Abstract
The Rho/Rho-kinase signaling pathway plays an important role in cerebral ischemia/reperfusion injury. However, very few studies have examined in detail the changes in the Rho/Rho-kinase signaling pathway in chronic cerebral ischemia. In this study, rat models of chronic cerebral ischemia were established by permanent bilateral common carotid artery occlusion and intragastrically administered 9 mg/kg fasudil, a powerful ROCK inhibitor, for 9 weeks. Morris water maze results showed that cognitive impairment progressively worsened as the cerebral ischemia proceeded. Immunohistochemistry, semi-quantitative RT-PCR and western blot analysis showed that the expression levels of Rho-kinase, its substrate myosin-binding subunit, and its related protein alpha smooth muscle actin, significantly increased after chronic cerebral ischemia. TUNEL staining showed that chronic cerebral ischemia could lead to an increase in neuronal apoptosis, as well as the expression level of caspase-3 in the frontal cortex of rats subjected to chronic cerebral ischemia. Fasudil treatment alleviated the cognitive impairment in rats with chronic cerebral ischemia, and decreased the expression level of Rho-kinase, myosin-binding subunit and alpha smooth muscle actin. Furthermore, fasudil could regulate cerebral injury by reducing cell apoptosis and decreasing caspase-3 expression in the frontal cortex. These findings demonstrate that fasudil can protect against cognitive impairment induced by chronic cerebral ischemia via the Rho/Rho-kinase signaling pathway and anti-apoptosis mechanism.
Keywords: nerve regeneration, chronic cerebral ischemia, fasudil, Rho-kinase, alpha smooth muscle actin, myosin-binding subunit, cognitive impairment, caspase-3, apoptosis, neural regeneration
Introduction
The Rho/Rho-kinase signaling pathway functions using four key signaling molecules: Rho protein, Rho-kinase, myosin phosphase, and alpha smooth muscle action (α-SMA). Rho, a member of the small-molecular-weight GTPase superfamily, can regulate cell actin restructuring through its downstream effector ROCK (Arias et al., 2009; Antoniu, 2012), and participates in cell migration, movement, apoptosis, gene transcription, nerve regeneration, and other biological processes. ROCK is a kind of serine-threonine protein kinase that consists of two cell subtypes, ROCK1 and ROCK2, and relates Rho with Rho-GTP. The expression of ROCK1 is widespread in the brain and muscle, whereas ROCK2 is only abundant in the brain, muscle, cardio tissue and placenta (Tan et al., 2011; Shalin et al., 2012). ROCK plays an important role in smooth muscle contraction by regulating the phosphorylation of myosin light chain. Additionally, several vascular active substances that interact with ROCK have been identified in many cellular functions (gene expression, cytokinesis, cell adhesion and migration), such as angiotensin II (AT-II), endothelin 21 (ET 21), and platelet-derived growth factor (PDGF) (Ishikurak et al., 2005; Shimokawa and Rashid 2007; Shin et al., 2008; Yano et al., 2008). ROCK has been identified as an important target involved in a variety of diseases (Antoniu, 2012; Oh et al., 2013). ROCK regulates neural cell migration, proliferation, survival, axon guidance, and regeneration (Gonzalez-Forero et al., 2013), and has been found to be closely related to the pathogenesis of several nervous system disorders. Recent studies showed the potential therapeutic use of ROCK inhibitors in the treatment of Alzheimer's disease (Hou et al., 2012; Raad et al., 2012), Parkinson's disease (Tonges et al., 2012; Villar-Cheda et al., 2012), stroke (Koumura et al., 2011), epilepsy (İnan and Büyükafşar, 2008), chronic pain (Yoshimi et al., 2010), autoimmune neuritis (Pineda et al., 2011), and cell rupture and apoptosis (Chen et al., 2013). Under ischemic, hypoxic, and inflammatory condition, the expression of ROCK significantly increased in the brain, and the inhibition of ROCK can alleviate ischemia/reperfusion impairments (Olson, 2008; Arias et al., 2009).
Chronic cerebral ischemia refers to cerebral blood flow perfusion inadequacy over a long time period (Horvath, 2001). It leads to pathological cerebral metabolic disorders and functional decline, and contributes greatly to the pathophysiological process of vascular dementia, Binswanger disease and Alzheimer's disease. The expression of Rho protein and ROCK increases greatly in animal models of acute cerebral injury, but it has not been determined in rats with cognitive dysfunction induced by chronic cerebral ischemia. Fasudil, a typical ROCK inhibitor, has been shown to exert neuroprotective effects in cerebral ischemic injury (Yamashita et al., 2007), vasoconstriction (Namkoong et al., 2009), and spinal muscular atrophy (SMA; Bowerman et al., 2012). However, few studies have investigated whether fasudil can alleviate cognitive dysfunction following chronic cerebral ischemia, and its relationship with the Rho/ROCK signaling pathway is unclear.
In our study, we aimed: (1) to employ rat models of permanent, bilateral common carotid artery occlusion to evaluate cognitive dysfunction induced by chronic cerebral ischemia; (2) to charify the role of Rho/Rho-kinase pathway in chronic cerebral ischemia-induced cognitive dysfunction; and (3) to investigatete the neuroprotective effects and mechanism of Fasudil on cognitive dysfunction in chronic cerebral ischemia.
Materials and Methods
Animals
Male 2-month-old Wistar rats weighing 250–280 g were purchased from the Experimental Animal Center of Jilin University, China (certificate No. SYXK (Ji) 2008-0010/0011). All rats were housed in clean polypropylene cages with 12-hour light/dark cycles for at least 1 week before the experiments. The rooms were equipped with air conditioning equipment to maintain the temperature at 23 ± 2°C and the humidity at 50 ± 5%. Food and water were provided ad libitum. Animal use and all experimental procedures were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by Jilin University Experimental Animal Ethics Committee in China. All efforts were made to minimize animal discomfort and reduce the number of animals used.
Establishment of chronic cerebral ischemia models and drug administration
The rats included in our study exhibited no apparent motor dysfunction, visual impairment or indigestion. Rats with poor swimming ability or intellectual retardation were excluded with the Morris water maze test before grouping. All rats were autopsied and no pathological lesions were found. Of the total 150 rats, 30 were included in the sham-operated group, and the remaining 120 rats received permanent common carotid artery occlusion (2-vessel occlusion) (de la Torre, 2000). Rectal temperature was maintained at 37–38°C using a heated blanket during the whole surgical procedure. While the bilateral carotid arteries were occluded, the rat tail was cut to induce the sudden increase in blood pressure, which can lead to heart failure and death. Thirty rats died from ischemic seizure mostly within 72 hours after 2-vessel occlusion. Thirty rats in the sham-operated group, 60 rats in the ischemia group and 30 rats in the fasudil group were included in the final analysis. After surgery, penicillin (20 million units/kg body weight) was intramuscularly injected daily for 3 consecutive days. Fasudil solution (fasudil was provided by Tianjin Chase Sun Pharmaceutical Co. Ltd., China (approval No. H20040356; 30 mg/bottle) was intragastrically administered at 9 mg/kg into rats in the fasudil group, once daily for 20 days starting less than 48 hours after operation. 25% Dimethyl sulfoxide solution was used as a vehicle in the ischemia group and the sham group. The rats in the above three groups were executed for analysis at 3, 6 and 9 weeks after operation. For the Morris water maze test, 10 rats were chosen for analysis at each time point from the sham-operated group and from the fasudil group, while 20 rats were chosen at each time point from the ischemia group. For western blot assay, RT-PCR, and immunohistochemical staining, 10 rats in each group were used at each time point.
Assessment of learning and memory abilities
A place navigation test was performed using the Morris water maze, which comprises a circular pool, automatic camera and computer analysis system (Olympus, Tokyo, Japan). Before the test, the circular pool wall was marked with four equally-spaced entry points, dividing the circular pool into four quadrants. A platform (diameter 11 cm) was placed at the center of one quadrant and immersed 1 cm beneath the water surface during trials. Milk powder was mixed into the pool water to prevent rats from seeing the platform. The water temperature (26 ± 1°C), light intensity, external cues in the room, and water opacity were rigorously controlled and kept unchanged throughout the behavioral test. The rat's head was stained before the test to allow the camera to track and record the rat's movement.
The main procedures in the place navigation test were as follows. The rats were released into the water facing the pool wall at one of the four entry points. The time lapsed until the rat reached the hidden platform was recorded as the escape latency, and the distance traveled before finding the platform was recorded as the swimming distance. Each rat was allowed 120 seconds to find the platform, and could remain there for a further 30 seconds (the standing time indicates that the platform is its target). If a rat could not reach platform before the 120-second cutoff, escape latency was recorded as 120 seconds, and the researcher would lead the rat to the platform. Escape latency and swimming distance were used to evaluate the rats’ learning and memory abilities.
Collection of specimens
Rats were anesthetized with 10% chloral hydrate (300 mg/kg) via intraperitoneal injection, followed by intracardial perfusion with 0.1 M PBS (pH 7.4) mixed with 4% paraformaldehyde at 30°C. The rats were sacrificed by decapitation at the preset time points. Frontal lobes and hippocampi were removed immediately on dry ice, wrapped with aluminum foil and then preserved in liquid nitrogen at −70°C. Serial coronal sections were cut from the frontal lobes and hippocampi and every section was 4 μm thick. One of every three sections was selected and mounted onto a slide for staining.
Immunohistochemical staining
Briefly, the paraffin-embedded sections were dewaxed with xylene and dehydrated with a graded alcohol series. Subsequently, sections were incubated in 3% (w/v) H2O2 for 15 minutes, and washed with PBS three times for 5 minutes each. Then, antigen retrieval was carried out with 10 mM sodium citrate buffer. The sections were treated with peroxidase for 10–15 minutes in blocking solution to block endogenous peroxidase, and then in 5% goat serum for 10 minutes to block non-specific antibody binding. Overnight incubation with rabbit anti-microtubule-associated protein 2 (MAP2) polyclonal antibody (1:100; Boster, Wuhan, Hubei Province, China), rabbit anti-rat α-SMA primary monoclonal antibody and rabbit anti-caspase-3 primary polyclonal antibody (1:100; Boster) was performed in humidified boxes at 4°C. PBS was used as a negative control. After that, tissue specimens were incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibodies (1:2,000; Boster) at 37°C for 30 minutes, and with streptomycin avidin-peroxidase solution at 37°C for 30 minutes. Then, staining was developed with 3,3′-diaminobenzidine (DAB) solution for 5–10 minutes. Tissues were rinsed in PBS three times for 5 minutes each between each step, and then dyed in hematoxylin. Sections were subsequently mounted, dehydrated, coverslipped, and examined under an optical microscope (Olympus, Tokyo, Japan). Immunohistochemistry was analyzed with an HPLAS-1000 high-definition color pathology graphic analysis system (Olympus). Five different fields of view (200× magnification) were selected randomly for each section. The number of positively-stained cells was the mean of five different fields of view.
Semi-quantitative RT-PCR analysis
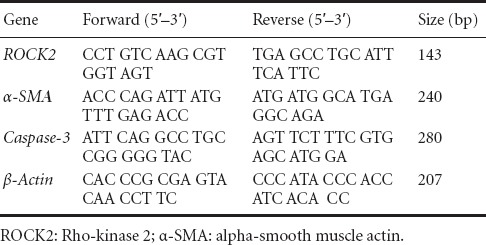
The mRNA levels of Rho-kinase2 (ROCK2), α-SMA, and caspase-3 were measured using semi-quantitative RT-PCR. Primers (Sangon Biotech Co., Ltd., Shanghai, China) were designed according to the nucleotide sequences using Premier 5.0 software (Premier Biosoft International, Palo Alto, CA, USA). Total RNA was extracted from tissues (50–100 mg) taken from the frontal lobe with Trizol. RNA concentration and purity were evaluated by spectrometry on the basis of optical density measurements at 260 and 280 nm. Using the extracted RNA as template, cDNA synthesis was performed in a 20-μL reaction mixture using reverse transcriptase. The reverse-transcribed products were preserved at −20°C until use. cDNA (2 μL) from this mixture was used for RT-PCR amplification. The amplification conditions for Rho-kinase 2 were: predenature at 94°C for 5 minutes; 30 cycles of denaturing at 94°C for 30 seconds; annealing at 47°C for 40 seconds; extension at 72°C for 2 minutes; and a final extension at 72°C for 5 minutes. Amplification conditions for α-SMA, caspase-3 and β-actin were similar to those for ROCK2, except that the annealing temperature for β-actin (Sangon Biotech Co., Ltd.) was 60°C (Table 1). The amplification products were quantified following 2% agarose gel electrophoresis. After scanning with a gel image analysis system (Tanon Science & Technology Co., Ltd., Shanghai, China), Bandscan (Tanon Science & Technology Co., Ltd.) was used to analyze band gray scale and to calculate the ratio of target gene band intensity to that of the corresponding β-actin band to determine the level of mRNA expression.
Table 1.
Primers and expected sizes of PCR products
Western blot analysis
Samples preserved in liquid nitrogen were rapidly ground, followed by PBS washing and centrifugation (centrifugal radius 13.5 cm) twice, at 1,000 r/min for 5 minutes. The cell pellet was then topped with 150 μL cell lysis buffer (comprising 50 mM Tris-Hcl, pH 7.6,150 mM NaCl, 1% NP-40, 0.5 sodium deoxycholate, 0.1% sodium dodecyl sulphate, 1 mM ethylenediamine tetraacetic acid, 1 mM phenylmethylsulfonyl fluoride, 2 μg/mL leupeptin, 0.5 mM dithiothreitol, and 1–2 μg/mL aprotinin). Cell lysates were homogenized for 30 minutes at 4°C and centrifuged (centrifugal radius 9.35 cm) at 12,000 r/min for 2 minutes at 4°C. The supernatants obtained were saved and used as the total protein extract. Protein concentrations were quantified with the Biorad protein assay and stored at −20°C. Protein samples were separated on SDS-PAGE mini gels at 120 V until bromophenol blue reached the bottom of the separation gel. Equivalent amounts of total protein for each sample were loaded (20 μL). They were subsequently transferred electrophoretically to a nitrocellulose membrane by applying a 110 V current at 4°C for 1.5 hours. The membrane was immersed in TBS for 12 minutes and then stained with Ponceau stain for 6 minutes. After blocking with 5% skimmed milk powder for 1 hour at room temperature, the membrane was hybridized with rabbit anti-ROCK2 primary polyclonal antibody and rabbit anti-rat myosin-binding subunit (MBS) primary monoclonal antibody (Boster) diluted in 0.2% Tris-buffered saline solution overnight at 4°C. Subsequently, they were washed with Tris-buffered saline solution, three times for 10 minutes each. The immunoblot was revealed with an ECL western blot detection kit (Amersham Pharmacia Biotech, Buckinghamshire, UK). Densitometry analysis was performed using ImageJ software (National Institutes of Health, Rockville, MD, USA). The results are expressed as the ratio of the target protein band intensity to that for β-actin.
TUNEL staining
The sections of cerebral frontal cortex are dewaxed with xylene, and rehydrated with a graded series of ethanol (100, 95, 90, 80, and 70%) and distilled water. Each step was processed for 5 minutes at room temperature. After being rinsed with PBS for 10 minutes, the sections were incubated with proteinase K working solution (20 μg/mL in PBS) for 7 to 10 minutes at 37°C. The sections were then rinsed with PBS three times for 5 minutes each. A TUNEL reaction mixture with 10 μL of enzyme solution and 490 μL of label solution was prepared and used to equilibrate components at room temperature. Each section was incubated with 50 μL of TUNEL reaction mixture in a humidified chamber for 60 minutes at 37°C. The sections were rinsed with PBS three times for 5 minutes each, incubated with 1 μg/mL Hoechst 33342 in PBS for 10 minutes at room temperature to stain the nuclei, rinsed with PBS three times for 5 minutes each, mounted with Fluoromount-G and stored at 4°C until imaging. The images were captured under fluorescence microscopy (Eclipse microscope, model E800; Nikon Instruments Inc., Melville, NY, USA) equipped with a digital camera (SPOT; Diagnostic Instrument, Sterling Heights, MI). Excitation at 494 nm induces the Fluorescein for the TUNEL-positive cells, while excitation at 350 nm induces Hoechst 33342. Software was used by Simple PCI version 6.0 software (Compix Inc., Cranberry Township, PA, USA).
Statistical analysis
Data were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA), and are expressed as the mean ± SD. Differences were considered significant at P < 0.05, which was assessed using one-way analysis of variance and the Student's t-test.
Results
Learning and memory abilities of rats with chronic cerebral ischemia
The place navigation test showed that the escape latency and swimming distance in the cerebral ischemia group were obviously longer than in the sham-operated group (P < 0.05; Figure 1), and the most changes happened at 9 weeks after operation (P < 0.05; Figure 1). Fasudil treatment significantly reduced the escape latency and swimming distance compared with the ischemia group (P < 0.05; Figure 1).
Figure 1.
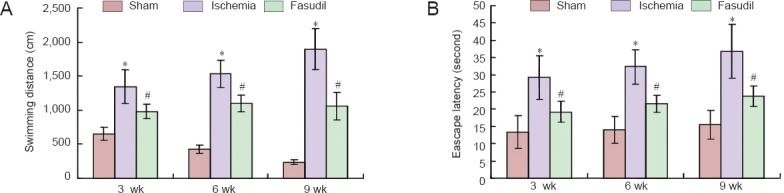
Learning and memory abilities of chronic cerebral ischemia rats and the effects of fasudil treatment (Morris water navigation test).
(A, B) Swimming distance (A) and escape latency (B) in the place navigation test. *P < 0.05, vs. sham-operated (sham) group; #P < 0.05, vs. ischemia group. There were 10 rats at each time point in the sham and fasudil groups, and 20 rats at each time point in the ischemia group. Data are expressed as the mean ± SD and were analyzed using one-way analysis of variance and the Student's t-test. wk: Weeks.
mRNA and protein expression levels of Rho-kinase in the frontal cortex and hippocampus of chronic cerebral ischemia rats
Semi-quantitative reverse-transcription (RT)-PCR and western blot assay showed that mRNA and protein expression of ROCK2 was weak in the sham-operated group. However, the expression level rose at 3 weeks after 2VO, reached a peak at 6 weeks, and then declined at 9 weeks, but remained above the sham-operated level (P < 0.05; Figure 2). After fasudil administration, the mRNA and protein expression of ROCK2 was decreased, but remained higher than that in the sham-operated group (P < 0.05; Figure 2).
Figure 2.
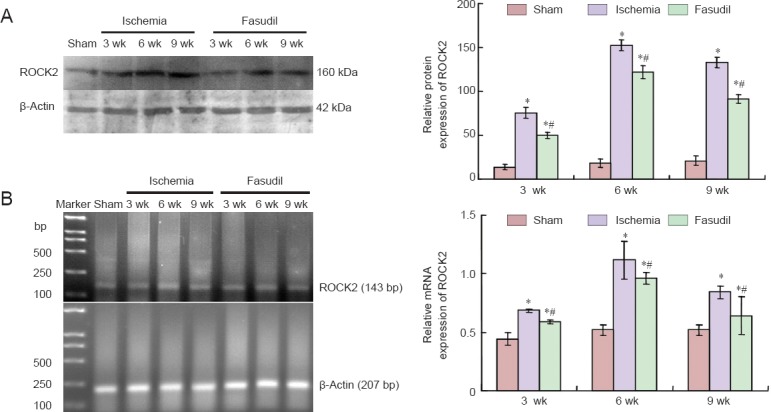
Protein and mRNA expression of Rho-kinase in the frontal cortex of chronic cerebral ischemia rats and the effects of Fasudil treatment.
(A) Protein expression of Rho-kinase 2 (ROCK2) was determined by western blot assay in the three [sham-operated (sham), ischemia, fasudil] groups at 3, 6, and 9 wk after surgery. (B) mRNA expression of ROCK2 was determined by RT-PCR in the three (sham, ischemia, fasudil) groups at 3, 6, and 9 wk after surgery. The mRNA and protein results are expressed as the ratio of the target gene or protein band intensity to that for β-actin. There were 10 rats at each time point per group. Data are presented as the mean ± SD and analyzed using one-way analysis of variance and the Student's t-test. *P < 0.05, vs. sham group; #P < 0.05, vs. ischemia group. wk: Weeks.
Western blot analysis showed that the protein expression of myosin-binding subunit significantly increased following ischemia (P < 0.05), but decreased after fasudil treatment (P < 0.05; Figure 3). The changes paralleled the ROCK2 expression levels determined by RT-PCR.
Figure 3.
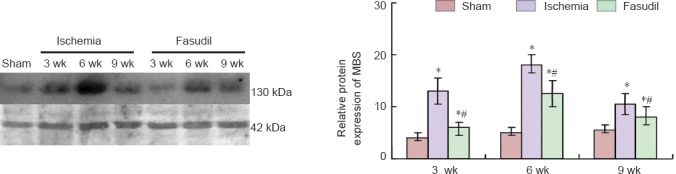
Protein expression of myosin-binding subunit (MBS) in the frontal cortex of chronic cerebral ischemia rats and the effects of fasudil treatment.
The protein expression of myosin-binding subunit in the three [sham-operated (sham), ischemia, fasudil] groups at 3, 6, and 9 weeks (wk) after surgery was determined. The results are expressed as the ratio of the target protein band intensity to that for β-actin. Data are expressed as the mean ± SD and were analyzed using one-way analysis of variance and the Student's t-test. *P < 0.05, vs. sham group; #P < 0.05, vs. ischemia group.
The mRNA expression and immunoreactivity of α-SMA in the frontal cortex of rats with chronic cerebral ischemia
Immunohistochemical staining showed that α-SMA-immunoreactive cells were visible at 3 weeks after cerebral ischemia, subsequently increased and reached a peak at 9 weeks, which declined after intervention by fasudil but remained above the sham-operated group level (P < 0.05; Figure 4A).
Figure 4.
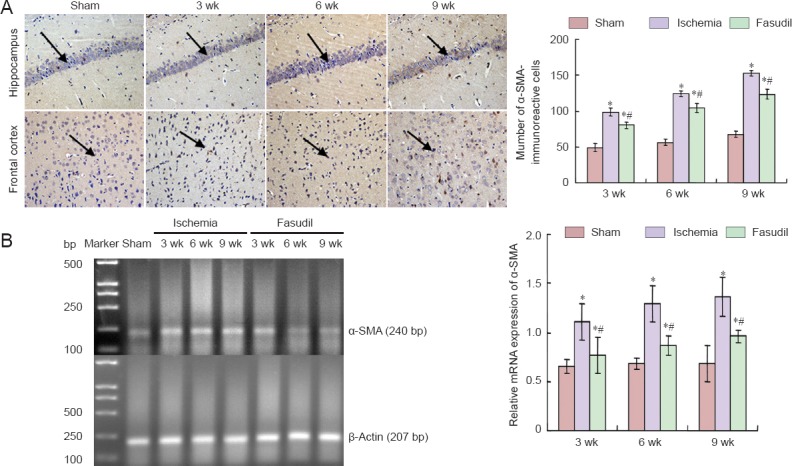
Immunoreactivity and mRNA expression of alpha-smooth muscle actin (α-SMA) in the frontal cortex of rats with chronic cerebral ischemia and the effects of fasudil treatment.
(A) Left: Representative photomicrographs of α-SMA-immunoreactive cells (200×). Right: Quantification of α-SMA immunoreactivity. Immunoreactivity of α-SMA was determined at 3, 6, and 9 weeks (wk) after surgery. Arrows show α-SMA-immunoreactive cells. (B) mRNA expression of α-SMA determined by RT-PCR in the three [sham-operated (sham), ischemia, fasudil] groups at 3, 6, 9 wk after surgery. The mRNA results are expressed as the ratio of the target gene band intensity to that for α-actin. There were 10 rats at every time point per group. Data are presented as the mean ± SD and analyzed using one-way analysis of variance and the Student's t-test. *P < 0.05, vs. sham group; #P < 0.05, vs. ischemia group.
RT-PCR demonstrated that mRNA expression level of α-SMA remained stable in the sham-operated group. In the ischemia group, the mRNA expression level of α-SMA was greater than that in sham-operated group (P < 0.05), then increased progressively and reached the peak at 9 weeks, which declined after intervention by fasudil but remained above the sham-operated group level (P < 0.05) (Figure 4B).
Cell apoptosis in the frontal cortex of rats with chronic cerebral ischemia
TUNEL staining showed that apoptotic cells in the frontal cortex of rats following ischemia (P < 0.05; Figure 5), which rose at 3 weeks, reached a peak at 6 weeks, and declined progressively at 9 weeks. We also found that apoptosis declined significantly after fasudil treatment (P < 0.05; Figure 5).
Figure 5.
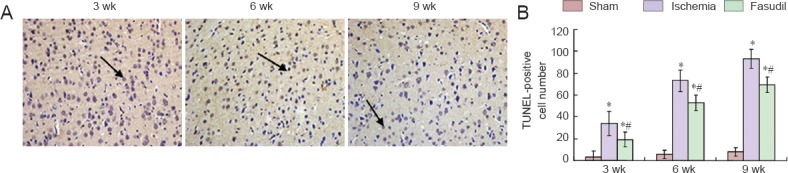
Cell apoptosis in the cerebral frontal cortex in rats following chronic cerebral ischemia (TUNEL staining).
(A) Representative photomicrographs of TUNEL-positive cells in rats in the sham and ischemia groups at 3, 6, 9 weeks (wk) after surgery (200×). Arrows showed TUNEL-positive cells. (B) The number of TUNEL-positive cells/200-fold field in the frontal cortex. Data are presented as the mean ± SD and analyzed using one-way analysis of variance and the Student's t-test. *P < 0.05, vs. sham operated (sham) group; #P < 0.05, vs. ischemia group.
The mRNA expression and immunoreactivity of caspase-3 in the frontal cortex of rats with chronic cerebral ischemia
Immunohistochemical staining showed that the immunoreactivity of caspase-3 in the frontal cortex appeared visible 3 weeks after 2-VO, reached a peak at 6 weeks following ischemia, and declined significantly in the fasudil group compared with the ischemia group (P < 0.05; Figure 6A).
Figure 6.
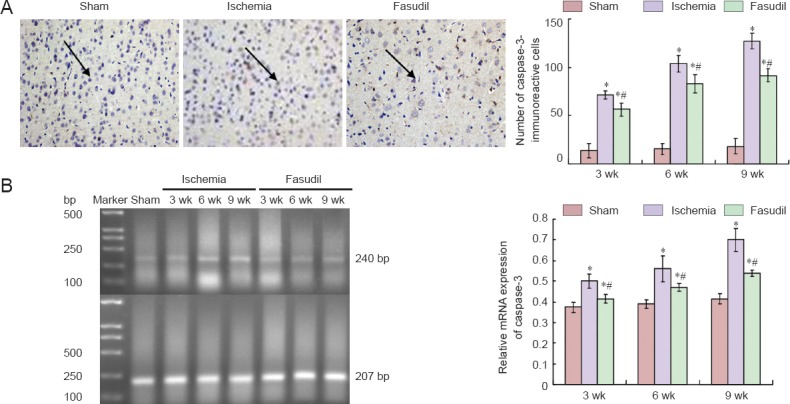
Immunoreactivity and mRNA expression of caspase-3 in the frontal cortex of rats following chronic cerebral ischemia and the effects of fasudil treatment.
(A) Left: Representative photomicrographs of caspase-3-immunoreactive cells (arrows) in rats in the sham and ischemia groups at 3, 6, 9 weeks (wk) after surgery. Right: Quantification of caspase-3 immunoreactivity. (B) mRNA expression of caspase-3. The mRNA results are expressed as the ratio of the target gene band intensity to that for β-actin. There were 10 rats at each time point per group. Data are expressed as the mean ± SD and analyzed using one-way analysis of variance and the Student's t-test. *P < 0.05, vs. sham operated (sham) group; #P < 0.05, vs. ischemia group.
RT-PCR demonstrated that mRNA expression of caspase-3 highly increased following cerebral ischemia (P < 0.05)(Figure 6B), and was significantly decreased after fasudil treatment (P < 0.05; Figure 6B).
The MAP2 immunohistochemical changes in the frontal cortex and hippocampus of rats with chronic cerebral ischemia
In the sham-operated group, the number and distribution of neurons in the frontal cortex and hippocampus were normal; the nucleus was round and the nucleolus was clear. But in the ischemia group, with ischemia time, the pathological changes of neurons were significantly visible, the number and distribution were rare, cytoplasm disappeared, staining was enhanced and karyopyknosis was observed. These findings were apparent in the frontal cortex. The immunoreactivity of MAP2, which was mainly in the dentrites of neurons, was widespread in the rat brain in the sham-operated group. In the ischemia group, the MAP2-immunoreactive neurons were observed in the frontal cortex, and the signal intensities were progressively increased with ischemia time. The dendrites of the MAP2-immunoreactive cells were bent, intermittent, scattered and disordered (Figure 7).
Figure 7.
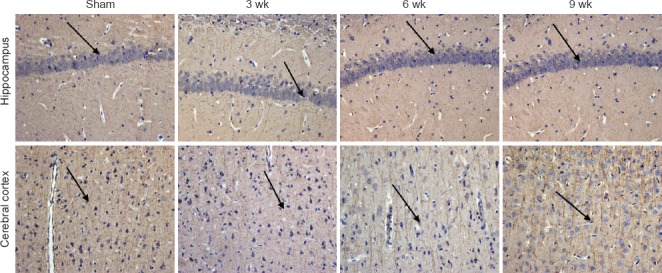
Representative photomicrographs of microtubule-associated protein 2 (MAP2)-immunoreactive cells in the hippocampus and frontal cortex of rats following chronic cerebral ischemia (200×).
MAP2-immunoactive cells in rats in the sham and ischemia groups at 3, 6, 9 weeks after surgery. Arrows indicate MAP2-immunoreactive cells.
Discussion
Chronic cerebral ischemia can result in persistent or progressive cognitive dysfunction (Yoshizaki et al., 2008). Since being established in 1992, the 2-VO method has become a classic way of inducing chronic cerebral ischemia diseases (de la Torre, 2000). MAP2 is an important component of the microtubule cytoskeleton, the decreased immune activity of which leads to neuronal necrosis (Jalava et al., 2007). Lingwood et al. (2008) and Prieto-Gomez et al. (2008) previously confirmed that MAP2 is an important sign of acute cerebral ischemia, but had not performed any studies on this topic in chronic cerebral ischemia. Our study showed that MAP2 expression in neurons and dendrites was progressively enhanced with ischemia time. The results indicate that MAP2 expression can be used as a classical morphological indicator to evaluate the existence of chronic cerebral ischemia.
Rho and ROCK activities generally increase in cerebrovascular disease, not only in the vascular smooth muscle and endothelial cells, but also in inflammatory cells and neurons (Hasan et al., 2012). The Rho/ROCK pathway produces an effect on the function of smooth muscle cells through their cell-mediated signal transduction system (Tiftik et al., 2008). Rho regulates the cell recombinant protein actin skeleton through its downstream effector ROCK, which is widely involved in cell migration, apoptosis, gene transcription and nerve regeneration (Antoniu, 2012). ROCK is a downstream product of Rho, with two cell subtypes, ROCK1 and ROCK2, the latter mainly existing in the central nervous system in places like hippocampal pyramidal neurons and the cerebral cortex. Weiss et al. (2007) has shown that ROCK affects cell signal transduction system through various channels, and is involved in the regulation of smooth muscle cell proliferation and restenosis. Its mechanisms were confirmed, such as promoting MLC phosphorylation, increasing endothelial permeability, and downregulating the expression of endothelial nitric oxide synthase (Huang et al., 2009; Popoff and Geny, 2009). Problems with the above mechanisms could lead to blood supply disorders of the brain, which affect memory and learning abilities.
There have been numerous studies on the mechanism of the Rho/ROCK signaling pathway in acute cerebral ischemia, but few on that in chronic ischemia (Zhang et al., 2015). We detected the expression of ROCK proteins and mRNA in the frontal cortex of rats subjected to a 2-VO operation. The results indicated that ROCK2 expression in the brain tissue of rats with cognitive dysfunction induced by chronic cerebral ischemia increased gradually with ischemia time, which is consistent with the degree of postoperative cognitive dysfunction.
Myosinphosphatase is an activated ROCK substrate composed of three subunits: MBS, a catalytic subunit and a small subunit of unknown function (Totsukawa et al., 2000). MBS is the first confirmed ROCK substrate. ROCK upregulates phosphorylation levels of MLC through two methods: by phosphorylating MLC directly, and by making MLCP inactive via the phosphorylation of MBS (Wang et al., 2009). In our study, western blot assay was used to detect the protein expression of MBS, which was considered to be a sign of ROCK activation. We found that the protein expression of MBS was visible at each time point in the ischemia group, peaked at 6 weeks, and then declined, and the expression change was similar to that of ROCK. These results indicate that after chronic cerebral ischemia, ROCK expression increased, leading to cognitive dysfunction.
There is evidence that ischemic hypoxia causes not only neuronal necrosis (Pender et al., 2014) but also neuronal apoptosis (Chen et al., 2009). Wang et al. (2009) had demonstrated that apoptosis plays an important role in neuron depigmentation induced by cerebral ischemia. Activated caspase-3 can directly induce free radical generation by mitochondria, and can accelerate mitochondrial dysfunction and cytochrome c release, ultimately leading to cell apoptosis (Sun et al., 2009). In the present study, caspase-3 took part in the pathological course of neuronal injury and greatly contributed to neuronal apoptosis in the frontal cortex after ischemia/reperfusion (Wu et al., 2009). In rat models of global cerebral ischemia, caspase-3 mRNA and protein expression in the frontal cortex was detected 72 hours after cerebral ischemia, accompanied by an increase in protein activity (Xu et al., 2009). In the present study, at 3 weeks after cerebral ischemia, caspase-3 immunoreactivity and mRNA expression increased, and its space-time distribution and dynamic changes were consistent with apoptosis cells. It also appeared in the frontal cortex and reached its peak at the same time. These findings indicate that caspase-3 expression is closely related to ischemic neuron injury.
In the present study, the learning and memory abilities of rats subjected to 2-VO were significantly improved after fasudil treatment. Western blot assay and RT-PCR results showed that ROCK2 protein and mRNA expression was decreased after fasudil treatment in rats subjected to 2-VO. Fasudil completely binds to ATP-binding sites, leading to a loss of ROCK activity and the inhibition of myosin light chain kinase (Fukushima et al., 2005). Fasudil is known to be a selective protein kinase inhibitor of the Rho/ROCK signal pathway in acute cerebral ischemia. However, its function has not been classified in chronic cerebral ischemia. The results from this study show that chronic cerebral hypoperfusion injury can lead to cognitive dysfunction, and that fasudil significantly relieved this phenomenon, indicating that ROCK plays a role in the occurrence of chronic cerebral hypoperfusion injury.
We also found that the expression of MBS and α-SMA was downregulated by Fasudil at each observation time point in rats subjected to 2-VO operation. Furthermore, cognitive impairment was improved after Fasudil treatment. The results suggest that the mechanism by which Fasudil improves cognitive dysfunction may be associated with the inhibition of ROCK2 expression and MBS and α-SMA phosphorylation. TUNEL staining results showed that apoptotic neurons increased under the condition of chronic cerebral ischemia, but apoptosis index decreased significantly after Fasudil treatment. These results suggest that fasudil plays an important role in inhibiting apoptosis induced by chronic cerebral ischemia.
Therefore, we can draw the following conclusions. (1) The model of cognitive dysfunction induced by chronic cerebral ischemia can be successfully established by 2-VO operation, and MAP2 immunohistochemistry is a reliable indicator of evaluating chronic cerebral impairment. (2) The expression and activation of ROCK play an important role in chronic cerebral ischemia, which leads to cognitive dysfunction, manifested as increased expression of MBS and related protein α-SMA; the Rho/Rho-kinase pathway is downregulated by fasudil. (3) The inhibition of ROCK by fasudil has a neuroprotective effect on chronic cerebral ischemia through an anti-apoptotic mechanism in the brain in vivo. In conclusion, our research provides experimental support for the pharmacological application of fasudil for the treatment of cognitive impairment induced by chronic cerebral ischemia.
Acknowledgments
We would like to express our gratitude to Gui-zhen Zhang from the Department of Center Laboratory, China-Japan Union Hospital, Jilin University, China for technical support.
Footnotes
Funding: This study was supported by the Natural Science Foundation of Jilin Province of China, No. 200705272, 20140414028GH.
Conflicts of interest: None declared.
Copyedited by Jackson C, Raye W, Li CH, Song LP, Zhao M
References
- Antoniu SA. Targeting RhoA/ROCK pathway in pulmonary arterial hypertension. Expert Opin Ther Targets. 2012;16:355–363. doi: 10.1517/14728222.2012.671811. [DOI] [PubMed] [Google Scholar]
- Arias HR, Richards VE, Nq D, Ghafoori ME, Le V, Mousa SA. Role of non-neuronal nicotinic acetylcholine receptors in angiogenesis. Int J Biochem Cell Biol. 2009;41:1441–1451. doi: 10.1016/j.biocel.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Bowerman M, Murray LM, Boyer JG, Anderson CL, Kothary R. Fasudil improves survival and promotes skeletal muscle development in a mouse model of spinal muscular atrophy. BMC Med. 2012;10:24. doi: 10.1186/1741-7015-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liu L, Yin J, Luo Y, Huang S. Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int J Biochem Cell Biol. 2009;41:1284–1295. doi: 10.1016/j.biocel.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Chen M, Liu A, Ouyang Y, Huang Y, Chao X, Pi R. Fasudil and its analogs: a new powerful weapon in the long war against central nervous system disorders. Expert Opin Invest Drugs. 2013;22:537–550. doi: 10.1517/13543784.2013.778242. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Impaired cerebromicrovascular perfusion. Summary of evidence in support of its causality in Alzheimer's disease. Ann N Y Acad Sci. 2000;924:136–152. doi: 10.1111/j.1749-6632.2000.tb05572.x. [DOI] [PubMed] [Google Scholar]
- Fukushima M, Nakamuta M, Kohjima M, Kotoh k, Enjoji M, Kobayashi N, Nawata H. Fasudil hydrochloride hydrate, a Rho-kinase (ROCK) inhibitor, suppresses collagen production and enhances collagenase activity in hepatic stellate cells. Liver lnt. 2005;25:829–838. doi: 10.1111/j.1478-3231.2005.01142.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Forero D, Montero F, Garcia-Morales V, Dominguez G, Gomez-Perez L, Garcia-Verdugo JM, Moreno-Lopez B. Endogenous Rho-kinase signaling maintains synaptic strength by stabilizing the size of the readily releasable pool of synaptic vesicles. J Neurosci. 2013;32:68–84. doi: 10.1523/JNEUROSCI.3215-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan Z, Palani K, Rahman M, Zhang S, Syk I, Jeppsson B, Thorlacius H. Rho-kinase signaling regulates pulmonary infiltration of neutrophils in abdominal sepsis via attenuation of CXC chemokine formation and Mac-1 expression on neutrophils. Shock. 2012;37:282–288. doi: 10.1097/SHK.0b013e3182426be4. [DOI] [PubMed] [Google Scholar]
- Horvath S. The pathological and clinical consequences of chronic cerebral hypoperfusion. Orv Hetil. 2001;142:323–329. [PubMed] [Google Scholar]
- Hou Y, Zhou L, Y QD, Du XP, Li M, Yuan M, Zhou ZW. Changes in hippocampal synapses and learning-memory abilities in a streptozotocintreated rat model and intervention by using fasudil hydrochloride. Neuroscience. 2012;200:120–129. doi: 10.1016/j.neuroscience.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Huang L, Li Q, Li H, He Z, Cheng Z, Chen J, Guo L. Inhibition of intracellular Ca 2+ release by a Rho-kinase inhibitor for the treatment of ischemic damage in primary cultured rat hippocampal neurons. Eur J Pharmacol. 2009;602:238–244. doi: 10.1016/j.ejphar.2008.11.053. [DOI] [PubMed] [Google Scholar]
- İnan SY, Büyükafşar K. Antiepileptic effects of two Rho-kinase inhibitors, Y-27632 and fasudil, in mice. Br J Pharmacol. 2008;155:44–51. doi: 10.1038/bjp.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikura K, Fujita H, Hida M, Awazu M. Trapidil inhibits platelet-derived growth factor-induced migration via protein kinase A and RhoA/Rho-associated kinase in rat vascular smooth muscle cells. Eur J Pharmacol. 2005;515:28–33. doi: 10.1016/j.ejphar.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Jalava NS, Lopez-Picon FR, Kukko-Lukjanov TK, Holopainen IE. Changes in microtubule-associated protein-2 (MAP2) expression during development and after status epilepticus in the immature rat hippocampus. Int J Dev Neurosci. 2007;25:121–131. doi: 10.1016/j.ijdevneu.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Koumura A A, Hamanaka J, Kawasaki K, Tsuruma K, Shimazawa M, Hozumi I, Inuzuka T, Hara H. Fasudil and ozagrel in combination show neuroprotective effects on cerebral infarction after murine middle cerebral artery occlusion. J Pharmacol Exp Ther. 2011;338:337–344. doi: 10.1124/jpet.110.177675. [DOI] [PubMed] [Google Scholar]
- Lingwood BE, Healy GN, Sullivan SM, Pow DV, Colditz PB. MAP2 provides reliable early assessment of neural injury in the newborn piglet model of birth asphyxia. J Neurosci Methods. 2008;171:140–146. doi: 10.1016/j.jneumeth.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Luo L, Jan LY, Jan YN. Rho family GTP-binding proteins in growth cone signaling. Curr Opin Neurobiol. 1997;7:81–86. doi: 10.1016/s0959-4388(97)80124-9. [DOI] [PubMed] [Google Scholar]
- Namkoong S, Kim CK, Cho YL, Kim JH, Lee H, Ha KS, Choe J, Kim PH, Won MH, Kwon YG, Shim EB, Kim YM. Forskolin increases angiogenesis through the coordinated cross-talk of PKA-dependent VEGF expression and Epac-mediated PI3K/Akt/eNOS signaling. Cell Signal. 2009;21:906–915. doi: 10.1016/j.cellsig.2009.01.038. [DOI] [PubMed] [Google Scholar]
- Oh KS, Mun J, Cho JE, Lee S, Yi KY, Lim CJ, Lee JS, Park WJ, Lee BH. Discovery of novel scaffolds for Rho kinase 2 inhibitor through TRFRET-based high throughput screening assay. Comb Chem High Throughput Screen. 2013;16:37–46. doi: 10.2174/1386207311316010006. [DOI] [PubMed] [Google Scholar]
- Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol. 2008;20:242–248. doi: 10.1016/j.ceb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pender MP, Caurhes PA, Pfluger CM, Burrows SR. Deficiency of CD8+ effector memory T cells is an early and persistent feature of multiple sclerosis. Mult Scler. 2014;20:1825–1832. doi: 10.1177/1352458514536252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda AA, Minohara M, Kawamura N, Matsushita T, Yamasaki R, Sun X, Piao H, Shimokawa H, Kira J. Preventive and therapeutic effects of the selective Rho-kinase inhibitor fasudil on experimental autoimmune neuritis. J Neurol Sci. 2011;306:115–120. doi: 10.1016/j.jns.2011.03.031. [DOI] [PubMed] [Google Scholar]
- Popoff MR, Geny B. Multifaceted role of Rho, Rac, Cdc42 and Ras in intercellular junctions, lessons from toxins. Biochim Biophys Acta. 2009;1788:797–812. doi: 10.1016/j.bbamem.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Prieto-Gomez B, Velazquez-Paniagua M, Cisneros LO, Reyes-Vazquez C, Jimenez-Trejo F, Reyes ME, Mendoza-Torreblanca J, Gutierrez-Ospina G. Melatonin attenuates the decrement of dendritic protein MAP-2 immuno-staining in the hippocampal CA1 and CA3 fields of the aging male rat. Neurosci Lett. 2008;448:56–61. doi: 10.1016/j.neulet.2008.10.041. [DOI] [PubMed] [Google Scholar]
- Raad M, El Tal T, Gul R, Mondello S, Zhang Z, Boustany RM, Guingab J, Wang KK, Kobeissy F. Neuroproteomics approach and neurosystems biology analysis: ROCK inhibitors as promising therapeutic targets in neurodegeneration and neurotrauma. Electrophoresis. 2012;33:3659–3668. doi: 10.1002/elps.201200470. [DOI] [PubMed] [Google Scholar]
- Sahai E, Ishizaki T, Narumiya S, Treisman R. Transformation mediated by RhoA requires activity of ROCK kinases. Curr Biol. 1999;9:136–145. doi: 10.1016/s0960-9822(99)80067-0. [DOI] [PubMed] [Google Scholar]
- Shahin R, Alqtaishat S, Taha MO. Elaborate ligand-based modeling reveal new submicromolar Rho kinase inhibitors. J Comput Aided Mol Des. 2012;26:249–266. doi: 10.1007/s10822-011-9509-y. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Rashid M. Development of Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol Sci. 2007;28:296–302. doi: 10.1016/j.tips.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Shin DM, Kang J, Ha J, Kang HS, Park SC, Kim IG, Kim SJ. Cystamine prevents ischemia-reperfusion injury by inhibiting polyamination of RhoA. Biochem Biophys Res Commun. 2008;365:509–514. doi: 10.1016/j.bbrc.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Sun M, Zhao Y, Gu Y, Xu C. Inhibition of nNOS reduces ischemic cell death through down-regulating calpain and caspase-3 after experimental stroke. Neurochem Int. 2009;54:339–346. doi: 10.1016/j.neuint.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Tan HB, Zhong YS, Cheng Y, Shen X. Rho/ROCK pathway and neural regeneration: a potential therapeutic target for central nervous system and optic nerve damage. Int J Ophthalmol. 2011;4:652–657. doi: 10.3980/j.issn.2222-3959.2011.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiftik RN, Erol A, Cnar MG, Kubat H, Ark M, Ulker S, Büyükafşar K. Nitric oxide does not downregulate Rho-kinase (ROCK-2) expression in rat coronary endothelial cells. J Cardiovasc Pharmacol. 2008;51:140–147. doi: 10.1097/FJC.0b013e31815e4089. [DOI] [PubMed] [Google Scholar]
- Tonges L, Frank T, Tatenhorst L, Saal KA, Koch JC, Szego EM, Bahr M, Weishaupt JH, Lingor P. Inhibition of rho kinase enhances survival of dopaminergic neurons and attenuates axonal loss in a mouse model of Parkinson's disease. Brain. 2012;135:3355–3370. doi: 10.1093/brain/aws254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar-Cheda B, Dominguez-Meijide A, Joglar B, Rodriguez-Perez AI, Guerra MJ, Labandeira-Garcia JL. Involvement of microglial RhoA/Rhokinase pathway activation in the dopaminergic neuron death. Role of angiotensin via angiotensin type 1 receptors. Neurobiol Dis. 2012;47:268–279. doi: 10.1016/j.nbd.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Wang SM, Yang WL. Circulating hormone adrenomedullin and its binding protein protect neural cells from hypoxia-induced apoptosis. Biochim Biophys Acta. 2009;1790:361–367. doi: 10.1016/j.bbagen.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zheng XR, Riddick N, Bryden M, Baur W, Zhang X, Surks HK. ROCK Isoform Regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res. 2009;104:531–540. doi: 10.1161/CIRCRESAHA.108.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Frischknecht K, Greutert H, Payeli S, Steffel J, Lüscher TF, Carrel TP, Tanner FC. Different migration of vascular smooth muscle cells from human coronary artery bypass vessels. Role of Rho/ROCK pathway. J Vasc Res. 2007;44:149–156. doi: 10.1159/000099141. [DOI] [PubMed] [Google Scholar]
- Wu JS, Cheung WM, Tsai YS, Chen YT, Fong WH, Tsai HD, Chen YC, Liou JY, Shyue SK, Chen JJ, Chen YE, Maeda N, Wu KK, Lin TN. Ligand-activated peroxisome proliferator-activated receptor-gamma Protects Against Ischemic cerebral Infarction and neuronal apoptosis by 14-3-3 epsilon Upregulation. Circulation. 2009;119:1124–1134. doi: 10.1161/CIRCULATIONAHA.108.812537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Liu Y, Wang L, He J, Zhang H, Chen X, Li Y, Yang J, Tao J. Gambogic acid induces apoptosis by regulating the expression of Bax and Bcl-2 and enhancing caspase-3 activity in human malignant melanoma A375 cells. Int J Dermatol. 2009;48:186–192. doi: 10.1111/j.1365-4632.2009.03946.x. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Kotani Y, Nakajima Y, Shimazawa M, Yoshimura S, Nakashima S. Fasudil, a Rho kinase (ROCK) inhibitor, protects against ischemic neuronal damage in vitro and in vivo by acting directly on neurons. Brain Res. 2007;1154:215–224. doi: 10.1016/j.brainres.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Yano K, Kawasaki K, Hattori T, Tawara S, Toshima Y, Ikeqaki I, Sasaki Y, Satoh S, Asano T, Seto M. Demonstration of elevation and localization of Rho-kinase activity in the brain of a rat model of cerebral infarction. Eur J Pharmacol. 2008;594:77–83. doi: 10.1016/j.ejphar.2008.07.045. [DOI] [PubMed] [Google Scholar]
- Yoshimi E, Yamamoto H, Furuichi Y, Shimizu Y, Takeshita N. Sustained analgesic effect of the Rho kinase inhibitor AS1892802 in rat models of chronic pain. J Pharmacol Sci. 2010;114:119–122. doi: 10.1254/jphs.10158sc. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K, Adachi K, Kataoka S, Watanabe A, Tabira T, Takahashi K, Wakita H. Chronic cerebral hypoperfusion induced by right unilateral common carotid artery occlusion causes delayed white matter lesions and cognitive impairment in adult mice. Exp Neurol. 2008;210:585–591. doi: 10.1016/j.expneurol.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhang JJ, Han ZM. Efficacy of Rho kinase inhibitor on cognitive impairment induced by chronic cerebral hypoperfusion in rats. Int J Clin Exp Med. 2015;8:2435–2440. [PMC free article] [PubMed] [Google Scholar]


