Abstract
Post-stroke depression is associated with reduced expression of brain-derived neurotrophic factor (BDNF). In this study, we evaluated whether BDNF overexpression affects depression-like behavior in a rat model of post-stroke depression. The middle cerebral artery was occluded to produce a model of focal cerebral ischemia. These rats were then subjected to isolation-housing combined with chronic unpredictable mild stress to generate a model of post-stroke depression. A BDNF gene lentiviral vector was injected into the hippocampus. At 7 days after injection, western blot assay and real-time quantitative PCR revealed that BDNF expression in the hippocampus was increased in depressive rats injected with BDNF lentivirus compared with depressive rats injected with control vector. Furthermore, sucrose solution consumption was higher, and horizontal and vertical movement scores were increased in the open field test in these rats as well. These findings suggest that BDNF overexpression in the hippocampus of post-stroke depressive rats alleviates depression-like behaviors.
Keywords: nerve regeneration, brain injury, brain-derived neurotrophic factor, lentivirus, post-stroke depression, depression-like behavior, hippocampus, cerebral ischemia, sucrose solution consumption, open field test, chronic unpredictable mild stress, western blot assay, neural regeneration
Introduction
Previous studies have shown that post-stroke depression (PSD) is one of the most frequent and important neuropsychiatric disorders associated with stroke, and the prevalence of PSD has increased from 28% to 56% (Haq et al., 2010). Depression and cognitive impairment in these patients aggravates the primary disease, and hinders recovery and the healing process (Sibon et al., 2012). With the incidence of PSD annually rising, cognitive impairment after PSD is attracting much more attention among researchers. PSD is caused by numerous factors, including biological, psychological and social factors, but the pathogenesis is still unclear (Li et al., 2014). Current studies on the molecular pathogenesis of PSD are focused on three main areas: inflammation (Spalletta et al., 2006; Dantzer et al., 2011; Anisman and Hayley, 2012), neurotrophins (Zhang and Pardridge, 2006; Numakawa et al., 2013) and glutamate (Ising et al., 2005; Sanacora et al., 2012).
Neurotrophins are a group of molecules that promote the development and survival of neurons. In current research, brain-derived neurotrophic factor (BDNF) has attracted extensive attention. A member of the nerve growth factor family, BDNF, is expressed in both central and peripheral nervous systems in mammals, particularly in the cortex and hippocampus. Clinical studies have shown that a reduction in BDNF levels in cerebrospinal fluid is associated with age-related cognitive decline (Li et al., 2009). In addition, perturbations in BDNF signaling are associated with other neurological diseases, including major depression (von Bohlen und Halbach, 2010). Indeed, the levels of serum BDNF are decreased in PSD patients (Zhou et al., 2011). Animal models of depression indicate that BDNF deficiency is strongly associated with depression, and antidepressants can reverse this change (Zhang and Pardridge, 2006). Cognitive impairment in a rat model of PSD is aggravated by a decrease in BDNF expression in the hippocampus (Song, 2012). To examine whether BDNF overexpression can improve neuronal function, BDNF lentiviral vectors were constructed by a number of labs (Tom et al., 2013; Bahi et al., 2014). Knockdown of BDNF in the rat hippocampus has also been performed (Taliaz et al., 2013).
In the present study, a BDNF lentiviral vector was injected into the hippocampus of a rat model of PSD, and the effect of BDNF overexpression on depression-like behavior was analyzed.
Materials and Methods
Animals
Specific-pathogen-free adult male Sprague-Dawley rats weighing 250 ± 20 g were supplied by the Laboratory Animal Center of Zhejiang University in China (animal license No. SYXK (Zhe) 2007-0098). The animals were housed in clean cages and maintained at 22 ± 2°C with a constant 12-hour light/dark schedule. The animals were allowed free access to food and water. This project was approved by the Medical Ethics Committee of Jinhua Polytechnic (Jinhua, Zhejiang Province, China).
Experimental groups and model establishment
To evaluate behaviors, sucrose solution consumption and open-field test behavior were assessed. Thirty-two rats were equally and randomly divided into four groups as follows: control (sham-operated), PSD, PSD + lentivirus (LV)-green fluorescent protein (GFP) and PSD + LV-BDNF. Rats that died after surgery or injection were replaced with new animals. In the PSD, PSD + LV-GFP and PSD + LV-BDNF groups, a rat model of stroke using right middle cerebral artery occlusion was established via embolism (Wang et al., 2008). Based on the Longa 6-point scoring scale, rats with postoperative neurological scores ≥ 1 and < 4 after 24 hours were selected (0, no injury; 1, extension disorder of the left forelimb; 2, circling to the left; 3, falling to the left when walking; 4, unconscious; 5, death). Rats with middle cerebral artery occlusion were housed individually, and subjected to chronic unpredictable mild stress with seven different stimuli (water deprivation, fasting, wet litter, behavioral restriction, electric shock to the foot, tail clamping and forced ice-water swimming). Chronic unpredictable mild stress was started 7 days following surgery and lasted for 4 weeks. Animals in the PSD + LV-GFP and PSD + LV-BDNF groups were injected with lentivirus after chronic unpredictable mild stress, while the control and PSD groups were not. The timeline of various procedures is shown in Figure 1.
Figure 1.

The timeline of experimental procedures.
MCAO: Middle cerebral artery occlusion; CUMS: chronic unpredictable mild stress.
Lentivirus for the overexpression of BDNF
In this study, the three-plasmid-based lentiviral expression system was used. The lentiviral shuttle plasmid pLV-UbC-GFP-3FLAG was provided by Sunbio Biotech (Shanghai, China). Using the In-Fusion PCR cloning system, BDNF cDNA was inserted into the EcoRI site to produce pLV-UbC-GFP-3FLAG-BDNF. According to a standard method, viruses were propagated in 293T cells (No. CRL-11268, ATCC, Manassas, VA, USA) by co-transfecting pLV-UbC-GFP-3FLAG-BDNF with psPAX2 and pMD2.G plasmids. The titer of the recombinant virus was determined by real-time PCR assay. Sequences of the primers are given in Table 1.
Table 1.
Primer sequences for WPRE, BDNF and GAPDH, and the product lengths
Stereotaxic injection
For stereotaxic surgery, rats were anesthetized with pentobarbital sodium (50 mg/kg) and installed in a stereotaxic frame (Huaibei Zhenghua, Anhui Province, China). Using a precision Hamilton micro-syringe with a 26 G needle, 1 μL viral solution was bilaterally infused into the CA3 region of the hippocampus, 4.8 mm posterior to the bregma, ± 2.5 mm lateral to the medial suture, 3.5 mm ventral to the skull surface. All the injections were performed based on coordinates in The Rat Brain in Stereotaxic Coordinates by George Paxinos and Charles Watson. Rats in the PSD + LV-GFP group were injected with the lentiviral vector pLV-UbC-GFP-3FLAG, and rats in the PSD + LV-BDNF group were injected with the lentiviral vector pLV-UbC-GFP-3FLAG-BDNF.
Histochemistry
Seven days after viral injection, rats were anesthetized with pentobarbital sodium, and then perfused with 500 mL of 0.9% saline, followed by 4% paraformaldehyde solution. The brains were then removed and placed in 4% paraformaldehyde solution for at least 24 hours, then transferred to a 30% sucrose solution. Brains were frozen, sectioned coronally, mounted on glass slides, stained with 5 mg/L 4′,6-diamidino-2-phenylindole (DAPI) (Sigma, St. Louis, MO, USA), and examined by fluorescence microscopy (BX53; Olympus, Tokyo, Japan).
Western blot assay
The right hippocampal tissue was harvested and placed in cold radioimmunoprecipitation assay buffer with fresh 2 mM phenylmethylsulfonylfluoride (Beyotime Bio, Nantong, Jiangsu Province, China). Tissue blocks were ground on ice for 15 minutes, and centrifuged at 14,000 × g for 30 minutes at 4°C. Protein concentration was determined with a BCA Protein Quantification Kit (Beyotime Bio, Haimen, Jiangsu Province, China). Before loading onto a 12% sodium dodecyl sulfate-polyacrylamide gel, equal amounts of protein were boiled with 5× loading buffer for 5 minutes. Electrophoresis was performed at 80 V for 30 minutes and 120 V for 120 minutes. Separated proteins were transferred onto a polyvinylidene difluoride membrane at 120 V for 120 minutes. Membranes were blocked with 5% non-fat dry milk overnight at 4°C, and then incubated with primary antibody, rabbit polyclonal anti-BDNF (1:1,000; Sigma), overnight at 4°C. Equal loading of protein was confirmed by subsequent β-actin immunoblots (1:1,000; Sigma). Immunodetection was performed by electrochemiluminescence (Pierce, Rockford, IL, USA) after incubation with horseradish peroxidase-conjugated goat anti-rabbit or rabbit anti-mouse (1:3,000; Jackson ImmunoResearch, West Grove, PA, USA) antibody for 1 hour at 37°C. After the X-ray films were developed and photographed, Quantity One system (Bio-Rad, Hercules, CA, USA) was used to analyze gray values of BDNF and β-actin bands. The gray value ratio of BDNF to β-actin was calculated, and then the ratio of other groups was compared with the control group.
Real-time quantitative PCR
The left hippocampal tissue was ground in liquid nitrogen. Total RNA was extracted by Trizol extraction (Invitrogen Life Technologies, Carlsbad, CA, USA). The purity and concentration were determined by spectrophotometry. Subsequently, 1 μg total RNA was reverse-transcribed into cDNA using the Bestar qPCR RT Kit (DBI Bioscience, Ludwigshafen, Germany). Specific primers for rat BDNF and GAPDH (internal control) were designed by Primer Premier 6 and synthesized by Sangon Biotech (Shanghai, China). The primer sequences are given in Table 1. SsoAdvanced SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) was used for real time quantitative PCR that was performed with a Bio-Rad CFX96 detection system. The reaction was incubated at 95°C for 30 seconds, followed by 40 cycles of 95°C for 5 seconds and 57°C for 30 seconds. After the reaction, the Ct value of each sample was subtracted from the value of the internal control gene to generate the ΔCt. The 2–ΔΔCt values were then compared (Pfaffl, 2001).
Sucrose solution consumption
Prior to the test, rats were deprived of water for 24 hours, and then provided with 1% sucrose solution for 1 hour. Sucrose solution consumption (g/100 g) was calculated as the consumption (g) during 1 hour/body weight (g) × 100.
Open field test
The open box used was 100 cm × 100 cm × 50 cm in size. The bottom of the box was divided into 25 equal-area grids with white lines. Rats were placed in the central grid of the box. The horizontal and vertical movement of the rats was recorded. Behavioral assessment lasted 3 minutes. The horizontal movement score was based on the frequency of crossing the square, and the vertical movement score was based on the frequency of rearing (removing two forelimbs from the ground > 1 cm or clinging to the walls). Before each experiment, the box was completely cleaned. The behavioral assessment was conducted in a quiet room, from 8:00–12:00 a.m., by two observers. The data from the two observers were averaged before final analysis.
Statistical analysis
Data are expressed as the mean ± SEM, and were analyzed with SPSS 16.0 software (IBM, Armonk, NY, USA). One-way analysis of variance followed by Tukey's post hoc test was used to determine statistical significance. P < 0.05 was considered to indicate a statistically significant difference.
Results
Lentivirus-mediated GFP expression in the hippocampus
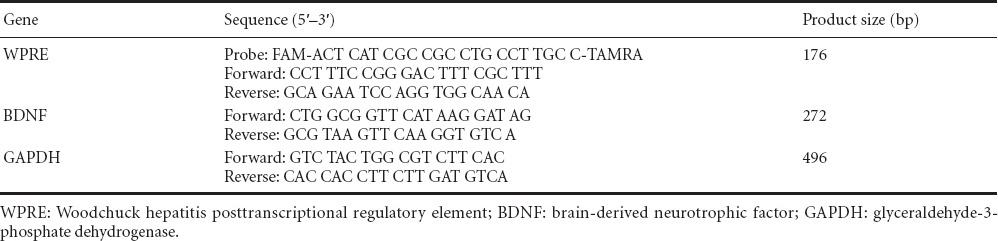
Tissue sections were stained with DAPI 7 days after lentiviral vector injection, and observed by fluorescence microscopy. Vectors were injected into the hippocampus (Figure 2D). The fluorescence emitted by GFP (encoded within the vector) was used to confirm the site of injection (Figure 2B, C).
Figure 2.
Images of representative slices from the hippocampus of rats injected with lentivirus.
(A) The cell nuclei were stained with DAPI (blue, arrow). (B) The distribution of lentivirus, as revealed by GFP expression (green, arrow). (C) Merged image of A and B. (D) GFP fluorescence (arrow) showing that the hippocampus was accurately targeted by the lentivirus injection. Scale bars: 50 μm. DAPI: 4′,6-Diamidino-2-phenylindole; GFP: green fluorescent protein.
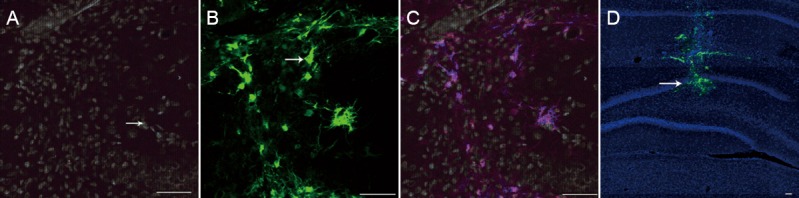
BDNF mRNA expression in the hippocampus of rats with PSD
BDNF mRNA expression levels in the hippocampus were lower in the PSD (0.17 ± 0.02), PSD + LV-GFP (0.20 ± 0.03) and PSD + LV-BDNF (0.41 ± 0.05) groups compared with the control group 7 days after injection (P < 0.05). BDNF mRNA levels were higher in the PSD + LV-BDNF group than in the PSD + LV-GFP group (P < 0.05). No significant difference was observed between the PSD and PSD + LV-GFP groups (P > 0.05; Figure 3).
Figure 3.
BDNF mRNA expression in the hippocampus of PSD rats at 7 days after lentiviral injection (real-time quantitative PCR).
*P < 0.05, vs. control group; #P < 0.05, vs. PSD + LV-BDNF group. Data are presented as the mean ± SEM with five rats in each group. Statistical analysis was done using one-way analysis of variance and Tukey's post hoc test. PSD: Post-stroke depression; LV: lentivirus; GFP: green fluorescent protein; BDNF: brain-derived neurotrophic factor.
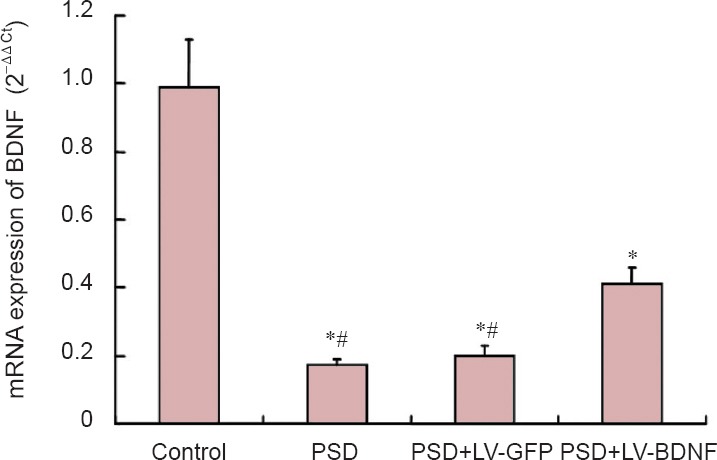
BDNF protein expression in the hippocampus of rats with PSD
BDNF protein expression levels in the hippocampus were lower in the PSD, PSD + LV-GFP and PSD + LV-BDNF groups compared with the control group 7 days after injection (P < 0.05). BDNF protein expression was greater in the PSD + LV-BDNF group than in the PSD + LV-GFP group (P < 0.05). No significant difference was observed between the PSD and PSD + LV-GFP groups (P > 0.05; Figure 4).
Figure 4.
BDNF protein expression in rat hippocampus at 7 days post injection of lentivirus by western blot assay.
(A) Molecular weight of BDNF is 27 kDa, and β-actin protein is 42 kDa. Bands for the four groups are shown (control, PSD, PSD + LV-GFP and PSD + LV-BDNF). (B) The gray value ratio of the BDNF band to the β-actin band was calculated, and the relative expression was compared with the control group. *P < 0.05, vs. control group; #P < 0.05, vs. PSD + LV-GFP group. The data are presented as the mean ± SEM with five rats in each group. Statistical analysis was performed using one-way analysis of variance and Tukey's post hoc test. PSD: Post-stroke depression; LV: lentivirus; GFP: green fluorescent protein; BDNF: brain-derived neurotrophic factor.
The effects of hippocampal lentivirus-mediated BDNF expression on sucrose solution consumption in rats with PSD
Before surgery, no significant difference was found in sucrose solution consumption between the control and other groups (P > 0.05). Sucrose solution consumption was lower in the PSD, PSD + LV-GFP and PSD + LV-BDNF groups compared with the control group after chronic unpredictable mild stress 7 days post injection (P < 0.05). Sucrose solution consumption was higher in the PSD + LV-BDNF group than in the PSD + LV-GFP group (P < 0.05). No significant difference was detected between the PSD and PSD + LV-GFP groups (P > 0.05; Table 2).
Table 2.
Changes in sucrose solution consumption (g/100 g body weight)
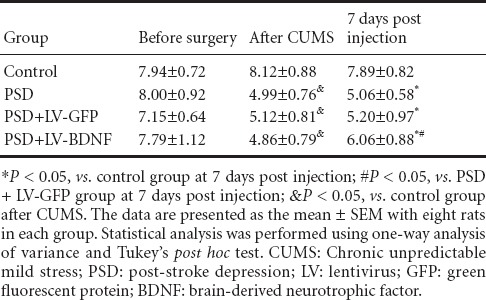
The effects of hippocampal lentivirus-mediated BDNF expression on depression-like behaviors in rats with PSD
There was no significant difference in the horizontal and vertical movement scores between the control and other groups before surgery (P > 0.05). The horizontal and vertical movement scores were lower in the PSD, PSD + LV-GFP and PSD + LV-BDNF groups compared with the control group after chronic unpredictable mild stress 7 days post injection (P < 0.05). The horizontal and vertical movement scores were higher in the PSD + LV-BDNF group than in the PSD + LV-GFP group (P < 0.05). No significant difference was observed between the PSD and PSD + LV-GFP groups (P > 0.05; Figure 5).
Figure 5.
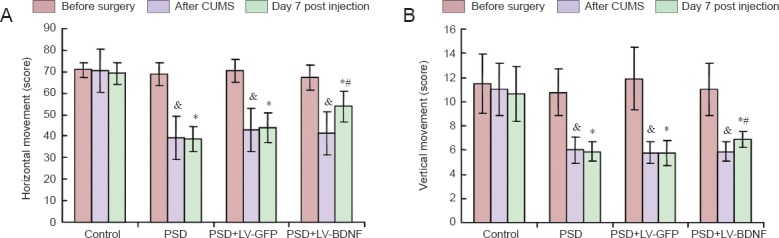
Effects of BDNF on depression-like behaviors in rats with PSD.
(A) The horizontal movement scores for the four groups (control, PSD, PSD + LV-GFP and PSD + LV-BDNF) at three time points (before surgery, after CUMS and on day 7 post injection). (B) Vertical movement scores. *P < 0.05, vs. control group at 7 days post injection; &P < 0.05, vs. PSD + LV-GFP group at 7 days post injection; *P < 0.05, vs. control group after CUMS. The data are presented as the mean ± SEM. Statistical analysis was performed using one-way analysis of variance and Tukey's post hoc test. CUMS: Chronic unpredictable mild stress; PSD: post-stroke depression; LV: lentivirus; GFP: green fluorescent protein; BDNF: brain-derived neurotrophic factor.
Discussion
A strong association has been found between BDNF and neurogenesis in in vitro and in vivo studies (Scharfman et al., 2005; Li et al., 2012). In addition, other studies have shown that antidepressants improve depression-like behaviors by promoting hippocampal neurogenesis (Santarelli et al., 2003; Dranovsky and Hen, 2006). In the present study, we investigated whether overexpression of BDNF in the hippocampus improves depression-like behaviors in a rat model of PSD.
Animals with PSD exhibit depression-like behaviors, including a decline in sucrose preference and reduced activities in the open-field test, indicative of anhedonia and a reduction in autonomous acts and exploring behavior. Moreover, the results indicate that the PSD model was successfully generated. Reduced BDNF protein and mRNA expression was found in PSD animals, consistent with other studies (Cheng et al., 2013; Zhang et al., 2012, 2014).
Although the mechanisms underlying PSD are not entirely clear, it has been shown that depression is associated with decreased expression of BDNF in the hippocampus. In this study, a lentivirus vector for overexpression of BDNF was injected into the hippocampus of PSD animals, and sucrose solution consumption and open field behaviors were assessed. The results provide direct evidence that increasing BDNF protein levels in the hippocampus alleviates behaviors associated with depression. Sucrose solution consumption and vertical and horizontal movement were greater in the PSD + LV-BDNF group compared with the PSD and PSD + LV-GFP groups, suggesting that depression-like behaviors were ameliorated by targeted BDNF expression in the hippocampus.
We conjecture that BDNF enhances the survival and growth of neurons in the hippocampus. BDNF plays an important role in learning and memory function, and promotes the survival and growth of a variety of neurons (Mertz et al., 2000). In animal studies, the association between depressive behaviors and reduced hippocampal neurogenesis has been extensively reported (Monteggia et al., 2007; Adachi et al., 2008; Taliaz et al., 2010). Studies show that neurogenesis in the hippocampus is regulated by the BDNF-TrkB signaling pathway and the N-methyl-D-aspartate receptor (Angelucci et al., 2004). BDNF protects against glutamate toxicity to hippocampal neurons, and this effect is mediated by the phosphatidylinositol 3-kinase and the Ras/mitogen-activated protein kinase signaling pathways (Almeida et al., 2005).
This study indicates that BDNF overexpression in the hippocampus of PSD rats may improve depression-like behaviors. However, experiments on BDNF knockout and knockdown in specific subregions of the mouse brain have produced conflicting results, although knockout of TrkB in neural progenitor cells blocks the proliferation of newborn neurons (Bergami et al., 2008; Taliaz et al., 2010). Because reduction of BDNF expression in the hippocampus impacts neuronal plasticity, synaptic structure and function, it is reasonable to speculate that low BNDF levels result in decreased neurogenesis in the hippocampus, thereby leading to PSD. However, the molecular mechanisms of depression and the association with BDNF remain unclear. In the present study, we did not examine whether overexpression of BDNF enhances neurogenesis, and whether this in turn underlies the improvement in depression-like behaviors. Furthermore, stereotaxic injection is not feasible in humans for clinical treatment, and intranasal administration may be a better option. It has been reported that intranasal BDNF can exert neuroprotective effects in rats with ischemic stroke (Jiang et al., 2012). Thus, whether intranasal BDNF can improve depressive behaviors in animals with PSD should be investigated in future studies, in addition to the relationship between BDNF and neurogenesis.
Footnotes
Funding: This study was supported by a grant from the Experimental Animal Science and Technology Project of Zhejiang Province in China, No. 2012C37083.
Conflicts of interest: None declared.
Copyedited by Patel B, Maxwell R, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry. 2008;63:642–649. doi: 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, Carvalho RF, Carvalho AP, Duarte CB. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Mathe AA, Aloe L. Neurotrophic factors and CNS disorders: findings in rodent models of depression and schizophrenia. Prog Brain Res. 2004;146:151–165. doi: 10.1016/s0079-6123(03)46011-1. [DOI] [PubMed] [Google Scholar]
- Anisman H, Hayley S. Inflammatory factors contribute to depression and its comorbid conditions. Sci Signal. 2012;5:pe45. doi: 10.1126/scisignal.2003579. [DOI] [PubMed] [Google Scholar]
- Bahi A, Chandrasekar V, Dreyer JL. Selective lentiviral-mediated suppression of microRNA124a in the hippocampus evokes antidepressants-like effects in rats. Psychoneuroendocrinology. 2014;46:78–87. doi: 10.1016/j.psyneuen.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Bergami M, Rimondini R, Santi S, Blum R, Gotz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Su Q, Shao B, Cheng J, Wang H, Wang L, Lin Z, Ruan L, ZhuGe Q, Jin K. 17beta-Estradiol attenuates poststroke depression and increases neurogenesis in female ovariectomized rats. Biomed Res Int 2013. 2013 doi: 10.1155/2013/392434. 392434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Wei N, Lu T, Zhu J, Xu G, Liu X. Intranisal brain-derived neurotrophic factor protects brain from ischemic insult via modulating localinflammation in rats. Neuroscience. 2012;172:398–405. doi: 10.1016/j.neuroscience.2010.10.054. [DOI] [PubMed] [Google Scholar]
- Haq SU, Symeon C, Agius M, Brady R. Screening for depression in post stroke patients. Psychiatr Danub. 2010;22(Suppl 1):S33–35. [PubMed] [Google Scholar]
- Ising M, Kunzel HE, Binder EB, Nickel T, Modell S, Holsboer F. The combined dexamethasone/CRH test as a potential surrogate marker in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1085–1093. doi: 10.1016/j.pnpbp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Li G, Peskind ER, Millard SP, Chi P, Sokal I, Yu CE, Bekris LM, Raskind MA, Galasko DR, Montine TJ. Cerebrospinal fluid concentration of brain-derived neurotrophic factor and cognitive function in non-demented subjects. PLoS One. 2009;4:e5424. doi: 10.1371/journal.pone.0005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ling S, Yang Y, Hu Z, Davies H, Fang M. Systematic hypothesis for post-stroke depression caused inflammation and neurotransmission and resultant on possible treatments. Neuro Endocrinol Lett. 2014;35:104–109. [PubMed] [Google Scholar]
- Li Y, Yui D, Luikart BW, McKay RM, Li Y, Rubenstein JL, Parada LF. Conditional ablation of brain-derived neurotrophic factor-TrkB signaling impairs striatal neuron development. Proc Natl Acad Sci U S A. 2012;109:15491–15496. doi: 10.1073/pnas.1212899109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz K, Koscheck T, Schilling K. Brain-derived neurotrophic factor modulates dendritic morphology of cerebellar basket and stellate cells: an in vitro study. Neuroscience. 2000;97:303–310. doi: 10.1016/s0306-4522(99)00585-0. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Adachi N, Richards M, Chiba S, Kunugi H. Brain-derived neurotrophic factor and glucocorticoids: reciprocal influence on the central nervous system. Neuroscience. 2013;239:157–172. doi: 10.1016/j.neuroscience.2012.09.073. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Sibon I, Lassalle-Lagadec S, Renou P, Swendsen J. Evolution of depression symptoms following stroke: a prospective study using computerized ambulatory monitoring. Cerebrovasc Dis. 2012;33:280–285. doi: 10.1159/000334663. [DOI] [PubMed] [Google Scholar]
- Spalletta G, Bossu P, Ciaramella A, Bria P, Caltagirone C, Robinson RG. The etiology of poststroke depression: a review of the literature and a new hypothesis involving inflammatory cytokines. Mol Psychiatry. 2006;11:984–991. doi: 10.1038/sj.mp.4001879. [DOI] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry. 2010;15:80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaz D, Nagaraj V, Haramati S, Chen A, Zangen A. Altered brain-derived neurotrophic factor expression in the ventral tegmental area, but not in the hippocampus, is essential for antidepressant-like effects of electroconvulsive therapy. Biol Psychiatry. 2013;74:305–312. doi: 10.1016/j.biopsych.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Tom VJ, Sandrow-Feinberg HR, Miller K, Domitrovich C, Bouyer J, Zhukareva V, Klaw MC, Lemay MA, Houle JD. Exogenous BDNF enhances the integration of chronically injured axons that regenerate through a peripheral nerve grafted into a chondroitinase-treated spinal cord injury site. Exp Neurol. 2013;239:91–100. doi: 10.1016/j.expneurol.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bohlen und Halbach O. Involvement of BDNF in age-dependent alterations in the hippocampus. Front Aging Neurosci. 2010;2:36. doi: 10.3389/fnagi.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Zhang ZJ, Guo YJ, Teng GJ, Chen BA. Hippocampal neurogenesis and behavioural studies on adult ischemic rat response to chronic mild stress. Behav Brain Res. 2008;189:9–16. doi: 10.1016/j.bbr.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Zhang L, Luo J, Zhang M, Yao W, Ma X, Yu SY. Effects of curcumin on chronic, unpredictable, mild, stress-induced depressive-like behaviour and structural plasticity in the lateral amygdala of rats. Int J Neuropsychopharmacol. 2014;17:793–806. doi: 10.1017/S1461145713001661. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pardridge WM. Blood-brain barrier targeting of BDNF improves motor function in rats with middle cerebral artery occlusion. Brain Res. 2006;1111:227–229. doi: 10.1016/j.brainres.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Wu LN, Song JG, Li WQ. Correlations between cognitive impairment and brain-derived neurotrophic factor expression in the hippocampus of post-stroke depression rats. Molr Med Rep. 2012;6:889–893. doi: 10.3892/mmr.2012.1009. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Lu T, Xu G, Yue X, Zhu W, Ma M, Liu W, Zhu S, Liu X. Decreased serum brain-derived neurotrophic factor (BDNF) is associated with post-stroke depression but not with BDNF gene Val66Met polymorphism. Clin Chem Lab Med. 2011;49:185–189. doi: 10.1515/CCLM.2011.039. [DOI] [PubMed] [Google Scholar]


