Abstract
The main active components extracted from Panax notoginseng are total saponins. They have been shown to inhibit platelet aggregation, increase cerebral blood flow, improve neurological behavior, decrease infarct volume and promote proliferation and differentiation of neural stem cells in the hippocampus and lateral ventricles. However, there is a lack of studies on whether total saponins of Panax notoginseng have potential benefits on immature neuroblasts in the olfactory bulb following ischemia and reperfusion. This study established a rat model of global cerebral ischemia and reperfusion using four-vessel occlusion. Rats were administered total saponins of Panax notoginseng at 75 mg/kg intraperitoneally 30 minutes after ischemia then once a day, for either 7 or 14 days. Total saponins of Panax notoginseng enhanced the number of doublecortin (DCX)+ neural progenitor cells and increased co-localization of DCX with neuronal nuclei and phosphorylated cAMP response element-binding/DCX+ neural progenitor cells in the olfactory bulb at 7 and 14 days post ischemia. These findings indicate that following global brain ischemia/reperfusion, total saponins of Panax notoginseng promote differentiation of DCX+ cells expressing immature neuroblasts in the olfactory bulb and the underlying mechanism is related to the activation of the signaling pathway of cyclic adenosine monophosphate response element binding protein.
Keywords: nerve regeneration, total saponins of Panax notoginseng, cerebral ischemia/reperfusion, immature neurons, neurogenesis, doublecortin, olfactory bulb, neural regeneration
Introduction
The subventricular zone of the lateral ventricle is a neurogenic niche because it can maintain neurogenesis throughout life (Luskin, 1993; Alvarez-Buylla and Garcıa-Verdugo, 2002; Ponti et al., 2013). Precursors from the subventricular zone migrate toward the olfactory bulb following the rostral migratory stream pathway where they are functionally integrated into circularity networks (Winner et al., 2002; Ma et al., 2009; Nissant et al., 2009; Macklis, 2012). Doublecortin (DCX) is an endogenous microtubule-associated protein with an immature phenotype and is a good marker of neuroblasts (Francis et al., 1999; Brown et al., 2003; Couillard-Despres et al., 2005; Saaltink et al., 2012). Transient cerebral ischemia or brain trauma can stimulate endogenous neurogenesis in certain areas of the brain including the olfactory bulb, and this contributes to neuronal recovery to varying degrees (Zhang et al., 2001; Iwai et al., 2003; Choi et al., 2010; Pan et al., 2013). However, the capacity of endogenous self-repair is limited, so it is imperative to find either candidate drugs or other strategies to induce and amplify endogenous adult precursors following global cerebral ischemia.
Panax notoginseng is a traditional Chinese medicine widely used in China to remove blood stasis. Total saponins of Panax notoginseng (TSPN) are the bioactive constituents of Panax notoginseng (Ng, 2006), and are responsible for inhibiting apoptosis and caspase activation (Li et al., 2009), enhancing endogenous brain-derived neurotrophic factor expression, reducing cytokines and blood-brain barrier permeability, and stimulating neural stem cell proliferation and differentiation in the hippocampus (Si et al., 2011). Although the olfactory bulb is an area that displays neurogenesis and is an important part of the rostral migratory stream, there are no reports on whether TSPN could enhance neurogenesis in the olfactory bulb following global cerebral ischemia/reperfusion (I/R). This study evaluated the effects of TSPN on immature neuroblasts in the olfactory bulb of a rat model of transient global cerebral I/R induced by four-vessel occlusion.
Materials and Methods
Establishment of transient global cerebral I/R models
A total of 80 specific-pathogen-free male Sprague-Dawley rats weighing 250–290 g and aged 8 weeks were obtained from the Animal Center of Xiangya School of Medicine, Central South University, China (license No. SCXK (Xiang) 2009-0012). All animal procedures conformed to the National Institutes of Health Guide for the care and use of laboratory animals, and all procedures were approved by the Ethics Committee of Central South University, China. All efforts were made to minimize animal discomfort and reduce the number of animals used.
Four-vessel occlusion was used to induce cerebral ischemia (Pulsinelli and Brierley, 1979). Briefly, all rats were anesthetized with 1% sodium pentobarbital diluted in distilled water (50 mg/kg, intraperitoneally) in a sterile room. The rats were positioned in Kopf stereotaxic apparatus (David KOPF Instruments, Tujunga, CA, USA) and the vertebral arteries were irreversibly occluded by electrocoagulation. A non-absorbable suture was looped around the carotid arteries. On the following day, rats were re-anesthetized and carotid arteries were disconnected carefully to avoid damage to the vagus nerve, and then occluded with micro-arterial clamps for 30 minutes. Rats lost their righting reflex during I/R and this was achieved by de-clamping the arteries. During the surgery, rectal temperature was monitored and maintained at 37 ± 0.5°C with a rectal probe and a heat lamp was used to ensure the body temperature at 37°C. Eight rats died, so the remaining 72 animals were equally and randomly divided into the TSPN group and vehicle group.
TSPN administration
The rats in the TSPN group were administered TSPN (reagent No. M004538, high-performance liquid chromatography ≥ 98%, Chengdu Maikaxi Chemical Co., Ltd., Chengdu, China) intraperitoneally 30 minutes after brain ischemia. The dose of TSPN was 75 mg/kg suspended in 0.9% sodium salt 10 g/L, once per day for 1, 7 and 14 days after reperfusion. Rats in the vehicle group were treated with an equal volume of sodium salt, one injection per day until the rats were sacrificed at either 1, 7 or 14 days post ischemia.
Tissue preparation
Rats in both groups were intraperitoneally anesthetized with a high dose of sodium pentobarbital (400 mg/kg) and then perfused transcardially with saline followed by 4% paraformaldehyde. The brains were post-fixed at 4°C overnight, and were treated in gradual concentrations of sucrose (15% and 30%) until they sank. Coronal sections were prepared across the olfactory bulb (Paxinos and Watson, 2005) in a cryostat (Thermo Shandon Limited, UK). Twelve sets of 30-μm sections were cut and collected for western blot assay. For double immunofluorescence, 12 sets of 8-μm sections were also collected by thaw-mounting on positively charged microslides.
Immunohistochemistry and immunofluorescent labeling
Immunohistochemistry was carried out using the avidin-biotin complex method. Sections were treated with 3% H2O2 for 30 minutes at 24 ± 1°C, and pre-incubated for 1 hour, followed by incubation with goat anti-DCX (1:2,000; Santa Cruz Biotechnology, Dallas, TX, USA) overnight at 4°C. Sections were further reacted with rabbit anti-goat IgGs (Boster, Wuhan, Hubei Province, China) at 1:400 for 2 hours at 24 ± 1°C and subsequently with avidin-biotin complex reagents for an additional 2 hours. Immunoreactivity was visualized using 0.003% hydrogen peroxide and 0.05% 3,3′-diaminobenzidine.
Immunofluorescent labeling was carried out as follows: sections were immersed in PBS containing 5% donkey serum and 0.1% Triton X-100 for 1 hour, treated with goat anti-DCX (1:2,000), mouse anti-neuronal nuclei (NeuN) (1:4,000; Merck Millipore, Darmstadt, Germany), rabbit anti-phosphorylated cyclic adenosine monophosphate response element binding protein (p-CREB) (1:2,000; Cell Signaling, Boston, MA, USA) overnight at 4°C, followed by a 2-hour reaction with Alexa-Fluor 488 and Alexa-Fluor 594-donkey anti-goat, anti-mouse, anti-rabbit IgGs (1:200, Invitrogen, Carlsbad, CA, USA). Sections were counterstained with bisbenzimide (1:50,000; Hoechst 33342).
Immunostained sections were examined and imaged on a microscope equipped with a digital camera and imaging system (BX40, Olympus, Tokyo, Japan). The sections of the olfactory bulb used for comparison were on the same horizontal plane. Because the DCX+ cells in the olfactory bulb were in a cluster, the mean optical density of images was captured using an identical photographic setting and compared using NIH Image J (NIH, MD, USA). Images at 40× magnification were taken for morphological analyses of positive cells. Co-localization of cells in the olfactory bulb was calculated in a set of sections. In each section, five fields were captured with a 20× objective lens. The total DCX+ cells and co-localized cells were added together for each section, brain, and group. The formula for co-localization rate was: co-localized DCX+ cells/total DCX+ cells × 100%.
Western blot assay
The olfactory bulb was snap-frozen in liquid nitrogen. Frozen tissues were homogenized in a cocktail buffer, including phosphatase inhibitors and protease inhibitors (Roche Applied Science, Mannheim, Germany). The samples were then centrifuged at 1,200 × g for 15 minutes at 4°C. Bicinchoninic acid assay was used to measure the protein concentrations from supernatants. A total of 20 μg protein was loaded in each lane and separated by 12% Bis-Tris sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Subsequently, the blotted proteins were transferred to nitrocellulose membrane. The membrane was blocked using 10% nonfat milk for 2 hours at 24 ± 1°C, then incubated overnight with goat anti-DCX (1:1,000, Santa Cruz Biotechnology) and mouse anti-GAPDH (1:10,000; Boster). The membrane was washed and incubated with horseradish peroxidase-conjugate rabbit anti-goat or anti-mouse (1:5,000; Merck Millipore, Merck KGaA, Germany) for 2 hours at 24 ± 1°C, followed by exposure to photographic films for 1 to 5 minutes. Western blot band intensity (integrated optical density) was measured using NIH Image J. GAPDH was used as an internal reference.
Statistical analysis
All data are expressed as the mean ± SD, and were analyzed using Prism GraphPad5.0 (GraphPad Software Inc., La Jolla, CA, USA). Comparisons were conducted using one-way analysis of variance followed by Bonferroni post hoc tests. A value of P ≤ 0.05 was considered statistically significant.
Results
TSPN up-regulated the number of DCX+ cells in the olfactory bulb following global cerebral I/R
DCX has been used as a marker for immature neurons, and DCX+ cells populate in the piriform cortex and olfactory bulb of rabbits, cats, rats, guinea pigs and non-human primates (Xiong et al., 2008; Cai et al., 2009; Zhang et al., 2009; Klempin et al., 2011; He et al., 2014; Zeng et al., 2014). In the present study, global cerebral I/R affected DCX labeling, with varied shapes and sizes of cells detectable mainly around the granule cell layer, internal plexiform layer, and mitral cell layer. Dendrite-like processes chained in a cluster in the granule cell layer (Figure 1A3, B3). There was no significant difference in the mean optical density of DCX in the granule cell layer in vehicle or TSPN groups 1 day post ischemia (P > 0.05). In comparison with the vehicle group, TSPN significantly increased the mean optical density of DCX+ cells in the granule cell layer at 7 and 14 days post ischemia (day 7: P < 0.05; day 14: P < 0.001; Figure 1C). Furthermore, the mean optical density of DCX+ cells was significantly higher in the TSPN group than in the vehicle group in the internal plexiform layer and mitral cell layer (P < 0.0001, F = 16.8, degrees of freedom (df) = 5,24; P < 0.0001, F = 10.7, df = 5,24). However, there was no significant difference in the internal plexiform layer and mitral cell layer between the TSPN and vehicle groups 1 day post ischemia (P > 0.05). At 7 and 14 days after ischemia, the mean optical density of DCX+ cells in the internal plexiform layer was significantly higher in the TSPN group than in the vehicle group (day 7: P < 0.05; day 14: P < 0.01). The mean optical density of DCX+ cells in the mitral cell layer was significantly higher in the TSPN group than in the vehicle group at 14 days post ischemia (P < 0.05; Figure 1D and E).
Figure 1.
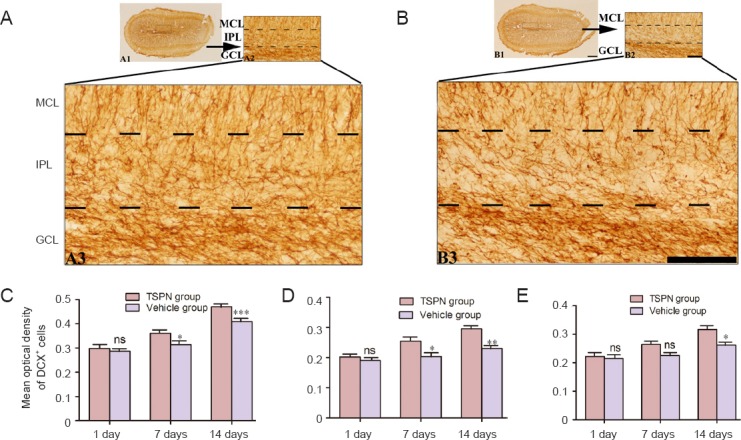
Effects of TSPN on DCX immunoreactivity in the olfactory bulb of adult rats following global cerebral ischemia/reperfusion.
Representative images are from the olfactory bulb in TSPN group (A1–A3) or vehicle group (B1–B3) in rats surviving 14 days. (A3, B3) The montage of images was photographed with a 40× objective lens to illustrate DCX immunoreactivity and morphology. (A1, B1) Scale bar: 50 μm; (A2, B2) scale bar: 100 μm; (A3, B3) scale bar: 200 μm. (C–E) DCX immunoreactivity in GCL, IPL and MCL, respectively. All data are expressed as the mean ± SD (six rats per group), and comparisons were conducted using one-way analysis of variance followed by Bonferroni post hoc tests. *P < 0.05, **P < 0.01, ***P < 0.001, vs. vehicle group; ns: P > 0.05, vs. vehicle group. TSPN: Total saponins of Panax notoginseng; MCL: mitral cell layer; IPL: internal plexiform layer; GCL: granule cell layer; DCX: doublecortin.
TSPN elevated DCX protein levels in the olfactory bulb following global cerebral I/R
DCX protein levels in the olfactory bulb formation were determined by western blot assay. Immunoblotted DCX signal increased in the TSPN group compared with the vehicle group at 7 and 14 days post ischemia (Figure 2A). The mean optical density of DCX protein level at 7 and 14 days post ischemia was significantly higher in the TSPN group than in the vehicle group (day 7: P < 0.001; day 14: P < 0.001), but not at 1 day post ischemia (P > 0.05; Figure 2B).
Figure 2.
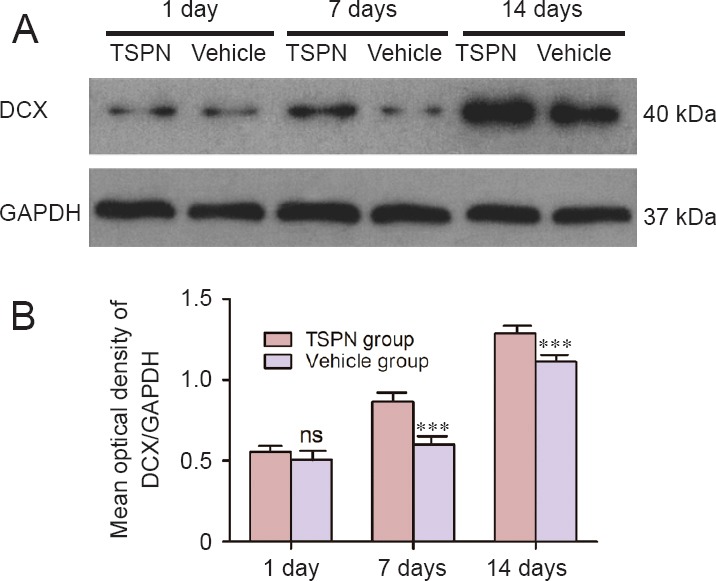
Representative western blot assay (A) and quantification of DCX protein levels (B) in the olfactory bulb after global cerebral ischemia/reperfusion.
DCX levels in the TSPN group were significantly higher than that in the vehicle group at 7 and 14 days post ischemia. All data are expressed as the mean ± SD (six rats per group), and comparisons were conducted using one-way analysis of variance followed by Bonferroni post hoc tests. ***P < 0.001, vs. vehicle group; ns: P > 0.05, vs. vehicle group. TSPN: Total saponins of Panax notoginseng; DCX: doublecortin; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
TSPN enhanced co-localization of DCX with NeuN in the olfactory bulb following global cerebral I/R
There were a higher number of DCX+ cells in the granule cell layer than in any other layer between the vehicle and TSPN groups (Figure 1). We therefore focused on the granule cell layer. DCX+ cells expressing NeuN were detected in the olfactory bulb at all time points post ischemia (Figure 3A), and the ratios of DCX+ cells co-expressing NeuN to the total DCX-labeled cells were higher in the TSPN group than in the vehicle group at both 7 and 14 days post ischemia (day 7: P < 0.01; day 14: P < 0.001), but not at 1 day (P > 0.05; Figure 3B).
Figure 3.
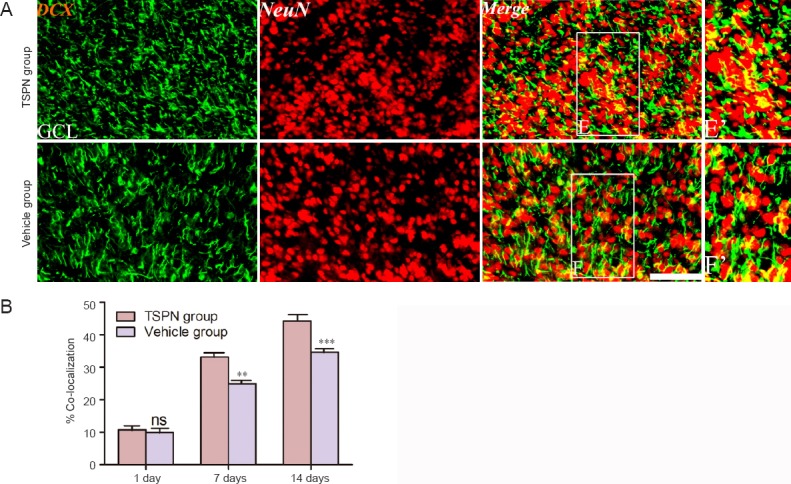
Double immunofluorescence staining for DCX and NeuN (A) and quantitative data (B) in the olfactory bulb of adult rats at 14 days post ischemia.
E’ and F’ are enlarged images of boxes in E and F, respectively. Scale bar: 100 μm in E and F; scale bar: 50 μm in E’ and F’. All data are expressed as the mean ± SD (six rats per group), and comparisons were conducted using one-way analysis of variance followed by Bonferroni post hoc tests. **P < 0.01, ***P < 0.001, vs. vehicle group. ns: P > 0.05, vs. vehicle group. TSPN: Total saponins of Panax notoginseng; GCL: granule cell layer; DCX: doublecortin; NeuN: neuronal nuclei.
TSPN induced immature neuroblasts in the olfactory bulb through phosphorylation of cyclic adenosine monophosphate response element binding protein (CREB) following global cerebral I/R
To further understand the underlying mechanism by which TSPN regulated immature neurons in the olfactory bulb, we tested the activation of p-CREB in the olfactory bulb following global cerebral ischemia using immunohistochemistry. A number of p-CREB/DCX double-labeled cells were found in both groups at various time points (Figure 4A). Moreover, the percentage of p-CREB/DCX double-labeled cells significantly increased in the TSPN group compared with the vehicle group at 7 days (P < 0.01) and at 14 days (P < 0.001), but not at 1 day post ischemia (P > 0.05; Figure 4B).
Figure 4.
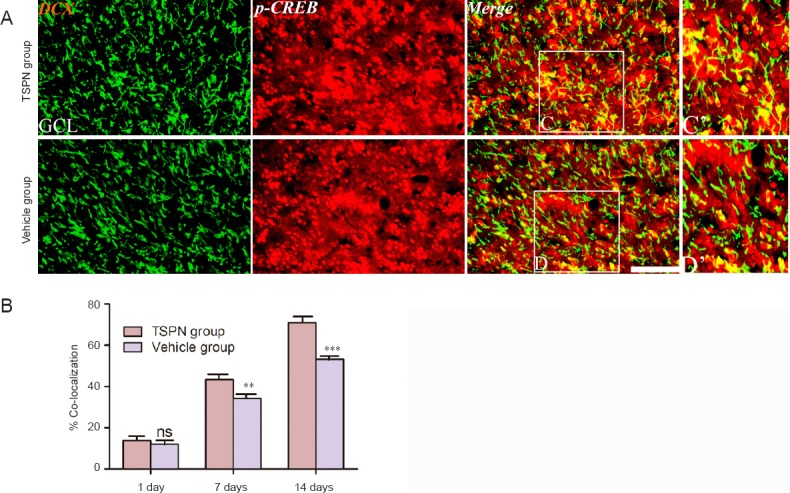
Double immunofluorescence staining for DCX and p-CREB (A) and quantitative data (B) in the olfactory bulb of adult rats at 14 days post ischemia.
C’ and D’ are enlarged images of boxes in C and D, respectively. Scale bar: 100 μm in C and D; scale bar: 50 μm in C’ and D’. All data are expressed as the mean ± SD (six rats per group), and comparisons were conducted using one-way analysis of variance followed by Bonferroni post hoc tests. **P < 0.01, ***P < 0.001, vs. vehicle group. ns: P > 0.05, vs. vehicle group. TSPN: Total saponins of Panax notoginseng; p-CREB: phosphorylated cyclic adenosine monophosphate response element binding protein; GCL: granule cell layer; DCX: doublecortin.
Discussion
We examined TSPN effects on immature neuroblasts in an animal model of global cerebral I/R. TSPN evidently enhanced DCX-expressing immature neurons in the olfactory bulb. Moreover, TSPN noticeably increased DCX/NeuN co-expression and p-CREB/DCX+ cells, which suggests that TSPN can promote neurochemical differentiation of immature neural cells in the olfactory bulb following global ischemia by up-regulating the p-CREB signaling pathway.
Neurogenesis is a process whereby precursor cells divide into immature neurons and then differentiate into neurons with a mature phenotype (Lazarini et al., 2014). Immature neurons expressing DCX are of great significance to brain plasticity and development (Nacher et al., 2001; Bloch et al., 2011) and are involved in cytoskeletal changes (Kutsuna et al., 2014) and structural plasticity, and are thought to occur at synaptic and neuritic levels under physiological conditions (Luo, 2002; He et al., 2014). Neurogenesis can be accelerated after brain ischemia in the adult rat, monkey, human cerebrum including the olfactory bulb (Jin et al., 2001b; Tonchev et al., 2003; Gould, 2007; Kutsuna et al., 2014). In our study, the increased numerical density of immature DCX-expressing neurons indicates an increased population of newborn immature neurons and dendritic processes. To further confirm the immunohistochemistry result, western blots were carried out to detect DCX protein levels. Densitometry was consistent with the immunofluorescence, suggesting that TSPN increased the population of immature neurons in the olfactory bulb following global cerebral I/R.
It is well known that when newly generated precursor cells in the adult brain begin developing and maturing biochemically and morphologically (Brown et al., 2003), newly generated neurons in the olfactory bulb are crucial for olfactory function (Gheusi et al., 2000; Lledo and Saghatelyan, 2005; Kermen et al., 2010). NeuN is a mature neuronal marker (Mullen et al., 1992; Sarnat et al., 1998) and is both apparent and increased during hippocampal neurogenesis. Therefore, the extent of DCX/NeuN co-expression may implicate the neurochemical maturity of immature olfactory DCX-expressing neurons. Results from this study showed a significant increase in NeuN co-expression among the DCX+ neurons in the olfactory bulb of global cerebral ischemia rats following intraperitoneally injecting TSPN at 7 and 14 days. These findings suggest that TSPN could enhance and drive the neurochemical differentiation of immature neurons in the olfactory bulb toward the phenotype of maturation.
CREB is an important nuclear transcription factor, and experiments in vivo and vitro demonstrated that CREB signaling pathways play a vital role in the neurogenesis and survival of immature neuroblasts in the olfactory bulb (Giachino et al., 2005), subventricular zone (Gampe et al., 2011; Herold et al., 2011) and hippocampus (Jagasia et al., 2009; Kim et al., 2010). Activated CREB is involved in many biological functions such as enhancement of neuronal regeneration, synapse formation and spatial learning (Walton et al., 1996; O’Connell et al., 2000; Rajan et al., 2015). In transient ischemic adult rodents, high levels of p-CREB existed (Walton et al., 1996; Tanaka et al., 2000; Jin et al., 2001a) in immature DCX-expressing neurons in the dentate gyrus (Nakagawa et al., 2002). Cell counting showed that there were a large number of p-CREB/DCX+ neural progenitor cells in the olfactory bulb of the two groups at different time points, which suggested engagement of the CREB signaling pathway in adult neurogenesis and recruitment of immature DCX-expressing neurons for brain repair following global cerebral ischemia. Furthermore, TSPN apparently increased the number of the DCX+ cells expressing p-CREB in the olfactory bulb of rats surviving 7 and 14 days, implying that TSPN could modulate the newborn immature neuroblasts by up-regulating the p-CREB signaling pathway.
In summary, TSPN could enhance regeneration and maturation of DCX+ cells expressing immature neuroblasts in the olfactory bulb of adult rats through activating the CREB signaling pathway following global cerebral I/R.
Footnotes
Funding: This study was supported by the Hunan Provincial Innovation Foundation for Postgraduate in China, No. CX2014B099 (to XH); the Science Foundation of Hunan Provincial Education Department of China, No. 11C1264 (to FJD), 13C958(to XH).
Conflicts of interest: None declared.
Copyedited by Paul P, Park M, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Alvarez-Buylla A, Garcıa-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch J, Kaeser M, Sadeghi Y, Rouiller EM, Redmond DE, Brunet JF. Doublecortin-positive cells in the adult primate cerebral cortex and possible role in brain plasticity and development. J Comp Neurol. 2011;519:775–789. doi: 10.1002/cne.22547. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Cai Y, Xiong K, Chu Y, Luo DW, Luo XG, Yuan XY, Struble RG, Clough RW, Spencer DD, Williamson A, Kordower JH, Patrylo PR, Yan XX. Doublecortin expression in adult cat and primate cerebral cortex relates to immature neurons that develop into GABAergic subgroups. Exp Neurol. 2009;216:342–356. doi: 10.1016/j.expneurol.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Yoo KY, Park OK, Lee CH, Kim SK, Hwang IK, Lee YL, Shin HC, Won MH. Relation among neuronal death, cell proliferation and neuronal differentiation in the gerbil main olfactory bulb after transient cerebral ischemia. Cell Mol Neurobiol. 2010;30:929–938. doi: 10.1007/s10571-010-9522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- Gampe K, Brill MS, Momma S, Götz M, Zimmermann H. EGF induces CREB and ERK activation at the wall of the mouse lateral ventricles. Brain Res. 2011;1376:31–41. doi: 10.1016/j.brainres.2010.11.040. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci U S A. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachino C, De Marchis S, Giampietro C, Parlato R, Perroteau I, Schütz G, Fasolo A, Peretto P. cAMP response element-binding protein regulates differentiation and survival of newborn neurons in the olfactory bulb. J Neurosci. 2005;25:10105–10118. doi: 10.1523/JNEUROSCI.3512-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- He X, Zhang XM, Wu J, Fu J, Mou L, Lu DH, Cai Y, Luo XG, Pan A, Yan XX. Olfactory experience modulates immature neuron development in postnatal and adult guinea pig piriform cortex. Neuroscience. 2014;259:101–112. doi: 10.1016/j.neuroscience.2013.11.056. [DOI] [PubMed] [Google Scholar]
- Herold S, Jagasia R, Merz K, Wassmer K, Lie DC. CREB signalling regulates early survival, neuronal gene expression and morphological development in adult subventricular zone neurogenesis. Mol Cell Neurosci. 2011;46:79–88. doi: 10.1016/j.mcn.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Iwai M, Sato K, Kamada H, Omori N, Nagano, Shoji M, Abe K. Temporal profile of stem cell division, migration, and differentiation from subventricular zone to olfactory bulb after transient forebrain ischemia in gerbils. J Cereb Blood Flow Metab. 2003;23:331–341. doi: 10.1097/01.WCB.0000050060.57184.E7. [DOI] [PubMed] [Google Scholar]
- Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC. GABA-CREB signalling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Mao X, Simon R, Greenberg D. Cyclic AMP response element binding protein (CREB) and CREB binding protein (CBP) in global cerebral ischemia. J Mol Neurosci. 2001a;16:49–56. doi: 10.1385/JMN:16:1:49. [DOI] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001b;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermen F, Sultan S, Sacquet J, Mandairon N, Didier A. Consolidation of an olfactory memory trace in the olfactory bulb is required for learning-induced survival of adult-born neurons and long-term memory. PLoS One. 2010;5:e12118. doi: 10.1371/journal.pone.0012118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Yang M, Cho J, Kim SH, Kim JC, Shin T, Moon C. Promotion of cAMP responsive element-binding protein activity ameliorates radiation-induced suppression of hippocampal neurogenesis in adult mice. Toxicol Res. 2010;26:177–183. doi: 10.5487/TR.2010.26.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempin F, Kronenberg G, Cheung G, Kettenmann H, Kempermann G. Properties of doublecortin-(DCX)-expressing cells in the piriform cortex compared to the neurogenic dentate gyrus of adult mice. PLoS One. 2011;6:e25760. doi: 10.1371/journal.pone.0025760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuna N, Yamashita A, Eriguchi T, Oshima H, Suma T, Sakatani K, Yamamoto T, Yoshino A, Katayama Y. Acute stress exposure preceding transient global brain ischemia exacerbates the decrease in cortical remodeling potential in the rat retrosplenial cortex. Neurosci Res. 2014;78:65–71. doi: 10.1016/j.neures.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Lazarini F, Gabellec MM, Moigneu C, de Chaumont F, Olivo-Marin JC, Lledo PM. Adult neurogenesis restores dopaminergic neuronal loss in the olfactory bulb. J Neurosci. 2014;34:14430–14442. doi: 10.1523/JNEUROSCI.5366-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Deng CQ, Chen BY, Zhang SP, Liang Y, Luo XG. Total saponins of Panax Notoginseng modulate the expression of caspases and attenuate apoptosis in rats following focal cerebral ischemia-reperfusion. J Ethnopharmacol. 2009;121:412–418. doi: 10.1016/j.jep.2008.10.042. [DOI] [PubMed] [Google Scholar]
- Lledo P-M, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005;28:248–254. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Luo L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev Biol. 2002;18:601–635. doi: 10.1146/annurev.cellbio.18.031802.150501. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Ma DK, Kim WR, Ming Gl, Song H. Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann N Y Acad Sci. 2009;1170:664–673. doi: 10.1111/j.1749-6632.2009.04373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklis Jeffrey D. Human adult olfactory bulb neurogenesis? Novelty is the best policy. Neuron. 2012;74:595–596. doi: 10.1016/j.neuron.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nacher J, Crespo C, McEwen BS. Doublecortin expression in the adult rat telencephalon. Eur J Neurosci. 2001;14:629–644. doi: 10.1046/j.0953-816x.2001.01683.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, Tsuji S, Duman RS. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J Neurosci. 2002;22:9868–9876. doi: 10.1523/JNEUROSCI.22-22-09868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng) J Pharm Pharmacol. 2006;58:1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- Nissant A, Bardy C, Katagiri H, Murray K, Lledo PM. Adult neurogenesis promotes synaptic plasticity in the olfactory bulb. Nat Neurosci. 2009;12:728–730. doi: 10.1038/nn.2298. [DOI] [PubMed] [Google Scholar]
- O’Connell C, Gallagher HC, O’Malley A, Bourke M, Regan CM. CREB phosphorylation coincides with transient synapse formation in the rat hippocampal dentate gyrus following avoidance learning. Neural Plast. 2000;7:279–289. doi: 10.1155/NP.2000.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A, Li M, Gao JY, Xue ZQ, Li Z, Yuan XY, Luo DW, Luo XG, Yan XX. Experimental epidural hematoma causes cerebral infarction and activates neocortical glial and neuronal genesis in adult guinea pigs. J Neurosci Res. 2013;91:249–261. doi: 10.1002/jnr.23148. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. London: Academic Press; 2005. [Google Scholar]
- Ponti G, Obernier K, Guinto C, Jose L, Bonfanti L, Alvarez-Buylla A. Cell cycle and lineage progression of neural progenitors in the ventricular-subventricular zones of adult mice. Proc Natl Acad Sci U S A. 2013;110:E1045–1054. doi: 10.1073/pnas.1219563110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- Rajan K, Thangaleela S, Balasundaram C. Spatial learning associated with stimulus response in goldfish Carassius auratus: relationship to activation of CREB signalling. Fish Physiol Biochem. 2015;41:685–694. doi: 10.1007/s10695-015-0038-9. [DOI] [PubMed] [Google Scholar]
- Saaltink D-J, Håvik B, Verissimo CS, Lucassen PJ, Vreugdenhil E. Doublecortin and doublecortin-like are expressed in overlapping and non-overlapping neuronal cell population: implications for neurogenesis. J Comp Neurol. 2012;520:2805–2823. doi: 10.1002/cne.23144. [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Nochlin D, Born DE. Neuronal nuclear antigen (NeuN): a marker of neuronal maturation in the early human fetal nervous system1. Brain Dev. 1998;20:88–94. doi: 10.1016/s0387-7604(97)00111-3. [DOI] [PubMed] [Google Scholar]
- Si YC, Zhang JP, Xie CE, Zhang LJ, Jiang XN. Effects of Panax notoginseng saponins on proliferation and differentiation of rat hippocampal neural stem cells. Am J Chin Med. 2011;39:999–1013. doi: 10.1142/S0192415X11009366. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Nagata E, Ito D, Suzuki S, Dembo T, Kosakai A, Fukuuchi Y. Persistent CREB phosphorylation with protection of hippocampal CA1 pyramidal neurons following temporary occlusion of the middle cerebral artery in the rat. Exp Neurol. 2000;161:462–471. doi: 10.1006/exnr.1999.7313. [DOI] [PubMed] [Google Scholar]
- Tonchev AB, Yamashima T, Zhao L, Okano H. Differential proliferative response in the postischemic hippocampus, temporal cortex, and olfactory bulb of young adult macaque monkeys. Glia. 2003;42:209–224. doi: 10.1002/glia.10209. [DOI] [PubMed] [Google Scholar]
- Walton M, Sirimanne E, Williams C, Gluckman P, Dragunow M. The role of the cyclic AMP-responsive element binding protein (CREB) in hypoxic-ischemic brain damage and repair. Brain Res Mol Brain Res. 1996;43:21–29. doi: 10.1016/s0169-328x(96)00144-1. [DOI] [PubMed] [Google Scholar]
- Winner B, Cooper-Kuhn CM, Aigner R, Winkler J, Kuhn HG. Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur J Neurosci. 2002;16:1681–1689. doi: 10.1046/j.1460-9568.2002.02238.x. [DOI] [PubMed] [Google Scholar]
- Xiong K, Luo DW, Patrylo PR, Luo XG, Struble RG, Clough RW, Yan XX. Doublecortin-expressing cells are present in layer II across the adult guinea pig cerebral cortex: partial colocalization with mature interneuron markers. Exp Neurol. 2008;211:271–282. doi: 10.1016/j.expneurol.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng JJ, He XH, Yan XX, Luo XG, Pan AH. Unilateral olfactory functional deprivation model in the left peripheral nostrils by electric cautery injury: olfactory bulb neurogenesis and transformation. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:231–238. [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Cai Y, Chu Y, Chen EY, Feng JC, Luo XG, Xiong K, Struble RG, Clough RW, Patrylo PR, Kordower JH, Yan XX. Doublecortin-expressing cells persist in the associative cerebral cortex and amygdala in aged nonhuman primates. Front Neuroanat. 2009;3:17. doi: 10.3389/neuro.05.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


