaCl, 5 KCl, 1.2 KH2 PO4, 1.3 MgSO4, 26 NaHCO3, 10 Na ascorbate, 10 dextrose, 2 CaCl2, pH 7.35, 321 mOsm in ddH2 O to induce the formation of vesicles and activate proteins that induce their accumulation at sites of plasmalemmal damage where they interact to seal plasmalemmal disruptions.
Six randomly-assigned “PEG-fused” animals received all four solutions described above and six randomly assigned “negative control” animals received all four solutions except that PEG was not added to solution (3). After application of these four solutions, the meninges were placed in their original position and muscles closed with 3-0 suture. The skin incision was closed with wound clips. Bladders were expressed twice per day until function returned.
Rats were behaviorally assessed for return of hindlimb function using the Basso, Beattie and Bresnahan (BBB) locomotor test (Basso et al., 1996) for 5 weeks postoperatively by a person blinded as to treatment. Data were analyzed using a two-tailed Student's t-test and two-way analysis of variance (ANOVA) with Bonferroni post-hoc test with significance set at P < 0.05.
Results obtained in applying a PEG-Fusion technology to repair severed spinal axons: No adverse effects were observed in applying the series of solutions to the dorsal surface of the spinal cord. The rats were behaviorally tested for 5 weeks. In the first week after injury, the mean BBB score of the negative control group was 3.17 ± 1.25 SEM. These mean scores were slightly higher than the PEG-fused group (2.83 ± 0.91 SEM). At 2–5 postoperative weeks, the mean score of the PEG group was 3–4 points higher than the negative control group. The scores of the PEG-fused group were significantly higher at 3–5 weeks after injury (P < 0.05; Figure 1). Using a two-way ANOVA with Bonferroni post-hoc test, the curves were significantly different (P < 0.001; Figure 1).
Implications of PEG-fusion technologies to repair severed spinal axons: CNS axonal severance is typically the most serious consequence of SCI and probably the most frequent CNS injury treated by clinicians (National Spinal Cord Injury Center, 2008). Many SCIs result from crush contusion transection or ablation, rather than cut, traumas in which the severed ends are connected by damaged glial sheaths and axolemmal membranes (Bittner et al., 2000; Kwon et al., 2010). This first attempt using PEG-fusion to restore behaviors after crush-severance did not produce behavioral recoveries as rapid or as dramatic as that produced by PEG-fusion of crushed, cut or ablated PNAs (Britt et al., 2010; Bittner et al., 2012; Riley et al., 2015). Borgens et al. (2002) have reported recovery of a spinal reflex after delayed application of PEG following a forceps crush injury. Nevertheless, these initial results suggest that our PEG-fusion technology could be used to enhance behavioral recovery after SCI involving a crush lesion to several millimeters in length. PEG-fusion success might be further increased by more rapid removal of the dura or devices that would increase the rate or extent of diffusion of the four PEG-fusion solutions to all portions of the spinal cord. PEG-fusion success might also be enhanced by PEG-fusion of allografts as outlined below.
Following cut injuries, severed spinal axons and other tissues typically separate by several millimeters and there are no tightly adhering tissues like the epineurium or perineurium that surround PNA axons through which micro-sutures might be put to bring cut ends in close apposition required for successful PEG-fusion. Furthermore, more extensive crush injuries would almost-certainly damage greater lengths of spinal axons in Ca2+-containing cerebrospinal fluids and such damage cannot be repaired without using autograft or allograft tissues to bridge the gap that is then PEG-fused at both ends as reported for similar lesions to PNAs (Britt et al., 2010; Bittner et al., 2012). PEG-fusion of donor allograft tissues dramatically restore lost behavioral functions following ablations of sciatic PNAs (Riley et al., 2015) and might have similar results to repair gaps or ablated segments of spinal axons.
It is possible that our current PEG-fusion technology could be modified to rapidly and permanently restore much behavior lost by cut- or crush severance or ablations of spinal axons, a result not readily obtained by any other chemical or surgical treatment published to date. Clinical translation of this PEG-fusion technology may be feasible because all components of the fusion protocol are readily available and FDA approved. Furthermore, other studies have shown cooling (Sea et al., 1995; Marzullo et al., 2002) and cyclosporin A (Sunio et al., 1997) can maintain peripheral nervous system (PNS) or CNS viable and PEG-fusable for 3–10 days. Success with allograft repair of PNS and/or CNS nerve gaps might well lead to establishment of donors and tissue banks for PNS (sciatic, ulnar, etc.) and CNS (spinal) nerves similar to those established for corneas, hearts, kidneys, or livers.
We would like to acknowledge Aleksej Zuzek for help with statistical analyses. GDB and JDP designed this study, performed experiments, and were responsible for data analysis. KKP participated in some experiments. All authors participated in paper writing and approved the final version of this paper. This study was supported by grants from the Lone Star Paralysis Foundation to GDB and JDP and by an NIH grant R01 NS081063 to GDB.
References
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;39:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Bittner GD, Fishman HM. Axonal sealing following injury. In: Ingoglia N, Murray M, editors. Nerve regeneration. New York: Marcel Dekker; 2000. pp. 337–369. [Google Scholar]
- Bittner GD, Ballinger ML, Raymond MA. Reconnection of severed nerve axons with polyethylene glycol. Brain Res. 1986;367:351–355. doi: 10.1016/0006-8993(86)91617-3. [DOI] [PubMed] [Google Scholar]
- Bittner GD, Keating CP, Kane JR, Britt JM, Spaeth CS, Fan JD, Zuzek A, Wilcott RW, Thayer WP, Winograd JM, Gonzalez-Lima F, Schallert T. Rapid, effective, and long-lasting behavioral recovery produced by microsutures, methylene blue, and polyethylene glycol after completely cutting rat sciatic nerves. J Neurosci Res. 2012;90:967–980. doi: 10.1002/jnr.23023. [DOI] [PubMed] [Google Scholar]
- Britt JM, Kane JR, Spaeth CS, Zuzek A, Robinson GL, Gbanaglo MY, Estler CJ, Boydston EA, Schallert T, Bittner GD. Polyethylene glycol rapidly restores axonal integrity and improves the rate of motor behavior recovery after sciatic nerve crush injury. J Neurophysiol. 2010;104:695–703. doi: 10.1152/jn.01051.2009. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Shi R, Bohnert D. Behavioral recovery from spinal cord injury following delayed application of polyethylene glycol. J. Exp Biol. 2002;205:1–12. doi: 10.1242/jeb.205.1.1. [DOI] [PubMed] [Google Scholar]
- Constantini S, Young W. The effects of methylprednisolone and the ganglioside GM1 on acute spinal cord injury in rats. J Neurosurg. 1994;80:97–111. doi: 10.3171/jns.1994.80.1.0097. [DOI] [PubMed] [Google Scholar]
- Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–128. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- Kakulas BA. A review of the neuropathology of human spinal cord injury with emphasis on special features. J Spinal Cord Med. 1999;22:119–124. doi: 10.1080/10790268.1999.11719557. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Sekhon LH, Fehlings MG. Emerging repair, regeneration and translational research advances for spinal cord injury. Spine. 2010;35(21 Suppl):S263–270. doi: 10.1097/BRS.0b013e3181f3286d. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Roy J, Lee JH, Okon E, Zhang H, Marx JC, Kindy MS. Magnesium chloride in a polyethylene glycol formulation as a neuro-protective therapy for acute spinal cord injury: preclinical refinement and optimization. J Neurotrauma. 2009;26:1379–1393. doi: 10.1089/neu.2009.0884. [DOI] [PubMed] [Google Scholar]
- Lore AB, Hubbell JA, Bobb DSJ, Ballinger ML, Loftin KL, Smith JW. Rapid induction of functional and morphological continuity between severed ends of mammalian or earthworm myelinated axons. J Neurosci. 1999;19:2442–2454. doi: 10.1523/JNEUROSCI.19-07-02442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Borgens R, Shi R. Polyethylene glycol immediately repairs neuronal membranes and inhibits free radical production after acute spinal cord injury. J Neurochem. 2002;83:471–480. doi: 10.1046/j.1471-4159.2002.01160.x. [DOI] [PubMed] [Google Scholar]
- Marzullo TC, Britt JM, Stavisky RC, Bittner GD. Cooling enhances in vitro survival and fusion-repair of severed axons taken from the peripheral and central nervous systems of rats. Neurosci Lett. 2002;327:9–12. doi: 10.1016/s0304-3940(02)00378-6. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Degeneration and Regeneration of the Nervous System. In: May RM, editor. London: Oxford University Press; 1928. pp. 1913–1914. [Google Scholar]
- Raza H, John A, Brown EM, Benedict S, Kambal A. Alterations in mitochondrial respiratory functions, redox metabolism and apoptosis by oxidant 4-hydroxynonenal and antioxidants curcumin and melatonin in PC12 cells. Toxicol Appl Pharmacol. 2008;226:161–168. doi: 10.1016/j.taap.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Riley DC, Bittner GD, Mikesh M, Cardwell NL, Pollins AC, Ghergherehchi CL, Bhupanapadu Sunkesula SR, Ha TN, Hall BT, Poon AD, Pyarali M, Boyer RB, Mazal AT, Munoz N, Trevino RC, Schallert T, Thayer WP. Polyethylene glycol-fused allografts produce rapid behavioral recovery after ablation of sciatic nerve segments. J Neurosci Res. 2015;93:572–583. doi: 10.1002/jnr.23514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas JC, John JM, Lee J, Gonzalez-Lima F. Methylene blue provides behavioral and metabolic neuroprotection against optic neuropathy. Neurotox Res. 2009;15:260–273. doi: 10.1007/s12640-009-9027-z. [DOI] [PubMed] [Google Scholar]
- Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- Sea T, Ballinger ML, Bittner GD. Cooling of peripheral myelinated axons retards Wallerian degeneration. Exp Neurol. 1995;133:85–95. doi: 10.1006/exnr.1995.1010. [DOI] [PubMed] [Google Scholar]
- Sexton KW, Pollins AC, Cardwell NL, Del Corral GA, Bittner GD, Shack RB, Nanney LB, Thayer WP. Hydrophilic polymers enhance early functional outcomes after nerve autografting. J Surg Res. 2012;177:392–400. doi: 10.1016/j.jss.2012.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavisky RC, Britt JM, Zuzek A, Truong E, Bittner GD. Melatonin enhances the in vitro and in vivo repair of severed rat sciatic axons. Neurosci Lett. 2005;376:98–101. doi: 10.1016/j.neulet.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Sunio A, Bittner GD. Cyclosporin A retards the wallerian degeneration of peripheral mammalian axons. Exp Neurol. 1997;146:46–56. doi: 10.1006/exnr.1997.6484. [DOI] [PubMed] [Google Scholar]


